Abstract
Objective:
To summarize available data on the effectiveness and safety of single-agent misoprostol for medical abortion in the first trimester.
Data Sources:
We searched Medline, CABI, Cochrane, EMBASE, LILACS, and the Web of Science, and ClinicalTrials.gov for English language studies that evaluated misoprostol alone for abortion of viable pregnancy in the first trimester.
Methods of Study Selection:
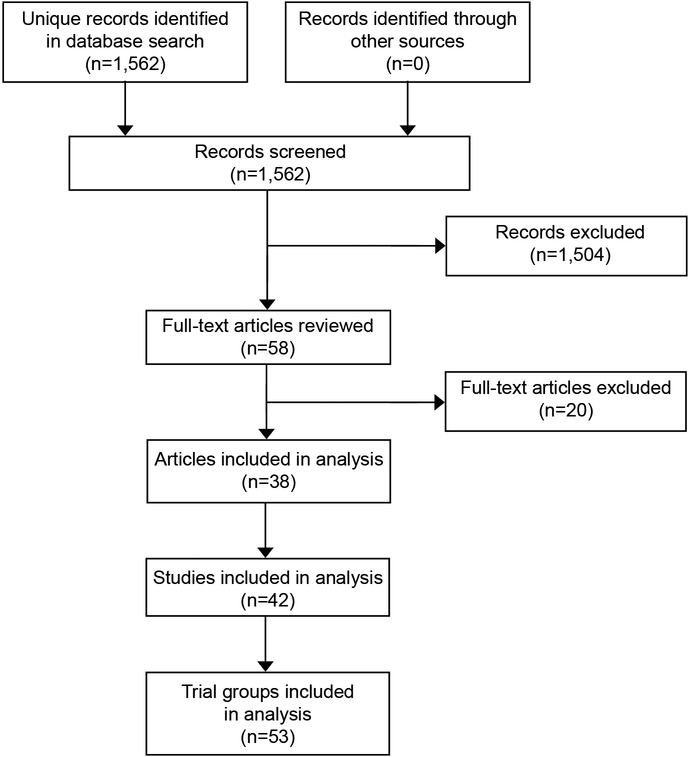
Our search yielded 1562 citations, of which 38 included data from 53 trial groups that met our inclusion and exclusion criteria.
Tabulation, Integration, and Results:
We abstracted data about each trial group, including study characteristics, treatment regimen, clinical protocol, number of women treated and followed, and numbers with outcomes of interest. We used meta-analytic methods and logistic regression to examine factors associated with surgical intervention after treatment. Among all 12,829 evaluable women, 2536 (meta-analytic estimate 22.0%, 95% CI 18.8%, 25.5%) had surgical uterine evacuation. Multiple factors were significantly associated with this proportion, including misoprostol amount per dose and route of administration, loss to follow-up rate, publication date, geographic region, number of misoprostol doses, duration of dosing, and time between dosing and evaluation. Of 6359 evaluable women, 384 (meta-analytic estimate 6.8%, 95% CI 5.3%, 8.5%) had ongoing pregnancy. At most 26 of 12,184 evaluable women (meta-analytic estimate 0.7%, 95% CI 0.4%, 1.0%)were transfused or hospitalized for abortion-related reasons. In trials that provided satisfaction data, most of women were satisfied or very satisfied with the treatment (meta-analytic estimate 78%, 95% CI 71%, 85%).
Conclusions:
Misoprostol alone is effective and safe and is a reasonable option for women seeking abortion in the first trimester. Research is indicated to further refine the regimen and to establish efficacy in the late first trimester.
Systematic Review Registration:
PROSPERO, CRD42018083589.
PRECIS
Treatment regimens that contain only misoprostol can be effective and safe for first-trimester medical abortion.
INTRODUCTION
For early medical abortion, the primary regimens recommended by current clinical guidelines include two drugs: mifepristone and misoprostol. Because mifepristone potentiates the abortifacient action of misoprostol, the combination is highly effective, resulting in complete abortion in more than 95% of women through 63 days of gestation1,2 and 93% between 64 and 70 days.2,3 However, mifepristone is costly and is unavailable in many settings. In the United States, although the drug is approved for marketing, the Food and Drug Administration has imposed restrictions on its distribution that substantially limit both patients’ and providers’ access to it.4 For women who cannot obtain mifepristone, use of misoprostol alone, which is inexpensive and is widely used for various obstetric and gastrointestinal indications, can serve as an important alternative option. A systematic review published in 2007 found that the efficacy of misoprostol single-agent regimens at gestational ages ≤63 days ranged from 84% to 96%,5 but since then, additional studies have been published. We performed this systematic review to summarize available data on the effectiveness and safety of medical abortion with misoprostol alone in the first trimester of pregnancy. The primary outcome of our analysis was surgical evacuation of the uterus to complete the abortion; secondary outcomes were viable ongoing pregnancy after taking the prescribed misoprostol regimen, transfusions and hospitalizations.
SOURCES
We registered our systematic review protocol on PROSPERO (CRD42018083589) before beginning data collection and followed MOOSE guidelines6 in reporting the results. With the assistance of a librarian, we searched six databases (Medline, CABI, Cochrane, EMBASE, LILACS, and the Web of Science,) on November 17, 2017, and ClinicalTrials.gov on June 20 2018, for English language studies that evaluated misoprostol alone for medical abortion of a known or presumed viable pregnancy in the first trimester. We did not exclude studies based on study design, date, or any other criteria. Our search strategy for Medline is indicated in Box 1.
Box 1. Search strategy for Medline.
(Early trimester OR less than ten weeks OR early pregnancy OR first trimester OR less than thirteen weeks) AND (Medical abortion OR medical termination OR oral medication abortion OR oral medication termination OR non-surgical abortion OR non-surgical termination OR elective abortion OR termination of pregnancy OR induced abortion OR induced termination of pregnancy) AND (misoprostol OR cytotec) AND (single agent OR single-agent OR alone OR only).
Search strategies for other databases were substantively similar. In addition, we reviewed the reference lists of relevant articles, and we contacted experts in the field for information about any published or unpublished trials not discovered in our search.
STUDY SELECTION
Two authors (MH and EGR) separately reviewed the title, abstract, and full text if necessary of each paper identified by the search to select all language reports of studies that included women with viable pregnancies who were treated in the first trimester (91 days or less) with misoprostol alone to cause abortion. The same two authors then reviewed each selected study together and systematically abstracted relevant data about these women into a custom database. We excluded women who received abortifacient drugs other than misoprostol, women who were treated in the second trimester, women who had missed abortions or non-viable pregnancies before treatment, and women who did not take any misoprostol after study enrollment. Some studies evaluated more than one misoprostol regimen; in our abstraction process, we recorded data about women who received each regimen in each study as a separate trial group.
The primary data abstracted included the number of women treated with the misoprostol regimen in each group, details of the misoprostol regimen (specifically, the number of misoprostol doses provided, the amount of misoprostol in each dose and route of administration, and the intervals between doses), abortion outcomes (specifically, whether surgical evacuation of the uterus was performed and whether the patient had a viable ongoing pregnancy at the time of surgery), the numbers of reported hospitalizations and transfusions, and information about patient satisfaction. We also recorded data about specified factors that we postulated could cause heterogeneity or bias in assessment of efficacy and safety, including information about the trial design, conduct, and publication, the maximum gestational age and other inclusion criteria, location of misoprostol administration (facility or home), follow-up rates, and timing and method of outcome assessment. We contacted some authors to obtain additional data or to clarify details about the studies. We used our judgment to interpret certain details in some reports and to correct apparent errors and inconsistencies.
We combined data across groups to estimate the proportion of patients who had surgical evacuation of the uterus to complete the abortion and viable ongoing pregnancy using meta-analytic methods, conducted in R, version 3.5.0, with the “metafor” package, version 2.0.7,8 We applied the Freeman-Tukey double arcsine transformation, and we report p-values from the chi-square test of heterogeneity and associated I2 statistic. We calculated estimates and 95% confidence intervals using the DerSimonian-Laird random-effects model.
To explore possible explanations for heterogeneity among trial groups in the proportions who had surgery, we examined associations between this outcome and selected characteristics of the trial groups. Many of these characteristics were highly correlated across trial groups, with numerous zero cells in cross-classifications. Therefore, we opted to present only unadjusted results. We used generalized estimating equations with a logistic link and an independence working correlation matrix to control for nesting of trial groups within paper. The response for the models was the ratio of the number of surgeries to the number of evaluable patients for each trial group (conducted in SAS, version 9.4, SAS Institute, Cary, NC, USA); thus model results were weighted by trial group sample size. We included all trial groups in our examination of misoprostol amount per dose and route of administration. We restricted our examination of other factors to groups treated with an initial dose of 800 mcg misoprostol administered vaginally, the most common combination, because most characteristics did not vary across groups treated with other dose-route combinations. We categorized each characteristic considering both clinical interest and the distribution of the data, and we estimated odds ratios and 95% confidence intervals. We tested for linear trends using linear contrasts of model parameters. We made no adjustment for multiple comparisons. In reviewing the results, we focused on associations that were both substantial (odds ratio>1.5 or <0.67) and significant (p<0.05).
To further evaluate the association between number of misoprostol doses and efficacy, we estimated the proportion of women reported to have had complete abortion after taking only the doses required for all women in that trial group before any doses that were contingent on abortion status. This analysis included only those trial groups that reported these data. We estimated unadjusted odds ratios with confidence intervals and tested for linear trend as described above for amount of misoprostol in the initial dose.
We assessed safety by computing the proportion of women across all trial groups who were reported to have been hospitalized or receive transfusions after treatment. We did not abstract data on non-serious side effects because ascertainment and reporting was not standardized across studies.
RESULTS
Our search yielded 1562 unique citations, of which 37 reported at least one group of women who were treated with misoprostol alone for abortion of viable pregnancy at ≤91 days of gestation9–45 and one additional group in which the maximum gestational age was 98 days46 (Figure 1). We included the last of these because most of the women in the study were ≤91 days; the mean gestational age was 64 days. We also identified one additional study (ClinicalTrials.gov Identifier: NCT02299401) that has to date been published only as an abstract, but the authors declined to provide final data for this review because of concern about jeopardizing the planned primary publication.
Figure 1.
Study selection flow diagram.
The 38 papers included 42 studies conducted in at least 16 countries over at least the past 24 years (Appendix 1, available online at http://links.lww.com/xxx). Of these studies, 21 were non-comparative case series studies, 7 were randomized trials or cohort studies comparing different misoprostol-only regimens, and 14 were randomized trials or cohort studies comparing misoprostol-only regimens to other abortion treatments (aspiration, methotrexate, or misoprostol combined with mifepristone, methotrexate, tamoxifen, letrozole, or laminaria).
The 42 studies included 53 trial groups of women treated with misoprostol alone (Table 1). The total number of treated subjects in all groups combined was 13,573. The two largest groups, both retrospective case series in anonymous Latin American countries where abortion was legally restricted, constituted 44% of this total.9,10 Over all groups, 744 women (5%) were lost before abortion outcome was ascertained. The proportion lost was 0–7% in 51 groups; the proportions in the other two groups, which were the two largest, were 10% and 13%. Our analysis included 12,829 evaluable women across all trial groups.
Table 1.
Characteristics of studies
| Trial groups N=53 |
Evaluable women N=12829 |
|||
|---|---|---|---|---|
| n | % | n | % | |
| 3–100 | 27 | 51% | 1247 | 10% |
| 101–500 | 19 | 36% | 3472 | 27% |
| 501–720 | 5 | 9% | 2766 | 22% |
| 2805–3225 | 2 | 4% | 5344 | 42% |
| Lost to follow-up | ||||
| None | 39 | 74% | 4484 | 35% |
| ≤10% | 12 | 23% | 3001 | 23% |
| >10% | 2 | 4% | 5344 | 42% |
| Publication date | ||||
| 1994–1999 | 14 | 26% | 1609 | 13% |
| 2000–2004 | 14 | 26% | 5093 | 40% |
| 2005–2009 | 18 | 34% | 5237 | 41% |
| 2010-present | 7 | 13% | 890 | 7% |
| Study design | ||||
| randomized trial | 23 | 43% | 3315 | 26% |
| non-randomized prospective | 24 | 45% | 3527 | 27% |
| non-randomized retrospective | 6 | 11% | 5987 | 47% |
| Region | ||||
| Latin or South America | 11 | 21% | 7664 | 60% |
| North America | 11 | 21% | 1023 | 8% |
| Asia | 17 | 32% | 980 | 8% |
| other or multiple | 14 | 26% | 3162 | 25% |
| Planned maximum gestational age | ||||
| 42–56 days | 19 | 36% | 4173 | 33% |
| 57–63 days | 17 | 32% | 4563 | 36% |
| 64–70 days | 7 | 13% | 3247 | 25% |
| ≥71 days | 10 | 19% | 846 | 7% |
| First misoprostol dose and route | ||||
| 200 mcg vaginal | 2 | 4% | 111 | 1% |
| 400 mcg vaginal | 4 | 8% | 160 | 1% |
| 600 mcg vaginal | 1 | 2% | 89 | 1% |
| 800 mcg vaginal | 31 | 58% | 10010 | 78% |
| 800 mcg buccal | 3 | 6% | 584 | 5% |
| 800 mcg sublingual | 2 | 4% | 1021 | 8% |
| 800 mcg oral | 4 | 8% | 119 | 1% |
| 1000 mcg vaginal | 1 | 2% | 300 | 2% |
| 400 mcg vaginal + 400 mcg sublingual | 1 | 2% | 149 | 1% |
| 400 mcg vaginal + 400 mcg oral | 1 | 2% | 5 | 0% |
| 800 mcg vaginal + 400 mcg buccal | 1 | 2% | 98 | 1% |
| 800 mcg vaginal + 400 mcg sublingual | 1 | 2% | 76 | 1% |
| 800 mcg oral + 400 mcg sublingual | 1 | 2% | 107 | 1% |
| Misoprostol moistened before vaginal administration* | ||||
| no or not stated | 20 | 47% | 6543 | 59% |
| Yes | 23 | 53% | 4455 | 41% |
| Number of required doses | ||||
| 1 | 34 | 64% | 3598 | 28% |
| 2 | 7 | 13% | 3374 | 26% |
| 3 | 9 | 17% | 5651 | 44% |
| 4 | 2 | 4% | 186 | 1% |
| 5 | 1 | 2% | 20 | 0% |
| Duration of required dosing | ||||
| 0 (only 1 required dose) | 34 | 64% | 3598 | 28% |
| 1–24h | 16 | 30% | 6251 | 49% |
| 25–48h | 1 | 2% | 2900 | 23% |
| 73h-7d | 2 | 4% | 80 | 1% |
| Total number of allowed doses | ||||
| 1 | 5 | 9% | 330 | 3% |
| 2 | 11 | 21% | 3069 | 24% |
| 3 | 25 | 47% | 8059 | 63% |
| 4 | 5 | 9% | 458 | 4% |
| 5 | 1 | 2% | 20 | 0% |
| 6 | 6 | 11% | 893 | 7% |
| Maximum duration of dosing if all allowed contingent doses were taken | ||||
| 0 (only 1 dose allowed) | 5 | 9% | 330 | 3% |
| 1–24h | 19 | 36% | 4995 | 39% |
| 25–48h | 13 | 25% | 5682 | 44% |
| 49–72h | 4 | 8% | 269 | 2% |
| 73h-7d | 9 | 17% | 1155 | 9% |
| >7d | 3 | 6% | 398 | 3% |
| Protocol permitted patient to take misoprostol at home | ||||
| all | 9 | 17% | 5188 | 40% |
| some | 15 | 28% | 5483 | 43% |
| none | 29 | 55% | 2158 | 17% |
| Evaluated by ultrasound prior to decision to perform surgery | ||||
| all patients | 46 | 87% | 10199 | 79% |
| some or no patients | 7 | 13% | 2630 | 21% |
| Earliest timing of decision re. surgery | ||||
| ≤24h | 2 | 4% | 128 | 1% |
| 25–48h | 7 | 13% | 438 | 3% |
| 49–72h | 12 | 23% | 4685 | 37% |
| 73h-7d | 16 | 30% | 1864 | 15% |
| >7d | 16 | 30% | 5714 | 45% |
Denominator for percents include only trial groups and evaluable women who took the first dose by the vaginal route.
The admission criteria for all studies were broad: in general, any woman requesting medical abortion who had no medical contraindication to the abortifacient drug treatment and whose gestational age was less than a specified maximum (42–98 days) was eligible. In 48 groups, which included 95% of evaluable women, gestational age was routinely determined by ultrasound. One study included only women aged ≤17 years,43 one included only women with ≥2 prior cesarean deliveries,26 and one included only women with gestational ages of 64–84 days.18
The 53 groups used a multitude of misoprostol regimens. In all groups, women were required to take a specified minimum number (1 to 5) of doses of misoprostol vaginally, buccally, orally, sublingually, or by a combination of routes (Table 1). In all but 4 groups,13,14,21 the initial dose was 800 mcg or was administered vaginally; the combination 800 mcg vaginally was used in 31 groups that collectively included 10,010 (78%) of the evaluable women. Misoprostol was moistened before vaginal insertion in 41% of evaluable women who took the drug by that route. In 39 of the 48 groups in which more than one dose was allowed, subsequent doses used the same amount per dose and route of misoprostol as the first dose. Multiple doses were administered 3–48 hours apart, such that the longest duration of the required treatment was 96 hours. In 35 groups (38% of evaluable women), if complete abortion had not occurred after the required doses, women were instructed to take additional contingent doses up to a specified maximum, after which a decision regarding surgical intervention was made. The maximum total number of allowed doses (required + contingent) in any group was 6, and the maximum duration of dosing if all allowed doses were taken was 14 days. Across all groups, most women were instructed to take no more than 3 doses within a maximum of 48 hours. Nine studies,18–24,43,45 7 of which were conducted by the same group of investigators, provided extra misoprostol to some or all women who were determined not to need surgery in order to evacuate “remains” from the uterus or for an unspecified reason, and one study provided a dose of misoprostol to all women who were scheduled for surgery.30 We did not count those extra doses in this analysis because they were given after the outcome had been determined and thus did not contribute to the outcome. In at least 24 groups (83% of evaluable women), women were allowed to take some or all of the misoprostol doses at home.
In 46 groups (79% of evaluable women), all women were assessed with ultrasound before the decision of whether to perform surgery, whereas in 6 groups, ultrasound was used only if clinically indicated, and in one group, abortions were provided by community health workers who apparently rarely used ultrasound.42 The earliest point at which surgical intervention was considered varied from 24 hours to 14 days after the first misoprostol dose. No paper provided explicit criteria for the decision to resort to surgical uterine evacuation, hospitalization or transfusion.
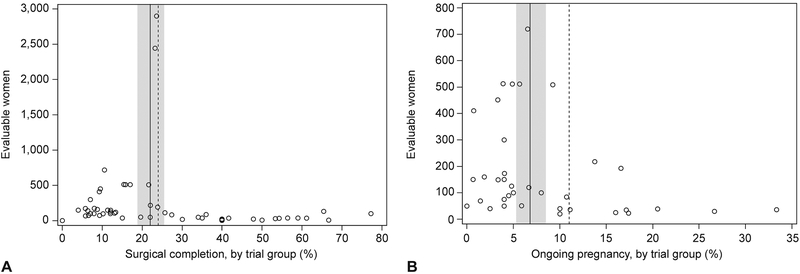
Among all 12,829 evaluable subjects (Table 1), 2,536 (20%) underwent surgical uterine evacuation (meta-analytic estimate 22.0%, 95% CI 18.8%, 25.5%). Across trial groups, the proportion with this outcome ranged from 0 to 77% (Figure 2, Panel A). More than 90% of the evaluable subjects were in trial groups in which the failure proportion was 24% or less.
Figure 2.
Group size by percent of women with surgical uterine evacuation (A) and ongoing pregnancy (B). Meta-analytic estimate of population proportion indicated by the solid line with 95% CI indicated by band. The trial groups to the left of the dashed lines contained 90% of patients.
We found strong evidence of heterogeneity across trial groups in the proportion of subjects who ultimately had surgery (p < 0.001, I2 = 94.2%). In unadjusted analyses, this proportion was significantly associated with characteristics of the initial misoprostol dose (Table 2). Among groups treated vaginally, the odds of surgery decreased with the amount of misoprostol in the initial dose (linear trend p<0.001); groups treated with ≥800 mcg had about one quarter the risk of surgery as groups treated with 200 mcg. Among groups treated with 800 mcg in the initial dose, oral administration was associated with nearly a 3-fold higher risk of surgery than vaginal administration, whereas risks were similar in groups who took the drug sublingually, buccally, and vaginally.
Table 2.
Surgical uterine evacuation by misoprostol dose and routea
| Evaluable women | Women who had surgical uterine evacuation | Adjusted ORb | |||
|---|---|---|---|---|---|
| N | % | OR | 95% CI | ||
| 200 mcg | 111 | 83 | 75% | 1 | |
| 400 mcg | 160 | 99 | 62% | 0.55 | (0.52, 0.57) |
| 600 mcg | 89 | 32 | 36% | 0.19 | (0.14, 0.26) |
| 800 mcg | 10010 | 1895 | 19% | 0.08 | (0.05, 0.12) |
| 1000 mcg | 300 | 21 | 7% | 0.03 | (0.02, 0.04) |
| First dose 800 mcg | |||||
| vaginal route | 10010 | 1895 | 19% | 1 | |
| buccal route | 584 | 104 | 18% | 0.93 | (0.49, 1.76) |
| sublingual route | 1021 | 191 | 19% | 0.99 | (0.76, 1.27) |
| oral route | 119 | 62 | 52% | 4.66 | (3.61, 6.01) |
| combinations of routes | 154 | 20 | 13% | 0.64 | (0.46, 0.89) |
Table includes 53 trial groups.
Confidence intervals are adjusted for clustering by paper.
In the 31 groups treated initially with 800 mcg vaginally, the proportion of women who had surgery was significantly associated with numerous group characteristics (Table 3). Some of these were details of the clinical protocol: the risk of surgery declined with increases in both the allowed number of misoprostol doses and the duration of dosing (linear trend p≤0.01 for both associations). Surgery was less common in groups in which the misoprostol was moistened before vaginal insertion and in groups in which the decision to perform surgery was delayed until 4–7 days after treatment. Surgery was also associated with other characteristics of the studies; for example, the two groups with loss rates >10% had higher surgery rates than groups with no loss, later studies had higher rates than earlier ones, and studies conducted in Asia and Latin America had higher rates than those conducted in North America. Many of the group characteristics were correlated with each other: for example, groups that were given more doses also had longer duration of dosing and were more likely to use moistened tablets if the route of administration was vaginal, and both of the two studies with >10% loss took place in Latin America and did not report using moistened tablets.
Table 3.
Surgical uterine evacuation by trial characteristics in groups treated with 800 mcg misoprostol vaginally
| Trial groups N=31 |
Evaluable women N=10010 | Women who had surgical uterine evacuation N=1895 |
ORa | ||||
|---|---|---|---|---|---|---|---|
| N | % | OR | 95% CI | ||||
| 3–100 | 15 | 823 | 193 | 23% | 1 | ||
| 101–500 | 11 | 2098 | 209 | 10% | 0.36 | 0.21 | 0.61 |
| 501–720 | 3 | 1745 | 242 | 14% | 0.53 | 0.30 | 0.93 |
| 2805, 3225 | 2 | 5344 | 1251 | 23% | 1.00 | 0.62 | 1.60 |
| Lost to follow-up in group | |||||||
| 0 | 22 | 2991 | 390 | 13% | 1 | ||
| >0 to 10% | 7 | 1675 | 254 | 15% | 1.19 | 0.81 | 1.75 |
| >10% | 2 | 5344 | 1251 | 23% | 2.04 | 1.56 | 2.66 |
| Publication date | |||||||
| 1994–1999 | 8 | 1342 | 164 | 12% | 1 | ||
| 2000–2004 | 12 | 4704 | 859 | 18% | 1.60 | 0.91 | 2.82 |
| 2005-present | 11 | 3964 | 872 | 22% | 2.03 | 1.39 | 2.96 |
| Study design | |||||||
| randomized | 12 | 1602 | 311 | 19% | 1 | ||
| prospective cohort or case series | 15 | 2603 | 284 | 11% | 0.51 | 0.35 | 0.74 |
| retrospective cohort or case series | 4 | 5805 | 1300 | 22% | 1.20 | 0.82 | 1.74 |
| Region | |||||||
| North America | 6 | 841 | 95 | 11% | 1 | ||
| Asia | 12 | 668 | 157 | 24% | 1.97 | 1.25 | 3.11 |
| Latin or South America | 8 | 7102 | 1426 | 20% | 2.41 | 1.16 | 5.03 |
| other or multiple | 5 | 1399 | 217 | 16% | 1.44 | 0.95 | 2.20 |
| Planned maximum gestational age | |||||||
| 42–56 days | 14 | 3905 | 743 | 19% | 1 | ||
| 57–63 days | 11 | 2742 | 380 | 14% | 0.68 | 0.43 | 1.09 |
| ≥63 days | 6 | 3363 | 772 | 23% | 1.27 | 0.85 | 1.89 |
| Misoprostol moistened before vaginal administration | |||||||
| no or not stated | 11 | 6093 | 1419 | 23% | 1 | ||
| yes | 20 | 3917 | 476 | 12% | 0.46 | 0.35 | 0.60 |
| Total number of allowed doses | |||||||
| 1 | 4 | 305 | 78 | 26% | 1 | ||
| 2 | 8 | 2862 | 656 | 23% | 0.87 | 0.47 | 1.60 |
| 3 | 16 | 6221 | 1106 | 18% | 0.63 | 0.30 | 1.31 |
| 4+ | 3 | 622 | 55 | 9% | 0.28 | 0.14 | 0.57 |
| Maximum duration of dosing if all allowed contingent doses were taken | |||||||
| 0 (only 1 dose allowed) | 4 | 305 | 78 | 26% | 1 | ||
| 1–24h | 9 | 3759 | 817 | 22% | 0.81 | 0.42 | 1.54 |
| 25–48h | 9 | 5138 | 910 | 18% | 0.63 | 0.29 | 1.37 |
| >48 h | 9 | 808 | 90 | 11% | 0.36 | 0.16 | 0.82 |
| Protocol permitted patient to take misoprostol at home | |||||||
| all or some | 13 | 8558 | 1663 | 19% | 1 | ||
| none | 18 | 1452 | 232 | 16% | 0.79 | 0.49 | 1.26 |
| Evaluated by ultrasound prior to decision to perform surgery | |||||||
| all patients | 29 | 8985 | 1729 | 19% | 1 | ||
| some or no patients | 2 | 1025 | 166 | 16% | 0.81 | 0.62 | 1.06 |
| Earliest timing of decision re. surgery | |||||||
| ≤72h | 10 | 4403 | 893 | 20% | 1 | ||
| 73h-7d | 8 | 1087 | 133 | 12% | 0.55 | 0.31 | 0.96 |
| >7d | 13 | 4520 | 869 | 19% | 0.94 | 0.59 | 1.49 |
Confidence intervals are adjusted for clustering by paper.
Data on abortion status after only the required misoprostol doses was available from 42 trial groups (Table 4). In these groups, each woman was evaluated to determine abortion completeness after she took all of the required doses but before any additional doses. Complete abortion was significantly more common after 3 doses than after only one, but no significant linear trend was apparent by number of doses from 1 to 5 (p=0.73).
Table 4.
Complete abortion after required misoprostol doses, by number of doses required
| Groups N=42 |
Evaluable women N=12072 |
Complete abortion without surgery N=9074 |
ORa | ||||
|---|---|---|---|---|---|---|---|
| Number of required misoprostol doses | n | n | n | % | OR | 95% CI | |
| 25 | 3232 | 2221 | 69% | ||||
| 2 | 6 | 3156 | 2343 | 74% | 1.31 | 0.98 | 1.75 |
| 3 | 8 | 5478 | 4355 | 79% | 1.77 | 1.30 | 2.40 |
| 4 | 2 | 186 | 141 | 76% | 1.43 | 0.49 | 4.16 |
| 5 | 1 | 20 | 14 | 70% | 1.06 | 0.89 | 1.27 |
Confidence intervals are adjusted for clustering by paper
In 36 groups, researchers noted the number of surgical interventions performed for ongoing pregnancy. Of the 6359 evaluable women in these groups (50% of the total), 384 (6%) had ongoing pregnancies (meta-analytic estimate 6.8%, 95% CI 5.3%, 8.5%,heterogeneity: p < 0.001, I2 = 81.7%). The proportion across groups ranged from 0 to 33%; more than 90% of the women were in groups in which no more than 11% of subjects had ongoing pregnancy (Figure 2, Panel B). The 384 ongoing pregnancies constituted 39% of the 989 medical abortion failures in these groups.
Across the 38 papers, 14 women were hospitalized for abortion-related reasons and 12 received transfusions. Excluding the studies in which women were or may have been hospitalized routinely throughout the abortion process,13,14,18,26,27,33,46 the sum of these numbers (26) constitutes at most 0.2% of the total 12,184 evaluable women (meta-analytic estimate 0.7%, 95% CI 0.4%, 1.0%). No deaths or ectopic pregnancies were reported.
Women in 20 groups provided information about satisfaction about the treatment regimen (Appendix 1, http://links.lww.com/xxx). In these groups, 2549 of 2961 women (86%; meta-analytic estimate 78%, 95% CI 71%, 85%) said that they were satisfied or very satisfied, and 2396 of 2832 (85%; meta-analytic estimate 76%, 95% CI 76%, 82%) said that they would use the method if needed in the future.
DISCUSSION
Data from 42 studies that included nearly 13,000 evaluable women indicate that misoprostol used alone can be effective and safe for inducing abortion in the first trimester. Across all studies, about 78% of women had complete abortions without recourse to surgery, and viable pregnancy was terminated in more than 93%. The reported incidence of serious complications requiring hospitalization or transfusion was at most 0.2%. Most women were satisfied with the treatment.
Our analysis identified some treatment characteristics that were associated with higher effectiveness. The chance of surgical uterine evacuation decreased significantly as the amount of misoprostol in the initial dose increased and was lower in trial groups that administered this dose vaginally, sublingually, or buccally rather than orally. Among groups in which the dose was 800 mcg vaginally, surgical intervention was substantially less common if women were permitted to take at least four doses, if these doses were taken over an interval of more than 48 hours, and if the tablets were moistened before insertion. Among all 53 groups, 20 were treated with at least three doses, the first of which consisted of at least 800 mcg misoprostol administered vaginally (moistened), sublingually, or buccally; of the 5338 evaluable women in these groups, 87% aborted without surgery.
The data reviewed here have many strengths: the studies were conducted in numerous diverse settings, the study populations were typical abortion clients unselected except with respect to gestational age, and follow-up rates were high. The variety of regimens and clinical protocols used in the 42 included studies enabled us to examine multiple factors that may contribute to the likelihood of surgical intervention following treatment with misoprostol alone. Our analysis of all of these studies provides insights not available from only the 7 individual studies published to date that directly compared different misoprostol-only regimens or protocols.
However, our analysis also had significant limitations. Two studies contributed 44% of the patients, and thus these studies dominated the analysis. We evaluated trial group characteristics, not data from individual women. Furthermore, we could examine only those characteristics that were reported consistently across studies, and because of the high degree of correlation between these characteristics across trial groups, we restricted our analysis primarily to groups treated with one particular misoprostol amount-route combination. We looked at each characteristic separately rather than simultaneously in an adjusted multivariable analysis, and as a result, the associations that we identified are certainly affected by confounding and should not be interpreted as proof of causality. For example, moistening of vaginally administered tablets was associated with higher numbers of allowed doses, and both were associated with lower surgery rates; our analysis did not establish the extent to which either factor may have been independently responsible for improved regimen effectiveness. Nevertheless, this finding is consistent with other data. In particular, wetting the tablets has been shown to improve vaginal absorption of misoprostol47 and enhance cervical dilation before surgical abortion,48 and two randomized trials have suggested that it decreases risk of surgical intervention in first trimester medical abortion.36,49 Our finding that surgery was less common in trial groups without loss to follow-up may be explained by the fact that indications for surgery (abortion failure, bleeding) prompt patients to seek care. It suggests that the true risk among all treated patients may be lower than our estimate.
Despite these limitations, currently available data suggest that misoprostol as a single agent is a reasonable option for women seeking abortion in the first trimester. This treatment is clearly less effective than standard regimens that also contain mifepristone,1,2 and thus enhanced vigilance should be recommended to detect potential failures. Nevertheless, misoprostol alone may be preferred by some women because it may be easier to obtain, less costly, or have other advantages. Further research is indicated to refine the regimen, addressing issues such as the optimal misoprostol amount per dose and dosing intervals if the drug is administered sublingually or buccally, which may be more convenient for women than vaginal insertion, and the efficacy of these regimens in the late first trimester.
Supplementary Material
Acknowledgments
Supported was supported by the National Institutes of Health through grant (WRHR K12; 5K12HD001271–18), by the Doris Duke Charitable Foundation, and by Gynuity Health Projects.
The authors thank Kristen Desanto for her help with the comprehensive literature review that was required for this publication.
Footnotes
Financial Disclosure
Mark A. Weaver has a consulting agreement with Gynuity Health Projects. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES
- 1.Raymond EG, Shannon C, Weaver MA, Winikoff B. First-trimester medical abortion with mifepristone 200 mg and misoprostol: a systematic review. Contraception 2013;87:26–37. [DOI] [PubMed] [Google Scholar]
- 2.Chen MJ, Creinin MD. Mifepristone With Buccal Misoprostol for Medical Abortion: A Systematic Review. Obstet Gynecol 2015;126:12–21. [DOI] [PubMed] [Google Scholar]
- 3.Abbas D, Chong E, Raymond EG. Outpatient medical abortion is safe and effective through 70 days gestation. Contraception 2015;92:197–9. [DOI] [PubMed] [Google Scholar]
- 4.Raymond EG, Blanchard K, Blumenthal PD, et al. Sixteen Years of Overregulation: Time to Unburden Mifeprex. N Engl J Med 2017;376:790–4. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Ruiz NL, Borgatta L, Yanow S, Kapp N, Wiebe ER, Winikoff B. Alternatives to mifepristone for early medical abortion. Int J Gynaecol Obstet 2007;96:212–8. [DOI] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 7.R Development Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 8.Viechtbauer W Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;36:1–48. [Google Scholar]
- 9.Aldrich T, Winikoff B. Does methotrexate confer a significant advantage over misoprostol alone for early medical abortion? A retrospective analysis of 8678 abortions. BJOG: An International Journal of Obstetrics & Gynaecology 2007;114:555–62. [DOI] [PubMed] [Google Scholar]
- 10.Billings DL. Misoprostol alone for early medical abortion in a Latin American clinic setting. Reproductive Health Matters 2004;12:57–64. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard K, Shochet T, Coyaji K, Nguyen Thi Nhu N, Winikoff B. Misoprostol alone for early abortion: an evaluation of seven potential regimens. Contraception 2005;72:91–7. [DOI] [PubMed] [Google Scholar]
- 12.Blum J, Raghavan S, Dabash R, et al. Comparison of misoprostol-only and combined mifepristone-misoprostol regimens for home-based early medical abortion in Tunisia and Vietnam. International Journal of Gynecology & Obstetrics 2012;118:166–71. [DOI] [PubMed] [Google Scholar]
- 13.Bogaert L, Misra A. Anthropometric characteristics and success rates of oral or vaginal misoprostol for pregnancy termination in the first and second trimesters. In: International journal of gynaecology and obstetrics; 2010:213–5. [DOI] [PubMed] [Google Scholar]
- 14.Bogaert LJv, Sedibe TM. Efficacy of a single misoprostol regimen in the first and second trimester termination of pregnancy. Journal of Obstetrics and Gynaecology 2007;27:510–2. [DOI] [PubMed] [Google Scholar]
- 15.Borgatta L, Mullally B, Vragovic O, Gittinger E, Chen A. Misoprostol as the primary agent for medical abortion in a low-income urban setting. Contraception 2004;70:121–6. [DOI] [PubMed] [Google Scholar]
- 16.Bugalho A, Faúndes A, Jamisse L, Usfá M, Maria E, Bique C. Evaluation of the effectiveness of vaginal misoprostol to induce first trimester abortion. In: Contraception; 1996:244–6. [DOI] [PubMed] [Google Scholar]
- 17.Bugalho A, Mocumbi S, Faundes A, David E. Termination of pregnancies of <6 weeks gestation with a single dose of 800 microg of vaginal misoprostol. Contraception 2000;61:47–50. [DOI] [PubMed] [Google Scholar]
- 18.Carbonell Esteve JL, Varela L, Velazco A, Cabezas E, Tanda R, Sanchez C. Vaginal misoprostol for late first trimester abortion. Contraception 1998;57:329–33. [DOI] [PubMed] [Google Scholar]
- 19.Carbonell Esteve JLL, Varela L, Velazco A, et al. Vaginal misoprostol 600 μg for early abortion. European Journal of Contraception and Reproductive Health Care 2000;5:46–51. [DOI] [PubMed] [Google Scholar]
- 20.Carbonell Esteve JLL, Varela L, Velazco A, et al. Early abortion with 800 μg of misoprostol by the vaginal route. Contraception 1999;59:219–25. [DOI] [PubMed] [Google Scholar]
- 21.Carbonell JL, Rodriguez J, Aragon S, et al. Vaginal misoprostol 1000 microg for early abortion. Contraception 2001;63:131–6. [DOI] [PubMed] [Google Scholar]
- 22.Carbonell JLL, Rodriguez J, Velazco A, et al. Oral and vaginal misoprostol 800 microg every 8 h for early abortion. Contraception 2003;67:457–62. [DOI] [PubMed] [Google Scholar]
- 23.Carbonell JL, Varela L, Velazco A, Fernandez C. The use of misoprostol for termination of early pregnancy. Contraception 1997;55:165–8. [DOI] [PubMed] [Google Scholar]
- 24.Carbonell JL, Varela L, Velazco A, et al. The use of misoprostol for abortion at < or = 9 weeks’ gestation. The European journal of contraception & reproductive health care : the official journal of the European Society of Contraception 1997;2:181–5. [DOI] [PubMed] [Google Scholar]
- 25.Creinin M, Vittinghoff E. Methotrexate and misoprostol vs misoprostol alone for early abortion. A randomized controlled trial. In: Jama; 1994:1190–5. [PubMed] [Google Scholar]
- 26.Daponte A, Nzewenga G, Dimopoulos KD, Guidozzi F. Pregnancy termination using vaginal misoprostol in women with more than one caesarean section. J Obstet Gynaecol 2007;27:597–600. [DOI] [PubMed] [Google Scholar]
- 27.Grapsas X, Liberis V, Vassaras G, Tsikouras P, Vlachos G, Galazios G. Misoprostol and first trimester pregnancy termination. Clin Exp Obstet Gynecol 2008;35:32–4. [PubMed] [Google Scholar]
- 28.Hertzen H, Piaggio G, Huong N, et al. Efficacy of two intervals and two routes of administration of misoprostol for termination of early pregnancy: a randomised controlled equivalence trial. In: Lancet (london, england); 2007:1938–46. [DOI] [PubMed] [Google Scholar]
- 29.Jain J, Dutton C, Harwood B, Meckstroth K, Mishell D. A prospective randomized, double-blinded, placebo-controlled trial comparing mifepristone and vaginal misoprostol to vaginal misoprostol alone for elective termination of early pregnancy. In: Human reproduction (oxford, england); 2002:1477–82. [DOI] [PubMed] [Google Scholar]
- 30.Jain J, Harwood B, Meckstroth K, Mishell D. Early pregnancy termination with vaginal misoprostol combined with loperamide and acetaminophen prophylaxis. In: Contraception; 2001:217–21. [DOI] [PubMed] [Google Scholar]
- 31.Jain J, Meckstroth K, Mishell D. Early pregnancy termination with intravaginally administered sodium chloride solution-moistened misoprostol tablets: historical comparison with mifepristone and oral misoprostol. In: American journal of obstetrics and gynecology; 1999:1386–91. [DOI] [PubMed] [Google Scholar]
- 32.Jain J, Meckstroth K, Park M, Mishell D. A comparison of tamoxifen and misoprostol to misoprostol alone for early pregnancy termination. Contraception 2000;60:353–6. [DOI] [PubMed] [Google Scholar]
- 33.Koopersmith T, Mishell D. The use of misoprostol for termination of early pregnancy. In: Contraception; 1996:238–42. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Antony Z, Kapur A, Togra M. Termination of pregnancy in first trimester - Medical option. In: Armed Forces medical journal, India; 2005:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee V, Ng E, Yeung W, Ho P. Misoprostol with or without letrozole pretreatment for termination of pregnancy: a randomized controlled trial. In: Obstetrics and gynecology; 2011:317–23. [DOI] [PubMed] [Google Scholar]
- 36.Ngai S, Tang O, Chan Y, Ho P. Vaginal misoprostol alone for medical abortion up to 9 weeks of gestation: efficacy and acceptability. In: Human reproduction (oxford, england); 2000:1159–62. [DOI] [PubMed] [Google Scholar]
- 37.Ngoc N, Blum J, Raghavan S, et al. Comparing two early medical abortion regimens: mifepristone+misoprostol vs. misoprostol alone. In: Contraception; 2011:410–7. [DOI] [PubMed] [Google Scholar]
- 38.Ozeren M, Bilekli C, Aydemir V, Bozkaya H. Methotrexate and misoprostol used alone or in combination for early abortion. In: Contraception; 1999:389–94. [DOI] [PubMed] [Google Scholar]
- 39.Prasad S, Kumar A, Divya A. Early termination of pregnancy by single-dose 800 microg misoprostol compared with surgical evacuation. In: Fertility and sterility; 2009:28–31. [DOI] [PubMed] [Google Scholar]
- 40.Singh K, Fong YF, Dong F. A viable alternative to surgical vacuum aspiration: repeated doses of intravaginal misoprostol over 9 hours for medical termination of pregnancies up to eight weeks. Bjog 2003;110:175–80. [PubMed] [Google Scholar]
- 41.Tang OS, Wong KS, Tang LCH, et al. Pilot study on the use of repeated doses of misoprostol in termination of pregnancy at less than 9 weeks of gestation. Advances in Contraception 1999;15:211–6. [DOI] [PubMed] [Google Scholar]
- 42.Tebbets C, Santana D, Ros Silvestre J, Redwine D. Building Bridges: A Case for Community Health Worker Provision of Misoprostol-Only Abortion in the First Trimester. Journal of Women’s Health 2017;17:17. [DOI] [PubMed] [Google Scholar]
- 43.Velazco A, Varela L, Tanda R, et al. Misoprostol for abortion up to 9 weeks’ gestation in adolescents. Eur J Contracept Reprod Health Care 2000;5:227–33. [DOI] [PubMed] [Google Scholar]
- 44.Wiebe ER, Trouton KJ, Lima R, et al. Misoprostol alone vs. methotrexate followed by misoprostol for early abortion. International Journal of Gynecology and Obstetrics 2006;95:286–7. [DOI] [PubMed] [Google Scholar]
- 45.Zikopoulos KA, Papanikolaou EG, Kalantaridou SN, et al. Early pregnancy termination with vaginal misoprostol before and after 42 days gestation. Human Reproduction 2002;17:3079–83. [DOI] [PubMed] [Google Scholar]
- 46.Derakhshan-Aydenloo S, Behroozi-Lak T, Broomand F. Evaluation of effect of letrozole prior to misoprostol in comparison with misoprostol alone in success rate of induced abortion. J Gynecol Obstet Hum Reprod 2017;06:06. [DOI] [PubMed] [Google Scholar]
- 47.Lee VC, Yung SS, Li RH, et al. A randomized comparison of pharmacokinetics of a single vaginal dose of dry misoprostol or misoprostol moistened with normal saline or with acetic acid. Human Reproduction 2011;26:2981–7. [DOI] [PubMed] [Google Scholar]
- 48.Kelekci S, Yilmaz B, Savan K. Misoprostol wetting with acetic acid for pre-abortion cervical priming. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 2004;85:188–9. [DOI] [PubMed] [Google Scholar]
- 49.Wiebe E Misoprostol administration in medical abortion. A comparison of three regimens. In: Journal of reproductive medicine; 2001:125–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.