Abstract
Background:
Compared to traditional risk factors, coronary artery calcium (CAC) scores improve prognostic accuracy for atherosclerotic cardiovascular disease (ASCVD) outcomes. However, the relative impact of statins on ASCVD outcomes stratified by CAC scores is unknown.
Objectives:
To determine if CAC can identify patients most likely to benefit from statin treatment.
Methods:
We identified consecutive subjects without pre-existing ASCVD or malignancy who underwent CAC scoring from 2002 to 2009 at Walter Reed. The primary outcome was first major adverse cardiovascular event (MACE), a composite of acute myocardial infarction, stroke, and cardiovascular death. The effect of statin therapy on outcomes was analyzed stratified by CAC presence and severity, after adjusting for baseline comorbidities with inverse probability of treatment weights based on propensity scores.
Results:
13,644 patients (mean age 50 years; 71% men) were followed for a median of 9.4 years. Comparing patients with and without statin exposure, statin therapy was associated with reduced risk of MACE in patients with CAC (adjusted subhazard ratio [aSHR] 0.76, 95% CI 0.60–0.95, p=0.015) but not in patients without CAC (aSHR 1.00, 95% CI 0.79–1.27, p=0.99). The effect of statin use on MACE was significantly related to the severity of CAC (p <0.0001 for interaction), with the NNT to prevent one initial MACE outcome over 10 years ranging from 100 (CAC 1–100) to 12 (CAC >100).
Conclusions:
In a large-scale cohort without baseline ASCVD, the presence and severity of CAC identified patients most likely to benefit from statins for the primary prevention of cardiovascular diseases.
Condensed Abstract:
Prior studies have shown that coronary artery calcium (CAC) screening improves risk prediction of atherosclerotic cardiovascular disease (ASCVD), but the true impact of statins on ASCVD outcomes stratified by CAC scores is unknown. In this retrospective cohort of 13,644 patients without pre-existing atherosclerotic cardiovascular disease or malignancy who underwent CAC scoring at Walter Reed Army Medical Center, increasing severity of CAC was associated with increased benefit from statin treatment for the prevention of cardiovascular morbidity and mortality. CAC presence and severity may help stratify patients most likely to benefit from statins.
Keywords: calcium score, cardiovascular risk, screening, primary prevention, atherosclerotic cardiovascular disease
Introduction
Current guidelines rely on age and traditional cardiovascular risk factors to estimate an individual’s risk for incident atherosclerotic cardiovascular disease (ASCVD) events to guide the use of statin therapy for primary prevention (1,2). Coronary artery calcium (CAC) scoring, a non-invasive measure of coronary artery atherosclerotic plaque burden, improves the accuracy of contemporary risk scores for predicting ASCVD outcomes (3,4), and has been suggested as a means to optimize patient selection for statin therapy (5,6). Patients with no detectable CAC are at very low risk for ASCVD outcomes, suggesting that the use of statins may not be warranted in these individuals (7). However, the relative impact of statin treatment stratified by CAC results is unknown, and current guidelines do not recommend widespread CAC testing, citing a lack of evidence regarding the relationship of CAC results on changes in preventive treatments and subsequent long-term ASCVD outcomes (8).
The sole study to date assessing the effect of statin therapy following CAC scoring, the St. Francis Heart Study, investigated the addition of atorvastatin 20 mg daily to aspirin in a randomized, placebo-controlled study of 1,005 asymptomatic subjects with severely elevated CAC relative to age (>80th percentile) (9). At a mean follow-up of 4.3 years, there was a trend in the statin group to reduced combined major adverse cardiovascular events (MACE) (6.9% vs 9.9%, p=0.08), with a significant reduction in MACE in the subset of patients with CAC >400 in a post-hoc analysis (8.7% vs 15.0%, p=0.046). Limitations of the study included an 18.4% dropout rate and a 14% crossover rate to statin therapy from the control arm, as well as the inclusion of coronary revascularizations in the primary endpoint.
Randomized controlled trials assessing CAC-guided prevention in a broad screening population have not been performed, likely due to concerns over trial size, costs, and the inherent difficulty establishing equipoise to withhold statins from patients at high risk for cardiovascular events due to a significantly elevated CAC score. We therefore performed a retrospective analysis of a large CAC registry to determine the effect of statin treatment on cardiovascular events.
Methods
Study Population and Data Collection
We identified 16,996 consecutive patients who underwent initial dedicated CAC testing by electron beam computed tomography (EBCT) between April 2002 and August 2009 at Walter Reed Army Medical Center (Washington, DC, USA). All subjects were >18 years of age at the time of CAC scanning. Baseline comorbidities were extracted using International Classification of Disease, Ninth Revision (ICD-9) codes from the Military Data Repository for any inpatient or outpatient diagnoses entered prior to the date of CAC scoring, as previously described (4). Baseline medications were extracted for the six-month period prior to the CAC score. Initial entry into the military health system and date of last encounter were determined for each patient.
Patients were excluded if they were foreign military members (n=275) or lacked any of the following: (1) 12 months in the military health care system prior to their initial CAC scan (n=282), (2) follow-up after their CAC scan (n=87), or (3) prescriptions filled during the study period (n=102). Patients were also excluded if they had pre-existing coronary artery disease, myocardial infarction, stroke or cerebral revascularization, peripheral vascular disease, or malignancy (n=2606) as identified using standard ICD-9 codes (Online Table 1). There were 13,644 patients analyzed. The local Institutional Review Board approved the study, and informed consent was not required due to the retrospective study design.
Calcium Scoring
For the measurement of CAC, EBCT was performed with Imatron C-150 and C300 LXP scanners (Imatron Corp., South San Francisco, California) and CAC scored per the Agatston method as previously described (10,11). Coronary calcium tests were conducted at the discretion of the ordering provider and results were reported in the electronic health record, per routine clinical care. Patients were classified as having no CAC (CAC 0) or positive CAC (CAC > 0), with further subdivision into CAC groups of 0, 1–100, 101–400 and greater than 400 (12).
Military Data Repository
The Military Data Repository (MDR) contains comprehensive administrative and medical care claims information (e.g., demographics, diagnoses, diagnostic and treatment procedures, prescriptions and vital status) for active duty military, retirees and other Department of Defense (DoD) healthcare beneficiaries and their dependents. The database includes both inpatient and outpatient services that are provided either at military treatment facilities worldwide or at civilian facilities paid by the DoD. Complete pharmacy data are available since October 1, 2001.
Outcome Measures and Follow-up
Subjects were assessed for a primary combined MACE outcome of cardiovascular mortality, (ICD-10 Codes I00-I78), incident myocardial infarction (ICD-9 Code 410) or stroke (ICD-9 Codes 430, 431, 433.x1, 434.x1, and 436), as previously described (4). Codes for stroke were limited to the primary diagnosis, and codes for MI were limited to the first two positions, consistent with prior studies by the Food and Drug Administration (13,14). These definitions are associated with a ≥90% positive predictive value for adjudicated stroke and MI outcomes in prior administrative claims databases (15–18). Among patients with >1 incidence of MACE, only the first was used in the analysis.
Death data, including cause of death, was extracted for all patients from the MDR and National Death Index and cross-referenced to the Veterans Affairs Beneficiary Identification Records Locator Subsystem (BIRLS) as well as the Social Security Death Index (19). Patients were followed until they no longer actively filled medications within the military health system, otherwise exited the system, died, or December 31, 2014, whichever was sooner.
Statin use
Statin use was classified as a binary variable by the presence (or absence) of at least one filled statin prescription at baseline or within five years after the CAC score and before a primary event or end of follow-up. To account for induction and latent periods (20), we used a 1-month lag for the initial statin prescription for statins initiated after the CAC score. In a sensitivity analysis, we classified statin users as those with filled prescriptions within 2 years, instead of five years, from their CAC score.
Inverse probability of treatment weighting (IPTW).
To reduce the impact of potential confounding variables, an inverse probability of treatment weighting method was used. First, a non-parsimonious, multivariable logistic regression model was created to obtain the probability of receiving statin treatment at baseline. The inverse of the probability of statin assignment was then used to create a weight for each patient (21). Independent variables for the logistic regression model were the presence of CAC, year of CAC score, the use of angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), beta blockers (BB), and aspirin (all assessed at baseline), the Charlson comorbidity score (22), male sex, age, baseline hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, tobacco dependence, and all 2×2 interactions. The presence of hypertension, hyperlipidemia, and diabetes mellitus were determined both by administrative codes as well as the baseline use of antihypertensives, lipid agents, or diabetes medications, respectively (Online Table 2). The presence of atrial fibrillation and current or history of tobacco use were determined by codes alone (Online Table 2), and the year of CAC score was included to account for changing practice patterns over time.
Variables were balanced between statin users and non-users over the entire study cohort. When evaluating statin use within CAC subgroups, all covariates were forced back into the multivariable time-to-event model alongside the propensity treatment weighting to limit residual confounding. The interaction of statin therapy among CAC subgroups was tested by inserting a CAC group*statin term in the model. The Fine-Gray model was used to account for the competing risk of non-cardiovascular death when assessing MACE-free survival (23). Cumulative incidence curves were obtained from the models by applying overall marginal frequencies and mean values for covariates. To determine the ability of CAC score to risk stratify patients for statin therapy, an incident MACE rate per 1000-person years and 10-year number needed to treat (NNT) were derived from the cumulative incidence function (CIF) extracted at 10 years for each of the CAC groups (24).
Additional Sensitivity and Subgroup Analyses
To assess the impact of prolonged use of statin therapy and medication compliance, we determined the proportion of time a patient was taking statin therapy during follow-up period and before the outcome of interest. The total number of pills filled during this period was divided by the number of days in the follow-up period. A threshold of >50% was used to indicate medication compliance (25). Patients with overlapping fills were limited to an on-hand-supply not exceeding 180 pills at time of dispensing (26). Compliant patients were subsequently compared to patients not on any statin therapy after applying IPTW to balance covariates as in the primary analysis.
To account for variation in statin use over time and adjust for immortal time bias (27,28), statin therapy was further analyzed using a time-varying covariate in a Cox regression model. Patients were considered on statin treatment after a prescription fill date for the number of days equal to their on-hand-supply plus a 28-day lag period (20,29,30). Covariates included in the model were identical to those used in the propensity analysis but without all 2×2 interactions.
A separate sensitivity analysis also explored the relationship between statin intensity and MACE in a Cox proportional hazards model after classifying statins into low-, medium- and high-intensity according to current lipid guidelines (2) (Online Appendix). In a post-hoc, subgroup analysis, we also conducted a propensity-weighted analysis restricted to patients with diabetes at baseline (Online Appendix).
Finally, in a post-hoc, exploratory analysis, we estimated each patient’s baseline ASCVD risk using the pooled cohort equation (8) by entering assumed values for systolic blood pressures and lipid profiles based on the presence (or absence) of hypertension and hyperlipidemia and whether or not the patient was receiving treatment with antihypertensives or anti-lipid therapy (Online Appendix). Patients were categorized into low ASCVD risk (<5%), intermediate risk (5–20%) and high risk. After IPTW within each group, hazards of MACE were compared across ASCVD risk category in patients with CAC, CAC 1–100, and CAC 101+.
Baseline characteristics and CAC scores were compared between statin-users and nonusers using standardized mean differences. A standardized mean difference less than 0.1 is considered a negligible difference between groups (31). For other comparisons, a 2-tailed value of p<0.05 was considered significant. Statistics were computed using SAS 9.4 (SAS Institute, Cary, North Carolina).
Results
Study Patients
After applying exclusion criteria, there were 13,644 consecutive patients (mean age 49.6 ± 8.4 years, 71% male) who underwent CAC screening from April 2002 to August 2009. They had a low burden of traditional ASCVD risk factors and 9,360 (69%) had no detectable CAC (Table 1). Approximately half of the patients (n=6886; 50.5%) were treated with statins at baseline or following their CAC score. Of these, 3,298 (47.9%) were prescribed statins in the 6 months prior to their CAC score. Of all statin prescriptions before first MACE or end of followup, 15.1% were of low-intensity, 65.7% were of medium-intensity, and 19.3% were of highintensity. Patients prescribed statins were on therapy for a median of 5.5 years, or 67.0% of their follow-up period. Patients prescribed a statin during the study were significantly more likely to be older, be male, have comorbidities including hypertension, hyperlipidemia, diabetes, and tobacco use, have a higher CAC score, and be on aspirin therapy at baseline. After IPTW, the groups were appropriately balanced on all variables (Table 1).
Table 1:
Baseline demographics, comorbidities*, and medications for patients stratified by statin use after CAC score and prior to five years or MACE
| No Statin (n = 6758) | Statin (n = 6886) | Absolute Standardized Difference† | ||
|---|---|---|---|---|
| Before IPTW | After IPTW | |||
| Age, years, mean (SD) | 48.1 (7.6) | 51.1 (8.9) | 0.36 | 0.03 |
| Year of CAC score, median [IQR] | 2005 [2003–2007] | 2005 [2003–2007] | 0.08 | <0.01 |
| Charlson score, mean (SD) | 0.02 (0.14) | 0.03 (0.21) | 0.09 | <0.01 |
| Male, n (%) | 4459 (66.0) | 5173 (75.1) | 0.20 | 0.01 |
| Diabetes Mellitus, n (%) | 241 (3.6) | 687 (10.0) | 0.26 | 0.01 |
| Hypertension, n (%) | 1538 (22.8) | 3105 (45.1) | 0.49 | 0.01 |
| Hyperlipidemia, n (%) | 1585 (23.5) | 5163 (75.0) | 1.20 | 0.01 |
| Any tobacco use, n (%) | 359 (5.3) | 612 (8.9) | 0.14 | <0.01 |
| Atrial Fibrillation, n (%) | 53 (0.8) | 100 (1.5) | 0.06 | 0.01 |
| Race‡ | 0.07 | 0.06 | ||
| White | 4855 (77.8) | 4826 (75.2) | ||
| Black | 945 (15.1) | 1081 (16.8) | ||
| Native American | 25 (0.4) | 24 (0.4) | ||
| Asian | 180 (2.9) | 179(2.8) | ||
| Other | 236 (3.8) | 312 (4.9) | ||
| Coronary Artery Calcium Score | ||||
| 0, n (%) | 5618 (83.1) | 3742 (54.3) | 0.68 | 0.05 |
| 1–100, n (%) | 944 (14.0) | 1933 (28.1) | ||
| 101–400, n (%) | 154 (2.3) | 800 (11.6) | ||
| 401+, n (%) | 42 (0.6) | 411 (6.0) | ||
| Baseline Medications | ||||
| Aspirin, n (%) | 476 (7.0) | 1710 (24.8) | 0.50 | <0.01 |
| Anti-hypertensive, n (%) | 995 (14.7) | 2346 (34.1) | 0.46 | 0.01 |
| ACE-I, n (%) | 383 (5.7) | 1203 (17.5) | 0.38 | 0.03 |
| ARB, n (%) | 139 (2.1) | 376 (5.5) | 0.18 | 0.03 |
| Beta-blocker, n (%) | 256 (3.8) | 639 (9.3) | 0.22 | 0.01 |
| Calcium channel blocker, n (%) | 189 (2.8) | 436 (6.3) | 0.17 | 0.01 |
| Diuretics, n (%) | 495 (7.3) | 1174 (17.1) | 0.30 | <0.01 |
| Insulin, n (%) | 7 (0.1) | 66 (1.0) | 0.12 | 0.01 |
| Non-insulin diabetic therapy | 43 (0.6) | 338 (4.9) | 0.26 | 0.07 |
| Fibrate or niacin, n (%) | 82 (1.2) | 259 (3.8) | 0.16 | 0.08 |
| Statins§, n (%) | - | 3298 (47.9) | ||
| Atorvastatin, n (%; median dose) | - | 504 (7.3; 20 mg) | ||
| Rosuvastatin, n (%; median dose) | - | 13 (0.2; 10 mg) | ||
| Fluvastatin, n (%; median dose) | - | 3 (0.0; 40 mg) | ||
| Lovastatin, n (%; median dose) | - | 10 (0.1; 20 mg) | ||
| Pravastatin, n (%; median dose) | - | 83 (1.2; 20 mg) | ||
| Simvastatin, n (%; median dose) | - | 2685 (39.0; 20 mg) | ||
| Follow-up, yrs, median [IQR] | 9.4 [7.2–11.2] | 9.4 [7.3–11.1] | ||
SD: Standard Deviation. IQR: Interquartile Range. CAC: Coronary artery calcium.
Associated ICD-9 codes for comorbid disorders are listed in Appendix. Diabetes Mellitus, Hypertension, and Hyperlipidemia defined as prior ICD-9 diagnosis or baseline diabetic, antilipid, or anti-hypertensive medical therapy.
Absolute Standardized difference = difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.20 (small effect size).
981 patients without race data.
Individual statin information based on most recent prescription prior to CAC score.
Outcomes
Over a median follow-up of 9.4 years (IQR 7.2–11.2), there were 532 patients (3.9%) who suffered a MACE including 191 with myocardial infarction (1.4%), 342 with stroke (2.5%), and 42 (0.3%) who had cardiovascular death. There were 209 deaths (1.5%) from any cause.
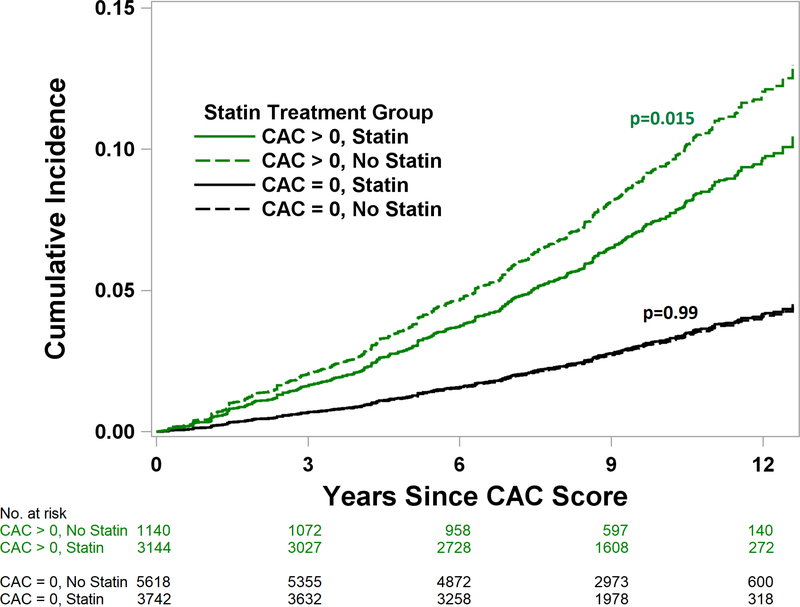
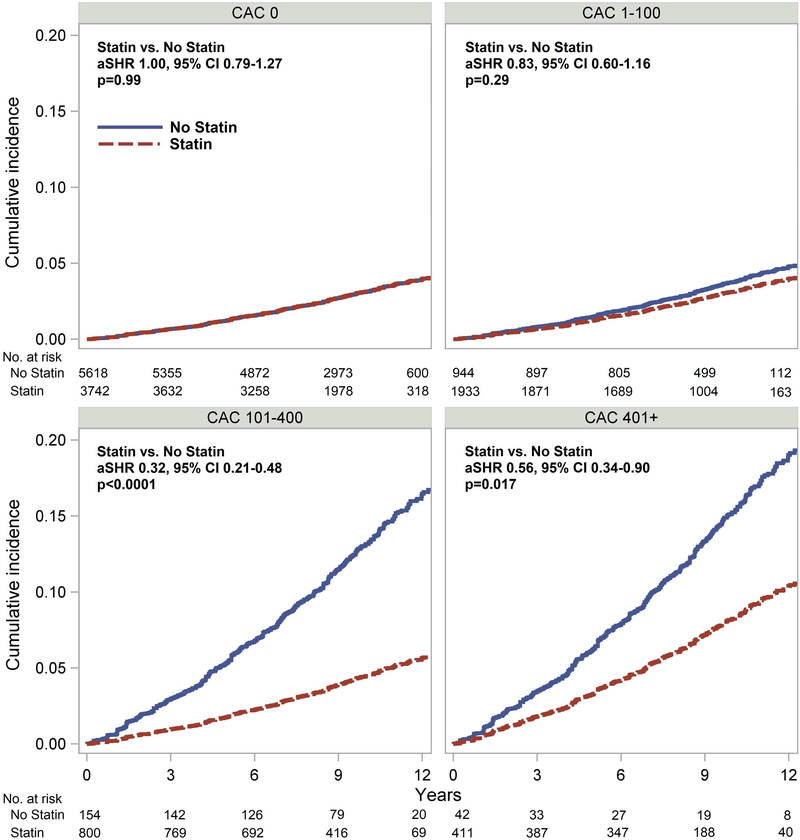
Patients with CAC who were prescribed a statin within five years of their CAC testing had a significantly lower risk of MACE (adjusted subhazard ratio (aSHR) 0.76, 95% CI 0.600.95, p=0.015), while patients without CAC had no MACE reduction with statin use (aSHR 1.00, 95% CI 0.79–1.27, p=0.99) (Figure 1). The effect of statin use on MACE was significantly related to the severity of CAC (p for interaction <0.001; Central Illustration; Table 2), with patients having CAC >100 associated with the most benefit. Using a 2-year cutoff for statin prescription in a sensitivity analysis yielded similar results (Online Appendix).
Figure 1.
Cumulative Incidence of MACE Stratified by Statin Treatment and CAC Presence. Patients with CAC who were prescribed a statin had a significantly reduced risk of MACE (aSHR 0.76, 95% CI 0.60–0.95, p=0.015), while patients without CAC had no associated MACE reduction (aSHR 1.00, 95% CI 0.79–1.27, p=0.99). p=0.097 for interaction between statin treatment and CAC presence. aSHR – adjusted subhazard ratio. MACE – major adverse cardiovascular event. CAC – coronary artery calcium.
Central Illustration.
Cumulative Incidence of MACE Stratified by Statin Treatment and CAC Severity. Benefit of statin therapy was significantly related to CAC group (p<0.0001 for interaction), with benefit in patients with CAC >100 but not in patients with CAC < 100. aSHR – adjusted subhazard ratio. MACE – major adverse cardiovascular event. CAC – coronary artery calcium.
Table 2:
Sub-hazard Ratios for MACE among CAC groups
| Statin vs No Statin* | > 50% compliance* | |||
|---|---|---|---|---|
| aSHR (95% CI) | p value | aSHR (95% CI) | p value | |
| CAC = 0 | 1.00 (0.79–1.27) | 0.99 | 0.66 (0.49–0.88) | 0.0046 |
| CAC > 0 | 0.76 (0.60–0.95) | 0.015 | 0.76 (0.59–0.98) | 0.031 |
| CAC 1–100 | 0.83 (0.60–1.16) | 0.29 | 0.78 (0.53–1.15) | 0.21 |
| CAC 101–400 | 0.32 (0.21–0.48) | <0.0001 | 0.32 (0.20–0.51) | <0.0001 |
| CAC 401+ | 0.56 (0.34–0.90) | 0.017 | 0.59 (0.35–0.99) | 0.044 |
| CAC > 100 vs CAC < 100 | 0.46 (0.31–0.67) | <0.0001 | 0.61 (0.40–0.93) | 0.021 |
Groups compared after IPTW and adjusting for competing hazard of non-cardiovascular death.
aSHR = adjusted subhazard ratio. CI=Confidence Interval.
Presence or absence of statin prescription within 5 years of CAC before MACE or end of follow-up.
>50% compliance with statin therapy during follow-up period vs patients with no statin exposure
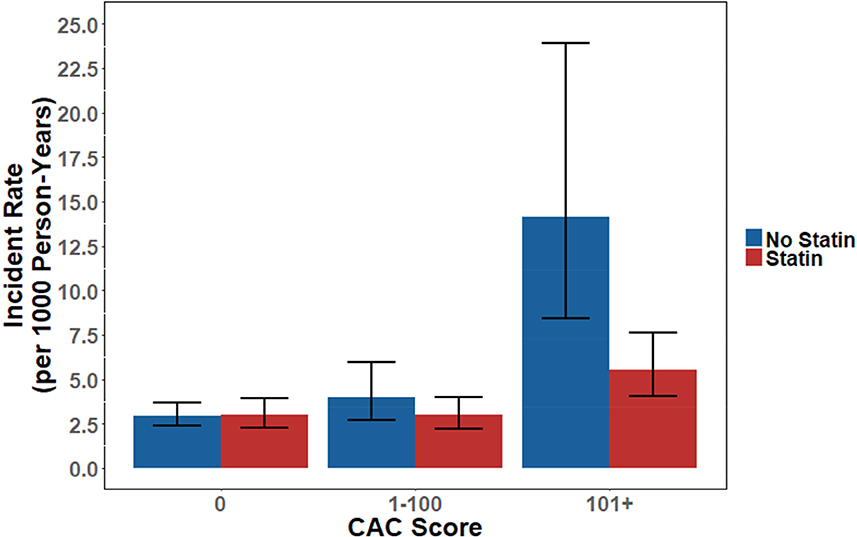
In the 10-year NNT analysis, there was no significant effect of statins among patients without any CAC. Patients with a CAC of 1–100 had a trend towards benefit (NNT = 100, p=0.095), while patients with a CAC >100 derived significant benefit with a NNT of 12 (p<0.0001) (Table 3). These differences in observed benefit can also be visualized by the comparative incident MACE rate derived through ten years of follow-up (Online Figure 1).
Table 3:
NNT to prevent first occurrence of MACE through 10 years
| CAC Score | Therapy | N | MACE | CIF* | ARR | NNT (NNH) | aSHR† | p value |
|---|---|---|---|---|---|---|---|---|
| 0 | No Statin | 5618 | 114 | 0.0295 | (0.03%) | (3571) | 1.01 | 0.94 |
| Statin | 3742 | 100 | 0.0298 | |||||
| 1–100 | No Statin | 944 | 32 | 0.0401 | 1.00% | 100 | 0.75 | 0.095 |
| Statin | 1933 | 76 | 0.0301 | |||||
| 101+ | No Statin | 196 | 32 | 0.1409 | 8.53% | 12 | 0.38 | <0.0001 |
| Statin | 1211 | 123 | 0.0556 |
CIF – Cumulative incidence function. ARR – Absolute Risk Reduction. NNT – Number needed to treat. NNH – Number needed to harm. aSHR – adjusted subhazard ratio. MACE – Major adverse cardiovascular event. CAC – Coronary artery calcium.
Cumulative incidence of MACE at 10 years, calculated at observed marginal differences for covariates (means).
aSHR calculated at 10 years.
Statin Compliance
In a sensitivity analysis, patients with >50% compliance during follow-up (n=4415) were compared to patients with no statin treatment (n=6758). The groups were appropriately balanced on all variables after IPTW (Online Table 3). Adjusting for the competing hazard of noncardiovascular death, statin treatment was associated with reduced MACE for the entire study subgroup (aSHR 0.56, 95% CI 0.45–0.69, p<0.0001) (Table 2). The benefit of statin therapy was related to CAC severity (p=0.028); patients with CAC >100 had a greater reduction in MACE (aSHR 0.61, 95% CI 0.40–0.93, p=0.021) compared to patients with CAC <100.
Time-Dependent Analysis
As a time-dependent variable, statin therapy was an independent predictor among all patients for reduced MACE in the Cox proportional hazard model (aHR 0.64, 95% CI 0.52–0.78, p<0.0001). There was no significant interaction between statin treatment and CAC group. Multivariable predictors of increased MACE in the Cox model were increasing CAC, increasing age, use of beta blockers at baseline, hyperlipidemia or tobacco use at baseline, and earlier CAC screening year (Online Table 4).
Strength Analysis
In a separate sensitivity analysis, statin intensity was an independent predictor of improved MACE free survival in the multivariable Cox regression model across all patients (p=0.0012) (Online Table 5). Compared to CAC <100, patients with CAC >100 had greater reduction in MACE from the highest strength tercile of statin compared to no statin (p=0.03) (Online Appendix.)
Benefit Across ASCVD Risk Categories
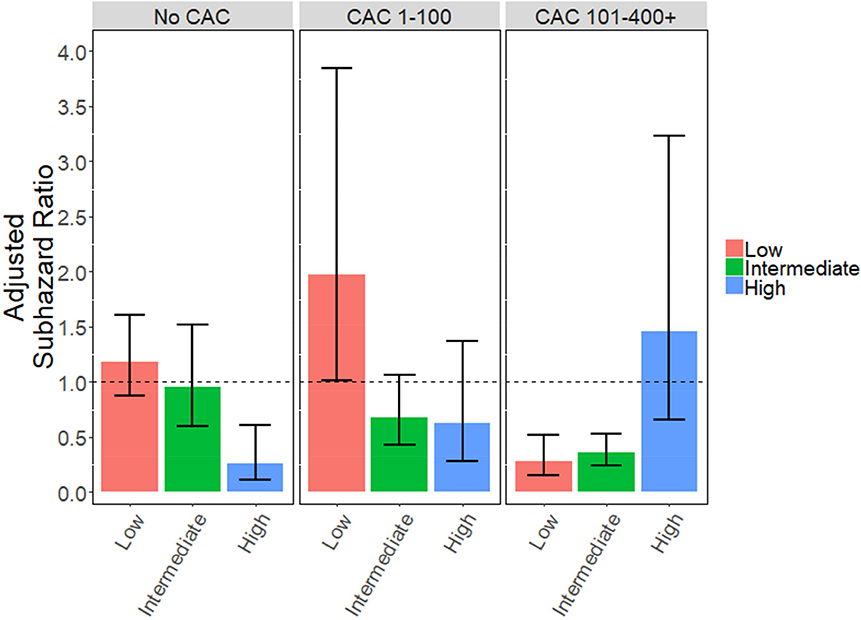
In a post-hoc, exploratory analysis using estimated values for the systolic blood pressure and lipids as detailed in the online appendix, patients with no CAC and otherwise high ASCVD risk (>20%) had a 74% relative reduction in the hazard of MACE with statin therapy (aSHR 0.26, 95% CI 0.11–0.61, p<0.01), but there was no benefit of statin therapy in patients with no CAC and low or intermediate baseline ASCVD risk (Online Appendix; Online Figures 2 and 3). Conversely, patients with CAC 101+ had a 64%−71% relative reduction in the hazard of MACE even with low (<5%; aSHR 0.29, 95% CI 0.16–0.52, p<0.0001) or intermediate (5–20%; aSHR 0.36, 95% CI 0.24–0.53, p<0.0001) ASCVD risk scores (Online Figure 2). There was no observed benefit for statins in patients with elevated CAC (101+) and high ASCVD risk (n=185), but the relatively low number of patients in the non-statin group limited the analysis (23 not on statins, 162 on statins). Based on the overall trend of the data, it does appear that this group is likely an outlier. These results should be interpreted with caution given the reliance on assumed variable values.
Discussion
In this large, observational study, the presence and severity of CAC identified patients most likely to derive long-term benefit from statin treatment. Among this relatively lower-risk cohort, CAC >100 was consistently associated with a greater reduction in the hazard for MACE with statin therapy relative to CAC <100 (Central Illustration). Patients without any CAC had no benefit from statin treatment in the primary propensity analysis. To our knowledge, our study is the largest to evaluate the effectiveness of statin treatment in patients with CAC, and the only study to directly compare the direct benefit of statin use between CAC groups. Many have argued for the potential use of CAC to help identify patients with increased benefit from statins (5,32) and improve shared decision-making (33), although lack of direct data showing the utility of CAC in selecting patients for statin treatment has prevented widespread use or a stronger recommendation in clinical guidelines. Our study helps provide valuable information on the effect of statin therapy in a real-world population without known ASCVD who underwent CAC scoring.
Prior Primary Prevention Trials and Estimates
Prior studies and models have attempted to estimate the benefit of statin treatment in patients stratified by CAC (33–35). An analysis of 5534 Multi-Ethnic Study of Atherosclerosis (MESA) participants found that CAC>100 identified patients with highest risk for cardiovascular events, potentially selecting patients most likely to benefit from statin therapy (34). Using a subgroup of the MESA cohort (n=4085) that would have qualified for one of 7 previous statin randomized controlled trials, mathematical models estimated the 10-year NNT for MACE for CAC 0, 1–100, and 101+ as 87, 37 and 19, respectively (33), based on an average relative risk reduction of 30% for statin therapy in the referenced primary prevention trials (36,37). The 30% reduction reflected inclusion of revascularization in the combined endpoint, while the relative risk reduction was 25% (absolute risk reduction 2.9%) when limiting the endpoint to nonfatal and fatal cardiovascular disease in the Cochrane meta-analysis (36). From the MESA data, a CAC level >100 was chosen to be an appropriate discriminator to select patients with the greatest absolute risk reduction, and thus the greatest benefit, from statin therapy.
CAC and Statin Effect Modification
While these previous estimates were based on a stable relative risk reduction across all patients, our study found that the presence of CAC was associated with varying statin impact. Patients with no CAC showed no benefit from statin therapy in our primary propensity weighted analysis, while patients with any CAC had an associated 24% reduction in MACE, which is comparable to the Cochrane meta-analysis (36). The absolute risk reduction for nonfatal and fatal CVD was 2.9% in the Cochrane study, which fell between the CAC 1–100 (absolute risk reduction (ARR) 1%) and CAC 101–400 groups in our study (ARR 10%). As with previous estimates, a CAC threshold >100 continued to be a discriminator for selecting patients most likely to benefit from statin therapy. Patients with CAC of 1–100 only had a trend towards statin benefit, though our study was likely underpowered for this subgroup analysis given their low MACE rate. Taken in total, our study may be the first to show the ability of a screening test to potentially tailor a statin treatment strategy.
No previous study has supported the ability of a biomarker or test to discriminate patients that will or will not benefit from statin therapy (38), and it has generally been assumed that statins provide a consistent relative risk reduction across the general population. CAC, theoretically, is an ideal candidate as a potential discriminator, since it directly measures coronary atherosclerosis resulting from the patient’s entirety of previous exposures and risk factors. It is therefore plausible that a patient with no coronary artery calcium would not show the same benefit in cardiovascular event reduction as a patient with proven atherosclerosis. Certainly, a CAC of 0 has repeatedly been shown to confer a very low annualized risk of MACE, including the Walter Reed cohort (4).
Prolonged Statin Therapy
In our sensitivity analysis, we did observe a potential benefit with prolonged statin therapy (statin therapy for >50% of follow-up period) even in the group without CAC (aSHR 0.66, 95% CI 0.49–0.88). Time-dependent analysis also showed benefit with statin therapy across all groups in the multivariate model without propensity weighting. Thus, a modest benefit of prolonged statin therapy may still exist among patients with a CAC of 0, but the absolute benefit would be small given their cumulative incidence of MACE (after accounting for noncardiovascular death) was only 3.0% at 10 years. Statins may still be warranted in certain subpopulations in the absence of CAC when other compelling risk factors are present (e.g., very high LDL-C), and patients with no CAC but high baseline ASCVD risk (>20%) did benefit from statin therapy in our post-hoc exploratory analysis using estimated risk variables.
Limitations
We recognize several important limitations in our study. Given the retrospective design, patients were not pre-assigned to statin therapy. We used propensity weighting to attempt to adjust for baseline covariates, though we cannot rule out residual confounding. Since patients were included in the statin treatment group if they received a prescription in the initial interval following CAC screening, their post-baseline assignment introduces some artifact into the cumulative incidence function curve. Reassuringly, results were consistent in our sensitivity analysis using two years instead of five years as the cutoff for statin assignment.
As with any large observational study using administrative claim data, there also remains a risk of inaccurate covariate or outcomes assessment. For the outcome of MACE, ICD-9 codes for MI and stroke have been shown to have ≥90% PPV for representing adjudicated clinical MI and stroke events (15,16), though the risk of imprecise outcome accounting remains. While all deaths and their causes were ascertained using the NDI, it is possible that some deaths may have been misclassified (cardiovascular versus non-cardiovascular).
Coding for covariates are inherently less sensitive, and to attempt to partially address this limitation, we did utilize baseline medication data to augment ICD-9 coding for diagnosis of applicable comorbid disorders of hypertension, hyperlipidemia and diabetes mellitus. Tobacco dependence is often undercoded (39), though the addition of smoking status only had a marginal effect on the efficacy of statin therapy in another population (40). Although we used a propensity score, we cannot fully eliminate confounders and selection bias and were unable to account for the relative severity of comorbid disorders or calculate ASCVD risk scores because the Walter Reed CAC Cohort does not contain measured blood pressure and lipid values.
As in any prevention study, we cannot rule out a healthy user bias (41), whereby a patient that is more likely to receive preventive therapy may also be more likely to engage in other healthy activities that reduce their chance for MACE, such as exercise or a healthy diet. In our study, all patients were willing to undergo CAC scoring as preventive testing, though some patients may still have been more likely to agree to statin therapy. The related healthy adherer effect may have also influenced the results of our sensitivity analysis looking at prolonged statin therapy (41). Finally, it should be noted that the study population was from a single tertiary medical center and involves subjects with broad, comprehensive access to medical care, which may limit generalizability.
Implications and Path Forward
Overall, these results support the guidance of the recent SCCT consensus statement using a CAC threshold of 100 for treatment (5), though further studies are still needed for confirmation of these results. Until we have further studies, a threshold of 100 does appear to be an appropriate cutoff to select patients at greatest benefit for statin therapy from the general population. Providers should consider the SCCT statement, along with the overall patient risk profile, in patient shared-decision making.
Conclusions
In this large, long-term, retrospective analysis of the Walter Reed cohort, increasing severity of CAC was associated with increased benefit from statin treatment for the prevention of MACE, with greatest benefit in patients with CAC > 100. In our primary, propensity-weighted analysis, patients with CAC=0 had no benefit from statin therapy in mean follow-up of nearly 10 years. Calcium scoring, therefore, shows significant potential to help select patients most likely to benefit from statin therapy.
PERSPECTIVES.
Competency in Medical Knowledge: Among primary prevention patients, quantification of the Agatston CAC score can help identify patients with questionable (CAC=0) or substantial (CAC >100) benefit from statin therapy.
Competency in Patient Care: Patients who have uncertain benefit from statin therapy based on traditional risk models may be offered coronary artery calcium scoring for help in shared decision making.
Translational Outlook: Future research is needed to confirm the ability of CAC screening to aid in building a statin treatment strategy.
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- DoD
Department of Defense
- ICD-9
International Classification of Disease, Ninth Edition
- ICD-10
International Classification of Disease, Tenth Edition
- MACE
major adverse cardiovascular events
- MDR
Military Data Repository
- MI
myocardial infarction
- NDI
National Death Index
ONLINE APPENDIX
METHODS
Strength Sensitivity Analysis
To analyze the effect of dose intensity of statin treatment, the average strength of statin usage was computed for each patient by classifying prescribed statins into highintensity, medium-intensity, or low-intensity as defined by current lipid guidelines (1). High-intensity statins and medium-intensity statins were assigned a relative strength of 1.93 and 1.34, respectfully, compared to low-intensity statins based on the comparative average LDL reduction of 56%, 39%, and 29% (2,3). The strength coefficient was then multiplied by the number of pills prescribed, totaled among all prescriptions, and divided by the days in the follow-up period to determine an average strength of statin per day. Patients on statins were ranked into terciles based on their average statin strength and compared alongside patients with no statin therapy in a multivariable Cox Regression model. Covariates included in the model were identical to those used in the main analyses.
Statin strength was further analyzed in a time-dependent variable in a Cox regression model similar to the time-dependent analysis in the main paper. For this analysis, statin strength was set to the strength of the most recent fill. Patients were considered on statin treatment after a prescription fill date for the number of days equal to their on-hand-supply plus a 28-day lag period (4–6). Patients with overlapping fills were limited to an on-hand-supply not exceeding 180 pills at time of dispensing (7). Covariates included in the model were again identical to those used in the main analyses.
Baseline Statin Use
We also explored the impact of baseline statin use on the hazard of MACE. Restricting the analysis to patients classified as statin users within five years of their CAC screening and prior to MACE, we compared patients with and without baseline statin use. Inverse probability of treatment weighting (IPTW) was used to adjust for baseline covariates as in the primary analysis. Identical to the main model, independent variables for the logistic regression model were the presence of CAC, year of CAC score, the use of angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), beta blockers (BB), and aspirin (all assessed at baseline), the Charlson comorbidity score (24, 25), male sex, age, baseline hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, tobacco dependence, and all 2×2 interactions.
Compliant vs Non-compliant Statin Users
We further compared patients with >50% statin exposure to those with less than 50% statin exposure during the follow-up period, using IPTW to account for differences in baseline covariates between the two groups as in the primary analysis.
Two-year Analysis
As a sensitivity analysis, a two-year, as opposed to five-year, cutoff was used for discrimination of statin use. As such, statin use was classified by the presence (or absence) of at least one filled statin prescription at baseline or within two years after the CAC score and before a primary event or end of follow-up. Groups were compared after IPTW to account for baseline covariates identically to the primary analysis.
Diabetes Mellitus
Restricting the analysis to the subgroup of patients with baseline diabetes mellitus, we again compared patients with and without statin use after IPTW. Statin use was defined, as in the main analysis, as the presence (or absence) of a filled prescription before major adverse cardiovascular event (MACE) or end of follow-up within five years following their CAC screening test and before outcome of MACE or end of follow-up. Given the subgroup analysis and reduced size of the cohort, independent variables for the logistic regression model were the presence of CAC, year of CAC score, the use of angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), beta blockers (BB), and aspirin (all assessed at baseline), the Charlson comorbidity score, male sex, age, baseline hypertension, hyperlipidemia, tobacco dependence, use of diabetes medications, and use of non-statin medications (fibrate or niacin) without the inclusion of 2×2 interactions.
Estimation of Baseline Atherosclerotic Cardiovascular Disease (ASCVD) Risk and MACE
In a post-hoc, exploratory analysis, we attempted to determine the impact of CAC screening among low risk (<5%), intermediate risk (5–20%), and high baseline ASCVD risk patients (>20%). In essence, we wanted to test the hypothesis that patients with a CAC of 0 but an otherwise high ASCVD risk score would be more likely to benefit from statin therapy. Given the limitations of our data and lack of direct blood pressure and cholesterol measurements, assumed values for systolic blood pressure and cholesterol levels were input into the pooled cohort equation (PCE) (8) according to the algorithm below. Patients with hyperlipidemia on cholesterol treatment were estimated to have the same cholesterol as a patient without a diagnosis of hyperlipidemia, as the PCE does not account for treatment of cholesterol, only the level of cholesterol.
Patients with hypertension and not on antihypertensives were assigned a systolic blood pressure of 145.
Patients with hypertension and on treatment with antihypertensives were assigned a systolic blood pressure of 135.
Patients without hypertension were assigned a systolic blood pressure of 125.
All patients were assigned an HDL of 50 and triglyceride level of 100.
Patients with hyperlipidemia not on treatment were assigned a total cholesterol of 210.
All other patients were assigned a total cholesterol of 170 and an HDL of 50. Given the crude nature of our assumptions, we also used a range of numbers for blood pressure and cholesterol levels to observe the impact of using different values on the overall results in patients with CAC of 0.
All patients were assigned an HDL of 50 and triglyceride level of 100 in each model.
Patients without hyperlipidemia or those on treatment for hyperlipidemia were assigned a total cholesterol value of 170.
Patients with hyperlipidemia, not on treatment, were assigned total cholesterols of 210 and 230 (corresponding to LDL levels of 140 and 160).
Patients with hypertension, not on treatment, were assigned systolic blood pressures of 145 and 155.
Patients with hypertension, on treatment, were assigned systolic blood pressures of 130 and 140.
Patients without hypertension were assigned systolic blood pressures of 120 and 130.
For each model iteration, inverse probability of treatment weighting was used to balance baseline covariates between statin users and non-users within each of the ASCVD risk categories (low, intermediate and high). The same variables were input into the model as in the main analysis, but without 2×2 interactions given the smaller size of the subgroups.
RESULTS
Strength Sensitivity Analysis
Statin intensity was an independent predictor of improved MACE free survival in the multivariable Cox regression model adjusting for the competing risk of non-cardiac death (p=0.0012) (Online Table 5). There was no significant interaction with CAC group and tercile of statin strength overall (p=0.18), though patients with CAC > 100 had greater benefit than patients with CAC < 100 from the highest strength tercile of statin compared to no statin (adjusted subhazard ratio [aSHR] 0.54, 95% CI 0.31–0.93, p = 0.03).
In time-dependent analysis, statin strength remained an independent predictor of MACE free survival in the proportional hazards model (aSHR 0.76, 95% CI 0.66–0.88, p=0.0002) (Online Table 6). The strength variable did not interact significantly with CAC group (p=0.95).
Baseline Statin Use
Among patients classified as statin users, patients with baseline statin use were compared to patients with statin exposure only after CAC screening. After IPTW, the groups were appropriately balanced on all variables with the exception of baseline hyperlipidemia (Online Table 7). There was no difference between baseline statin use and hazard of MACE (aSHR 1.001, 95% CI 0.81–1.24, p=0.99). There was no interaction between baseline statin use and severity of CAC (p=0.49 for interaction). Baseline hyperlipidemia could not be balanced given its co-linearity with baseline statin use.
Compliant vs Non-compliant Statin Users
Comparing patients with and without compliant statin exposure (>50%) after IPTW, compliant statin therapy was associated with reduced risk of MACE in all patients (aSHR 0.76, 95% CI 0.63–0.91) without a significant interaction with presence of CAC (p=0.42 for interaction). After IPTW, the groups were appropriately balanced on all variables (Online Table 8).
Two-year Analysis
Comparing patients with and without statin exposure defined at 2 years after IPTW, statin therapy was associated with reduced risk of MACE in patients with CAC 101+ (aSHR 0.60, 95% CI 0.43–0.83) but not in patients in other CAC groups (CAC 0 aSHR 1.05, 95% CI 0.82–1.34; CAC 1–100 aSHR 0.97, 95% CI 0.69–1.36), p for interaction = 0.054. After IPTW, the groups were appropriately balanced on all variables (Online Table 9).
Diabetes Mellitus
In patients with diabetes at baseline (n=928) using IPTW to adjust for baseline covariatess, statin therapy was associated with reduced risk of MACE in patients with CAC (aSHR 0.33, 95% CI 0.19–0.56) but not in patients without CAC (aSHR 0.90, 95% CI 0.35–2.31), p for interaction = 0.073. The groups were appropriately balanced on all baseline variables after IPTW (Online Table 10).
Baseline ASCVD Risk and Impact of CAC
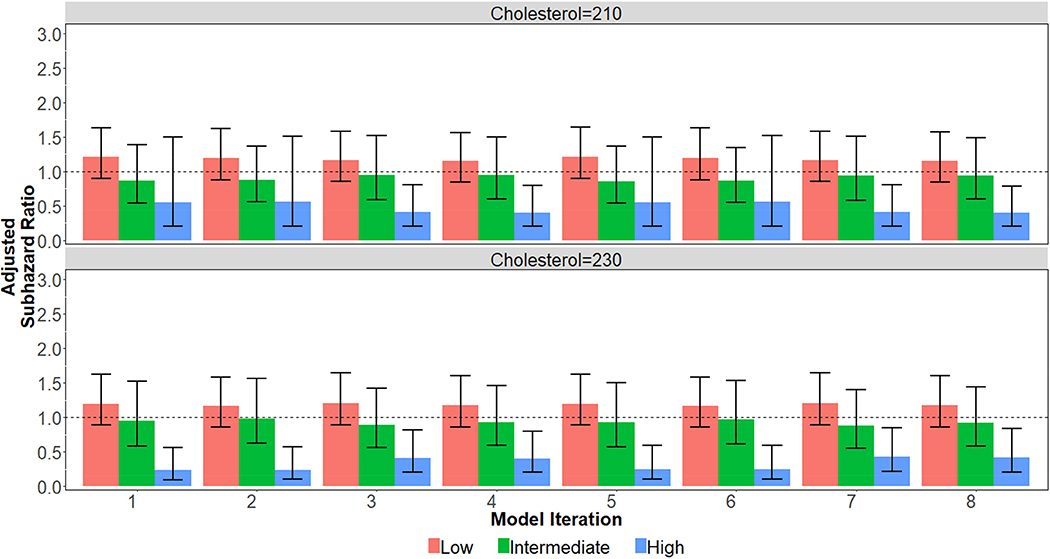
Using the crude estimation of risk variables as stated in the methods, patients with no CAC did benefit from statins if their ASCVD was otherwise high (>20%; aSHR 0.26, 95% CI 0.11–0.61, p<0.01), but not if they had low or intermediate baseline ASCVD risk (Online Figure 2). Similarly, patients with CAC 101+ benefited from statin therapy even with low (<5%; aSHR 0.29, 95% CI 0.16–0.52, p<0.0001) or intermediate (5–20%; aSHR 0.36, 95% CI 0.24–0.53, p<0.0001) ASCVD risk scores. There was no benefit shown for statins in patients with elevated CAC (101+) and high ASCVD risk (n=185), but the relatively low number of patients in the non-statin group limited the analysis (23 not on statins, 162 on statins). Based on the overall trend of the data, it does appear that this group is likely an outlier.
Using a range of values for patient blood pressure and cholesterol yielded similar results (Online Figure 3). Patients without CAC but with high ASCVD risk benefitted from statin use across the majority of the models, including all models with a more elevated cholesterol (cholesterol = 230) assigned to patients with hyperlipidemia and not on treatment. These results should be interpreted with caution given the reliance on estimated/assumed risk factor variables. After inverse probability of treatment weighting, all covariates were balanced within each subgroup in each model iteration at an absolute standardized difference of 0.20 or less, with over 90% being balanced less than 0.10. Online Table 1. ICD-9 Codes of Baseline Conditions Leading to Exclusion
Online Figure 1.
Incident MACE Rate by Statin Treatment and CAC Severity The incident MACE rate for each CAC and treatment group were derived from the CIF of MACE through 10 years. There was no difference between statin treated patients in those without CAC (p=0.94) or those with CAC 1–100 (p=0.095), but there was a significant difference in those with CAC over 100 (p<0.0001).
Online Figure 2.
Statin Use and Hazard of MACE Stratified by ASCVD Risk Score and CAC Severity
Patients were classified as low baseline ASCVD risk (<5%), intermediate risk (5–20%) or high risk (>20%) using assumed risk variables and the pooled cohort equation (PCE).
MACE – major adverse cardiovascular event. CAC – coronary artery calcium.
Online Figure 3.
Statin Use and Hazard of MACE in Patients with CAC=0 Stratified by ASCVD Risk Score Using Multiple Risk Variable Estimates
Multiple Models were run calculating ASCVD risk scores with varying estimates for risk factor variables (See Figure Legend).
MACE – major adverse cardiovascular event. CAC – coronary artery calcium. SBP – systolic blood pressure
Online Table 1.
ICD-9 Codes of Baseline Conditions Leading to Exclusion
| Exclusion Criterion | ICD-9 or Procedure Codes |
|---|---|
| Myocardial infarction | 410.x |
| History of Myocardial Infarction | 412.x |
| Coronary artery disease | 429.2 HCPCS: G8033-G8041 |
| Coronary Revascularization |
ICD-9: V45.81, V45.82, V45.88 HCPCS: 00566, 33510–33519, 33521–33523, 33530, 33533–33536, 33572, 92920, 92921, 92924, 92925, 92928, 92929, 92933, 92934, 92937, 92938, 92941, 92943, 92944, 92973, 92975, 92977, 92980–92982, 92984, 92995, 92996, C9600, C9601, C9602, C9603, C9604, C9605, C9606, C9607, C9608, G0290, G0291, G8158, G8159, G8161-G8167, G8170G8172 PRC: 00.66, 17.55, 36.0, 36.1, 36.2, 36.3 |
| Ischemic heart disease | 414.x, 429.7 |
| Other heart disease | 429.5, 429.6, 429.9 |
| Stroke | 430, 431, 433.x1, 434.x1, 436 |
| Cerebrovascular Disease including Transient Ischemic Attack | 432.x, 433.x0, 434.x0, 435.x, 437.x, 438.x |
| Cerebral Revascularization |
PRC: 00.61–00.65, 38.01, 38.02, 38.11, 38.12, 39.74 HCPCS: 35301, 35390, 35501, 35506–33509, 35601, 35606, 35642, 35701, 61711 |
| Peripheral Vascular Disease | 249.7, 250.7, 440.2, 440.3, 440.4, 443.1, 443.81, 443.9 |
| Peripheral Revascularization |
PRC: 38.13, 38.15, 38.16, 38.18, 39.22, 39.23, 39.24, 39.25, 39.26, 39.29, 39.50, 39.90 HCPCS: 34001, 34051, 34101, 34111, 34151, 34201, 34203, 35311, 35321, 35331, 35341, 35351, 35355, 35361, 35363, 35371, 35372, 35381, 35450, 35452, 35454, 35456, 35458, 35459, 35460, 35470–35475, 35480–35485, 35490–35495, 35511, 35512, 35515, 355516, 35518, 35521–35523, 35525, 35526, 35531, 35533, 35535–35541, 35546, 35548, 35549, 35551, 35556, 35558, 35560, 35563, 35565, 35566, 35570, 35571, 35583, 35585, 35587, 35612, 35616, 35621, 35623, 35626, 35631–35634, 35636–35638, 35641, 35646, 35647, 35650, 35651, 35654, 35656, 35661, 35663, 35665, 35666, 35671, 35700, 35721, 35741, 35879, 35881 |
| Malignancy | 140.x-165.x, 170.x-172.x, 174.x-176.x, 179.x-209.x, 238.77, 338.3, 789.51 |
| Malignancy Procedure |
PRC: 00.10, 41.09, 99.25, 99.85 HCPCS: 77261–77263, 77280, 77285, 77290, 77295, 77299–77301, 77305, 77310, 77315, 77321, 77326–77328, 77331–77334, 77336, 77338, 7737077373, 77380, 77381, 77399, 77401–77404, 7740677409, 77411–77414, 77416–77423, 77425, 77427, 77430–77432, 77435, 77470, 77499, 77520, 77522, 77523, 77525, 77600, 77605, 77610, 77615, 77620, 77750, 77761–77763, 77776–77778, 77781–77787, 77789–77791, 77799, 96400–96402, 96405, 96406, 96408–96417, 96420, 96422, 96423, 96425, 96440, 96445, 96450, 96542, 96545, 96549, C8953-C8955, C9414-C9437, G0210-G0212, G0215, G0223G0228, G0231, G0233, G0234, G0252-G0254, G0292, G0296, G0355, G0359, G0361, G8371G8374, G8376, G8377, G8380, G8381, G8389, G8464, G8465, G9021-G9032, G9050-G9054, G9063-G9067, G9069-G9117 |
HCPCS = Healthcare Procedure Coding System; ICD-9 = International Classification of Diseases-9; PRC = ICD-9 procedural code
Online Table 2.
ICD-9 Codes of Comorbid Conditions
| Comorbidity | ICD-9 Code(s) |
|---|---|
| Hypertension | 401.x, 402.x, 403.x, 404.x, 405.x, 997.91 |
| Hyperlipidemia | 272.0, 272.2, 272.4 |
| Diabetes Mellitus | 250.x HCPCS: G0108, G0109, G0245G0247, G8015-G8026, G8332-G8336, G8385, G8386, G8390 |
| Tobacco Dependence | 305.1, 989.84, V15.82 HCPCS: D1320, G0375, G0376, G8093, G8094, G8402, G8403, G8453-G8455, G9016 |
| Atrial Fibrillation | 427.3 PRC: 37.33, 37.34, 37.36 HCPCS: G8183, G8184 |
Abbreviations as in Online Table 1.
Online Table 3:
Baseline demographics, comorbidities*, and medications for patients with no statin use and with use of statin > 50% of follow-up period and before MACE
| No Statin (n = 6758) | 50% compliance (n = 4415) | Absolute Standardized Difference† | ||
|---|---|---|---|---|
| Before IPTW | After IPTW | |||
| Age, years, mean (SD) | 48.1 (7.6) | 52.4 (8.9) | 0.53 | 0.05 |
| Year of CAC score, median [IQR] | 2005 [2003–2007] | 2005 [2003–2007] | 0.02 | 0.01 |
| Charlson score, mean (SD) | 0.02 (0.14) | 0.03 (0.22) | 0.09 | 0.02 |
| Male, n (%) | 4459 (66.0) | 3307 (74.9) | 0.20 | 0.03 |
| Diabetes Mellitus, n (%) | 241 (3.6) | 505 (11.4) | 0.30 | 0.02 |
| Hypertension, n (%) | 1538 (22.8) | 2206 (50.0) | 0.59 | 0.02 |
| Hyperlipidemia, n (%) | 1585 (23.5) | 3518 (79.7) | 1.36 | 0.03 |
| Any tobacco use, n (%) | 359 (5.3) | 351 (8.0) | 0.11 | 0.01 |
| Atrial Fibrillation, n (%) | 53 (0.8) | 76 (1.7) | 0.08 | 0.00 |
| Race‡ | 0.07 | 0.07 | ||
| White | 4855 (77.8) | 3222 (79.0) | ||
| Black | 945 (15.1) | 555 (13.6) | ||
| Native American | 25 (0.4) | 11 (0.3) | ||
| Asian | 180 (2.9) | 103 (2.5) | ||
| Other | 236 (3.8) | 190 (4.7) | ||
| Coronary Artery Calcium Score | ||||
| 0, n (%) | 5618 (83.1) | 2131 (48.3) | 0.83 | 0.07 |
| 1–100, n (%) | 944 (14.0) | 1324 (30.0) | ||
| 101–400, n (%) | 154 (2.3) | 619 (14.0) | ||
| 401+, n (%) | 42 (0.6) | 341 (7.7) | ||
| Baseline Medications | ||||
| Aspirin, n (%) | 476 (7.0) | 1231 (27.9) | 0.57 | 0.01 |
| Anti-hypertensive, n (%) | 995 (14.7) | 1726 (39.1) | 0.57 | 0.02 |
| ACE-I, n (%) | 383 (5.7) | 923 (20.9) | 0.46 | 0.06 |
| ARB, n (%) | 139 (2.1) | 268 (6.1) | 0.20 | 0.04 |
| Beta-blocker, n (%) | 256 (3.8) | 484 (11.0) | 0.28 | 0.01 |
| Calcium channel blocker, n (%) | 189 (2.8) | 317 (7.2) | 0.20 | 0.01 |
| Diuretics, n (%) | 495 (7.3) | 848 (19.2) | 0.36 | 0.00 |
| Insulin, n (%) | 7 (0.1) | 51 (1.2) | 0.13 | 0.01 |
| Non-insulin diabetic therapy | 43 (0.6) | 264 (6.0) | 0.30 | 0.08 |
| Fibrate or niacin, n (%) | 82 (1.2) | 172 (3.9) | 0.17 | 0.08 |
SD: Standard Deviation. IQR: Interquartile Range. CAC: Coronary artery calcium.
Associated ICD-9 codes for comorbid disorders are listed in Appendix. Diabetes Mellitus, Hypertension, and Hyperlipidemia defined as prior ICD-9 diagnosis or baseline diabetic, antilipid, or anti-hypertensive medical therapy.
Absolute Standardized difference = difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.10.
851 patients without race data.
Online Table 4:
Multivariate predictors of MACE in time-dependent analysis of statin use
| aSHR (95% CI) | p Value | |
|---|---|---|
| Statin treatment | 0.64 (0.52–0.78) | < 0.001 |
| CAC Group | <0.0001 | |
| 1–100 | 1.14 (0.89–1.44) | |
| 101–400 | 2.00 (1.48–2.69) | |
| > 400 | 3.51 (2.55–4.82) | |
| Age (per 10 year increase) | 1.69 (1.50–1.90) | <0.0001 |
| Beta-blocker* | 1.50 (1.14–1.98) | 0.0042 |
| Hyperlipidemia* | 1.42 (1.15–1.74) | <0.001 |
| Tobacco* | 1.75 (1.32–2.31) | <0.0001 |
| Year of CAC (per 1 year increase) | 0.94 (0.89–0.99) | 0.022 |
| ACE or ARB* | 1.18 (0.91–1.52) | 0.21 |
| Aspirin* | 0.91 (0.72–1.15) | 0.43 |
| Male sex | 1.18 (0.96–1.45) | 0.13 |
| Hypertension* | 1.04 (0.81–1.32) | 0.78 |
| Diabetes Mellitus* | 0.98 (0.73–1.33) | 0.92 |
| Charlton Comorbidity Score* | 1.14 (0.88–1.48) | 0.32 |
| Statin*CAC interaction (CAC=0 vs CAC > 0) | - | 0.82 |
aSHR – adjusted subhazard ratio. MACE –Major adverse cardiovascular event. CAC – Coronary artery calcium.
Multivariable predictors of MACE in Cox proportional hazards model treating statin as a time-dependent variable.
Risk factors, beta-blockers, aspirin, and ACE-I or ARB use assessed at baseline.
Online Table 5:
Multivariate predictors of MACE in statin strength analysis by terciles
| aSHR (95% CI) | p Value | |
|---|---|---|
| Statin strength | 0.0012 | |
| None (Reference) | - | |
| 1st tercile | 0.74 (0.53–1.03) | |
| 2nd tercile | 0.61 (0.43–0.88) | |
| 3rd tercile | 0.45 (0.29–0.70) | |
| CAC Group | <0.0001 | |
| 0 (Reference) | - | |
| 1–100 | 1.02 (0.67–1.56) | |
| 101–400 | 4.11 (2.42–6.95) | |
| > 400 | 4.80 (2.50–9.23) | |
| Age (per 10 year increase) | 1.63 (1.45–1.84) | <0.0001 |
| Beta-blocker* | 1.54 (1.16–2.04) | 0.0029 |
| Hyperlipidemia* | 1.69 (1.36–2.10) | <0.0001 |
| Tobacco* | 1.75 (1.35–2.39) | <0.0001 |
| Year of CAC (per 1 year increase) | 0.92 (0.88–0.97) | 0.037 |
| ACE or ARB* | 1.21 (0.93–1.56) | 0.16 |
| Aspirin* | 0.93 (0.73–1.17) | 0.53 |
| Male sex | 1.19 (0.97–1.47) | 0.096 |
| Hypertension* | 1.03 (0.81–1.32) | 0.81 |
| Diabetes Mellitus* | 1.00 (0.74–1.36) | 0.98 |
| Charlton Comorbidity Score* | 1.13 (0.86–1.47) | 0.39 |
aSHR – adjusted subhazard ratio. MACE –Major adverse cardiovascular event. CAC – Coronary artery calcium.
Multivariable predictors of MACE in Cox proportional hazards model treating the strength of statin as a time-dependent variable.
Risk factors, beta-blockers, aspirin, and ACE-I or ARB use assessed at baseline.
Online Table 6:
Multivariate predictors of MACE in time-dependent analysis of statin strength
| aSHR (95% CI) | p Value | ||||
|---|---|---|---|---|---|
| Statin strength (per 1 unit increase) | 0.76 (0.66–0.88) | 0.0002 | |||
| CAC Group | <0.0001 | ||||
| 1–100 | 1.13 (0.89–1.44) | ||||
| 101–400 | 2.00 (1.49–2.69) | ||||
| > 400 | 3.54 (2.57–4.88) | ||||
| Age (per 10 year increase) | 1.68 (1.48–1.89) | <0.0001 | |||
| Beta-blocker* | 1.51 (1.14–1.99) | 0.0039 | |||
| Hyperlipidemia* | 1.40 (1.14–1.72) | 0.0012 | |||
| Tobacco* | 1.75 (1.32–2.32) | <0.0001 | |||
| Year of CAC (per 1 year increase) | 0.94 (0.89–0.99) | 0.026 | |||
| ACE or ARB* | 1.18 (0.91–1.52) | 0.21 | |||
| Aspirin* | 0.91 (0.72–1.15) | 0.42 | |||
| Male sex | 1.18 (0.96–1.45) | 0.12 | |||
| Hypertension* | 1.03 (0.81–1.31) | 0.82 | |||
| Diabetes Mellitus* | 1.00 (0.74–1.34) | 0.97 | |||
| Charlton Comorbidity Score* | 1.14 (0.88–1.47) | 0.33 | |||
aSHR – adjusted subhazard ratio. MACE –Major adverse cardiovascular event. CAC – Coronary artery calcium.
Multivariable predictors of MACE in Cox proportional hazards model treating the strength of statin as a time-dependent variable.
Risk factors, beta-blockers, aspirin, and ACE-I or ARB use assessed at baseline.
Online Table 7:
Baseline demographics, comorbidities*, and medications for statin users stratified by baseline statin use prior to CAC screening
| Statin at Baseline (n = 3298) | Statin Only After CAC Screening (n = 3588) | Absolute Standardized Difference† | ||
|---|---|---|---|---|
| Before IPTW | After IPTW | |||
| Age, years, mean (SD) | 51.4 (9.1) | 50.8 (8.6) | 0.06 | 0.02 |
| Year of CAC score, median [IQR] | 2005 [2004–2007] | 2004 [2003–2007] | 0.19 | 0.09 |
| Charlson score, mean (SD) | 0.04 (0.24) | 0.02 (0.17) | 0.11 | 0.08 |
| Male, n (%) | 2513 (76.2) | 2660 (74.1) | 0.05 | 0.01 |
| Diabetes Mellitus, n (%) | 448 (13.6) | 239 (6.7) | 0.23 | 0.01 |
| Hypertension, n (%) | 1742 (52.8) | 1363 (38.0) | 0.30 | 0.08 |
| Hyperlipidemia, n (%) | 3298 (100.0) | 1865 (52.0) | 1.36 | 0.81 |
| Any tobacco use, n (%) | 302 (9.2) | 310 (8.6) | 0.02 | 0.05 |
| Atrial Fibrillation, n (%) | 55 (1.7) | 45 (1.3) | 0.03 | 0.01 |
| Race‡ | 0.06 | 0.10 | ||
| White | 2319 (75.0) | 2507 (75.3) | ||
| Black | 540 (17.5) | 541 (16.2) | ||
| Native American | 11 (0.4) | 13 (0.4) | ||
| Asian | 74 (2.4) | 105 (3.2) | ||
| Other | 147 (4.8) | 165 (5.0) | ||
| Coronary Artery Calcium Score | ||||
| 0, n (%) | 1821 (55.2) | 1921 (53.5) | 0.03 | 0.01 |
| 1–100, n (%) | 911 (27.6) | 1022 (28.5) | ||
| 101–400, n (%) | 361 (10.9) | 439 (12.2) | ||
| 401+, n (%) | 205 (6.2) | 206 (5.7) | ||
| Baseline Medications | ||||
| Aspirin, n (%) | 1157 (35.1) | 553 (15.4) | 0.46 | 0.07 |
| Anti-hypertensive, n (%) | 1426 (43.2) | 920 (25.6) | 0.38 | 0.09 |
| ACE-I, n (%) | 786 (23.8) | 417 (11.6) | 0.32 | 0.02 |
| ARB, n (%) | 243 (7.4) | 133 (3.7) | 0.16 | 0.05 |
| Beta-blocker, n (%) | 376 (11.4) | 263 (7.3) | 0.14 | 0.04 |
| Calcium channel blocker, n (%) | 259 (7.9) | 177 (4.9) | 0.12 | 0.04 |
| Diuretics, n (%) | 722 (21.9) | 452 (12.6) | 0.25 | 0.12 |
| Insulin, n (%) | 51 (1.5) | 15 (0.4) | 0.11 | 0.14 |
| Non-insulin diabetic therapy | 257 (7.8) | 81 (2.3) | 0.26 | 0.11 |
| Fibrate or niacin, n (%) | 133 (4.0) | 126 (3.5) | 0.03 | 0.09 |
SD: Standard Deviation. IQR: Interquartile Range. CAC: Coronary artery calcium.
Associated ICD-9 codes for comorbid disorders are listed in Online Table 2. Diabetes Mellitus, Hypertension, and Hyperlipidemia defined as prior ICD-9 diagnosis or baseline diabetic, anti-lipid, or anti-hypertensive medical therapy.
Absolute Standardized difference = difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.20 (small effect size).
464 patients without race data.
Online Table 8:
Baseline demographics, comorbidities*, and medications for patients with < 50% and > 50% statin exposure during the follow-up period and before MACE
| Less than 50% Compliance (n = 2471) | Greater than 50% Compliance (n = 4415) | Absolute Standardized Difference† | ||
|---|---|---|---|---|
| Before IPTW | After IPTW | |||
| Age, years, mean (SD) | 48.61 (8.23) | 52.45 (8.89) | 0.45 | 0.02 |
| Year of CAC score, median [IQR] | 2005 [2003–2007] | 2005 [2003–2007] | 0.15 | <0.01 |
| Charlson score, mean (SD) | 0.03 (0.19) | 0.03 (0.22) | <0.01 | -0.01 |
| Male, n (%) | 1866 (75.5) | 3307 (74.9) | 0.01 | <0.01 |
| Diabetes Mellitus, n (%) | 182 (7.4) | 505 (11.4) | 0.14 | <0.01 |
| Hypertension, n (%) | 899 (36.4) | 2206 (50.0) | 0.28 | <0.01 |
| Hyperlipidemia, n (%) | 1645 (66.6) | 3518 (79.7) | 0.30 | <0.01 |
| Any tobacco use, n (%) | 261 (10.6) | 351 (8.0) | 0.09 | 0.01 |
| Atrial Fibrillation, n (%) | 24 (1.0) | 76 (1.7) | 0.07 | 0.02 |
| Race‡ | 0.25 | 0.24 | ||
| White | 1604 (68.5) | 3222 (79.0) | ||
| Black | 526 (22.5) | 555 (13.6) | ||
| Native American | 13 (0.6) | 11 (0.3) | ||
| Asian | 76 (3.2) | 103 (2.5) | ||
| Other | 122 (5.2) | 190 (4.7) | ||
| Coronary Artery Calcium Score | ||||
| 0, n (%) | 1611 (65.2) | 2131 (48.3) | 0.39 | 0.03 |
| 1–100, n (%) | 609 (24.6) | 1324 (30.0) | ||
| 101–400, n (%) | 181 (7.3) | 619 (14.0) | ||
| 401+, n (%) | 70 (2.8) | 341 (7.7) | ||
| Baseline Medications | ||||
| Aspirin, n (%) | 479 (19.4) | 1231 (27.9) | 0.20 | 0.01 |
| Anti-hypertensive, n (%) | 620 (25.1) | 1726 (39.1) | 0.30 | 0.01 |
| ACE-I, n (%) | 280 (11.3) | 923 (20.9) | 0.26 | 0.01 |
| ARB, n (%) | 108 (4.4) | 268 (6.1) | 0.08 | 0.03 |
| Beta-blocker, n (%) | 155 (6.3) | 484 (11.0) | 0.17 | 0.02 |
| Calcium channel blocker, n (%) | 119 (4.8) | 317 (7.2) | 0.10 | 0.01 |
| Diuretics, n (%) | 326 (13.2) | 848 (19.2) | 0.16 | <0.01 |
| Insulin, n (%) | 15 (0.6) | 51 (1.2) | 0.06 | 0.01 |
| Non-insulin diabetic therapy | 74 (3.0) | 264 (6.0) | 0.15 | 0.03 |
| Fibrate or niacin, n (%) | 87 (3.5) | 172 (3.9) | 0.02 | 0.03 |
SD: Standard Deviation. IQR: Interquartile Range. CAC: Coronary artery calcium.
Associated ICD-9 codes for comorbid disorders are listed in Appendix. Diabetes Mellitus, Hypertension, and Hyperlipidemia defined as prior ICD-9 diagnosis or baseline diabetic, antilipid, or anti-hypertensive medical therapy.
Absolute Standardized difference = difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.10.
464 patients without race data.
Online Table 9:
Baseline demographics, comorbidities*, and medications for patients stratified by statin use after CAC score and prior to two years or MACE
| Statin (n = 5589) | No Statin (n = 8055) | Absolute Standardized Difference† | ||
|---|---|---|---|---|
| Before IPTW | After IPTW | |||
| Age, years, mean (SD) | 51.4 (9.0) | 48.4 (7.7) | 0.35 | 0.04 |
| Year of CAC score, median [IQR] | 2005 [2003–2007] | 2005 [2003–2007] | 0.11 | 0.01 |
| Charlson score, mean (SD) | 0.04 (0.21) | 0.02 (0.15) | 0.09 | 0.05 |
| Male, n (%) | 4247 (76.0) | 5385 (66.9) | 0.20 | 0.01 |
| Diabetes Mellitus, n (%) | 607 (10.9) | 321 (4.0) | 0.26 | 0.02 |
| Hypertension, n (%) | 2689 (48.1) | 1954 (24.3) | 0.51 | 0.01 |
| Hyperlipidemia, n (%) | 4596 (82.2) | 2152 (26.7) | 1.34 | <0.01 |
| Any tobacco use, n (%) | 526 (9.4) | 445 (5.5) | 0.15 | <0.01 |
| Atrial Fibrillation, n (%) | 89 (1.6) | 64 (0.8) | 0.07 | 0.02 |
| Race‡ | 0.05 | 0.07 | ||
| White | 3940 (75.3) | 5741 (77.3) | ||
| Black | 890 (17.0) | 1136 (15.3) | ||
| Native American | 19 (0.4) | 30 (0.4) | ||
| Asian | 147 (2.8) | 212 (2.9) | ||
| Other | 236 (4.5) | 312 (4.2) | ||
| Coronary Artery Calcium Score | ||||
| 0, n (%) | 2840 (50.8) | 6520 (80.9) | 0.71 | 0.05 |
|
1–100, n (%) |
1625 (29.1) | 1252 (15.5) | ||
|
101–400, n (%) |
732 (13.1) | 222 (2.8) | ||
| 401+, n (%) | 392 (7.0) | 61 (0.8) | ||
| Baseline Medications | ||||
| Aspirin, n (%) | 1549 (27.7) | 637 (7.9) | 0.54 | 0.01 |
| Anti-hypertensive, n (%) | 2078 (37.2) | 1263 (15.7) | 0.50 | 0.02 |
| ACE-I, n (%) | 1081 (19.3) | 505 (6.3) | 0.40 | 0.03 |
| ARB, n (%) | 337 (6.0) | 178 (2.2) | 0.19 | 0.04 |
| Beta-blocker, n (%) | 579 (10.4) | 316 (3.9) | 0.25 | 0.01 |
| Calcium channel blocker, n (%) | 380 (6.8) | 245 (3.0) | 0.17 | 0.01 |
| Diuretics, n (%) | 1049 (18.8) | 620 (7.7) | 0.33 | 0.02 |
| Insulin, n (%) | 63 (1.1) | 10 (0.1) | 0.13 | 0.02 |
| Non-insulin diabetic therapy | 310 (5.5) | 71 (0.9) | 0.27 | 0.06 |
| Fibrate or niacin, n (%) | 215 (3.8) | 126 (1.6) | 0.14 | 0.11 |
SD: Standard Deviation. IQR: Interquartile Range. CAC: Coronary artery calcium.
Associated ICD-9 codes for comorbid disorders are listed in Online Table 2. Diabetes Mellitus, Hypertension, and Hyperlipidemia defined as prior ICD-9 diagnosis or baseline diabetic, anti-lipid, or anti-hypertensive medical therapy.
Absolute Standardized difference = difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.20 (small effect size).
981 patients without race data.
Online Table 10:
Baseline demographics, comorbidities*, and medications in diabetic patients stratified by statin use after CAC score and prior to five years or MACE
| Statin (n = 687) | No Statin (n = 241) | Absolute Standardized Difference† | ||
|---|---|---|---|---|
| Before IPTW | After IPTW | |||
| Age, years, mean (SD) | 55.3 (8.8) | 51.3 (8.3) | 0.47 | 0.01 |
| Year of CAC score, median [IQR] | 2005 [2004–2007] | 2005 [2003–2007] | 0.09 | 0.02 |
| Charlson score, mean (SD) | 0.20 (0.51) | 0.12 (0.38) | 0.16 | 0.03 |
| Male, n (%) | 468 (68.1) | 130 (53.9) | 0.29 | 0.02 |
| Hypertension, n (%) | 553 (80.5) | 128 (53.1) | 0.61 | 0.03 |
| Hyperlipidemia, n (%) | 597 (86.9) | 111 (46.1) | 0.96 | 0.04 |
| Any tobacco use, n (%) | 78 (11.4) | 14 (5.8) | 0.20 | 0.17 |
| Atrial Fibrillation, n (%) | 11 (1.6) | 2 (0.8) | 0.07 | 0.11 |
| Race‡ | 0.10 | 0.10 | ||
| White | 412 (64.4) | 138 (63.3) | ||
| Black | 175 (27.3) | 61 (28.0) | ||
| Other | 53 (8.3) | 19 (8.7) | ||
| Coronary Artery Calcium Score | ||||
| 0, n (%) | 320 (46.6) | 179 (74.3) | 0.66 | 0.01 |
| 1–100, n (%) | 199 (29.0) | 51 (21.2) | ||
| 101–400, n (%) | 98 (14.3) | 6 (2.5) | ||
| 401+, n (%) | 70 (10.2) | 5 (2.1) | ||
| Baseline Medications | ||||
| Aspirin, n (%) | 320 (46.6) | 50 (20.7) | 0.57 | 0.05 |
| Anti-hypertensive, n (%) | 485 (70.6) | 86 (35.7) | 0.75 | 0.04 |
| ACE-I, n (%) | 343 (49.9) | 40 (16.6) | 0.76 | 0.15 |
| ARB, n (%) | 87 (12.7) | 14 (5.8) | 0.24 | 0.07 |
| Beta-blocker, n (%) | 133 (19.4) | 20 (8.3) | 0.32 | 0.04 |
| Calcium channel blocker, n (%) | 103 (15.0) | 17 (7.1) | 0.26 | 0.02 |
| Diuretics, n (%) | 222 (32.2) | 41 (17.0) | 0.36 | 0.11 |
| Insulin, n (%) | 65 (9.5) | 7 (2.9) | 0.27 | 0.16 |
| Non-insulin diabetic therapy | 338 (49.2) | 43 (17.8) | 0.70 | 0.01 |
| Fibrate or niacin, n (%) | 56 (8.2) | 13 (5.4) | 0.11 | 0.13 |
SD: Standard Deviation. IQR: Interquartile Range. CAC: Coronary artery calcium.
Associated ICD-9 codes for comorbid disorders are listed in Appendix. Diabetes Mellitus, Hypertension, and Hyperlipidemia defined as prior ICD-9 diagnosis or baseline diabetic, anti-lipid, or anti-hypertensive medical therapy.
Absolute Standardized difference = difference in means or proportions divided by standard error; imbalance defined as absolute value greater than 0.10.
70 patients without race data
References for Online Appendix
- 1.Stone NJ, Robinson J, Lichtenstein AH et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013. [DOI] [PubMed] [Google Scholar]
- 2.Smith MEB, Lee NJ, Haney E, Carson S Drug Class Reviews Drug Class Review: HMG-CoA Reductase Inhibitors (Statins) and Fixed-dose Combination Products Containing a Statin: Final Report Update 5. Portland (OR): Oregon Health & Science University, Portland, Oregon., 2009. [PubMed] [Google Scholar]
- 3.Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothman KJ Induction and latent periods. Am J Epidemiol 1981;114:253–9. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen LH, Lokkegaard E, Andreasen AH, Keiding N Using prescription registries to define continuous drug use: how to fill gaps between prescriptions. Pharmacoepidemiol Drug Saf 2008;17:384–8. [DOI] [PubMed] [Google Scholar]
- 6.Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 2017;377:2228–2239. [DOI] [PubMed] [Google Scholar]
- 7.Lauffenburger JC, Shrank WH, Bitton A, et al. Association between patientcentered medical homes and adherence to chronic disease medications: A cohort study. Ann Intern Med 2017;166:81–88. [DOI] [PubMed] [Google Scholar]
- 8.Goff DC Jr., Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
Disclosures: The authors have no relationships with industry relevant to this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piepoli MF, Hoes AW, Agewall S et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson J, Lichtenstein AH et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934. [DOI] [PubMed] [Google Scholar]
- 3.Yeboah J, McClelland RL, Polonsky TS et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012;308:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell J, Paisley R, Moon P, Novak E, Villines T. Coronary Artery Calcium Score and Long-term Risk of Death, Myocardial Infarction and Stroke: The Walter Reed Cohort Study. JACC Cardiovasc Imag. 2017. November 10. doi: 10.1016/j.jcmg.2017.09.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Hecht H, Blaha MJ, Berman DS et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017;11:157–168. [DOI] [PubMed] [Google Scholar]
- 6.Nasir K, Bittencourt MS, Blaha MJ et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 7.Blaha MJ, Cainzos-Achirica M, Greenland P et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC Jr., Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol 2005;46:166–72. [DOI] [PubMed] [Google Scholar]
- 10.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol 2005;46:807–814. [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Nasir K, McClelland RL et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2009;53:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 2010;304:411–8. [DOI] [PubMed] [Google Scholar]
- 14.Graham DJ, Reichman ME, Wernecke M et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–64. [DOI] [PubMed] [Google Scholar]
- 15.Tirschwell DL, Longstreth WT Jr . Validating administrative data in stroke research. Stroke 2002;33:2465–70. [DOI] [PubMed] [Google Scholar]
- 16.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
- 17.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf 2008;17:20–6. [DOI] [PubMed] [Google Scholar]
- 18.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 2005;36:1776–81. [DOI] [PubMed] [Google Scholar]
- 19.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol 2002;12:462–8. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ. Induction and latent periods. Am J Epidemiol 1981;114:253–9. [DOI] [PubMed] [Google Scholar]
- 21.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol. 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Statistic Assoc 1999;94:496–509. [Google Scholar]
- 24.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Shin DW, Yun JM et al. Medication Adherence and the Risk of Cardiovascular Mortality and Hospitalization Among Patients With Newly Prescribed Antihypertensive Medications. Hypertension 2016;67:506–12. [DOI] [PubMed] [Google Scholar]
- 26.Lauffenburger JC, Shrank WH, Bitton A, et al. Association between patient-centered medical homes and adherence to chronic disease medications: A cohort study. Ann Intern Med 2017;166:81–88. [DOI] [PubMed] [Google Scholar]
- 27.Prentice RL, Kalbfleisch JD, Peterson AV Jr., Flournoy N, Farewell VT, Breslow NE The analysis of failure times in the presence of competing risks. Biometrics 1978;34:541–54. [PubMed] [Google Scholar]
- 28.Mantel N, Bayar D. Evaluation of response-time data involving transient states: An illustration using heart-transplant data. J Am Stat Assoc 1974;69:81–86. [Google Scholar]
- 29.Nielsen LH, Lokkegaard E, Andreasen AH, Keiding N. Using prescription registries to define continuous drug use: how to fill gaps between prescriptions. Pharmacoepidemiol Drug Saf 2008;17:384–8. [DOI] [PubMed] [Google Scholar]
- 30.Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O. Contemporary Hormonal Contraception and the Risk of Breast Cancer. N Engl J Med 2017;377:2228–2239. [DOI] [PubMed] [Google Scholar]
- 31.Mamdani M, Sykora K, Li P et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005;330:960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasir K, Bittencourt MS, Blaha MJ et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657–68. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen MB, Falk E, Li D et al. Statin Trials, Cardiovascular Events, and Coronary Artery Calcification: Implications for a Trial-Based Approach to Statin Therapy in MESA. JACC Cardiovasc Imaging 2017.
- 34.Martin SS, Blaha MJ, Blankstein R et al. Dyslipidemia, coronary artery calcium, and incident atherosclerotic cardiovascular disease: implications for statin therapy from the multiethnic study of atherosclerosis. Circulation 2014;129:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaha MJ, Budoff MJ, DeFilippis AP et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet 2011;378:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor F, Huffman MD, Macedo AF et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013:Cd004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, MacFadyen JG, Fonseca FAH et al. Number Needed to Treat With Rosuvastatin to Prevent First Cardiovascular Events and Death Among Men and Women With Low Low-Density Lipoprotein Cholesterol and Elevated High-Sensitivity C-Reactive Protein. Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes. 2009;2:616–623. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, McEvoy JW, Nasir K et al. Critical review of high-sensitivity C-reactive protein and coronary artery calcium for the guidance of statin allocation: head-to-head comparison of the JUPITER and St. Francis Heart Trials. Circ Cardiovasc Qual Outcomes 2014;7:315–22. [DOI] [PubMed] [Google Scholar]
- 39.Huo J, Yang M, Tina Shih Y-C. Sensitivity of Claims-Based Algorithms to Ascertain Smoking Status More Than Doubled with Meaningful Use. Value in Health 2017. [DOI] [PubMed] [Google Scholar]
- 40.Nayan M, Hamilton RJ, Finelli A, Austin PC, Kulkarni GS, Juurlink DN. The value of complementing administrative data with abstracted information on smoking and obesity: A study in kidney cancer. Canadian Urological Association J. 2017;11:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrank WH, Patrick AR, Alan Brookhart M. Healthy User and Related Biases in Observational Studies of Preventive Interventions: A Primer for Physicians. J General Intern Med. 2011;26:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]