Abstract
Background & Aims:
Colorectal cancer (CRC) deaths occur when patients do not receive screening or have inadequate follow up of abnormal results, or when the screening test itself fails. We have few data on the contribution of each to CRC-associated deaths or factors associated with these events.
Methods:
We performed a retrospective cohort study of patients in the Kaiser Permanente Northern and Southern California systems (55–90 years old) who died from CRC from 2006 through 2012 and had ≥5 years of enrollment prior to diagnosis. We compared data from patients with a matched cohort of cancer-free patients in the same system. Receipt, results, indications, and follow up of CRC tests in the 10-year period prior to diagnosis were obtained from electronic databases and chart audits.
Results:
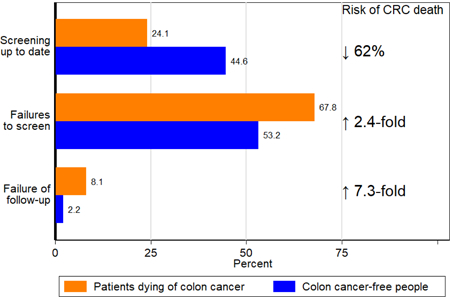
Among 1750 CRC deaths, 75.9% (n=1328) occurred in patients who were not up to date in screening and 24.1% (n=422) occurred in patients who were up to date. Failure to screen was associated with fewer visits to primary care physicians. Among 3486 cancer-free patients, 44.6% were up to date on their screening. Patients who were up to date in their screening had reduced risk of CRC death (odds ratio [OR], 0.38; 95% CI, 0.33–0.44). Failure to screen, or failure to screen at appropriate intervals, occurred in a 67.8% of patients who died from CRC vs 53.2% of cancer-free patients; failure to follow up on abnormal results occurred in 8.1% of patients who died from CRC vs 2.2% of cancer-free patients. CRC death was associated with higher odds of failure to screen or failure to screen at appropriate intervals (OR, 2.40; 95% CI, 2.07–2.77) and failure to follow up on abnormal results (OR, 7.26; 95% CI, 5.26–10.03).
Conclusions:
Being up to date on screening substantially reduces risk of CRC death. In 2 healthcare systems with high rates of screening, most people who died from CRC had failures in the screening process that could be rectified, such as failure to follow up on abnormal findings; these significantly increased risk for CRC death.
Keywords: colon cancer, adenoma, early detection, cancer prevention
Graphical Abstract
INTRODUCTION
Colorectal cancer (CRC) remains a leading cause of cancer death,1 with about 694,000 deaths worldwide in 2012 and projected to increase to 1.1 million deaths by 2030.2 Use of screening in average-risk persons beginning at age 50 with currently recommended strategies, including colonoscopy, sigmoidoscopy, and fecal testing, is effective at reducing the risk of death from CRC.3–6 Thus, most deaths from CRC are believed to result from breakdowns in screening processes, particularly failures to undergo screening or stay current with screening.7 However, deaths may also result from patients’ failures to undergo surveillance endoscopy at recommended intervals after adenoma removal4, 6 or to not receive timely follow-up testing for positive screening results,8 which has been shown to increase the risk of CRC diagnosis, including advanced-stage disease.9, 10 Deaths from CRC also occur in people who are screening up-to-date, because of lesions missed during screening or interval cancers that develop before the next scheduled screening.11 However, no studies to date have examined detailed screening histories and their relation to death from CRC.
Previous studies in cervical and breast cancer have helped to identify targets for reducing screening failures for those cancers,12–14 but similar information about CRC screening is lacking, particularly from settings with robust data systems and screening programs.15 This study characterized failures in the screening process continuum over an extended period of time in patients who died from CRC in two large integrated healthcare systems with high screening rates to help identify targets for interventions to further decrease CRC mortality rates in diverse settings. We also evaluated the screening histories of cancer-free patients and the association between being screening-up-to-date (by any test) and various types of failures in the screening process and the risk of death for CRC.
METHODS
Study Population and Setting
Data for this study were derived from screening-eligible members of Kaiser Permanente Northern California (KPNC) and Southern California (KPSC), two large integrated healthcare systems that provide care for approximately seven million members. Both health systems successfully implemented organized screening outreach programs that began between 2006 and 2008. The programs use fecal immunochemical testing (FIT) as the primary screening strategy, or colonoscopy by patient or provider request.16 CRC screening rates in these two health systems have increased over time, reaching over 80% in 2012, exceeding the national average of 58% in 2012 or 61% in 2015.16–19 This study was approved by the institutional review boards of KPNC, KPSC, and the University of Pennsylvania.
Data were collected as part of a large observational study that evaluated the effectiveness of screening in preventing CRC deaths, which has been fully described previously.20, 21 In that study, eligible patients were 55–90 years of age at the death date from CRC during 2006–2012 in KPNC or 2011–2012 in KPSC, and were enrolled in their respective health plans for ≥5 years prior to their CRC diagnosis date.21 The cohort was further restricted to those who died of adenocarcinomas because of strong evidence that detection of precancerous adenomas and early-stage cancers reduces the risk for death from these cancer types. We excluded those with a history of inflammatory bowel disease or a strong family history of CRC as defined previously.18,19 We also excluded patients with missing medical charts.
We restricted this study to people diagnosed between 2002–2012 because screening was less common in the general US population prior to that period and Medicare began coverage for colonoscopy in mid-2001.22 We included a CRC-free cohort of patients matched to those who died from CRC, using an incidence-density matching approach, on the diagnosis date on age, sex, study site, and years of enrollment in the health plan.20, 21 The diagnosis date was used as the reference date for ascertaining screening histories in both groups of patients.
Data collection
Electronic medical records, administrative, and tumor and vital status registry data were used to identify study patients and variables. Information on CRC diagnosis, tumor location and receipt of initial treatment were ascertained from tumor registries. We categorized tumors in the cecum, ascending colon, and transverse colon as right colon cancers, those in the descending, sigmoid, and rectosigmoid colon as left colon cancers. Rectal tumors were categorized separately and others as unspecified. Cause-specific vital status was obtained from KPNC and KPSC mortality files which were obtained from state and federal death registries that were in turn derived mostly from information in death certificates.21
Age, sex, race/ethnicity, healthcare utilization, and clinical histories (to construct the Charlson comorbidity score at the 5-year point prior to the diagnosis date) were obtained from electronic databases and medical records.20, 21 The number of outpatient visits to primary care physicians (PCP: family medicine, internal medicine, geriatrics, and obstetrics and gynecology) was enumerated in the 5-year period before CRC diagnosis, but excluded the 90-day period prior to diagnosis. Socioeconomic status (SES) was estimated using the percentage of people ≥25 years with less than a high school diploma in each patient’s census tract in the 2000 census.23, 24
The date, type, reason and results (number, size, types, and location of lesions, or positive or negative) of CRC tests (colonoscopy, sigmoidoscopy, barium enema (BE), and fecal tests) received in the 10-year period before diagnosis were collected from electronic data and chart audits performed by trained reviewers using standardized processes.21 The results of fecal tests (FIT or guaiac fecal occult blood tests (FOBT)) and other relevant laboratory abnormalities, such as iron deficiency anemia, triggering diagnostic testing were extracted from laboratory databases.
Classification of CRC Test Indication and Screening History
The indication for each CRC test was assigned as screening, surveillance, or diagnostic using a multi-step approach that included expert review of individual patients’ data on selected tests as described previously.21 Briefly, a test was considered surveillance if it followed adenoma or polyp finding from a previous endoscopic test, diagnostic if it was for the work-up of relevant symptoms or a prior positive, abnormal, or incomplete test, and screening if it was indicated as such in the chart.25
We classified patients’ testing histories by adapting the screening process framework of Zapka et al. for breast and cervical cancer12 with consideration for the multiplicity and different testing intervals for CRC screening, and the potential for screening to both detect cancers early and prevent disease through removal of precancerous lesions.19 Patients were classified as failures to screen (and screen at appropriate intervals), failure to follow-up an abnormal screening result, or failure of the screening test to prevent CRC death as defined below.
The classifications were based primarily on whether or not a patient was up-to-date by using the 2008 multi-society CRC screening guidelines,26 defined as having received a colonoscopy within 10 years, or sigmoidoscopy and/or BE within 5 years of CRC diagnosis. For FIT/FOBT, we used a 2-year interval because of evidence of effectiveness for this interval in clinical trials and its common use worldwide.3 Patients who received CRC testing for work-up of symptoms and had a negative result were considered screening up-to-date for the testing interval of that modality; for example, a person with a negative colonoscopy (that had adequate bowel preparation and was complete to the cecum) for a diagnostic indication of blood per rectum would be considered screening up-to-date for 10 years. Up-to-date status for surveillance was based on the performing physician’s recommendation or relevant guidelines, if not specified.11 When assigning follow-up intervals based on surveillance guidelines, and, in part, because we did not have histology data, we used information on the number and size of polyps detected and used the shorter of recommended intervals such as 5 years for 1–2 polyps. Patients with incomplete tests (e.g. inadequate bowel preparation for a colonoscopy) were considered up-to-date if the same or an appropriate alternative test was completed within six months of the original test date.
Thus, failures to screen (also referred to as “all failures to screen, combined”) was defined as not being up-to-date with screening on the CRC diagnosis date (or reference date for cancer-free patients), but excluded failure to follow-up on abnormal results. We further subdivided the failures to screen group into: 1) failure to ever screen, 2) failure to screen at appropriate intervals, and 3) failure to receive appropriate surveillance (Table 1 and Figure 1). The subcategories accounted for timely initiation of screening, rescreening at recommended intervals, and surveillance after polypectomy, respectively, as crucial processes in the screening continuum. Patients whose only relevant history was diagnostic testing in the six-month period prior to CRC diagnosis (considered part of cancer work-up) were classified as failure to ever screen. Failure to follow-up an abnormal screening result was defined as no follow-up diagnostic colonoscopy within 9 months after a positive fecal test;9, 10 for sigmoidoscopy or BE, the 9-month interval or as recommended by the performing provider, whichever was longer, was used. Failure of the screening test was defined as being up-to-date with screening including timely follow-up and surveillance.
Table 1:
Testing categories, definitions, and implications for patients dying of colorectal cancer, n=1,750
| CATEGORY | N (%) | Descriptions | Intervention |
|---|---|---|---|
| 1. Failure to screen or screen at appropriate interval |
1,187 (67.8) |
||
| 1a. Failure to screen |
591 (33.8) | No screening test during the 10-year period |
Improve access/uptake of screening |
| 1b. Failure to screen at appropriate intervals |
574 (32.8) | Receive screening but had clinically important gaps; For instance, initiated screening but not up to date on the diagnosis date, or did not initiate screening for several years after becoming age-eligible and had cancer diagnosis by first screening test (had colonoscopy >10 years, sigmoidoscopy >5 years, or FOBT >2 years prior to diagnosis; or unscreened 5–10 years after becoming age-eligible and cancer diagnosed by the only screening test received |
Close gaps in screening among screening eligible population; improve initiation of screening and regular re-screening; identify and target those who did not initiate screening or initiated, but did not continue |
| 1c. Failure to receive surveillance |
22 (1.3) | Initiated screening (>1 year before diagnosis) and had adenoma but did not have follow-up surveillance as recommended or appropriate follow-up was not recommended for patient |
Improve adherence to, or recommendations for, surveillance colonoscopy |
| 2. Failure to follow-up for positive screening |
141 (8.1) | Had screening test and was positive but had no timely follow-up visit or diagnostic testing |
Improve access to follow- up evaluation or the targeting of screening to those who are candidates for colonoscopy to minimize misuse |
| 3. Failure of the screening test |
422 (24.1) | Was up-to-date at the cancer diagnosis date, including those who had a positive test and had diagnostic testing. |
Improve the effectiveness of screening tests or of diagnostic testing after a positive result |
Note: Some patients classified as up-to-date with screening received a negative test for a diagnostic indication .
Figure 1.
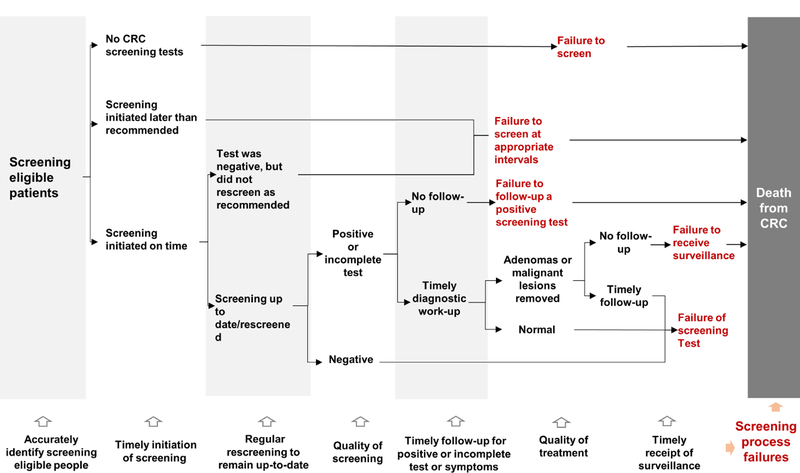
Colorectal cancer test exposure trajectories and failures in people who died from colorectal cancer, KPNC and KPSC 2006–2012
Patients experiencing ≥2 types of failures were classified based on the type closest to the cancer diagnosis date. For example, a person who delayed colonoscopy after a positive FIT, but who, at the delayed colonoscopy, had an polypectomy and subsequently failed to complete recommended surveillance, was classified as a failure to receive surveillance.
Statistical Analysis
Differences in the proportions of screening failure types were analyzed in patients who died of CRC and in matched cancer-free patients. Analyses were also performed according to diagnosis age (<55, 55–64, 65–74, 75–84, and 85+ years), sex, race/ethnicity (Non-Hispanic white, Non-Hispanic black, Hispanic, Asian/Pacific Islander, and Other), number of PCP visits (0, 1, 2 and 3+), diagnosis year, and tumor location (right, left, rectal, and unspecified) using Chi-square tests. We then used unconditional logistic regression models that simultaneously adjusted for the above-mentioned factors as well as comorbidity score and SES to assess, separately, associations with failure to ever screen and all failures to screen, combined; all test hypotheses were two-sided. We repeated these analyses restricted to patients with documented receipt of initial cancer treatment in tumor registry data.
We fitted three separate conditional regression models to evaluate the association between being screening up-to-date and failures in the screening process and risk of CRC deaths adjusting for race-ethnicity, SES, comorbidity score, and visits with PCP. In Model 1, we estimated the association between being screening up-to-date compared with a reference group comprised of all screening process failures. For context, we provide the estimate for the association between not being up-to-date and risk of CRC death. In Model 2, we used an indicator for all failures to screen, combined plus an indicator for failure to follow-up; being screening up-to-date was the reference group. In Model 3, we used separate indicators for each of the screening process failures (failure to ever screen, screen at appropriate intervals, receive surveillance, and follow-up on abnormal result); being up-to-date was the reference group. In sensitivity analyses, we used 2X2 contingency tables and unadjusted regression models to evaluate the association between failure of follow-up and risk of death among those with abnormal/positive results. Analyses were performed using STATA version 14.2 (StataCorp LP, College Station, Texas).
RESULTS
We identified a total of 1791 patients who died from adenocarcinoma of the colon and rectum between 2006 and 2012, and analyzed 1750 after exclusions for history of inflammatory bowel disease identified on chart audit (n=1), family CRC history (n=29), missing medical charts (n=3), and CRC diagnosis prior to 2002 (n=8). The average diagnosis age of the patients was 70 years and 49.5% were female, 67.0% non-Hispanic white, 12.0% non-Hispanic black, 9.4% Hispanic, and 8.9% Asian-Pacific Islander (Table 2). Most patients had a Charlson comorbidity score of zero. About 84.2% (n=1474) received initial cancer treatment, 5.4% (n=94) did not receive treatment, 1.4% (n=24) refused treatment, and 9.0% (n=158) had unknown treatment status. The characteristics of patients with documented receipt of initial treatment (n=1474) were similar to the overall study population (Supporting Table 1). The characteristics of the cancer-free matched patients (n=3486) are shown in Supporting Table 2.
Table 2:
Characteristics of patients who died from colorectal cancer according to testing categories, KPSC and KPNC 2006–2012
| Characteristics (row %) |
Failures to screen | Failure to follow-up for positive screening |
Failure of the screening testa |
Total | ||
|---|---|---|---|---|---|---|
| Failure to screen |
Failure to screen at appropriate intervals |
Failure to receive surveillance |
||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N | |
| TOTAL | 591 (33.8) | 574 (32.8) | 22 (1.3) | 141 (8.1) | 422 (24.1) | 1,750 |
|
Age at diagnosis (years) |
||||||
| <55 | 10 (43.5) | 9 (39.1) | 1 (4.3) | 0 (0.0) | 3 (13) | 23 |
| 55–64 | 211 (37.0) | 193 (33.9) | 1 (0.2) | 25 (4.4) | 140 (24.6) | 570 |
| 65–74 | 145 (28.7) | 172 (34.0) | 10 (2.0) | 57 (11.3) | 122 (24.1) | 506 |
| 75–84 | 185 (33.2) | 172 (30.9) | 9 (1.6) | 54 (9.7) | 137 (24.6) | 557 |
| 85+ | 40 (42.6) | 28 (29.8) | 1 (1.1) | 5 (5.3) | 20 (21.3) | 94 |
| Race/ethnicity | ||||||
| NH white | 388 (33.1) | 388 (33.1) | 19 (1.6) | 90 (7.7) | 287 (24.5) | 1,172 |
| NH black | 68 (32.4) | 74 (35.2) | 1 (0.5) | 16 (7.6) | 51 (24.3) | 210 |
| Hispanic | 61 (37.2) | 48 (29.3) | 1 (0.6) | 13 (7.9) | 41 (25) | 164 |
| Asian/PI | 61 (39.1) | 52 (33.3) | 0 (0.0) | 15 (9.6) | 28 (17.9) | 156 |
| Other | 13 (27.1) | 12 (25.0) | 1 (2.1) | 7 (14.6) | 15 (31.3) | 48 |
| Sex | ||||||
| Men | 289 (33.3) | 288 (33.2) | 10 (1.2) | 63 (7.3) | 218 (25.1) | 868 |
| Women | 124 (33.0) | 130 (34.6) | 4 (1.1) | 30 (8.0) | 88 (23.4) | 376 |
|
Percent with <HS education (quartiles)b |
||||||
| 1 | 124 (33.0) | 130 (34.6) | 4 (1.1) | 30 (8.0) | 88 (23.4) | 376 |
| 2 | 142 (33.7) | 136 (32.3) | 6 (1.4) | 35 (8.3) | 102 (24.2) | 421 |
| 3 | 158 (36.2) | 140 (32.0) | 6 (1.4) | 28 (6.4) | 105 (24) | 437 |
| 4 | 154 (32.2) | 157 (32.8) | 6 (1.3) | 46 (9.6) | 116 (24.2) | 479 |
| Missing | 13 (35.1) | 11 (29.7) | 0 (0.0) | 2 (5.4) | 11 (29.7) | 37 |
|
Health plan enrollment (years) |
||||||
| 5.0–7.4 | 100 (33.2) | 97 (32.2) | 3 (1.0) | 21 (7.0) | 80 (26.6) | 301 |
| 7.5–9.9 | 108 (34.3) | 116 (36.8) | 4 (1.3) | 24 (7.6) | 63 (20) | 315 |
| 10 or longer | 383 (33.8) | 361 (31.8) | 15 (1.3) | 96 (8.5) | 279 (24.6) | 1,134 |
|
Number of PCP Visitsc |
||||||
| 0 | 53 (65.4) | 23 (28.4) | 1 (1.2) | 2 (2.5) | 2 (2.5) | 81 |
| 1 | 26 (57.8) | 15 (33.3) | 0 (0.0) | 3 (6.7) | 1 (2.2) | 45 |
| 2 | 45 (46.9) | 31 (32.3) | 2 (2.1) | 7 (7.3) | 11 (11.5) | 96 |
| 3+ | 467 (30.6) | 505 (33.0) | 19 (1.2) | 129 (8.4) | 408 (26.7) | 1,528 |
| Charlson scored | ||||||
| 0 | 426 (35.9) | 400 (33.7) | 13 (1.1) | 79 (6.7) | 268 (22.6) | 1,186 |
| 1 | 79 (26.8) | 99 (33.6) | 4 (1.4) | 24 (8.1) | 89 (30.2) | 295 |
| 2+ | 86 (32.0) | 75 (27.9) | 5 (1.9) | 38 (14.1) | 65 (24.2) | 269 |
| Diagnosis year | ||||||
| 2002–2005 | 96 (36.2) | 90 (34.0) | 3 (1.1) | 15 (5.7) | 61 (23) | 265 |
| 2006–2008 | 233 (33.7) | 273 (39.5) | 9 (1.3) | 39 (5.6) | 137 (19.8) | 691 |
| 2009–2012 | 262 (33.0) | 211 (26.6) | 10 (1.3) | 87 (11.0) | 224 (28.2) | 794 |
| Tumor location | ||||||
| Rectal | 146 (36.8) | 143 (36.0) | 4 (1.0) | 22 (5.5) | 82 (20.7) | 420 |
| Left | 187 (44.5) | 129 (30.7) | 4 (1.0) | 33 (7.9) | 67 (16) | 397 |
| Right | 232 (26.3) | 292 (33.1) | 14 (1.6) | 83 (9.4) | 261 (29.6) | 882 |
| Unspecified | 26 (51.0) | 10 (19.6) | 0 (0.0) | 3 (5.9) | 12 (23.5) | 51 |
|
Receipt of initial therapy |
||||||
| Yes | 463 (31.4) | 516 (35.0) | 18 (1.2) | 115 (7.8) | 362 (24.6) | 1,474 |
| None | 40 (42.6) | 21 (22.3) | 2 (2.1) | 7 (7.4) | 24 (25.5) | 94 |
| Unknown | 10 (41.7) | 5 (20.8) | 0 (0.0) | 2 (8.3) | 7 (29.2) | 24 |
| Missing | 78 (49.4) | 32 (20.3) | 2 (1.3) | 17 (10.8) | 29 (18.4) | 158 |
Note: Percentages may not add to 100% due to rounding
Abbreviations: Kaiser Permanente Southern California (KPSC), Kaiser Permanente Northern California (KPNC), Non-Hispanic (NH), Primary Care Physician (PCP), High School (HS)
Legend:
Includes 24 patients who had a negative barium enema
Percentage of people 25 years or older with less than a high school diploma in the census tract based on the 2000 decennial census
Primary care physician (family medicine, internal medicine, geriatrics, and obstetrics and gynecology) outpatient encounters were enumerated in the 5-year period, but excluding the 90-day period, before the reference date.
Charlson comorbidity score at baseline defined as five years prior to the reference date, which accounted for the minimum enrollment requirement for inclusion in the study
CRC Deaths by testing history
The majority (75.9%) of patients dying from CRC in our study had an identifiable failure in the screening process (failure to screen (n=591, 33.8%), screen appropriate intervals (n=574, 32.8%), receive surveillance (n=22, 1.3%), or follow-up (n=141, 8.1%)), and 24.1% (n=422) were up-to-date on the diagnosis date (Table 1 & Table 2). Among those with failure of follow-up for abnormal result, 103 had a positive FOBT of whom 58 (57.3%) had a documented order for diagnostic colonoscopy (n=42), sigmoidoscopy (n=14), or BE (n=2); 60 of them received FOBTs after the positive FOBT, six had only BE, and two had only sigmoidoscopy (data are not shown).
Testing history and tumor location
Of the 1,750 CRCs, 50.4%, 22.7%, 24.0%, and 2.9% were in the right colon, left colon, rectum, and unspecified location, respectively (Table 2). Right colon cancers comprised 45.3% (n=538) of all failures to screen, combined, and 58.9% (n=83) of failures of follow-up. The majority of the tumors in patients who were screening up-to-date by any test or indication were located in the right colon, overall (61.8%, n=261), and regardless of the type of test (colonoscopy (58.6%, n=41/70), sigmoidoscopy (68.5%, n=100/146), and fecal tests (58.6%, n=106/181)) (Supporting Figure 1a and Figure 1b). Failures to screen was more common for rectal or left colon cancers than right colon cancers (p-value <0.01, Figure 2a and Supporting Figure 2a).
Figure 2.
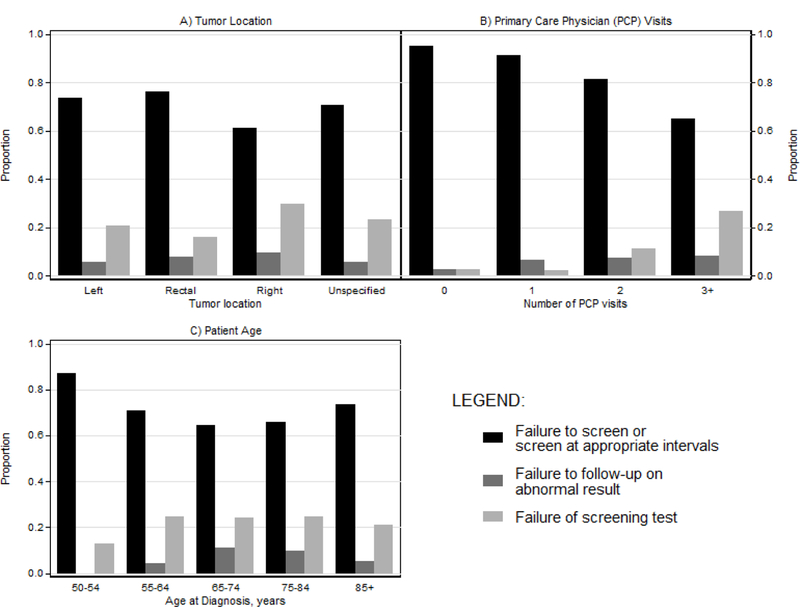
Colorectal cancer testing history by tumor location (a), primary care physician visits (b), and patient age (c) in patients who died from colorectal cancer, KPNC and KPSC 2006–2012
Associations with testing history
Most patients had at least one PCP visit. The proportions with failures to screen increased with decreasing numbers of PCP encounters (Figure 2b and Supporting Figure 2b). A higher proportion of younger patients (50–54 years) had failure to screen than patients ≥55 years (Figure 2c and Supporting Figure 2c). The proportion of patients in the combined failures to screen group was similar between those diagnosed during 2002–2005 (71.3%) and 2006–2008 (74.5%, p-value=0.31), but was lower in 2009–2012 (60.8%) (p-value <0.01).
In adjusted unconditional logistic regression analyses, compared with those who had ≥3 PCP encounters in the five years prior to CRC diagnosis, those who had two visits, one visit or no visits had 2.32 (95% confidence interval (CI): 1.35–3.99), 6.41 (95%CI: 2.25–18.30), and 12.12 (95%CI: 4.35–33.76) times higher odds, respectively, to have had failures to screen (Table 3). Compared with those 65–74 years, younger patients and those in the oldest age group (85+ years) were significantly more likely to have failures to screen. There was no statistically significant difference by race or sex. The likelihood of failures to screen was lower during 2009–2012 (odds ratio (OR)=0.45, 95%CI: 0.30–0.67) than during 2002–2005. Patterns were similar in analysis of failure to ever screen. Analyses restricted to those with documented treatment receipt did not change our findings (Supporting Table 3).
Table 3.
Logistic regression model predicting testing history according to sociodemographic and healthcare factors for patients dying of colorectal cancer, KPNC and KPSC 2006–2012a
| Characteristics | Adjusted Odds Ratios and 95% Confidence Intervals |
|
|---|---|---|
| Failure to ever screenb |
All failures to screen, combinedc |
|
| Race/Ethnicity | ||
| Non-Hispanic white | 1.0 (ref) | 1.0 (ref) |
| Non-Hispanic black | 0.92 (0.66–1.27) | 0.96 (0.69–1.34) |
| Hispanic | 1.13 (0.79–1.60) | 0.90 (0.63–1.30) |
| Asian/Pacific Islander | 1.25 (0.87–1.79) | 1.20 (0.81–1.77) |
| Other | 0.68 (0.35–1.33) | 0.54 (0.29–1.00) |
| Age at diagnosis, years | ||
| 50–54 | 1.61 (0.67–3.90) | 3.00 (0.86–10.5) |
| 55–64 | 1.44 (1.11–1.88) | 1.28 (0.98–1.67) |
| 65–74 | 1.0 (ref) | 1.0 (ref) |
| 75–84 | 1.36 (1.04–1.78) | 1.24 (0.95–1.62) |
| 85+ | 2.15 (1.34–3.46) | 2.24 (1.33–3.74) |
| Women (vs. men) | 0.99 (0.81–1.22) | 1.01 (0.82–1.24) |
|
Number of visits with a primary care physician |
||
| 0 | 4.32 (2.65–7.04) | 12.12 (4.35–33.76) |
| 1 | 3.30 (1.78–6.13 | 6.41 (2.25–18.30) |
| 2 | 2.01 (1.31–3.09) | 2.32 (1.35–3.99) |
| 3+ | 1.0 (ref) | 1.0 (ref) |
| Year of Diagnosis | ||
| 2002–2005 | 1.0 (ref) | 1.0 (ref) |
| 2006–2008 | 0.76 (0.53–1.09) | 0.96 (0.65–1.40) |
| 2009–2012 | 0.66 (0.45–0.97) | 0.45 (0.30–0.67) |
Abbreviations:
Kaiser Permanente Southern California (KPSC), Kaiser Permanente Northern California (KPNC)
Legend:
Simultaneously adjusted for all variable in the table, years of enrollment in the health plan, the Charlson comorbidity score, and % with less than high school education.
Patients who failed to receive any testing were compared to patients who failed to rescreen at appropriate intervals, to receive appropriate surveillance, failed to follow-up a positive screening test or were up-to-date with colorectal cancer screening
Patients who failed to screen or screen at appropriate intervals were compared to patients who failed to follow-up a positive screening test or were up-to-date with colorectal cancer screening
Screening histories of cancer-free patients
A total of 3,486 cancer-free patients were matched to the CRC deaths (Table 2b). We found similar associations between the selected characteristics and screening failures for matched cancer-free patients as patients who died from CRC (Supporting Table 2). For instance, compared with those with ≥3 PCP encounters, those who had two visits, one visit or no visits had 2.99 (95%CI: 1.91–4.70), 5.26 (95%CI: 2.39–11.56), and 7.29 (95%CI: 3.52–15.08) times higher odds, respectively, to have failures to screen.
Comparison of Screening histories of case and cancer-free patients
A lower proportion of patients dying from CRC than cancer-free patients were screening up-to-date (24.1% vs. 44.6%) on the diagnosis or reference date. In multivariable conditional logistic analysis that also adjusted for race-ethnicity, SES, comorbidity score and PCP visits, being screening up-to-date was associated with a 62% (OR=0.38, 95%CI: 0.33–0.44) lower risk of death from CRC. Reciprocally, patients who were not screening up-to-date had 2.61 (95%CI: 2.26–3.01) higher risks of CRC death.
A higher proportion of patients who died from CRC than cancer-free patients had a failure to ever screen (33.8% vs. 25.4%), screen at appropriate intervals (32.8% vs. 26.6%), or follow-up on abnormal results (8.1% vs. 2.2%). Similar proportions of CRC deaths and cancer-free patients had failure to receive surveillance (1.3% vs. 1.2%). Compared with cancer-free matched controls, patients who died of CRC had 2.40 (95%CI: 2.07–2.77) higher odds of all failures to screen (combined) and 7.26 (95%CI: 5.26–10.03) higher odds of failure to receive follow-up for abnormal results, relative to those who were screening up-to-date (Figure 3, and Supporting Table 4). That estimate was similar to the estimate from sensitivity analyses in the subgroup of patients at risk for failure of follow-up, comparing those who had failure of follow-up to those who received follow-up (OR 7.06, 95%CI: 4.30–11.58, Supporting Table 5).
Figure 3.

Association between screening patterns and death from colorectal cancer, KPNC and KPSC 2006–2012
Note: Estimates derived from three separate conditional logistic regression analysis of screening histories on risk of colorectal cancer death, adjusted for race-ethnicity, socioeconomic status, Charlson score, and primary care visits. Refer to Supporting Table 4 for sample sizes.
*This estimate is for all failures to screen, combined. The model included a separate indicator for failure to follow-up; being screening up-to-date was the reference group.
†This estimated the association between being screening up-to-date compared with a reference comprised of all screening process failures.
In analysis of the individual screening failures, CRC death was associated with 2.46 (95%CI: 2.08–2.91) higher odds of failure to ever screen, 2.36 (95%CI: 2.00–2.78) higher odds of failure to screen at appropriate intervals, and 2.15 (95%CI: 1.24–3.73) higher odds of failure to receive surveillance.
DISCUSSION
In this study of 1,750 patients who died from CRC, approximately 76% had identifiable failures in the screening process and 24% died of CRC despite being up-to-date with screening. We found that being screening up-to-date significantly lowered (by 62%) the risk of death from CRC. Conversely, failure to screen or screen at appropriate intervals, or failure to receive follow-up for abnormal results significantly increased risk for CRC death.
In this study, not being up-to-date in screening increased the risk of CRC death by nearly threefold. The most common type of screening process failure was a failure to ever screen or to screen at appropriate intervals and about 8% did not receive follow-up after an abnormal screen. We found that most patients had visits with PCPs, but a greater proportion of patients with no or few PCP encounters had a failure to screen or screen at appropriate intervals relative to those with more frequent encounters. Most patients who died from CRC despite being screening up-to-date had right-sided colon cancer, irrespective of the type of test received. Despite accounting for nearly one in five colonoscopies nationally,27 failure to receive adequate surveillance was observed in a relatively small proportion of patients dying from CRC (1.3%) and cancer-free individuals (1.2%).
Several randomized trials and observational studies have found screening to be effective in reducing the risk of CRC death.3, 20, 28–31 A modeling study suggested that a substantial proportion of US CRC deaths are in unscreened patients.15 In this study, we evaluated the entire CRC screening continuum to provide deeper insights into opportunities for interventions to minimize CRC deaths. For example, our finding that 34% of patients had no prior testing (failure to ever screen) suggests that improving access to and uptake of screening remains important in reducing CRC mortality (Table 1), even in populations with relatively high rates of screening. Multicomponent interventions such as eliminating access-related barriers and enhancing the ability of providers to deliver screening have been shown to improve uptake.32 In the current study, those with fewer PCP contacts had a higher likelihood of failures to screen than those with more contacts, suggesting that interventions to increase healthcare provider-patient engagement may help increase screening uptake. These findings are also supported by studies suggesting that physician recommendation is one of the strongest predictors of CRC screening uptake.
We previously reported that 84% of the screening exposures in people who died of CRC occurred within one year of diagnosis compared with 3% in matched cancer-free patients.21 This suggest that screening was received too late in the disease course to be protective, and reinforces the potential benefits of timely initiation of screening. In this study, about one-third of patients who died from CRC had not screened at appropriate intervals, pointing to the need for improvements in identifying those who may delay initiating screening and improving long term adherence to screening. Evidence on interventions to improve repeat screening is scant,34 though reminders have been shown to improve adherence to fecal testing,32 which has a more frequent testing interval (annually or biennially) than non-stool tests (sigmoidoscopy every 5 years and colonoscopy every 10 years).
Lack of follow-up after a positive fecal test is relatively common in many settings,8 including in clinical trials,35 and has been attributed to multiple barriers including lack of physician referral (either by PCPs or endoscopist), and non-adherence to referrals or non-receipt of colonoscopy due to structural or financial barriers.22, 36–38 In our study, the sizable proportion of patients (8%) dying from CRC who had an abnormal screening result without appropriate follow-up and its strong association (OR=7.26) with the risk of death from CRC suggests that interventions to improve timely follow-up after an abnormal result are important for optimizing the effectiveness of screening in preventing deaths from CRC. A recent systematic review suggests that patient navigation and provider reminders may improve follow-up after a positive fecal test, but evidence for other interventions is lacking and require more research.39
Patients who were up-to-date on screening had about 62% lower risk of death from CRC compared with those who were not up-to-date, which has not been reported previously. In our study, 24% of CRC deaths were not up-to-date on screening, which may be due initiating screening too late in the disease course, “de novo” interval cancers that developed between screening tests, or false negative test results (i.e., undetected cancers) that may be due to variations in test quality as measured by adenoma or polyp detection rate,9, 11,40, 41 or influences on the sensitivity of fecal tests such as excessive ambient temperature exposure.42 The published literature shows that all current screening modalities are less effective in the right colon than the more accessible left colon/rectum.20, 28, 43, 44 Compared to other testing groups, patients who were screening up-to-date were disproportionately represented in right colon cancers (62%). Thus, technological advances to improve the sensitivity of screening tests for right colon cancers remains an important target for decreasing CRC deaths.
Strengths of this study include the comprehensive evaluation of all eligible CRC deaths, the ability to evaluate the entire screening continuum, and the use of manual review of patient medical records to construct screening histories. However, we could not determine whether individual failures in the CRC screening process were due to patient, provider, health system, or test-related factors. Our findings were unchanged in analyses restricted to those with documented receipt of initial treatment, but we could not determine the quality or completeness of treatments following CRC diagnosis, which also influences mortality risk.45–47 Some patients may have received testing outside KPSC and KPNC, though incomplete data capture is rare in our population.20, 21 Also, we did not have information on the histology of polyps detected at endoscopy, which may result in misclassification of testing histories.
The proportions due to each of the screening failures likely vary across populations and settings, depending on the level of exposure to screening, approaches used for screening delivery, and the primary screening modality used. In KPNC, screening uptake increased from approximately 40% to over 80% during the time interval studied, compared to nearly 60% nationally towards the end of the study period.17, 48, 49 Thus, the proportion due to non-screening would likely be higher in settings and communities with lower screening uptake. Thus, our results may have limited generalizability to systems with low screening rates. However, our findings have broad applicability beyond the current setting. For example, the proportion due to non-screening in the current study was largest among those without regular PCP contacts, and is thus the likely predominant failure in settings with low exposure to screening. The distribution of failure types may vary over time as screening technology, quality, and delivery improve, which may lead to fewer failures of the screening tests. In our study, the proportion due to non-adherence to screening or failure of the screening test decreased over time (from 71%/74% before 2008 to 61% in 2009–2012) with implementation of organized screening in the healthcare systems suggesting a positive impact of system-wide outreach.
In conclusion, compared with those who were not up-to-date, being up-to-date on screening reduced the risk of dying from CRC by over 60%. In two health systems with high rates of screening, we observed that most patients dying from CRC had potentially modifiable failures of the screening process. Compared to cancer-free patients, those who died of CRC were more likely to have failed to ever screen, to screen at appropriate intervals, and to not follow-up an abnormal screening test. This study suggests that, even in settings with high screening uptake, access to and timely uptake of screening, regular re-screening, appropriate use of testing given patient characteristics, completion of timely diagnostic testing when screening is positive, and improving the effectiveness of screening tests, particularly for right colon cancer, remain important areas of focus for further decreasing CRC deaths.
Supplementary Material
Table 4.
Screening histories of colorectal cancer deaths and cancer-free patients, KPSC and KPNC 2006–2012
| Failure type (column %) | Total | No Screening | Colonoscopy | Sigmoidoscopy | Barium Enema | FOBT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n= 1750) |
Controls (n= 3486) |
Cases (n= 591) |
Controls (n= 884) |
Cases (n= 119) |
Controls (n= 409) |
Cases (n= 341) |
Controls (n=832) |
Cases (n= 68) |
Controls (n= 69) |
Cases (n= 631) |
Controls (n= 1292) |
||
| Failure to screen or screen at appropriate intervals |
Failure to ever screen |
591 (33.8) | 884 (25.4) | 591 (100) |
884 (100) | - | - | - | - | - | - | - | - |
| Failure to screen at appropriate intervals |
574 (32.8) | 929 (26.6) | - | - | 17 (14.3) | 11 (2.7) | 170 (49.9) | 348 (41.8) |
40 (58.8) | 53 (76.8) | 347 (55.0) |
517 (40.0) |
|
| Failure to receive surveillance |
22 (1.3) | 41 (1.2) | - | - | 21 (17.6) | 38 (9.3) | 1 (0.3) | 2 (0.2) | - | - | - | 1 (0.1) | |
| Failure to follow-up for abnormal test |
141 (8.1) | 76 (2.2) | - | - | 11 (9.2) | 19 (4.6) | 24 (7.0) | 15 (1.8) | 3 (4.4) | - | 103 (16.3) |
42 (3.3) | |
| Up to date on screening | 422 (24.1) | 1556 (44.6) |
- | - | 70 (58.8) | 341 (83.4) |
146 (42.8) | 467 (56.1) |
25 (36.8) | 16 (23.2) | 181 (28.7) |
732 (56.7) |
|
Acknowledgments
Grant Support: This study was supported by an award (number R01CA213645, U54CA163262) from the National Cancer Institute of the National Institutes of Health.
The views expressed here are those of the authors and do not represent any official position of the National Cancer Institute or National Institutes of Health. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. No part of this study has been presented in any form. Dr. Doubeni is a member of the US Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF.
Abbreviations:
- BE
barium enema
- CRC
colorectal cancer
- FIT
fecal immunochemical test
- FOBT
guaiac fecal occult blood test
- KPNC
Kaiser Permanente Northern California
- KPSC
Kaiser Permanente Southern California
- PCP
Primary care physician
- SES
socioeconomic status
- USPSTF
United States Preventive Services Task Force
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Corley is Editor-in-Chief of Gastroenterology and the remaining authors have no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–691. [DOI] [PubMed] [Google Scholar]
- 3.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008;103:1541–9. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–57. [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153:307–323. [DOI] [PubMed] [Google Scholar]
- 7.Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev 2014;23:1147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev 2016;25:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA 2017;317:1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: A systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin 2018;68:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zapka JG, Taplin SH, Solberg LI, et al. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev 2003;12:4–13. [PubMed] [Google Scholar]
- 13.Taplin SH, Ichikawa L, Yood MU, et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst 2004;96:1518–27. [DOI] [PubMed] [Google Scholar]
- 14.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst 2005;97:675–83. [DOI] [PubMed] [Google Scholar]
- 15.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann Epidemiol 2015;25:208–213 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin TR, Jamieson L, Burley DA, et al. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev 2011;33:101–10. [DOI] [PubMed] [Google Scholar]
- 17.Mehta SJ, Jensen CD, Quinn VP, et al. Race/Ethnicity and Adoption of a Population Health Management Approach to Colorectal Cancer Screening in a Community-Based Healthcare System. J Gen Intern Med 2016;31:1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatino SA, White MC, Thompson TD, et al. Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep 2015;64:464–8. [PMC free article] [PubMed] [Google Scholar]
- 19.de Moor JS, Cohen RA, Shapiro JA, et al. Colorectal cancer screening in the United States: Trends from 2008 to 2015 and variation by health insurance coverage. Prev Med 2018;112:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doubeni CA, Corley DA, Quinn VP, et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: a large community-based study. Gut 2018;67:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman M, Fletcher RH, Doria-Rose VP, et al. Observational methods to assess the effectiveness of screening colonoscopy in reducing right colon cancer mortality risk: SCOLAR. J Comp Eff Res 2015:1–11. [DOI] [PMC free article] [PubMed]
- 22.Doubeni CA, Corley DA, Zauber AG. Colorectal Cancer Health Disparities and the Role of US Law and Health Policy. Gastroenterology 2016;150:1052–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doubeni CA, Schootman M, Major JM, et al. Health status, neighborhood socioeconomic context, and premature mortality in the United States: The National Institutes of Health-AARP Diet and Health Study. Am J Public Health 2012;102:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doubeni CA, Jambaulikar GD, Fouayzi H, et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population--a retrospective cohort study. PLoS One 2012;7:e36392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fassil H, Adams KF, Weinmann S, et al. Approaches for classifying the indications for colonoscopy using detailed clinical data. BMC Cancer 2014;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–95. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman DA, Williams JL, Holub JL, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc 2014;80:133–43. [DOI] [PubMed] [Google Scholar]
- 28.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst 2011;103:1310–22. [DOI] [PubMed] [Google Scholar]
- 31.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017;389:1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med 2012;43:97–118. [DOI] [PubMed] [Google Scholar]
- 33.Guessous I, Dash C, Lapin P, et al. Colorectal cancer screening barriers and facilitators in older persons. Prev Med 2010;50:3–10. [DOI] [PubMed] [Google Scholar]
- 34.Green BB, Anderson ML, Cook AJ, et al. A centralized mailed program with stepped increases of support increases time in compliance with colorectal cancer screening guidelines over 5 years: A randomized trial. Cancer 2017;123:4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissfeld JL, Schoen RE, Pinsky PF, et al. Flexible sigmoidoscopy in the randomized prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial: added yield from a second screening examination. J Natl Cancer Inst 2012;104:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher DA, Jeffreys A, Coffman CJ, et al. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev 2006;15:1232–5. [DOI] [PubMed] [Google Scholar]
- 37.Baig N, Myers RE, Turner BJ, et al. Physician-reported reasons for limited follow-up of patients with a positive fecal occult blood test screening result. Am J Gastroenterol 2003;98:2078–81. [DOI] [PubMed] [Google Scholar]
- 38.Carlson CM, Kirby KA, Casadei MA, et al. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med 2011;171:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selby K, Baumgartner C, Levin TR, et al. Interventions to Improve Follow-up of Positive Results on Fecal Blood Tests: A Systematic Review. Ann Intern Med 2017;167:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkins L, Hunkeler EM, Jensen CD, et al. Factors influencing variation in physician adenoma detection rates: a theory-based approach for performance improvement. Gastrointest Endosc 2016;83:617–26 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedewa SA, Flanders WD, Ward KC, et al. Racial and Ethnic Disparities in Interval Colorectal Cancer Incidence: A Population-Based Cohort Study. Ann Intern Med 2017;166:857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal Immunochemical Test (FIT) for Colon Cancer Screening: Variable Performance with Ambient Temperature. J Am Board Fam Med 2016;29:672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- 44.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med 2013;158:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335–42. [DOI] [PubMed] [Google Scholar]
- 46.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–23. [DOI] [PubMed] [Google Scholar]
- 47.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedewa SA, Goodman M, Flanders WD, et al. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer 2015;121:3272–80. [DOI] [PubMed] [Google Scholar]
- 49.Levin TR, Corley DA, Jensen CD, et al. Effects of Organized Colorectal Cancer Screening on Cancer Incidence and Mortality in a Large, Community-based Population. Gastroenterology 2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


