Abstract
The social needs of organisms change as they mature. Yet, little is known about the mechanisms that subserve processing social interactions or how these systems develop. The medial extended amygdala (meEA) is comprised of the medial bed nucleus of the stria terminalis (BSTm) and the medial amygdala (MeA). This neural complex holds great promise for understanding how the social brain processes information. We assessed expression of the immediate early gene cFos and the enzyme tyrosine hydroxylase (TH) at three developmental time-points (postnatal day [PND] 2, 9, and 21) to determine how developing prairie voles process familial social contact, separation, and reunion. We demonstrate that (1) BSTm cFos responses were sensitive to separation from family units at PND 9 and PND 21, but not at PND 2; (2) MeA cFos responses were sensitive to reunion with the family, but only in PND 21 pups; (3) BSTm TH neurons did not exhibit differential responses to social condition at any age; and (4) MeA TH neurons responded strongly to social contact (remaining with family or following reunion), but only at PND 21. Our results suggest that the sub-units of the meEA become functionally responsive at different developmental time points, and are differentially activated in response to distinct social contexts. Overall, our results support the notion that interconnected regions of the meEA follow divergent developmental timelines and are sensitive to distinct properties of social contexts.
Keywords: prairie vole, tyrosine hydroxylase, medial extended amygdala, social behavior
Introduction
Much of what we understand about the mechanisms of social behavior is derived almost entirely from research on adult animals. From studies of social motivation and pair bonding to cooperation and emotional processing, the neural mechanisms that underlie social behaviors have been primarily explored in mature brains (Happé and Frith, 2014). The social (and asocial) needs of perinatal and juvenile offspring differ from those of adults, and these needs change substantially as young mature and become less vulnerable (Nelson et al., 2016). For instance, social exploration outside of the nest might be rewarding for sexually mature individuals, but stressful for defenseless perinatal young. Thus, as young develop, the ways in which their brains processes social cues are likely to transform throughout ontogeny (Rehling et al., 2012). Despite the potential for age-dependent differences in sociality, we know very little about when the neural systems that support social functioning develop, or where in the brain these processes functionally emerge.
The few studies that have examined the development of nonsocial and social neural functioning have demonstrated age-dependent neural responses. For example, in rats, physical restraint induces dramatically different expression patterns of cFos (an immediate early gene (IEG) and marker of neuronal activation) in the anterior olfactory nucleus, piriform cortex, tenia tecta, and amygdala of postnatal day (PND) 28 juveniles compared to adult PND 60 animals (Kellogg et al., 1998). Critically, neural responses to social stimuli are also refined with age. For example, compared to PND 28 juvenile hamsters, PND 58–66 adult males show divergent patterns of cFos expression in the ventral tegmental area (VTA), nucleus accumbens (NAcc) and medial prefrontal cortex after the presentation of female vaginal secretions, a biologically relevant cue for the sexually mature male (Bell et al., 2013). Furthermore, patterns of cFos responses differ across the brains of PND 7, PND 14, and PND 21 rat pups following exposure to a novel adult male rat (an important event for preweanling pups that are susceptible to infanticide), specifically within the paraventricular nucleus of the hypothalamus, the amygdala, the periaqueductal gray, and the locus ceruleus (Wiedenmayer and Barr, 2001). PND 7 rats do not mount discriminative neural responses to the adult stimulus, whereas older age groups show distinct, age-specific patterns of cFos expression following exposure to the social stimulus.
Together, these data demonstrate that the neural processing of postnatal experiences (stressful and/or social) changes across development, and within particular subregions of the brain. However, these few studies on developing brains are conducted within the second week of postnatal life, and thus we still know extremely little about the neural functioning of neonatal offspring. Furthermore, the majority of these data characterize brain responses to novel stimuli, and therefore the development of neural responses to the most common social partners of a young helpless animal, namely the parents and siblings, remains unclear. In the present study, we examine the development of social neural function beginning at a neonatal age (PND 2) in a neural circuit specifically involved in social behavior.
The extended amygdala represents a promising region of interest to investigate the ontogeny of social functioning. The extended amygdala is an important node of the social behavior network (Newman, 1999) that encompasses the reciprocally connected amygdaloid complex and bed nucleus of the stria terminalis (BST). The amygdaloid complex is composed of cytoarchitecturally and functionally distinct subregions (Johnston, 1923), which notably includes the medial amygdala (MeA) - a subregion of the amygdaloid complex that has been repeatedly implicated in fearful and emotional responses, olfactory information processing, and socio-sexual reward (Davis, 1992; Newman, 1999; Bergan et al., 2014). Indeed, disruptions to the MeA impair a variety of rodent social behaviors, including male sexual behavior, aggression, and parental care (Vochteloo and Koolhaas, 1987; Kirkpatrick et al., 1994), highlighting the importance of the MeA in social functioning.
Like the MeA, the BST can be anatomically and functionally divided into subcomponents, which each have sets of distinct and overlapping efferent and afferent projections to various amygdaloid nuclei (Coolen and Wood, 1998). Of particular interest here is the medial division (BSTm), which shares dense reciprocal projections with the MeA (Coolen and Wood, 1998; Pardo-Bellver et al., 2012). Traditionally, the BSTm and MeA comprise the medial extended amygdala (meEA), and share functional characteristics in response to social stimuli (Alheid, 2003). In rodents, these regions exhibit elevated cFos levels after agonistic encounters (Kollack-Walker and Newman, 1995), exposure to alarm pheromones (Kiyokawa et al., 2005), and predator odor (Dielenberg et al., 2001).
Notably, in some species, the meEA contains tyrosine hydroxylase (TH) neurons (Northcutt et al., 2007). TH is the rate-limiting enzyme in the biosynthesis of L-DOPA, the precursor molecule to catecholamines such as adrenaline, noradrenaline (NA), and dopamine (DA) (Kobayashi and Nagatsu, 2012). The largest population of TH cells, and most commonly studied, originates from the ventral tegmental area, and has been linked to rodent play behavior and social-dominance (Filipenko, Alekseyenko, Beilina, Kamynina, & Kudryavtseva, 2001; Northcutt & Nguyen, 2014). TH cell groups are not found within the rat meEA (Northcutt, Wang, & Lonstein, 2007), however, dense populations of TH neurons are found in the MeA and BST of the socially monogamous prairie vole (Microtus ochrogaster), and in adult animals these TH cell groups exhibit functional plasticity in response to the presentation of various conspecifics (Northcutt and Lonstein, 2009). Together, these data suggest that meEA TH cells may contribute to the expression of species-typical social behavior.
The molecular consequences of activating MeA and BSTm TH cells is unclear. In adult prairie voles, TH cells in the MeA and principal BST (pBST; dorsomedial to the BSTm) are not immunoreactive for aromatic L-amino acid decarboxylase (AADC; necessary for the conversion of L-DOPA to DA) or dopamine-beta-hydroxlyase (DBH; necessary for the conversion of L-DOPA to NA) (Ahmed et al., 2012). However, studies in rats have demonstrated that nearby AADC-producing cells can coordinate with TH cells to synthesize DA (Ugrumov et al., 2004). Furthermore, the multifaceted role of L-DOPA in neural modulation is relatively underappreciated (De Deurwaerdère et al., 2017), highlighting the importance of investigating TH neural function more broadly.
The species-specific expression of meEA TH cell groups and their sensitivity to the social environment in adult prairie voles, combined with the multiple molecular pathways by which TH can contribute to downstream peptide production, makes these cell groups promising regions to analyze in the context of encoding dynamic social environments. Furthermore, because these TH cell groups were discovered only in the last decade, and have only been analyzed in adult animals, there is still much to learn about their involvement in social behavior and development. Understanding the ontogeny of neural activity within these cells, and the conditions under which these cell groups become responsive will help elucidate their contributions to the social behavior of the developing animal. Furthermore, prairie voles have been touted as a model for the neurobiology of human social attachment behavior because they exhibit pair bonding behaviors and biparental care like humans (McGraw and Young, 2010). Thus, examining the functional development of TH systems within the meEA of the prairie vole could have substantial translational implications for social neuroscience.
We aimed to investigate how neuronal function within the BSTm and the MeA changes across developmental time. To do this we conducted an IEG study in prairie vole pups at three stages of early development (PND 2, 9, and 21). Utilizing an acute social isolation and reunion paradigm designed to induce stressful and/or emotional states, we examined meEA neural activity (assessed by cFos expression) and meEA TH neural activity (assessed by TH-cFos colocalization) after social environmental manipulation. To our knowledge, this study is the first to examine the ontogeny of meEA function and the development of TH neural function within this circuit. The interconnectedness of the BSTm and the MeA led us to hypothesize that the responsiveness of these regions may emerge simultaneously, and that they may exhibit similar neural response profiles. Either or both of these structures could be involved in the processing of social contexts, and we therefore did not predict a particular direction in their expected responses to each social context.
Materials and Methods
Subjects
All prairie vole pups used in this study were the first litter of the F2 generation of breeding pairs derived from wild-caught animals housed in our animal colony. We trapped all wild animals in Champagne County, Illinois, USA. Breeding pairs were established, and the first litter of each pair was culled to three subjects to control for variation in early social experience. Families were assigned unique Litter IDs to control for pup-relatedness in the statistical analyses (see below). Animals were housed under a 14L:10D light cycle in standard polycarbonate rodent cages (29 × 18 × 13cm) lined with Sani-chip bedding. Animals were provided nestlets, water, and standard rodent chow (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO, USA) ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Cornell University (2013–0102).
Design
To assess whether meEA function changes across development, we conducted an IEG experiment utilizing a 3 × 3 design (see Kelly et al., 2018). We tested animals that were at three different stages of early development: PND 2, PND 9, and PND 21. These ages were chosen because they reflect specific behavioral and physiological milestones of prairie vole development. At PND 2, pups are relatively immobile, incapable of thermoregulation, and entirely dependent on parental care for survival. At PND 9, pups open their eyes and are physically capable of environmental and social exploration. By PND 21, pups are weaned and can survive independently outside of the nest (McGuire & Novak, 1984).
Subjects underwent testing in one of three social conditions in which we manipulated contact with parents and siblings (see Fig. 1). Subjects were isolated from their family (Isolate, PND 2 - M:11, F:11; PND 9 - M:10, F:8; PND 21 - M:7, F:7), reunited with their family after isolation (Reunite, PND 2 - M:9, F:9; PND 9 - M:11, F:12; PND 21 - M:9, F:9), or interacted with their family under normal conditions (Together, PND 2 - M:10, F:10; PND 9 - M:7, F:14; PND 21 - M:10, F:9). This design was utilized to assess neural activity in response to emotionally salient and stressful social experiences. Social isolation from siblings and parents presumably represents a life-threatening context for young neonates, and thus is likely to produce a neural response.
Figure 1.
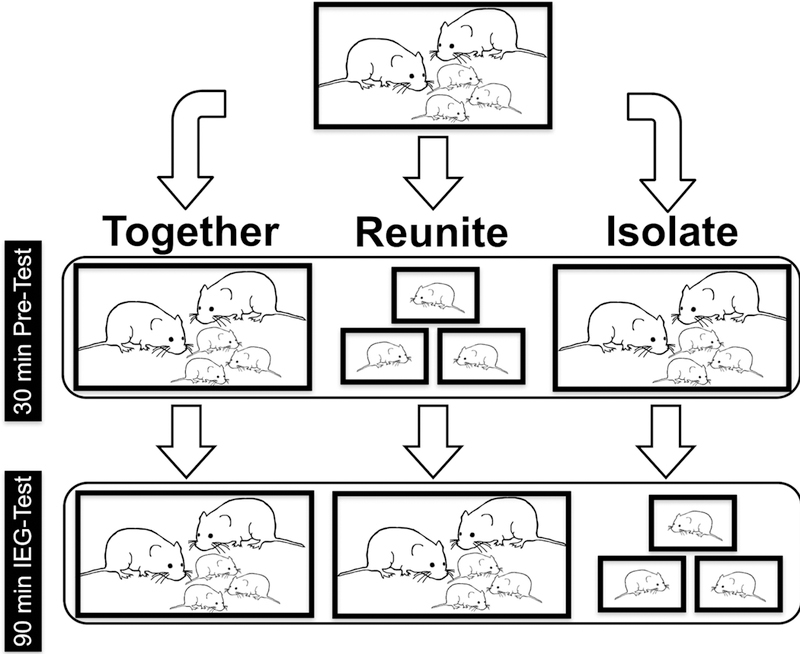
A top-to-bottom flowchart depicting experimental treatment conditions. Family units were randomly assigned to one of three conditions: Together, Isolate, or Reunite. All subjects started in identical housing conditions prior to the 30-minute pre-test phase. The same procedure was used for family units with pups of each age (PND 2, PND 9, or PND 21). See text for details.
Regions of Interest
Because previous studies implicate the MeA and BSTm in stress response and affiliation (Lim and Young, 2004; Choi et al., 2007; Pardo-Bellver et al., 2012), we sought to determine the age at which these brain regions become responsive to variation in social context. To this end, we quantified the immediate early gene product cFos within the meEA, because this protein is an established marker of neural activity that can be time-locked to experimental manipulations (Hoffman et al., 1993). Given that adult prairie voles exhibit changes in cFos activity within regions of the meEA as a function of social context (Northcutt and Lonstein, 2009), we also examined neural activity in TH cell groups within the meEA of developing pups by assessing cFos colocalization with TH neurons. Earlier work from Northcutt et al. (2007) identified the principal nucleus of the bed nucleus of the stria terminalis (pBST) as a TH neuron rich region within the extended amygdala of prairie voles. Although the pBST is not classically considered a component of the medial extended amygdala, it contributes to rodent sociosexual olfactory processing (Dong and Swanson, 2004) and responds to the presentation of various social stimuli (Northcutt and Lonstein, 2009). As such, we also measured pBST cFos activation and TH-cFos colocalization in relation to the experimental manipulations as described for the MeA and BSTm neural data.
Experimental Manipulations
Breeding pairs (n = 92) were formed and closely monitored until parturition. Litters were culled to three pups at birth. Families were then randomly assigned to one of nine experimental groups that spanned all possible combinations across age (PND 2/9/21) and social condition (Together, Isolate, and Reunite). All three pups in each litter remained with their families under normal colony conditions until they reached test-age.
The social manipulations were designed to assess how the neural profiles of the pups differed when they were removed from, or reunited with, their families at specific ages. The Together condition was intended to serve as a baseline (control) for neural activity when pups coexist with their family groups under normal conditions. Each manipulation consisted of a 30-min pre-test phase, followed by a 90-min IEG-test phase. The novel cages in all phases were lined with clean bedding, did not contain food or water, and were outfitted with transparent, perforated Plexiglas lids to allow for overhead video recording.
In the Together and Isolate conditions, parents and pups were transferred from their home cages to neutral, clean polycarbonate cages for the pre-test phase. After 30 mins, the Together families were moved as a group to a novel test-cage. In the Isolate condition, the pups were moved into individual novel test-cages. For the Reunite condition, pups were moved into individual novel cages for the 30 min pretest, after which they were reunited with each other and their parents in a novel cage for the 90 min IEG-test phase. All parents and pups experienced the same number of standardized handling experiences, and the same number of cage changes, regardless of experimental group. All subjects were sacrificed immediately following the IEG-test. Thus, the measured neural activity reflects the neurochemical changes that occurred when animals transitioned from pre-test conditions to the IEG-test phase.
We controlled for thermoregulatory and temperature differences across ages in phases where pups were isolated in a neutral cage. An infrared thermometer was used to take an average temperature reading under normal home cage pre-test conditions across five families at each age group. Electric heating pads were placed underneath the cage and heated to 36°C for PND 2 pups, 34°C for PND 9 pups, and 30°C for PND 21 pups.
Following perfusions, all pups were visually sexed. PCR amplification was used to detect male-specific SRY genes to confirm the sexes of the PND 2 and PND 9 animals, because at these ages it can be difficult to assess sex visually. Genomic DNA was extracted from tissue punches of lung and spleen samples using the DNEasy Blood and Tissue Kit and protocol (Qiagen, USA). SRY primer sequences were forward 5’- TTATGCTGTGGTCTCGTGGTC-3’; and reverse 5’- GCAGTCTCTGTGCCTCTTGG-3’. DNA segments were amplified in a thermocycler (Eppendorf realplex4) in 25 μL for 35 cycles (94 °C 30 s, 55 °C 1 min, and 72 °C 30 s). The amplified PCR products were separated on 2% agarose gels, and visualized under a UV transilluminator after staining (GelRed, Biotium, USA).
Histology and Immunocytochemistry
Immediately after the 90 min IEG-test, pups were deeply anesthetized by isoflurane and intracardially perfused with 0.1M phosphate-buffered saline (PBS, pH = 7.4), followed by 4% paraformaldehyde in PBS. Brains were extracted and post-fixed in 4% paraformaldehyde for 24 h, sunk in 30% sucrose for 48 h, then stored at −80°C until sectioning. Brains were mounted and coronally cryosectioned at 40 μm thickness into three series for PND 21 pups, and into two series for PND 2 and PND 9 pups (to account for smaller brain sizes). Tissue was stored at −80°C in cryoprotectant until immunocytochemical processing.
Antibody reporting
One series of tissue from each subject was immunofluorescently double-labeled for TH and cFos. Free-floating sections were rinsed twice for 30 min in PBS, and blocked for 1 h (PBS + 10% normal donkey serum + 0.03% Triton-X-100). The primary antibodies used were monoclonal mouse anti-TH (1:1000 μL, Millipore Corp, USA, Cat. # MAB318) and polyclonal rabbit anti-Fos (4:1000 μL, Millipore Corp, USA, Cat. # ABE457). Tissue was incubated in primary antibodies for 24 h, rinsed in PBS for 40 min, and incubated in secondary antibodies for 2 h. The secondary antibodies were donkey anti-mouse conjugated to Alexa Fluor 680 (6:1000 μL) and donkey anti-rabbit conjugated to Alexa Fluor 594 (4:1000 μL), with 5% donkey serum + 0.03% Triton-X-100 in PBS. Sections were rinsed in PBS overnight, mounted onto microscope slides, and cover-slipped with Prolong Gold antifade + DAPI nuclear stain (ThermoFisher Scientific).
Visualization and Quantification
Bilateral photomicrographs were taken at 10X on a Zeiss Axioimager II scope with an AxioCam MRm attachment, z-drive, and Apotome optical dissector (Carl Zeiss Inc., Gottingen, Germany) at two consecutive levels of the BSTm and MeA. Areas of interest were localized using neuroanatomical landmarks - the ventral side of the anterior commissure for the BSTm, and optic tract for the MeA (Fig. 2). Images were manually-counted for cFos and TH using the GNU Image Manipulation Program (GIMP, 2.8.22) and ImageJ (National Institutes of Health, Bethesda, MD). Monochromatic images were visually quantified for TH-immunoreactivity (-ir), cFos-ir, and colocalization of TH-ir and cFos-ir (TH-ir cells expressing Fos-ir). Inherently, brain sizes varied across age groups, and to a much lesser extent across individuals within age groups. To avoid unfairly biasing the number of cells per structure we could capture, we did not use a use a standard sized box to score a region of interest across all brains. Instead, we outlined and counted immunoreactive cells for the entire region of interest to account for brain size variation. This method had the advantage of providing an exhaustive metric that scales for age-dependent brain size differences across groups.
Figure 2.
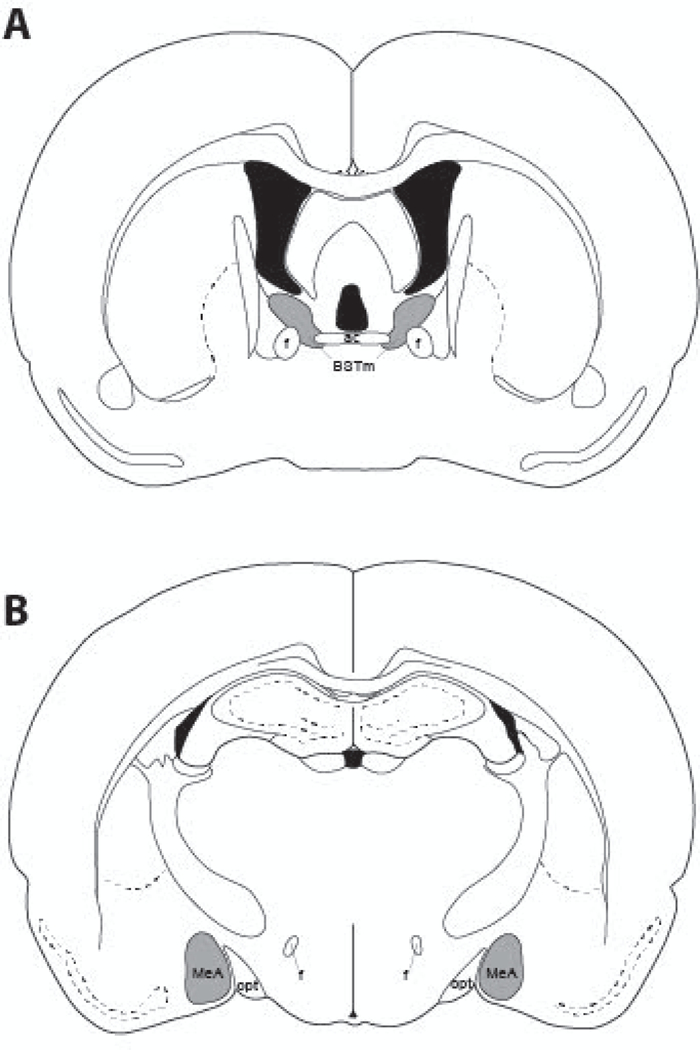
A diagram representing the location of cFos-ir and TH-ir neurons quantified in (A) the medial bed nucleus of the stria terminalis (BSTm), and (B) the medial amygdala (MeA). Regions where cells were quantified are shaded in gray. AC, anterior commissure; f, fornix; opt, optic tract.
Statistical Analysis
Cell counts were combined and averaged across rostral-caudal sections within each region of interest. To analyze the colocalization of TH-ir and cFos-ir neurons, the proportions of TH-ir neurons expressing cFos over the total number of TH-ir cells in each region were arcsine transformed. Cell count data were analyzed via Linear Mixed Models (LMM) in R v.3.2.1 (R Core Team, 2016), using the R package lme4 (Bates et al., 2015). LMMs are robust against slight departures from normality; model residuals were graphically assessed and either log-transformed or square-root-transformed to satisfy assumptions of normality when necessary. Social condition, Sex, and Age were included as fixed effects, while Litter ID was included as a random effect to control for genetic variation. p-values were derived from a likelihood ratio test within the R package lmerTest (Kuznetsova et al., 2017).
We hypothesized that isolation and reunion with families would induce unique neural profiles in pups as a function of their age. Our a priori interaction of interest was between the Age and Condition factors. When an Age × Condition interaction was significant, we conducted planned contrasts of social condition within age groups using the R package lsmeans (Lenth, 2016). All pairwise post-hoc tests were Bonferroni corrected in R to control for multiple comparisons, with a 0.05 α-level threshold for statistical significance.
We examined neural responses to variation in social context. We, therefore, did not explore the main effects of Age because the results lack context. As a result, we conducted analyses to specifically examine main effects and significant interactions involving Condition. We observed no three-way interactions of Age × Condition × Sex in any of the brain regions analyzed (all p’s > 0.05). Furthermore, we did not find any interactions of Sex × Age (all p’s > 0.05), and collapsed sex for the results presented below.
Results
TH-ir neurons.
In order to characterize the neuroanatomical development of TH-ir neurons in the prairie vole, we analyzed the raw number of TH-ir cells present in each region of interest.
The medial bed nucleus of the stria terminalis (BSTm).
We observed a main effect of Age (F(2, 155) = 23.93, p < 0.001) in the BSTm, where PND 21 animals had significantly more TH-ir neurons compared to both the PND 9 (t(155) = 5.21, p < 0.0001) and PND 2 (t(155) = 6.65, p < 0.0001) groups (Fig. 3A).
Figure 3.
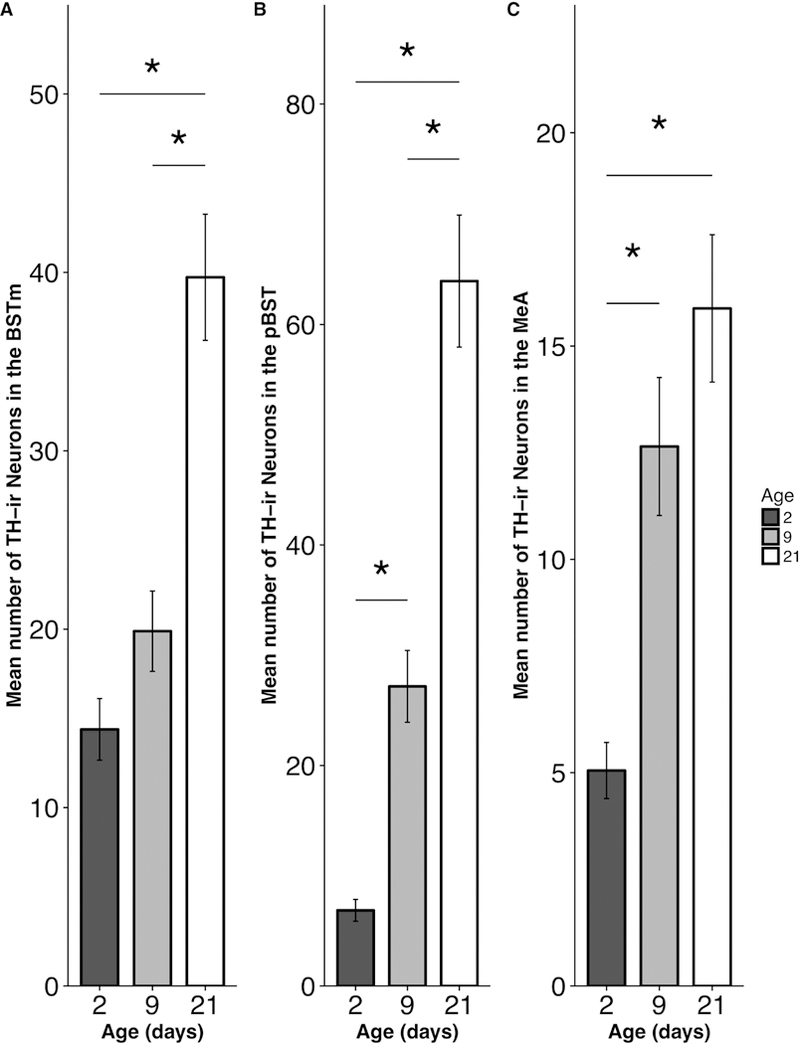
Mean (±SEM) number of TH-ir neurons as a function of pup age in the (A) medial portion of the bed nucleus of the stria terminalis (BSTm), (B) principal nucleus of the bed nucleus of the stria terminalis (pBST), and (C) medial amygdala (MeA). * p < 0.05.
The principle bed nucleus of the stria terminalis (pBST).
Analyses revealed a main effect of Age (F(2, 62.251) =32.125, p < 0.001) in the pBST. PND 21 animals had significantly more TH-ir neurons compared to both PND 9 (t(65.46) = 5.34, p < 0.0001) and PND 2 (t(60.59) = 7.86, p < 0.0001) groups. In addition, PND 9 animals has significantly more TH-ir cells than PND 2 pups (t(60.69) = 2.82, p = 0.019) (Fig. 3B).
The medial amygdala (MeA).
We found a main effect of Age (F(2, 73.07) = 14.81, p < 0.001) in the MeA, for which both PND 21 (t(72.51) = 5.28, p < 0.0001) and PND 9 pups (t(65.64) = 3.81, p = 0.0009) had significantly more TH-ir neurons than the PND 2 pups (Fig. 3C).
BSTm cFos responses
We observed a significant main effect of condition in the BSTm (F(2,78.52) = 6.08; p = 0.004), with subjects in the Isolate condition exhibiting significantly higher levels of cFos-ir compared to pups in the Together condition (p = 0.003). However, a significant Age × Condition interaction in the BSTm (F(4,78.39) = 2.49; p = 0.05; Fig. 4) suggests that neural responses in the older animals likely drives this main effect: Post-hoc comparisons revealed that Isolated PND 9 (t(90.79) = 2.71 ; p = 0.024) and PND 21 (t(97.6) = 3.38 ; p = 0.003) pups exhibited significantly more cFos-ir induction compared to pups in the Together condition. Isolated PND 9 pups also exhibited greater cFos-ir compared to pups in the Reunite condition (t(84.42) = 2.61 ; p = 0.032). We found that PND 2 pups did not show differences in cFos-ir across conditions (Together × Reunite t(59.81) = 0.192, Together × Isolate t(71.95) = 0.31; Reunite × Isolate t(64.3) = 0.5; all p’s > 0.05).
Figure 4.
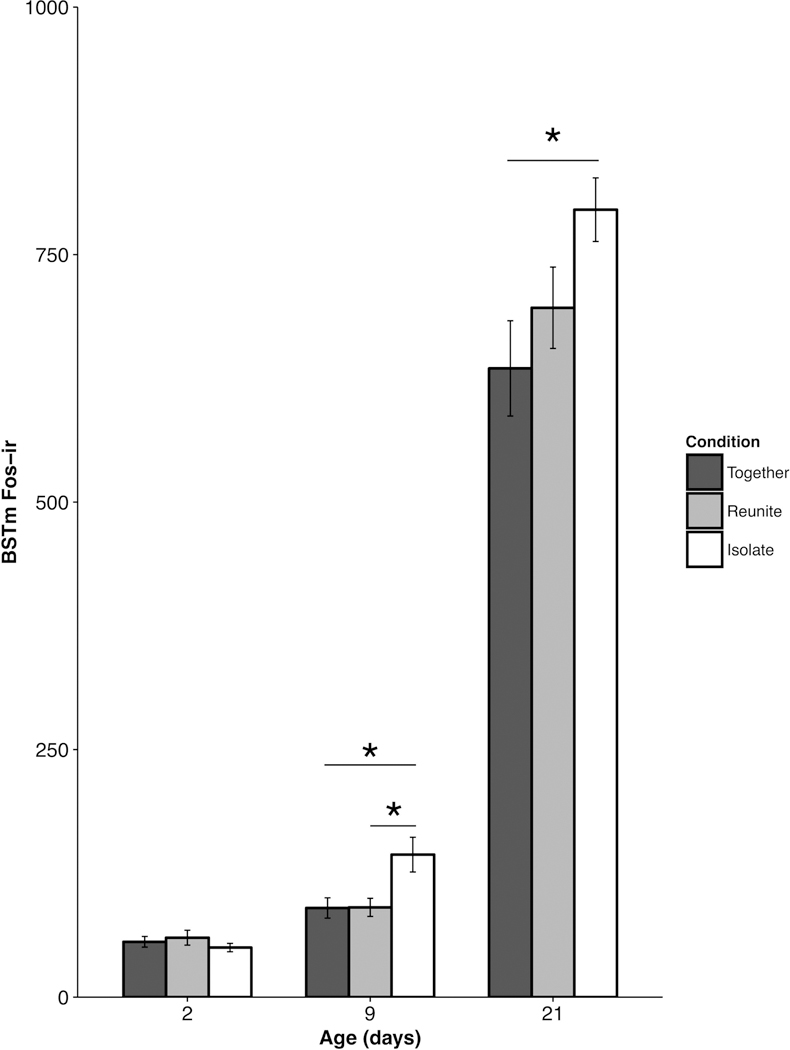
Mean (±SEM) cFos-ir as a function of pup age and experimental condition within the medial division of the bed nucleus of the stria terminalis (BSTm). * p < 0.05.
pBST cFos responses.
We did not observe a significant main effect of Condition (F(2, 74.17 ; p = 0.37 ) or an interaction of Age × Condition for cFos-ir in the pBST (F(4, 73.68 ) = 0.55 ; p = 0.7; Fig. 5), suggesting that this subdivision of the BST was not differentially sensitive to our experimental conditions at any stage of development.
Figure 5.
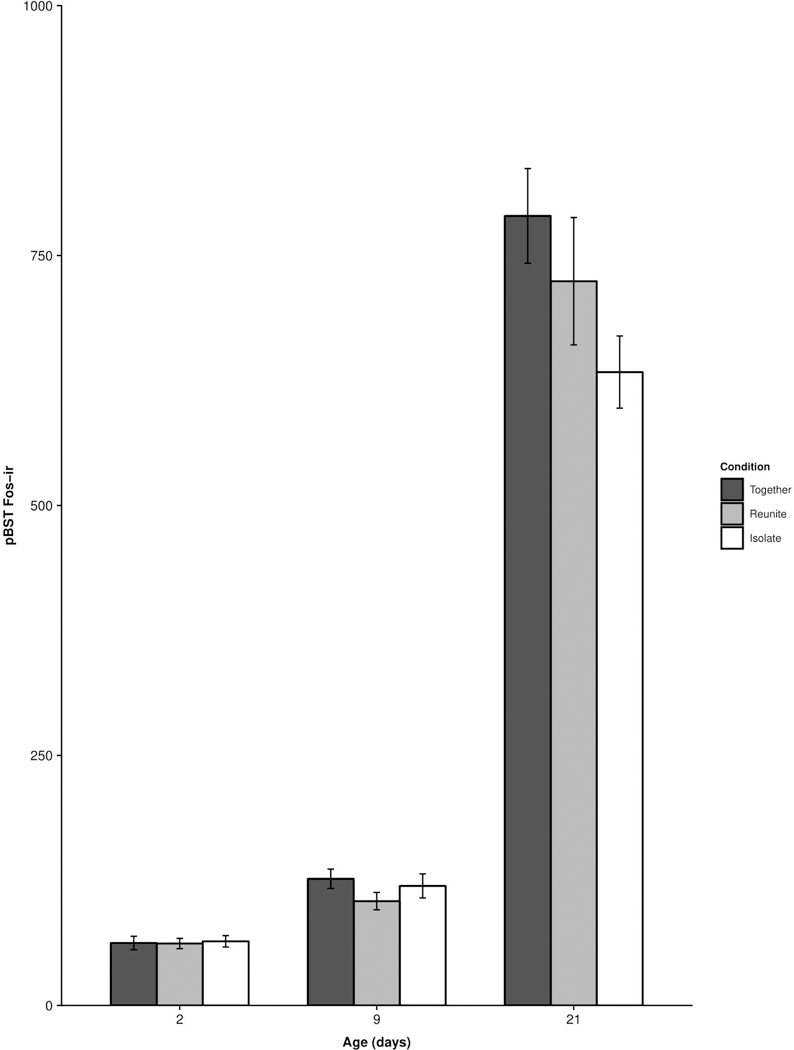
Mean (±SEM) cFos-ir as a function of pup age and experimental condition within the principal nucleus of the bed nucleus of the stria terminalis (pBST). No comparisons were statistically significant.
MeA cFos responses.
We observed a significant Age × Condition interaction for cFos-ir in the MeA (F(4, 51.86) = 3.36 ; p = 0.016; Fig. 6). Post-hoc comparisons revealed that PND 21 pups in the Reunite condition exhibited significantly more cFos-ir compared to PND 21 pups in the Together (t(56.98) = 2.51 ; p = 0.045) and Isolate (t(67.78) = 3.73 ; p = 0.001) conditions. Interestingly, neither the PND 2 nor PND 9 animals showed significantly different levels of cFos-ir as a function of social condition (Isolate × Reunite – PND 2 t(39.29) = 0.39 , PND 9 t(52.74) = 1.14; Isolate × Together – PND 2 t(45.43) = 0.085, PND 9 t(57.22) = 1.79; Reunite × Together – PND 2 t(35.45) = 0.452, PND 9 t(49.47) = 0.72; all p’s > 0.05).
Figure 6.
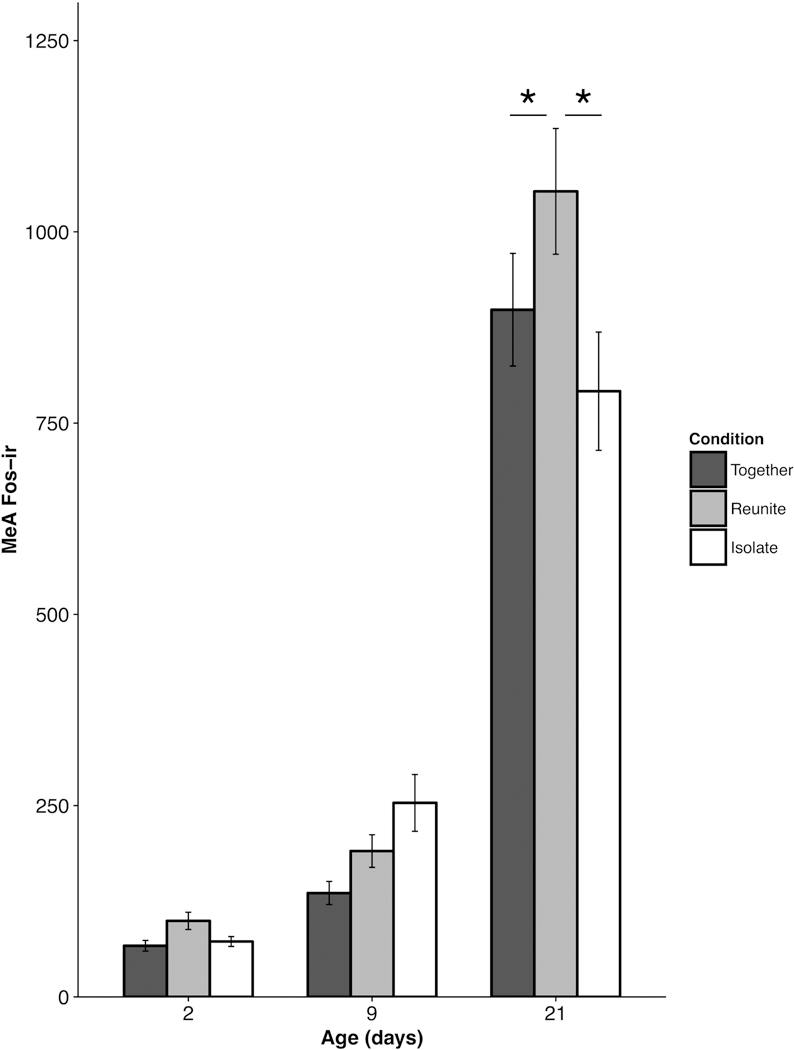
Mean (±SEM) cFos-ir as a function of pup age and experimental condition within the medial amygdala (MeA). * p < 0.05.
TH-cFos colocalization in the BSTm.
Analysis of the proportion of TH neurons expressing cFos in the BSTm yielded null results. We found no significant Age × Condition interaction for TH-cFos colocalization in the BSTm (F(4, 70.13) = 2.159; p = 0.083; Fig. 7).
Figure 7.
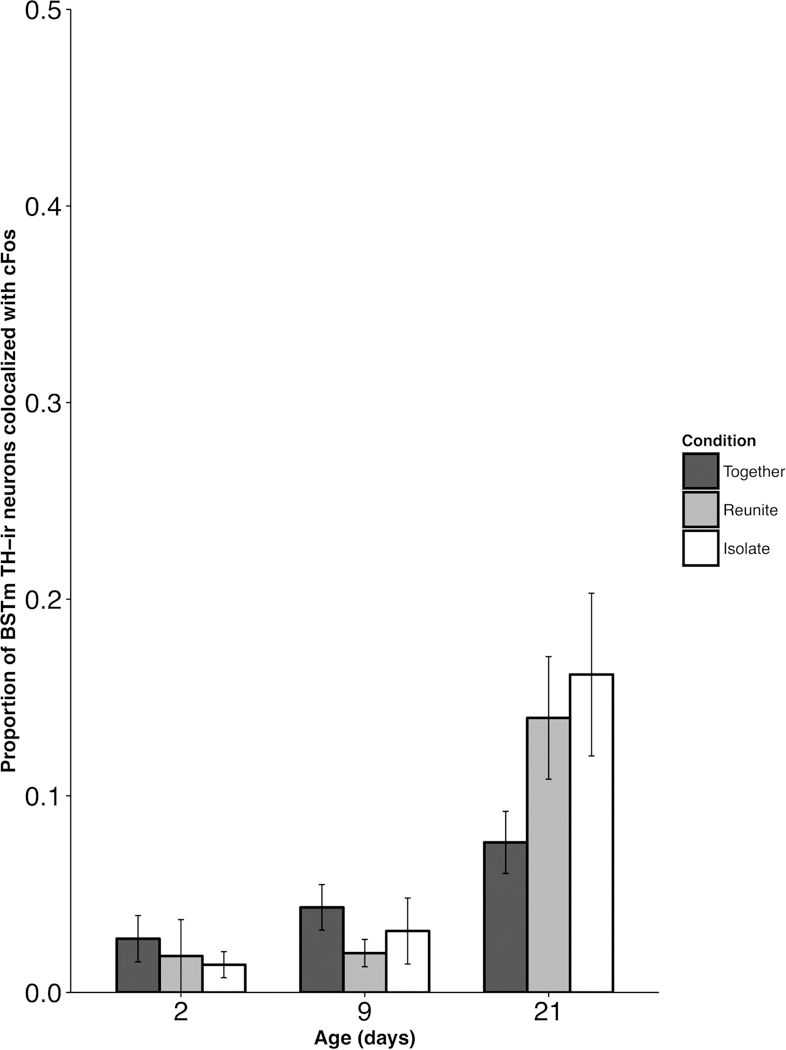
Mean (±SEM) proportion of tyrosine hydroxylase (TH)-ir neurons co-labeled with cFos-ir as a function of pup age and experimental condition within the medial division of the bed nucleus of the stria terminalis (BSTm). No comparisons were statistically significant.
TH-cFos colocalization in the pBST.
We did not find any significant main effect of Condition (F(2,75.81) = 2.13; p = 0.126), nor any significant Age × Condition interaction for TH-cFos colocalization in the pBST (F(4,75.28) = 0.96; p = 0.44; Fig. 8).
Figure 8.
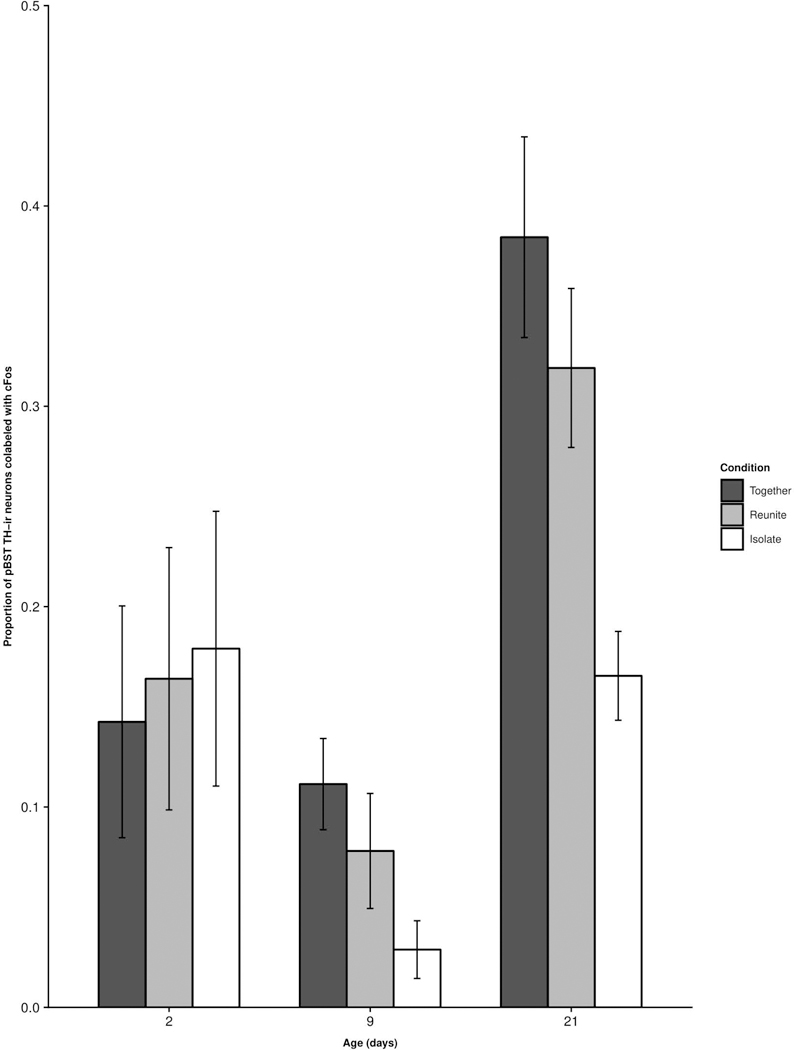
Mean (±SEM) proportion of TH-ir neurons co-labeled with cFos-ir as a function of pup age and experimental condition within the pBST. No comparisons were statistically significant.
TH-cFos colocalization in the MeA.
We observed a significant Age × Condition interaction for TH-cFos colocalization in the MeA (F(4,59.69) = 2.8275; p = 0.032; Fig. 9). Post-hoc comparisons revealed that in PND 21 pups, TH-cFos colocalization was significantly greater in pups in the Together (t(84.08) = 2.51; p = 0.042) and Reunite (t(75.91) = 3.127; p = 0.0075) conditions compared to those in the Isolate condition. Neither the PND 2 nor PND 9 pups showed differences in MeA TH-cFos colocalization as a function of social condition (Isolate × Reunite – PND 2 t(46.09) = 0.94, PND 9 t(60.53) = 0.98; Isolate × Together – PND 2 t(52.74) = 0.55, PND 9 t(65.37) = 0.89; Reunite × Together – PND 2 t(41.84) = 1.42, PND 9 t(57.14) = 0.043; all p’s > 0.05).
Figure 9.
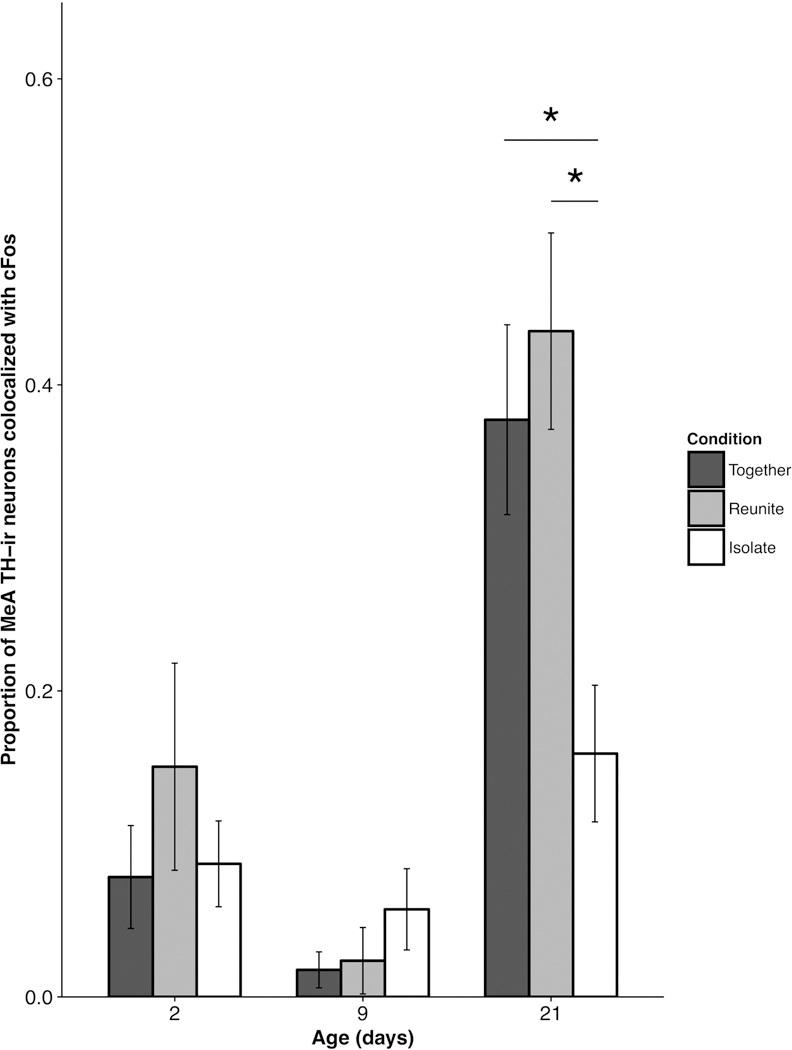
Mean (±SEM) proportion of tyrosine hydroxylase (TH)-ir neurons co-labeled with cFos-ir as a function of pup age and experimental condition within the medial amygdala (MeA). * p < 0.05.
Discussion
In the present study, we found that subregions within the meEA differentially responded to social context in an age-dependent manner. For both PND 9 and PND 21 animals, the BSTm demonstrated more general neuronal responsiveness (i.e., cFos expression) in animals experiencing the Isolate condition compared to those in the Together condition. These results indicate that the BSTm may be particularly sensitive to the presumably stressful experience of social isolation. Moreover, cFos responses within the MeA of PND 21 subjects were greatest in the Reunite condition compared to either the Together or Isolate conditions, suggesting that this region is sensitive to the emergence of social stimuli and/or potentially the rewarding context of being reunited with familial individuals after social isolation. However, the youngest age we examined (PND 2) did not show BSTm or MeA cFos response profiles that discriminated between social conditions, suggesting that the meEA becomes selectively responsive to changes in the social environment later in postnatal development.
Interestingly, the patterns we observed for cFos activity within the meEA were not identical to those we observed for TH neural activity within the meEA. Specifically, TH-cFos colocalization within the MeA was higher in both the Together and Reunite groups compared to the Isolate group (only in PND 21 animals, discussed below). These data suggest that there might be a specialized function for this sub-population of TH positive cells relative to other cell types in the MeA. However, additional data on the sensitivity of types of MeA cells to various environmental stimuli would be helpful to support this interpretation. Finally, although neurons of the BSTm were particularly responsive to social isolation (as indicated by cFos expression), TH neurons were not exclusively responsible for BSTm neuronal activation because we did not observe group differences for TH-cFos colocalization in the BSTm.
Altogether, these data provide evidence that 1) cell groups in the MeA and the BSTm differ in their sensitivities to aspects of the social environment, and 2) the social responsiveness of the BSTm appears to emerge earlier in prairie vole development (by PND 9) compared to that of the MeA (by PND 21). Below we discuss the ontogenetic differences in the timing and location of emerging functional cell groups across the meEA. Because significantly more developmental neurobiology has been characterized in rat pups, and rats share key developmental features with prairie voles (altriciality and a gestation period of 21 days; Smotherman and Robinson, 1987) we draw from the rat literature to provide a useful basis for understanding the ontogeny of prairie vole social development.
The duality of the meEA and the processing of social context.
Our results showed that the highest level of neuronal activation within the BSTm was detected under social isolation. In contrast, the neurons of the MeA only showed differential heightened activation in conditions in which the pups were in contact with caregivers. The implication of these results is that, within the meEA, the BSTm and the MeA appear to respond to distinct properties of the social environment.
Current understanding of the BST suggests that it acts as a relay center, receiving inputs from sensory and cortical structures and modulating steroid hormone and nonapeptide release via projections to the paraventricular nucleus of the hypothalamus (Choi et al., 2007). Thus, the BST could play a critical role in facilitating appropriate responses to different stressful contexts. Stressful novel experiences increase BST cFos counts in rat dams (Smith and Lonstein, 2008), whereas BST stimulation produces behavioral responses similar to those elicited by restraint stress (Casada and Dafny, 1991). Pharmacological synaptic blockade of the rat BST attenuates behavioral and autonomic responses to fear cues, indicating the importance of the BST in processing threatening contexts and subsequently producing appropriate behavioral and endocrine stress responses (Resstel et al., 2008). We found neurons of the BSTm are functionally sensitive to the absence of social contact (at PND 9 and PND 21). This outcome is consistent with documented functional roles of the BSTm in stress and anxiety-like responses, and suggests a broader role for the BSTm in processing stressful social contexts.
In contrast, the MeA was active particularly after the transition from brief isolation to reunion with family. This result provides ostensible evidence that the MeA plays a role in processing the exposure to social conspecifics. Supporting this interpretation is the longstanding association between MeA function and social affiliation and aggression in prairie voles (Wang and De Vries, 1993; Wang et al., 1997; Curtis and Wang, 2003). In male voles, MeA cFos expression is heightened after exposure to a sexually-receptive female, compared to cFos levels after exposure to a familiar sibling, or to isolation (Lim and Young, 2004). This suggests that the MeA appears to be sensitive to the presentation of social stimuli, and to the biological relevance of the conspecific. This interpretation is supported by the known functional role of the MeA in social recognition and social interest in rats (Gur et al., 2014). Furthermore, lesioning the MeA of male prairie voles decreases their levels of paternal care, and time spent in contact with a familiar female (Kirkpatrick et al., 1994). These results suggest that MeA function is necessary in facilitating adaptive behavioral responses to conspecifics, which is congruent with evidence that demonstrates selective representations of social stimuli within mouse MeA sub-circuitry (Bergan et al., 2014).
Alternatively, the neural activity we observed in the MeA might have resulted from manipulating the availability of general sensory information (olfactory, visual, or auditory cues). We think this is unlikely because if the mere presence of sensory cues were driving these neural responses, we would expect to see heightened levels of cFos expression in the Together condition in conjunction with the Reunite condition. Moreover, in female prairie voles, both the dorsal medial and the anterior medial nuclei of the amygdala show heightened cFos expression after exposure to male urine, compared to either saline or milk stimuli (Hairston et al., 2003). This result suggests that the prairie vole MeA is capable of responding selectively to the presentation of social odors, relative to odors more generally. Furthermore, rats and mice as young as PND 2 can be conditioned under an odor-aversion learning paradigm (Rudy and Cheatle, 1977) or odor-conditioned for food reinforcement (Armstrong, DeVito, & Cleland, 2006), suggesting that we should have seen similar neural responses in the PND 2 and PND 9 groups if the capacity for olfactory processing was driving the MeA cFos responses.
It is possible that simple exposure to any stimulus (regardless of its social or nonsocial properties) could explain the induction of cFos in the MeA in the Reunite condition. However, the MeA cFos responses of male hamsters and mice discriminate between the presentation of conspecific and heterospecific odors, and both social odors induce significantly greater amount of cFos relative to control unscented and peppermint stimuli (Meredith & Westbury, 2004; Samuelson & Meredith, 2009). These studies of MeA function suggest that the rodent MeA is highly sensitive to distinct social stimuli compared to nonsocial stimuli. Without including a non-social stimulus in our design, we cannot definitively conclude that our results are uniquely due to reunion with family, per se. Nonetheless, the ability to assess the social environment is inextricably tied to the mechanisms underlying sensory processing. Investigating how sensory modalities serve as potential routes by which the social environment is encoded is beyond the scope of the present work, but would be an important and valuable avenue of research that would elucidate how social contexts are processed in the developing brain.
Developmental timing of meEA response to social context.
It is interesting to consider the differential neural activation of the BSTm and MeA in response to social conditions with respect to some notable developmental benchmarks. We did not find discriminative patterns of cFos activation in either the BSTm or MeA in PND 2 animals, which was surprising given that separation from parents is likely to leave PND 2 pups at their greatest risk for predation, thermoregulatory vulnerability, or starvation. It is plausible that systems beyond the functional sensitivity of the extended amygdala are online to address these life-threatening challenges. For example, from the day of parturition until the weaning period, prairie vole pups emit calls in response to cooling (a consequence of isolation), which is believed to elicit retrieval responses from parents (Blake, 2002). Whether these calls are a physiological byproduct of the respiratory response to cold exposure (Blumberg and Alberts, 1990) or an evolutionarily adaptive communicative signal (Hofer and Shair, 1993), pup vocalizations may serve as a compensatory mechanism that mediates infant survival during a period in which isolation is not yet encoded by the meEA.
Importantly, our data do not suggest that the brains of neonatal pups are entirely incapable of reacting to context. Work in rats has shown that pups transiently upregulate cFos mRNA expression in the neocortex and midbrain 30 mins following parturition (Ringstedt et al., 1995), and PND 0 rats show significantly more cFos expression in the olfactory bulbs after the presentation of odors such as peppermint and propionic acid compared to clean air (Guthrie and Gall, 2003). These pieces of evidence indicate that the brains of even the youngest postnatal rat pups seem capable of responding to environmental changes. Our results depict an insensitivity of PND 2 cell groups within the meEA to social context, which is consistent with the hypothesis that the brains of perinatal pups lack the functional capacity for adult-like responses to changes in the social environment. The relatively delayed functional maturation of the MeA has been documented in other rodents. For example, rat pups show heightened MeA cFos expression when exposed to a threatening novel adult male at PND 21, but not at PND 7 (Wiedenmayer and Barr, 2001). One hypothesis posits that the hypo-activity of the amygdala in the neonatal rodent is not merely a result of neurobiological immaturity, but rather an evolutionarily adaptive mechanism that attenuates amygdala-dependent fear or aversion-learning during a critical sensitive period in which pups learn to form an attachment to their mothers (Landers and Sullivan, 2012). It follows that neonatal brains should not be considered mere immature versions of adult brains. Instead, by considering the developmental context, we may begin to appreciate the ways in which the particular neurobiology of infants facilitates adaptations to the demands of their changing environment (Alberts, 2008).
We found that neural responsiveness of the BSTm to distinct social contexts was first observable as early as PND 9, when the pups opened their eyes and became more mobile. In contrast, the MeA demonstrated differential responses to social contexts only at PND 21, during the developmental stage of weaning. These results suggest that subregions within the meEA exhibit differential functional development, with the BSTm beginning to discriminate between social contexts earlier in development relative to the MeA. It is unclear whether our findings stem from the underdevelopment of meEA brain structures, or immaturity of the afferent projections from sensory regions. In rats, projections from both the olfactory and accessory olfactory bulbs to the medial amygdala via the stria terminalis are seen in prenatal rats (Schwob and price 1978). Projections from the MeA to the BST are established prior to parturition, while reciprocal fibers are not seen in rat pups until PND1 (Cooke and Simerly, 2005). However, the density of projections between these regions of the meEA increase over the postnatal period, and only approach adult-typical densities around PND15. Consequently, it is plausible that structures outside of the meEA and upstream in the olfactory pathway become functionally responsive earlier in postnatal life to facilitate behavioral responses to environmental changes in preweanling animals. The functional consequences of differences in developmental timing for the BSTm and the MeA remain unclear. To this end, our data provide evidence that regions of the meEA are not homogenous in their functional developmental rates.
TH function within the meEA.
TH-ir cells are present in the BSTm, pBST, and MeA as early as PND 2 in prairie voles, indicating the potential for TH-dependent action in the meEA at an early age. Despite the presence of TH-ir neurons in the BSTm, we found no effects across Age and Social Context for TH-cFos co-localization in this region. On the other hand, MeA TH neurons in PND 21 brains responded strongly to social context, suggesting that family interactions at weaning elicit a TH-related neural response within the MeA. Notably, the sub-population of MeA neurons expressing TH demonstrated enhanced activation (indicated by TH-cFos co-localization) in the Reunite context (paralleling our cFos only data) and in the Together context (a departure from the cFos only data in this region). These results suggest the TH positive neurons of the MeA exhibit a general sensitivity to pro-social contexts. Other studies have shown that male prairie vole TH neural activity is greater following a variety of social interactions compared to social isolation (Northcutt & Lonstein, 2009), supporting the interpretation here that MeA TH activation reflects sensitivity to social interactions.
It is tempting to equate the presence of TH to dopamine action, because TH serves as a precursor to DA. Given that our data showed that MeA TH activation responded most intensely to prosocial contexts, it is even more tempting to conclude that TH in the MeA facilitates the rewarding properties (so often attributed to dopamine) under social contexts. Caution should be taken with this conclusion. The activation of TH-producing cells can relate to downstream dopaminergic neurotransmission, but it can also lead to subsequent production and exocytosis of adrenaline or noradrenaline (De Deurwaerdère et al., 2017). Furthermore, L-DOPA can be metabolized into non-catecholaminergic products (such as melanin precursors), interact with 5-HT neurons to modify extracellular serotonin (Navailles et al., 2013) or exhibit neurotransmitter-like properties as an end product itself (De Deurwaerdère et al., 2017). As such, the multifaceted potential of TH molecules (and L-DOPA action) obscures precise functional roles of TH neurons within the meEA. It is, therefore, unclear if the sensitivity of MeA TH neurons to familial stimuli is related to dopaminergic encoding of a social reward. AADC is required for dopamine synthesis, and MeA TH cells do not immunoreactively co-express AADC in adult prairie voles (Ahmed et al., 2012). We do not know if this is also true for pre-weaning prairie vole pups. On the other hand, AADC-expressing cells are found more generally in both the BST and the MeA of adult prairie voles (Ahmed et al., 2012), and neighboring AADC cells can coordinate with TH cells to facilitate DA synthesis in rats (Ugrumov et al., 2004; Ugrumov, 2009). Further studies are necessary to determine the specific products of TH neurons within the MeA of prairie voles, and how they might be involved in the encoding of social and/or rewarding contexts. Whether the TH activation we found in the MeA serves as a marker of DA activation, or the activation of other molecular pathways, the dynamic nature and multi-functional potential of TH is likely enabling TH-positive neurons in the MeA to differentially and appropriately respond to the social environment as animals mature. A deeper appreciation of the development of social behavior will only be achieved by identifying the specific ontogenetic windows during which time these cell groups become responsive to social contexts and exhibit adult-typical response profiles, and by understanding the abilities and limitations imposed by the development of this neurophysiology.
Conclusions
Our data provide evidence that the meEA exhibits functional differences across early development. Interestingly, the meEA of neonatal pups was not selectively responsive to variation in the social environment. However, meEA functioning of older pups (PND 9, age of eye-opening and independent locomotion; PND 21, age of weaning) was subregion-, age- and context-specific. Although the BSTm differentially responds to isolation by the second week of postnatal life, the MeA does not differentially respond to the presence of familial individuals (i.e., parents and siblings) until weaning-age. Furthermore, our findings suggest that TH neurons in the MeA do not differentially respond to social context until later in development (PND 21). As such, our results point towards heterogeneous ontogenies of functionality across, and TH circuitry within, the meEA. Despite the connectivity of the meEA, careful consideration of the specific subregions analyzed within the meEA should be taken given that individual nuclei have varying rates of structural, functional, and connective development (Goodson et al., 2004). Examining the development of social neural circuitry is important because it provides insight into the mechanisms that govern age-specific social phenotype. It can also identify developmental stages where changes in neuroanatomy and function occur, which might represent critical periods in development where the environment can most substantially impact behavioral profiles that persist into adulthood.
Acknowledgements
We would like to express gratitude to Chang Kim for his assistance with PCR, and to the Statistical Consulting Unit at Cornell University for their guidance with our statistical analyses. We are also thankful for support from the National Institute of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD081959 to AMK and HD79573 to AGO), the National Science Foundation IOS-1634027 to AGO, and the National Science Foundation Graduate Research Fellowship DGE-1650441 to LCH.
Footnotes
Conflicts of Interest
The authors declare we have no conflicting interests to disclose.
References
- Ahmed EI, Northcutt KV., Lonstein JS. 2012. L-Amino acid decarboxylase- and tyrosine hydroxylase-immunoreactive cells in the extended olfactory amygdala and elsewhere in the adult prairie vole brain. J Chem Neuroanat 43:76–85. [DOI] [PubMed] [Google Scholar]
- Alberts JR. 2008. The nature of nurturant niches in ontogeny. Philos Psychol 21:295–303. [Google Scholar]
- Alheid GF. 2003. Extended amygdala and basal forebrain. Ann N Y Acad Sci 985:185–205. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, DeVito LM, Cleland TA. 2006. One-trial associative odor learning in neonatal mice. Chem Senses 31:343–349. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Bell MR, Meerts SH, Sisk CL. 2013. Adolescent brain maturation is necessary for adult-typical mesocorticolimbic responses to a rewarding social cue. Dev Neurobiol 73:856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan JF, Ben-Shaul Y, Dulac C. 2014. Sex-specific processing of social cues in the medial amygdala. Elife 3:e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake BH. 2002. Ultrasonic calling in isolated infant prairie voles (Microtus ochrogaster) and montane voles (M. montanus). J Mammal 83:536–545. [Google Scholar]
- Blumberg MS, Alberts JR. 1990. Ultrasonic Vocalizations by Rat Pups in the Cold: An Acoustic By-Product of Laryngeal Braking? Behav Neurosci 104:808–817. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N. 1991. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull 27:207–212. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. 2007. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27:2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Simerly RB. 2005. Ontogeny of bidirectional connections between the medial nucleus of the amygdala and the principal bed nucleus of the stria terminalis in the rat. J Comp Neurol 489:42–58. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. 1998. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: Simultaneous anterograde and retrograde tract tracing. J Comp Neurol 399:189–209. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. 2003. Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster). Physiol Behav 80:95–101. [DOI] [PubMed] [Google Scholar]
- Davis M 1992. The Role Of The Amygdala In Fear And Anxiety. Annu Rev Neurosci 15:353–375. [DOI] [PubMed] [Google Scholar]
- Deurwaerdère P De, Giovanni G Di, Millan MJ. 2017. Expanding the repertoire of L-DOPA’s actions: A comprehensive review of its functional neurochemistry. Prog Neurobiol 151:57–100. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. 2001. “When a rat smells a cat”: The distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104:1085–1097. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. 2004. Projections from bed nuclei of the stria terminalis, posterior division: Implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol 471:396–433. [DOI] [PubMed] [Google Scholar]
- Filipenko ML, Alekseyenko OV., Beilina AG, Kamynina TP, Kudryavtseva NN. 2001. Increase of tyrosine hydroxylase and dopamine transporter mRNA levels in ventral tegmental area of male mice under influence of repeated aggression experience. Mol Brain Res 96:77–81. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. 2004. Chemoarchitectonic Subdivisions of the Songbird Septum and a Comparative Overview of Septum Chemical Anatomy in Jawed Vertebrates. J Comp Neurol 473:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R, Tendler A, Wagner S. 2014. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry 76:377–386. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Gall C. 2003. Anatomic mapping of neuronal odor responses in the developing rat olfactory bulb. J Comp Neurol 455:56–71. [DOI] [PubMed] [Google Scholar]
- Hairston JE, Ball GF, Nelson RJ. 2003. Photoperiodic and temporal influences on chemosensory induction of brain Fos expression in female prairie voles. J Neuroendocrinol 15:161–172. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. 2014. Annual research review: Towards a developmental neuroscience of atypical social cognition. J Child Psychol Psychiatry Allied Discip 55:553–577. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair HN. 1993. Ultrasonic Vocalization, Laryngeal Braking, and Thermogenesis in Rat Pups: A Reappraisal. Behav Neurosci 107:354–362. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MSS, Verbalis JG. 1993. c-Fos and Related Immediate Early Gene Products as Markers of Activity in Neuroendocrine Systems. Front Neuroendocrinol 14:173–213. [DOI] [PubMed] [Google Scholar]
- Johnston JB. 1923. Further contributions to the study of the evolution of the forebrain. J Comp Neurol 35:337–481. [Google Scholar]
- Kellogg CK, Awatramani GB, Piekut DT. 1998. Adolescent development alters stressor-induced fos immunoreactivity in rat brain. Neuroscience 83:681–699. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Ophir AG. 2018. Rapid nonapeptide synthesis during a critical period of development in the prairie vole: plasticity of the paraventricular nucleus of the hypothalamus. Brain Struct Funct 1–14. [DOI] [PMC free article] [PubMed]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. 1994. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav Neurosci 108:501–13. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. 2005. Mapping the neural circuit activated by alarm pheromone perception by c-Fos immunohistochemistry. Brain Res 1043:145–154. [DOI] [PubMed] [Google Scholar]
- Klejbor I, Luczynska A, Ludkiewicz B, Domaradzka-Pytel B, Morys J. 2003. The developmental pattern of c-fos expression in the rat thalamus following open-field stress stimulation. Pol J Vet Sci 6:201–207. [PubMed] [Google Scholar]
- Kobayashi K, Nagatsu T. 2012. Tyrosine Hydroxylase. Primer on the Autonomic Nervous System, Elsevier, p 45–47.
- Kollack-Walker S, Newman SW. 1995. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience 66:721–736. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff P, Christensen R. 2017. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Softw 82:1–26. [Google Scholar]
- Landers MS, Sullivan RM. 2012. The development and neurobiology of infant attachment and fear. Dev Neurosci 34:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV 2016. Least-Squares Means: The R Package lsmeans. J Stat Softw 69:1–33. [Google Scholar]
- Lim MM, Young LJ. 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125:35–45. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Young LJ. 2010. The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci 33:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, Novak M. 1984. A comparison of maternal behaviour in the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochrogaster) and pine vole (M. pinetorum). Anim Behav 32:1132–1141. [Google Scholar]
- Navailles S, Lagière M, Contini A, Deurwaerdère P De. 2013. Multisite Intracerebral Microdialysis to Study the Mechanism of L-DOPA Induced Dopamine and Serotonin Release in the Parkinsonian Brain. ACS Chem Neurosci 4:680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, Guyer AE. 2016. Developmental Cognitive Neuroscience [DOI] [PMC free article] [PubMed]
- Newman SW. 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 877:242–257. [DOI] [PubMed] [Google Scholar]
- Northcutt K, Nguyen J. 2014. Female juvenile play elicits Fos expression in dopaminergic neurons of the VTA. Behav Neurosci 128:178–86. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. 2009. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience 163:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt KV, Wang Z, Lonstein JS. 2007. Sex and species differences in tyrosine hydroxylase-synthesizing cells of the rodent olfactory extended amygdala. J Comp Neurol 500:103–115. [DOI] [PubMed] [Google Scholar]
- Pardo-Bellver C, Cádiz-Moretti B, Novejarque A, Martínez-García F, Lanuza E. 2012. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front Neuroanat 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2016. R: A language and environment for statistical computing R Found Stat Comput. [Google Scholar]
- Rehling A, Spiller I, Krause ET, Nager RG, Monaghan P, Trillmich F. 2012. Flexibility in the duration of parental care: Zebra finch parents respond to offspring needs. Anim Behav 83:35–39. [Google Scholar]
- Resstel LBM, Alves FHF, Reis DG, Crestani CC, Corrêa FMA, Guimarães FS. 2008. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience 154:869–876. [DOI] [PubMed] [Google Scholar]
- Ringstedt T, Tang L- Q, Persson H, Lendahl U, Lagercrantz H. 1995. Expression of c-fos, Tyrosine Hydroxylase, and Neuropeptide mRNA in the Rat Brain around Birth: Effects of Hypoxia and Hypothermia. Pediatr Res 37:15–19. [PubMed] [Google Scholar]
- Rudy JW, Cheatle MD. 1977. Odor-aversion learning in neonatal rats. Science (80- ) 198:845–846. [DOI] [PubMed] [Google Scholar]
- Smith CD, Lonstein JS. 2008. Contact with infants modulates anxiety-generated c-fos activity in the brains of postpartum rats. Behav Brain Res 190:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. 1987. Prenatal expression of species-typical action patterns in the rat fetus (Rattus norvegicus). J Comp Psychol 101:190–196. [PubMed] [Google Scholar]
- Ugrumov MV 2009. Non-dopaminergic neurons partly expressing dopaminergic phenotype: Distribution in the brain, development and functional significance. J Chem Neuroanat 38:241–256. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV, Melnikova VI, Lavrentyeva AV, Kudrin VS, Rayevsky KS. 2004. Dopamine synthesis by non-dopaminergic neurons expressing individual complementary enzymes of the dopamine synthetic pathway in the arcuate nucleus of fetal rats. Neuroscience 124:629–635. [DOI] [PubMed] [Google Scholar]
- Vochteloo JD, Koolhaas JM. 1987. Medial amygdala lesions in male rats reduce aggressive behavior: interference with experience. Physiol Behav 41:99–102. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. 1997. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res 767:321–332. [DOI] [PubMed] [Google Scholar]
- Wang Z, Vries GJ De. 1993. Testosterone effects on paternal behavior and vasopressin immunoreactive projections in prairie voles (Microtus ochrogaster). Brain Res 631:156–160. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. 2001. Developmental changes in c-fos expression to an age-specific social stressor in infant rats. Behav Brain Res 126:147–157. [DOI] [PubMed] [Google Scholar]


