Abstract
Mitochondria are a keystone of neuronal function, serving a dual role as sustainer of life and harbinger of death. While mitochondria are indispensable for energy production, a dysregulated mitochondrial network can spell doom for both neurons and the functions they provide. Traumatic brain injury (TBI) is a complex and biphasic injury, often affecting children and young adults. The primary pathological mechanism of TBI is mechanical, too rapid to be mitigated by anything but prevention. However, the secondary injury of TBI evolves over hours and days after the initial insult providing a window of opportunity for intervention. As a nexus point of both survival and death during this second phase, targeting mitochondrial pathology in TBI has long been an attractive strategy. Often these attempts are mired by efficacy-limiting unintended off-target effects. Specific delivery to and enrichment of therapeutics at their submitochondrial site of action can reduce deleterious effects and increase potency. Mitochondrial drug localization is accomplished using (1) the mitochondrial membrane potential, (2) affinity of a carrier to mitochondria-specific components (e.g. lipids), (3) piggybacking on the cells own mitochondria trafficking systems, or (4) nanoparticle-based approaches. In this review, we briefly consider the mitochondrial delivery strategies and drug targets that illustrate the promise of these mitochondria-specific approaches in the design of TBI pharmacotherapy.
Introduction
Mitochondrial dysfunction is at the core of many neuronal pathologies, serving as a nexus for neurons’ ongoing fate decision – survive or succumb. Traumatic brain injury (TBI) and similar acute central nervous system (CNS) injuries (ischemia, stroke) are no exception. TBI pathology is biphasic. The first phase centers on the direct physical force on brain tissue, but the second phase includes all the complex reactions of the sterile inflammatory response and discoordinated homeostasis that result from the initial mechanical injury (Prins et al., 2013; Werner and Engelhard, 2007). Ongoing mitochondrial dysregulation during this second phase provides a window of opportunity for intervention and is an attractive target for drug design. The need for effective pharmacotherapy is clear with 275k hospitalizations and 52k TBI-related deaths occurring in the United States annually (Faul et al., 2011). Further, individuals who survive severe TBI often face a lifetime of functional deficit, as well as other significant social and financial costs (Andelic et al., 2009; Coronado et al., 2011; Maas et al., 2008). As we currently lack useful neuroprotective therapies for TBI, new strategies must be considered that maximize the potential of past and prospective drug design efforts.
Development and implementation of rationally designed therapies specifically against TBI mitochondrial pathology have proved challenging. Numerous TBI-relevant and druggable mechanistic targets have been identified within mitochondria, but efforts to address this source of pathology have not translated into clinically effective therapy. Efficacy can be hindered by both the failure to attain adequate intramitochondrial drug concentration and occurrence of off-target effects on extramitochondrial machinery. To illustrate, TBI-induced DNA damage activates Poly(ADP-ribose) Polymerase (PARP1) that facilitates repair of nuclear DNA whereas mitochondrial PARP activation reduces mitochondrial DNA integrity and bioenergetic function (Satchell et al., 2003; Szczesny et al., 2014). Expanded on below, localization of PARP inhibitors to the mitochondria may be more effective than pancellular PARP inhibition. A mitochondrial partitioning strategy could reduce the negative effects of PARP activation in mitochondria while preserving the beneficial effects of PARP1 activation in nuclear DNA repair. Other TBI-relevant therapies might similarly benefit from mitochondrial localization. Generalizable strategies can facilitate highly specific accumulation of a drug in the mitochondria, potentially with submitochondrial specificity. The concept of subcellular site-specific drug partitioning has evolved over the recent past from a laboratory tool to viable therapeutic strategy. Here, “localization” refers to the spatial partitioning of active drug to the subcellular region/organelle relevant to the intended mechanistic site of action, the drug’s “target.” Mitochondrial drug localization can be accomplished using one of four strategies that capitalize upon (1) the mitochondrial membrane potential, (2) affinity to mitochondria-specific membrane lipids, (3) piggybacking on the cells own mitochondria trafficking systems, and (4) other permutations of these approaches that employ nanoparticle-based systems. This review will briefly discuss the potential of several mitochondrial drug localization strategies and describe mitochondrial targets thought to play a critical role in TBI pathology. We use several examples to illustrate how small molecule therapy could benefit from this approach.
Generalizable Methods of Mitochondrial Drug Localization
Mitochondria-localizing laboratory tools have existed for decades, but more recently these strategies have been adapted for clinical use. Mitochondrial drug localization is accomplished using any of four generalizable strategies. Efficient organelle-specific drug partitioning facilitates the colocalization of a therapy with its intended mechanistic target. Different mitochondrial localization methods also hint at the opportunity to target a drug to a specific submitochondrial region – enrichment of drug specifically in the matrix, inner or outer membrane, or within the intermembrane space. These targeting strategies are not without their own considerations. The partitioning efficiency of these approaches can be negatively influenced by the pathologic state they seek to treat while also altering the pharmacokinetic profile of the core therapeutic compound. Below, we review these four drug localization strategies, illustrated with several examples. Not all the methods to be discussed have been tested in the setting of TBI or acute CNS injury, but each holds promise for therapy design.
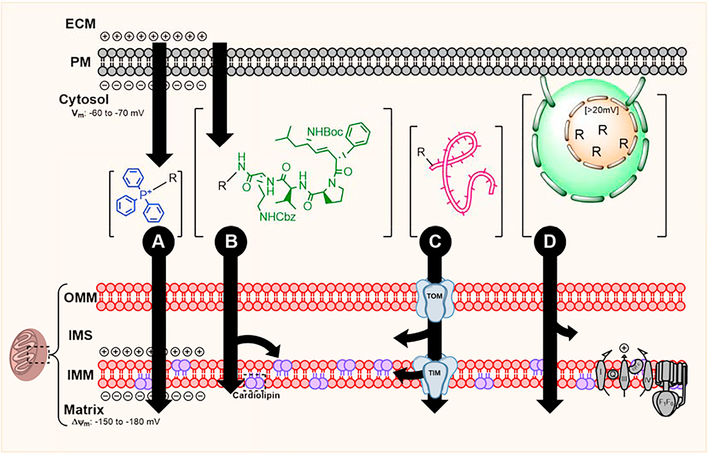
Membrane Potential-Driven.
Compared to other organelles, mitochondria are uniquely polarized with a trans-inner mitochondrial membrane (IMM) potential (Δψm) of −150 to −180 mV (Kamo et al., 1979). Lipophilic cations pass easily through the hydrophobic region of lipid bilayers because their positive charge is delocalized over a large area or shielded. As described by the Nernst equation, at 37°C these passively-transported cations accumulate up to 10x per 61.5 mV trans-membrane potential difference. The negative trans-plasma membrane resting potential of most cells aids in initial cytosolic uptake these compounds, with 90–95% of the intracellular cation localizing to the mitochondrial matrix at equilibrium (Liberman et al., 1969). Import efficiency increases in relation to the magnitude of potential difference. Therefore, cation-conjugated compounds possess a minor degree of cell-type selectivity, specifically to excitable cells (e.g. neurons, astrocytes, muscle cells) (Murphy and Smith, 2000; Ransom and Sontheimer, 1992). For neurons with a membrane resting potential of −70 mV, cation-conjugated drug accumulates 5 to 10-fold in cytosol versus extracellular fluid and 100 to 500-fold more in mitochondria versus cytosol at equilibrium. The final quantity of therapeutic compound found within mitochondria in vivo varies substantially based on cell type, affected by the properties of the conjugated drug, duration of treatment, cell volume, and number of mitochondria (Reily et al., 2013; Smith et al., 2003). However, explicitly because localization of mitochondrial-targeted cations relies primarily on passive electrochemical-driven transport, disease states associated with significant reduction in plasma membrane or Δψm present an unavoidable challenge to the efficiency of this approach.
As the prototypical mitochondria-targeting lipophilic cation, triphenylphosphonium (TPP) and its derivatives have been explored extensively (Fig 1-A) (Weissig et al., 1998). Most efforts utilizing TPP-driven drug localization have focused on delivery of lipophilic antioxidants (ubiquinone, tocopherol, quercetin) (Sheu et al., 2006) and superoxide dismutase mimetics (Dhanasekaran et al., 2005). For example, MitoQ is composed of ubiquinone covalently conjugated to TPP by a 10-carbon aliphatic chain linker (Kelso et al., 2001), and it was found to penetrate the blood-brain barrier (BBB) and enrich within neuronal mitochondria (McManus et al., 2011). Preclinical studies found MitoQ was superior compared to unconjugated ubiquinone in reducing indices of oxidative stress and functional defect in models of ischemic or oxidative injury (Adlam et al., 2005). While no studies have reported its clinical effectiveness in TBI, MitoQ was not found to be toxic to humans with prolonged exposure (Orsucci et al., 2011; Smith and Murphy, 2010; Snow et al., 2010). Like other lipophilic cations, TPP’s partitioning efficiency relies on an intact Δψm (Burns et al., 1995). TBI-induced electron transport chain (ETC) dysregulation often results in relative Δψm dissipation, effectively hampering the matrix localization efficiency of TPP-drug conjugates administered post-injury. MitoQ’s potency decreased nearly 25-fold when administered to cells with reduced Δψm (Smith and Murphy, 2010). Secondarily, the net movement of TPP and other lipophilic cations across the normally ion-impermeable IMM into the matrix risks “consuming” Δψm, presenting a dose-dependent risk of inducing or exacerbating ATP synthesis inhibition (Modica-Napolitano et al., 1984; Murphy, 2008). Despite these potential pitfalls, the success of MitoQ and similar agents demonstrates the potential of safe Δψm-driven approaches. Future drug design efforts should consider this method of delivery for use beyond antioxidants – for any small molecule enzyme inhibitors that could benefit from efficient mitochondrial matrix partitioning.
Fig. 1.
Methods of mitochondrial drug localization. (A) Membrane potential-driven localization uses a lipophilic cation, TPP. (B) Affinity-based localization uses the distinct composition of mitochondria to partition drug, hemi-gramicidin binds IMM-specific cardiolipin. (C) Intrinsic mitochondria protein trafficking systems, such as the TIM/TOM system, can deliver linear molecules. (D) Nanoparticles can be modified for specific mitochondrial localization and membrane fusion.
Affinity Driven.
Certain naturally-occurring antibiotics display strong affinity towards bacterial membranes and, owing to their shared evolutionary history, are similarly attracted to IMM components such as mitochondria-specific phospholipid, cardiolipin (CL) (Ji et al., 2012; Prenner et al., 1999). Unlike lipophilic cations that follow Δψm and partition primarily into the matrix, CL-avid compounds are thought to be Δψm-insensitive and localize to both sides of the IMM. Therefore, IMM or IMS-localized mechanistic targets might be better addressed by this method over traditional cationic strategies. The gramicidin S (GS) derivative, hemigramicidin (Leu-D-Phe-Pro-Val-Orn, “hemi-GS”), and Szeto-Schiller (SS) tripeptides are two prime examples of this localization strategy.
The hemi-GS pentapeptide (Fig 1-B) can be linked with a flexible tether to a wide variety of small molecules without compromising the payload’s biological activity and ability to interact with mitochondrial proteins (Wipf et al., 2005). Unlike its GS parent, hemi-GS does not permeabilize cell membranes and has no antibacterial activity as it lacks the requisite secondary structure (Escobales et al., 2014). Hemi-GS conjugates are highly effective at concentrating in mitochondria, primarily to the IMS and matrix. One of these conjugates, XJB-5–131 [hemi-GS-4-amino-TEMPO (4-amino-2,2,6,6-tetramethylpiperidine-N-oxyl)], was found to be non-toxic, rapidly penetrate the BBB, and enrich in mitochondria over 600-fold compared to cytosol in a Δψm-independent manner (Ji et al., 2012; Krainz et al., 2016). Hemi-GS is adaptable; varying the length and composition of the hemi-GS to payload linker region can modulate mitochondrial targeting efficiency, payload activity, molecular weight, lipophilicity, and polar surface area (Krainz et al., 2016). Preclinical studies demonstrated that XJB-5–131 is highly effective at reducing injury and preserving behavioral function following TBI and other CNS insults (Ji et al., 2012).
SS peptides are another potential method for affinity-based mitochondrial targeting. This family consists of peptides with <10 amino acid that share an alternating aromatic-cationic motif a 3+ net charge. Basic amino acids, arginine and lysine, provide two positive charges while the free amine of the N-terminus and aminated C-terminus allow for a final 3+ net charge at physiologic pH (Zhao et al., 2003). Experimental evidence suggests that these peptides predominately localize to the IMM (100-fold enrichment) and that despite their cationic nature, only 10–15% of that localization was attributed to a Δψm-driven mechanism (Szeto, 2008). Like hemi-GS, SS peptides’ selectively towards the IMM is reported at least to be partially driven by the presence of CL (Szeto, 2014). These molecules have been primarily used for their intrinsic antioxidant properties, but several studies have suggested their possible use as a payload delivery system (Cerrato et al., 2015).
Transporter Driven.
With the exception of 13 core ETC proteins, >99% of mitochondrial proteins are synthesized in the cytosol and transported into the mitochondria through mitochondrial transport machinery (Schmidt et al., 2010). Various linear therapeutic compounds may be similarly delivered by capitalizing upon these intrinsic trafficking systems (Fig 1-C). The best described and most common mitochondrial protein trafficking relies on the N-terminal mitochondrial targeting signal peptide (MTP) composed of 10–70 amino acids, while region-specific targeting is controlled by internal stop-transfer sequences (Claros and Vincens, 1996; Stojanovski et al., 2012). The MTP motif forms an amphiphilic helix – one face is enriched with hydrophobic leucine while the opposing side is positively charged (Abe et al., 2000). The translocase of the outer membrane (TOM) complex conducts the pre-protein (protein + MTP) to the outer mitochondrial membrane (OMM) or IMS. IMM or matrix-destined pre-proteins are further processed by the translocase of the inner membrane (TIM) complex in an active, energy- and Δψm-dependent manner (Wiedemann and Pfanner, 2017). Mitochondrial processing peptidase (MPP) and associated chaperones guide final pre-protein maturation (Gakh et al., 2002). The feasibility of MTP-conjugated drugs has been explored and found to be amenable to mitochondrial localization of most linear biomolecules. Although this method utilizes intrinsic, non-toxic small peptide carriers, unavoidable tradeoffs remain: 1) This transport mechanism uses a narrow (~2 nm) active channel; therefore, the transport is restricted by the size of macromolecules. Larger molecules, such as folded proteins, cannot be delivered into the matrix. 2) Transport is an ATP-dependent, active process. TBI-induced ATP depletion inhibits transport and therapy-delivery. 3) This method competitively inhibits the transport of the essential mitochondrial proteins. 4) Stability and tissue-penetration of the MTS-conjugate may prove challenging.
Few studies exist that use this localization method towards the design of clinical treatment, and no studies have been conducted in TBI. So far, efforts have focused on the delivery of peptides and oligonucleotides. MTP derived from mouse mitochondrial thiolase was shown to successfully deliver a 13-base nucleic acid sequence to mouse myoblast mitochondria (Flierl et al., 2003). Similarly, green fluorescent protein (Zhang et al., 1998), restriction enzyme Sma1 (Tanaka et al., 2002), and the human NADH ubiquinone oxidoreductase subunit 4 (ND4) gene (Yu et al., 2012) were all found to localize efficiently under MTS control. The final destination of these composite proteins is the matrix by default. Further refinement of the MTS (or alternative trafficking system) and therapeutic payload may mitigate some drawbacks and facilitate more specific submitochondrial targeting in the future.
Nanoparticle Delivery.
Mitochondriotropic liposomes and nanoparticles (NP) are the syntheses of previously described localization approaches, selectively transporting encapsulated drug into the mitochondria (Fig 1-D). However, these particle-based approaches are relatively new compared to previously discussed mitochondrial localization strategies.
Liposomes are self-assembling biodegradable particles (50 to 5,000 nm) composed of phospholipids and cholesterol (Pathak et al., 2015). They are organized into an aqueous core which encapsulate hydrophilic compounds while the outer lipid bilayer can host hydrophobic drugs (Marrache et al., 2013). MITO-Porter liposome demonstrated the mitochondrial targeting potential of liposomes by successfully ferrying cargo to OMM and into the IMS (Yamada et al., 2008). Unlike other cationic liposomes that enter cells primarily via clathrin-mediated endocytosis, MITO-Porter liposomes enter cells due to octa-arginine (R8)-stimulated macropinocytosis. The high positive charge density on the liposomal surface not only stimulates cellular uptake but also aids in efficient escape of intact liposomes from macropinosomes at both neutral and acidic pH. In comparison, other micropinocytosis-simulating motifs (e.g. octalysine, K8) are less efficient at facilitating liposome escape as pH decreases during vesicle maturation (El-Sayed et al., 2008). Free, intact liposomes localize to mitochondria based on the composition of their lipid bilayer, specifically the abundance of fusogenic lipids (especially sphingomyelin and phosphatidic acid) (Yamada et al., 2008). Although mitochondrial targeting of liposomes has been widely used in cancer research, there are currently no reported examples of liposomes that can both penetrate the BBB while simultaneously localizing to the mitochondria. Further, maintaining the structural integrity and preventing lipid hydrolysis is an ongoing challenge to both storage and administration of liposome-based therapies.
Polymeric NP serves as a highly adaptable alternative to liposomes. They too are biocompatible and biodegradable polymers manufactured to specific size, density, and charge to allow modulation of various properties, including BBB penetration and mitochondrial localization. Compared to liposomes where the cargo is burst-released into the mitochondria following membrane fusion, polymeric NP can be designed to precisely modulate the rate of drug release (Kamaly et al., 2013). NP containing hydrophobic polyester blocks demonstrate improved biostability in harsh physiological environments facilitating oral administration and may be more tolerable to variable storage conditions compared to liposomes. For example, polyethylene glycol is a typical hydrophilic block added to NP that (1) increases half-life in plasma by reducing reticuloendothelial system-based clearance and (2) serves as a scaffold for further modification with cationic and other mitochondrial-targeting moieties (Allen and Chonn, 1987; Pathak et al., 2015; Wongrakpanich et al., 2014). Specific to mitochondrial targeting, Marrache et al. observed that NP most efficiently localized to the mitochondria when 80–100 nm in diameter and carrying a zeta potential >22 mV. Indeed, TPP-conjugation augmented NP zeta potential and corresponding mitochondrial accumulation (Marrache and Dhar, 2012). Ghosh et al. found TPP-modified NP loaded with the antioxidant, quercetin, were orally active, penetrated the BBB, and accumulated in mitochondria while decreasing neuronal loss and improving mitochondrial function following cerebral ischemia-reperfusion (Ghosh et al., 2017).
Contribution of mitochondrial dysfunction to secondary injury after TBI
Neurons are dependent on mitochondria to supply ATP through oxidative phosphorylation (OxPhos), but they also rely on this organelle for both survival and death signaling, biosynthetic function (e.g. heme synthesis, iron-sulfur cluster synthesis), and other bioenergetic roles to survive and thrive (Hall et al., 2012). This system is highly coordinated; the overwhelming majority of the >1000 mitochondrial-localized proteins are encoded by the nuclear genome compared to the 13 protein coding genes provided by mitochondrial DNA (mtDNA). Accordingly, even small perturbations to mitochondrial coordination and function have potentially devastating consequences across the neurovascular unit and are central in TBI secondary injury. While endogenous mechanisms work towards repair or elimination of dysfunctional mitochondria, restorative forces are embattled by competing pro-death processes on multiple fronts – defeat is common, victory is pyrrhic. Mitochondrial drug localization might unlock a therapy’s true potential, but everything relies on choosing clinically effective targets and understanding when an intervention can provide maximum benefit both in isolation and as part of a multimodal treatment strategy. Below we provide a brief primer outlining selected TBI-relevant mitochondrial pathological mechanisms and their temporal relationship that aids in the design and use of mitochondrial localizing therapies (Fig 2). However, these pathways of mitochondrial dysfunction operate in a wide range of neurologic disease states making what will be discussed applicable to more than TBI.
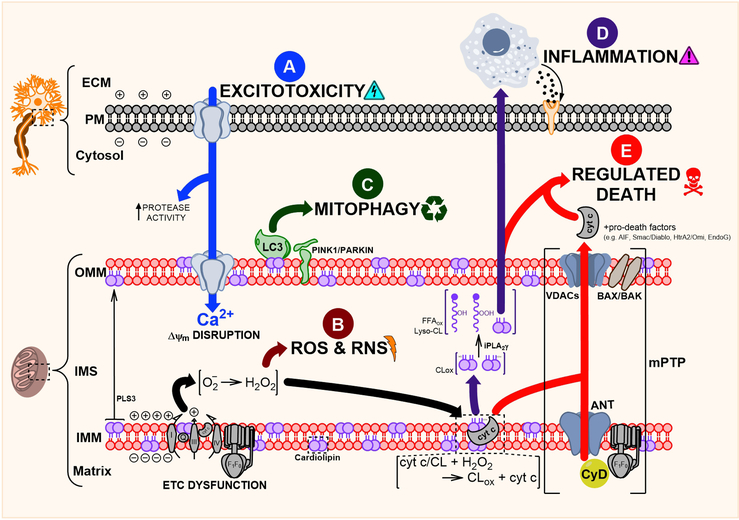
Fig. 2.
Mitochondria-focused mechanisms of neuronal injury following TBI. (A) Widespread neuronal depolarization results in the unregulated release of excitatory neurotransmitters and activation of their corresponding ion channels. Increased cytosolic Ca2þ is buffered, in part, by the mitochondria, disrupting Djm. (B) The ensuing ETC dysfunction causes protracted increases in ROS & RNS production. Injury becomes self-perpetuating as endogenous oxidant stress response systems are overwhelmed. (C) Damaged and nonfunctional mitochondria are actively eliminated through mitophagy. This system maintains a delicate balance between removing unsalvageable mitochondria and consolidating functional units. (D) Mitochondrial lipids, especially CL, are important sources of oxidized free fatty acid inflammatory mediators. CL is oxidized by the specific cyt c/CL peroxidase, and its oxidized acyl chains are liberated through the action of iPLA2g. (E) Permeabilization of the mitochondrial membranes and oxidation of CL prompts the release of pro-death factors e ultimately leading to neuronal death.
Prevention is the only way to stop primary mechanical TBI injury and the initial phases of secondary injury. The hyperacute injury is characterized by widespread neuronal depolarization resulting in a torrent of excitatory neurotransmitters (glutamate, aspartate). Resultant hyperactivation of N-methyl-D-aspartate (NMDA) receptors, α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptors, and various voltage-gated Na+ and Ca2+ channels drastically increases cytosolic Ca2+ (Arundine and Tymianski, 2004; Meldrum and Garthwaite, 1990). Protracted Ca2+ overload results in death through activation of catabolic proteases (e.g. calpains, caspases) (Saatman et al., 2010). Therefore, along with the smooth endoplasmic reticulum, mitochondria buffer excess cytosolic Ca2+ by the electrogenic activity of mitochondrial calcium uniporter (mCU), uncoupling proteins (UCP), H+/Ca2+ and Na+/Ca2+ exchangers, and mitochondrial-associated endoplasmic reticulum (Duchen, 2000; Raturi and Simmen, 2013; Waldeck-Weiermair et al., 2010; Williams et al., 2013). However, accumulation of mitochondrial Ca2+ increases oxidant production and comes at the long-term expense of ATP generation by reducing Δψm (Fig 2-A). With no window for intervention, the start of this process is currently unavoidable.
As a consequence of the mechanical injury and excitotoxity, initial significant bursts of mitochondrially generated reactive oxygen (ROS) and nitrogen (RNS) species occur too rapidly for intervention. However, ROS production remains high for several days following injury – making it an extremely popular therapeutic target. Oxidant production increases rapidly following TBI in parallel with the early Ca2+-facilitated hyperpolarization of the mitochondrial membrane, while longitudinal RNS production is fueled by the upregulation of inducible NO synthase (Fig 2-B) (Bayir et al., 2005). Antioxidant stress response elements in mitochondria can compensate for basal ROS production, but TBI overwhelms defensive reserves and potentiates mitochondrial dysfunction. Reactive oxidant production begins with the partial reduction of oxygen by a single electron, producing superoxide that is rapidly converted into stable hydrogen peroxide (H2O2) by mitochondrial manganese superoxide dismutase (MnSOD) (Anthonymuthu et al., 2016; Bayir et al., 2007). While superoxide’s effects are spatially constrained, it can interact with nitric oxide (NO) to rapidly form lethal peroxynitrite radicals (Gray and Carmichael, 1992; Kissner et al., 2003). Oxidative and nitrative stress leads to modification of nuclear and mitochondrial DNA, proteins, and lipids – disrupting physiologic function, potentiating injury, and interfering with beneficial injury resolution mechanisms (Bayir et al., 2007; Singh et al., 2006).
When the initial lines of mitochondrial defense are broken, neurons turn to mitophagy – selective autophagy of mitochondria – to remove liabilities, consolidate functional units, and free resources for eventual reconstruction (Fig 2-C). Under physiologic conditions, the occasional rogue mitochondria are fused with their healthy comrades to restore function. Unsalvageable mitochondria are set adrift by mitochondrial fission and degraded by mitophagy. These processes are controlled dynamically; 1) fusion is guided primarily by mitofusins (MFN1, MFN2) and the cardiolipin (CL)-binder Dominant Optic Atrophy 1 (OPA1) (Song et al., 2009), while 2) fission is mediated by Dynamin-related protein 1 (DRP1) and its multiple upstream recruitment mediators (Fis1, Mff) (Loson et al., 2013). TBI-induced damage is recognized early (<24 h pos-tinjury) by PTEN-induced kinase 1 (PINK1) and Parkin whose accumulation on the OMM facilitates the recruitment of autophagy protein LC3 (Chu et al., 2013). Simultaneous phospholipid scramblase 3 (PLS3)-mediated externalization of CL helps anchor LC3 to damaged mitochondrial units. The utility of mitophagy modulators appears particularly context- and time-dependent. Dysregulation of this system – too much or too little at a given time – exacerbates injury and makes for a difficult therapeutic target (Galluzzi et al., 2016). For example, promotion of autophagy/mitophagy following spinal cord injury promoted axonal regeneration and motor recovery (Wang et al., 2014), while other studies demonstrated autophagy inhibition could reduce brain edema and behavioral function following controlled cortical impact (Cui et al., 2015). When these repair and recycle processes fail to properly compensate for ongoing mitochondrial dysfunction, death becomes the only escape.
The IMS houses several important pro-apoptotic factors whose cytosolic release can trigger caspase-dependent and caspase-independent cell death (Galluzzi et al., 2009). Beyond its role in mitophagy, CL serves an additional function in apoptotic signaling. Δψm disruption and Ca2+-mediated transporter activation, including the action of PLS3, result in mobilization of CL (Chu et al., 2013; Kagan et al., 2016; Schug and Gottlieb, 2009). When in the presence of H2O2 and absence of LC3 binding, CL interacts with partially unfolded cytochrome (cyt) c forming a H2O2-fueled CL-specific peroxidase complex (Hanske et al., 2012; Kagan et al., 2005). Oxidized CL (CLox) can no longer interact with cyt c, which is released into the cytosol promoting apoptosis (Kagan et al., 2005). Further hydrolysis of CLox by calcium-independent phospholipase A2 (e.g. iPLA2γ) generates a suite of potentially pro-inflammatory and immunomodulatory oxidized free fatty acids (FFAox) (Fig 2-D) (Tyurina et al., 2014). While inflammation can provide benefit through removal of cellular debris (via macrophage, microglia recruitment) and promotion of injury resolution, inappropriate immune activity exacerbates injury. The role and net benefit or harm of mitochondrial-derived FFAox in TBI progression is unexplored.
Early increases in Δψm induce ROS production, but eventually potential-dissipating influences, like that of fatty-acid-dependent UCP, prevail. While Δψm reduction by UCP activation is effective in reducing lesion volume and preserving neurologic function after TBI (Pandya et al., 2007), concurrent mitochondrial outer membrane permeabilization (MOMP) progresses neurons towards inescapable death. MOMP results from Bax/Bak-mediated conformational changes in voltage-dependent anion channels (VDACs) (Chipuk et al., 2006; Kalkavan and Green, 2018; Kim et al., 2003). These OMM pores further dissipate Δψm and, along with the action of CLox, prompt the release of pro-apoptotic factors including cyt c, Smac/Diablo, HtrA2/Omi, EndoG, and apoptosis inducing factor (AIF) from the IMS (Galluzzi et al., 2009). Mitochondrial Ca2+ accumulation and Δψm alteration drive the assembly of the mitochondrial permeability transition pore (mPTP) complex – consisting of cyclophilin D (CyD) in the matrix, adenosine nucleotide transporter (ANT) at the IMM, and VDAC at the outer mitochondrial membrane (OMM) (Tsujimoto and Shimizu, 2007). Specifically, oxidation of ANT’s key thiol group induces conformational changes permitting CyD binding (McStay et al., 2002). The ANT-CyD complex likely binds VDAC, bringing the mitochondrial membranes in close proximity and creating a continuous flow between matrix, IMS, and cytosol. Other proteins including peripheral ATP synthase, benzodiazepine receptor, hexokinase, creatine kinase, and members of the Bcl-2 family (Bax, Bak) have been associated with mPTP regulation though their roles are less clear (Bernardi, 2013; Karch and Molkentin, 2014). Assembly of the MOMP and mPTP is followed by osmotic swelling and lysis of mitochondria; the loss of the mitochondria’s bioenergetic function and release of pro-death factors assures neuronal demise (Fig 2-E) (Kitsis and Molkentin, 2010).
Utility of Mitochondrial Targeting in Existing and Prospective Therapies
Few specific mitochondria localizing therapies have been explored in TBI. An extensive array of various untargeted compounds and their effects on mitochondrial pathology in experimental TBI and related acute brain injuries have been reviewed elsewhere (Xiong et al., 2009; Yonutas et al., 2016). Many preclinical successes fail during clinical translation. A combinatorial treatment strategy, simultaneously targeting multiple pathways of cellular and mitochondrial dysfunction, may ultimately be needed to benefit patients with TBI. However, finding and testing compatible therapeutic combinations will be challenging. The likelihood of success can be increased by improving the specificity and efficacy of each component of a mono- or poly-mechanism targeted treatment. Below, we present four recognized TBI therapeutic targets to discuss the potential utility of specific drug localization to mitochondria. Therapies against mitochondriagenerated oxidants or to provide alternative energy sources have been well described over the past decade and continue to improve. Therefore, here we will focus consideration on several enzyme inhibitors that can benefit from future application of mitochondria partitioning strategies.
Electron and Oxidant Scavenging.
The most recognized use of mitochondrial drug localization for clinical use is focused on the elimination of mitochondria-derived oxidants. The local concentrations of endogenous antioxidants are typically controlled by the same mechanisms regulating their spatial distribution (Shull et al., 1991), but this system is ill-equipped to tackle rapid TBI-induced mitochondrial oxidant production increases. Mitochondrial oxidative stress can interfere with key energy production complexes, including pyruvate dehydrogenase (PDH), exacerbating ATP depletion while further limiting repair capacity. Energy supplementation using neuron-metabolizable alternative biofuels (ketones, acetyl-L-carnitine) permit glycolysis-independent acetyl-CoA production for TCA cycle input while also supporting GSH synthesis (Scholpa and Schnellmann, 2017). Alternatively, GSH precursors modified to improve BBB penetration and bioavailability have shown great promise to improve functional outcome following CNS trauma. Large-scale preclinical studies demonstrated that aminated NAC (NACA) could not only successful cross BBB, but also chelate copper (a potential source of non-enzymatic ROS production) and scavenge free radicals ultimately preserving mitochondrial bioenergetics (e.g. aminated N-acetylcysteine, NACA) (Pandya et al., 2014; Patel et al., 2014). However, despite the direct contribution of oxidative stress in TBI secondary injury pathogenesis, the untargeted delivery of antioxidants has shown no or limited efficacy in clinical trials (Razmkon et al., 2011). Part of the problem may arise from the extremely rapid induction of oxidant injury that begins immediately following the primary mechanical injury. Unavoidable delays in access to definitive care might limit the clinical utility of anti-ROS interventions. Untargeted antioxidants may fail to achieve sufficient mitochondrial concentrations (Murphy and Smith, 2000). Conversely, untargeted supplementation may lead to high concentrations of extramitochondrial antioxidants, in sufficient quantities to interfere with physiologic extramitochondrial ROS signaling and the pro-survival roles provided (Cochemé and Murphy, 2010). As the reaction rate of primary free radicals is mostly diffusion-controlled, the quenching efficiency of free radical scavengers is affected by their physical proximity to the free radical source (Halliwell et al., 1992). Compared to a pancellular treatment, mitochondrial targeting offers explicit proximity-based advantage, improving specificity and potency. Recently, nitroxides possessing stable nitroxyl radicals, such as 4-amino TEMPO and carboxy-Proxyl, have been shown to be particularly amenable to specific mitochondrial targeting and electron scavenging owing to their unique self-renewing redox cycling mechanism. These properties make mitochondria-targeted nitroxides particularly promising candidates for TBI-related ROS/RNS suppression compared to past attempts (e.g. MitoQ, ebselen, MnSOD mimetic) (Fink et al., 2007). We found that XJB-5–131 administration early after experimental TBI virtually abolished CL oxidation and reduced lesion volume. Neurobehavioral testing revealed the XJB5–131-treated group recovered motor and cognitive function faster, and the improvements were enduring, measured up to a month following injury (Ji et al., 2012).
Targeting mPTP Opening.
Few have attempted to localize enzymatic inhibitors to mitochondria, but the benefits of such an approach should be further explored. Arguably one of the most effective drugs aimed at mitigation of mitochondrial dysfunction following TBI is cyclosporin A (CsA). CsA binds CyD in the mitochondrial matrix disrupting its interaction with IMM-localized ANT, thus inhibiting mPTP formation. CsA administration can improve Δψm and reduce intramitochondrial Ca2+ and oxidant production following TBI (Broekemeier et al., 1989; Yonutas et al., 2016). These mitochondria changes were paralleled by a reduction in neuronal death and lesion volume, along with corresponding improvements in cognitive function, synaptic plasticity, and BBB integrity (Kilbaugh et al., 2011; Okonkwo and Povlishock, 1999; Riess et al., 2001; Scheff and Sullivan, 1999; Sullivan et al., 2000). While early administration of CsA provides maximum benefit (Sullivan et al., 2011), significant protective effects have been observed up to 24 hours post-TBI (Sullivan et al., 2000). Phase III trials are still needed, but preliminary studies of CsA in clinical TBI show measurable improvements of functional outcome, cerebral metabolism, and cerebral perfusion (Hatton et al., 2008; Mazzeo et al., 2008). CsA is typically well tolerated but carries a notable risk of serious immune, cardiovascular, renal, and hepatic toxicities (Empey et al., 2006; Hatton et al., 2008; Mazzeo et al., 2009; Mazzeo et al., 2006). Further, it poorly penetrates the BBB and requires high systemic dosing to be detectable in cerebrospinal fluid (Brophy et al., 2013; Okonkwo et al., 2003).
Critically, there are several theoretical benefits to targeting and trapping CsA in the mitochondria with its TBI-relevant target, CyD. First, the most common intended use of CsA its for is ability to suppress T-cell activation via inhibition of calcineurin (Li et al., 2011; Pundir et al., 2017). Unfortunately, hospital-acquired infections are frequent occurrences following moderate and severe TBI – especially in patients with a history of chronic illness, multiple traumas, or recent surgery (Kourbeti et al., 2011; Schirmer-Mikalsen et al., 2013) – while TBI itself is linked to significant early (<24 h) and protracted (>4 d) decreases in circulating T lymphocytes (Mrakovcic-Sutic et al., 2010). By partitioning CsA to the mitochondrial compartment – safely sequestered from cytosolic/nuclear-localized calcineurin – the consequences of CsA-associated immunosuppression might be reduced. CsA analogs without off-target inhibition of calcineurin (NIM-811) are currently being tested (Mbye et al., 2009; Mbye et al., 2008; Readnower et al., 2011). However, use of mitochondrial targeting strategies may accomplish the same goal more readily in terms of development time and expense. Finally, the choice of localization strategy might affect overall efficacy. NP-based mitochondrial targeting strategies might aid CsA’s poor BBB penetration (McManus et al., 2011), while lipophilic cation or IMM-affinity driven localization techniques can maximize CsA accumulation at its intended site of action.
Targeting CL Oxidation.
Preventing the release of the pro-apoptotic signals from the mitochondria is yet another potential target in TBI. The majority of efforts focus on directly preventing the release of cyt c into the cytosol. Mitochondrial-targeted, imidazole conjugated stearic acid (TPP-ISA) and oleic acid (TPP-IOA) were shown to inhibit apoptosis following radiation injury (Atkinson et al., 2011). The imidazole moiety present in these fatty acids serve as a coordinating ligand for cyt c’s heme, thereby preventing its peroxidase activity when bound to CL (Jiang et al., 2014). This can reduce CLox-derived FFAox release, along with their associated inflammatory effects. Alternative strategies have attempted to convert the oxidizable CL pool into non-oxidizable CL that are resistant to cyt c-mediated oxidation and apoptosis. The formation of non-oxidizable CL with mitochondrial-targeted oleic acid supplementation was shown to suppress actinomycin D-induced apoptosis (Tyurina et al., 2012).
Targeting mt-PARP.
PARP1 serves a pivotal role in base excision repair of damaged nuclear DNA by recruiting and modifying histones, topoisomerases, and DNA polymerases (Dantzer et al., 2006), including damage resulting from TBI. However, PARP1-mediated accumulation of poly(ADP-ribose) polymers following severe cytologic injury results in regulated cell death (Fatokun et al., 2014). While PARP inhibitors showed strong benefit in experimental TBI, they failed to translate clinically (Stoica et al., 2014). Recently, the role of mitochondria-localized PARP (mt-PARP) has been the subject of study (Masmoudi et al., 2006; Masmoudi and Mandel, 1987). mt-PARP activation is thought to be responsible for the TBI-induced differential PARylation of a wide range of mission-critical mitochondrial proteins (Lai et al., 2008). Several of these modifications have been observed before significant nuclear PARP activation – stimulated by recognition of mtDNA injury and Protein Kinase A (PKA)-mediated phosphorylation (Brunyanszki et al., 2014; Brunyanszki et al., 2016).
Injury-induced mt-PARP consequences are divided into two categories, NAD+ consumption and PARylation of mitochondrial proteins. First, as limited mitochondrial NAD+ stores are consumed by mtPARP, ATP production and NAD+/NADH-dependent reactions are inhibited (Stein and Imai, 2012). Failure to maintain Δψm follows as cytosolic NAD+ cannot directly or rapidly replenish the mitochondrial pool. Instead, NAD+ must be resynthesized from precursors in an ATP-consuming process – undesirable at a time where ATP production is already faltering. Second, PARylation of AIF, ETC complexes, and mtDNA repair enzymes negatively affect their catalytic activity and stimulate regulated cell death pathways (Fatokun et al., 2014; Zhou et al., 2006). For example, in contrast to nuclear PAR signaling, mt-PARP activity is linked to reduced mtDNA integrity through inhibition of the mitochondria-specific DNA repair proteins, endo/exonuclease G, and polymerase-γ (Szczesny et al., 2014; Van Houten et al., 2016). These effects collectively potentiate mitochondrial dysfunction and increase mt-PARP activation in a vicious cycle. Therefore, clinically effective PARP inhibitors might be those that are mitochondrially targeted – maximizing mitochondrial benefit and minimizing nuclear harm.
Conclusion
TBI pathology is complex and often heterogeneous. Convergent pathways throughout affected neurons ensure they meet an unfortunate end. Mitochondria are the common denominator, centrally contributing to energetic failure, the release of pro-death signals, and inflammation. This sets mitochondria apart as a prime TBI pharmacotherapy target. Past attempts to intervene in mitochondrial pathology have stumbled due to efficacy-limiting effects on extra-mitochondrial pathways, poor accumulation of drug at its mechanistic target, and other unintended consequences. However, specific localization of a drug to its mechanistic target in the mitochondria might alleviate some of these concerns. Localization can be accomplished by taking advantage of the mitochondrial matrix potential, affinity towards the IMM, intrinsic trafficking machinery, or nanoparticles. Each of these methods is not without potential downsides, and our understanding of their safety and utility in humans is lacking. These methods have a proven track record in a diverse range of disease models in vitro and in vivo, and they can be readily adapted to accommodate their payload. Subcellular drug targeting to mitochondria or other organelles will become an increasingly important consideration in future drug design. This approach offers an opportunity to revive past pharmacological failures and design novel neuroprotective therapies.
TBI prompts mitochondrial dysfunction; no clinically effective therapies exist
Generalizable strategies allow specific drug targeting of mitochondrial pathology
Colocalization therapy with its target may improve potency and specificity
Mitochondrial-localization can improve new and old therapies in TBI
Acknowledgements
This work was supported by NIH Grants NS061817, NS076511, NS084604, P01HL114453, and U19AI068021.
Terms/Abbreviations:
- ANT
Adenosine Nucleotide Transporter
- AMPA
α-Amino-3-Hydroxy-5-Methyl-4-Isoxazoleproprionic acid
- BBB
Blood Brain Barrier
- Ca2+
Calcium
- iPLA2γ
Calcium-independent Phospholipase A2
- CL
Cardiolipin
- mitochondrial targeting moiety
Carrier
- CBF
Cerebral Blood Flow
- CNS
Central Nervous System
- CSF
Cerebrospinal Fluid
- cyt c
Cytochrome c
- CyD
Cyclophilin D
- CsA
Cyclosporin A
- DNP
2,4-dinitrophenol
- OPA1
Dominant Optic Atrophy 1
- DRP1
Dynamin-related Protein 1
- ETC
Electron Transport Chain
- GSH
Glutathione
- GPx
Glutathione Peroxidase
- GS
Gramicidin S
- hemi-GS
Hemigramicidin
- H2O2
Hydrogen Peroxide
- IMM
Inner Mitochondrial Membrane
- IMS
Intermembrane Space
- mtHsp
LC3, Mitochondrial Heat Shot Protein
- mPTP
Mitochondrial Permeability Transition Pore
- mtPARP
Mitochondrial Poly(ADP-ribose) Polymerase
- MPP
Mitochondria Processing Peptide
- Δψm
Mitochondrial Membrane Potential
- MnSOD
Mitochondrial Manganese Superoxide Dismutase
- MTP
Mitochondrial Targeting Signal Peptide
- MFN
Mitofusin
- NP
Nanoparticles
- NO
Nitric Oxide
- NMDA
N-methyl-D-aspartate
- OMM
Outer Mitochondrial Membrane
- CLox
Oxidized Cardiolipin
- FFAox
Oxidized Free Fatty Acids
- active drug attached to carrier
Parkin, Payload
- PARP1
Poly(ADP-ribose) Polymerase
- PINK1
PTEN-induced kinase 1
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SS
Szeto-Schiller
- drug’s binding/interaction partner
Target
- delivering a drug to a specific subcellular location, interchangeable with “localization”
Targeting
- TIM
Translocase of the Inner Membrane
- TOM
Translocase of the Outer Membrane
- TBI
Traumatic Brian injury
- TPP
Triphenylphosphonium
- UCP
Uncoupling Proteins
- VDAC
Voltage-dependent Anion Channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Shodai T, Muto T, Mihara K, Torii H, Nishikawa S. i., Endo T, Kohda D, 2000. Structural Basis of Presequence Recognition by the Mitochondrial Protein Import Receptor Tom20. Cell 100, 551–560. [DOI] [PubMed] [Google Scholar]
- Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA, 2005. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb j 19, 1088–1095. [DOI] [PubMed] [Google Scholar]
- Allen TM, Chonn A, 1987. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett 223, 42–46. [DOI] [PubMed] [Google Scholar]
- Andelic N, Hammergren N, Bautz-Holter E, Sveen U, Brunborg C, Roe C, 2009. Functional outcome and health-related quality of life 10 years after moderate-to-severe traumatic brain injury. Acta Neurol Scand 120, 16–23. [DOI] [PubMed] [Google Scholar]
- Anthonymuthu TS, Kenny EM, Bayir H, 2016. Therapies Targeting Lipid Peroxidation in Traumatic Brain Injury. Brain Res 1640, 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M, 2004. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cellular and Molecular Life Sciences CMLS 61, 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Kapralov AA, Yanamala N, Tyurina YY, Amoscato AA, Pearce L, Peterson J, Huang Z, Jiang J, Samhan-Arias AK, Maeda A, Feng W, Wasserloos K, Belikova NA, Tyurin VA, Wang H, Fletcher J, Wang Y, Vlasova II, Klein-Seetharaman J, Stoyanovsky DA, Bayîr H, Pitt BR, Epperly MW, Greenberger JS, Kagan VE, 2011. A mitochondria-targeted inhibitor of cytochrome c peroxidase mitigates radiation-induced death. Nature communications 2, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Borisenko GG, Tyurina YY, Janesko KL, Vagni VA, Billiar TR, Williams DL, Kochanek PM, 2005. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J Cereb Blood Flow Metab 25, 673–684. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM, 2007. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem 101, 168–181. [DOI] [PubMed] [Google Scholar]
- Bernardi P, 2013. The mitochondrial permeability transition pore: a mystery solved? Frontiers in Physiology 4, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekemeier KM, Dempsey ME, Pfeiffer DR, 1989. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264, 7826–7830. [PubMed] [Google Scholar]
- Brophy GM, Mazzeo AT, Brar S, Alves OL, Bunnell K, Gilman C, Karnes T, Hayes RL, Bullock R, 2013. Exposure of cyclosporin A in whole blood, cerebral spinal fluid, and brain extracellular fluid dialysate in adults with traumatic brain injury. J Neurotrauma 30, 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunyanszki A, Olah G, Coletta C, Szczesny B, Szabo C, 2014. Regulation of mitochondrial poly(ADP-Ribose) polymerase activation by the beta-adrenoceptor/cAMP/protein kinase A axis during oxidative stress. Mol Pharmacol 86, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunyanszki A, Szczesny B, Virag L, Szabo C, 2016. Mitochondrial poly(ADP-ribose) polymerase: The Wizard of Oz at work. Free Radic Biol Med 100, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RJ, Smith RAJ, Murphy MP, 1995. Synthesis and Characterization of Thiobutyltriphenylphosphonium Bromide, a Novel Thiol Reagent Targeted to the Mitochondrial Matrix. Arch Biochem Biophys 322, 60–68. [DOI] [PubMed] [Google Scholar]
- Cerrato CP, Pirisinu M, Vlachos EN, Langel U, 2015. Novel cell-penetrating peptide targeting mitochondria. Faseb j 29, 4589–4599. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Bouchier-Hayes L, Green DR, 2006. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ 13, 1396–1402. [DOI] [PubMed] [Google Scholar]
- Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE, 2013. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros MG, Vincens P, 1996. Computational method to predict mitochondrially imported proteins and their targeting sequences. The FEBS Journal 241, 779–786. [DOI] [PubMed] [Google Scholar]
- Cochemé HM, Murphy MP, 2010. Can antioxidants be effective therapeutics. Curr Opin Investig Drugs 11, 426–431. [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD, 2011. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. MMWR Surveill Summ 60, 1–32. [PubMed] [Google Scholar]
- Cui CM, Gao JL, Cui Y, Sun LQ, Wang YC, Wang KJ, Li R, Tian YX, Cui JZ, 2015. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol Med Rep 12, 2323–2328. [DOI] [PubMed] [Google Scholar]
- Dantzer F, Ame JC, Schreiber V, Nakamura J, Menissier-de Murcia J, de Murcia G, 2006. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol 409, 493–510. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B, 2005. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med 39, 567–583. [DOI] [PubMed] [Google Scholar]
- Duchen MR, 2000. Mitochondria and calcium: from cell signalling to cell death. The Journal of Physiology 529, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed A, Khalil IA, Kogure K, Futaki S, Harashima H, 2008. Octaarginine- and octalysine-modified nanoparticles have different modes of endosomal escape. J Biol Chem 283, 23450–23461. [DOI] [PubMed] [Google Scholar]
- Empey PE, McNamara PJ, Young B, Rosbolt MB, Hatton J, 2006. Cyclosporin A disposition following acute traumatic brain injury. J Neurotrauma 23, 109–116. [DOI] [PubMed] [Google Scholar]
- Escobales N, Nunez RE, Jang S, Parodi-Rullan R, Ayala-Pena S, Sacher JR, Skoda EM, Wipf P, Frontera W, Javadov S, 2014. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J Mol Cell Cardiol 77, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatokun AA, Dawson VL, Dawson TM, 2014. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol 171, 2000–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado V, Dellinger AM, 2011. Traumatic brain injury in the United States: national estimates of prevalence and incidence, 2002–2006. Injury Prevention 16, A268. [Google Scholar]
- Fink MP, Macias CA, Xiao J, Tyurina YY, Jiang J, Belikova N, Delude RL, Greenberger JS, Kagan VE, Wipf P, 2007. Hemigramicidin–TEMPO conjugates: Novel mitochondria-targeted anti-oxidants. Biochemical Pharmacology 74, 801–809. [DOI] [PubMed] [Google Scholar]
- Flierl A, Jackson C, Cottrell B, Murdock D, Seibel P, Wallace D, 2003. Targeted delivery of DNA to the mitochondrial compartment via import sequence-conjugated peptide nucleic acid. Molecular Therapy 7, 550–557. [DOI] [PubMed] [Google Scholar]
- Gakh O, Cavadini P, Isaya G, 2002. Mitochondrial processing peptidases. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1592, 63–77. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Blomgren K, Kroemer G, 2009. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 10, 481–494. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G, 2016. Autophagy in acute brain injury 17, 467. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Sarkar S, Choudhury ST, Ghosh T, Das N, 2017. Triphenyl phosphonium coated nano-quercetin for oral delivery: Neuroprotective effects in attenuating age related global moderate cerebral ischemia reperfusion injury in rats. Nanomedicine 13, 2439–2450. [DOI] [PubMed] [Google Scholar]
- Gray B, Carmichael AJ, 1992. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by electron spin resonance. Biochem J 281 ( Pt 3), 795802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CN, Klein-Flügge MC, Howarth C, Attwell D, 2012. Oxidative phosphorylation, not glycolysis, powers pre- and postsynaptic mechanisms underlying brain information processing. J Neurosci 32, 8940–8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM, Cross CE, 1992. Free radicals, antioxidants, and human disease: where are we now? The Journal of laboratory and clinical medicine 119, 598–620. [PubMed] [Google Scholar]
- Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV, 2012. Conformational properties of cardiolipin-bound cytochrome c. Proc Natl Acad Sci U S A 109, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton J, Rosbolt B, Empey P, Kryscio R, Young B, 2008. Dosing and safety of cyclosporine in patients with severe brain injury. J Neurosurg 109, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, 2012. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci 15, 1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Bakan A, Kapralov AA, Silva KI, Huang Z, Amoscato AA, Peterson J, Garapati VK, Saxena S, Bayir H, 2014. Designing inhibitors of cytochrome c/cardiolipin peroxidase complexes: mitochondria-targeted imidazole-substituted fatty acids. Free Radical Biology and Medicine 71, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Jiang J, Huang Z, Tyurina YY, Desbourdes C, Cottet-Rousselle C, Dar HH, Verma M, Tyurin VA, Kapralov AA, Cheikhi A, Mao G, Stolz D, St Croix CM, Watkins S, Shen Z, Li Y, Greenberg ML, Tokarska-Schlattner M, Boissan M, Lacombe ML, Epand RM, Chu CT, Mallampalli RK, Bayir H, Schlattner U, 2016. NDPK-D (NM23-H4)-mediated externalization of cardiolipin enables elimination of depolarized mitochondria by mitophagy. Cell Death Differ 23, 1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG, 2005. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1, 223–232. [DOI] [PubMed] [Google Scholar]
- Kalkavan H, Green DR, 2018. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ 25, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC, 2013. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci U S A 110, 6506–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobatake Y, 1979. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol 49, 105–121. [DOI] [PubMed] [Google Scholar]
- Karch J, Molkentin JD, 2014. Identifying the components of the elusive mitochondrial permeability transition pore. Proceedings of the National Academy of Sciences 111, 10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP, 2001. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276, 4588–4596. [DOI] [PubMed] [Google Scholar]
- Kilbaugh TJ, Bhandare S, Lorom DH, Saraswati M, Robertson CL, Margulies SS, 2011. Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J Neurotrauma 28, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, He L, Lemasters JJ, 2003. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304, 463–470. [DOI] [PubMed] [Google Scholar]
- Kissner R, Nauser T, Kurz C, Koppenol WH, 2003. Peroxynitrous acid--where is the hydroxyl radical? IUBMB Life 55, 567–572. [DOI] [PubMed] [Google Scholar]
- Kitsis RN, Molkentin JD, 2010. Apoptotic cell death “Nixed” by an ER-mitochondrial necrotic pathway. Proc Natl Acad Sci U S A 107, 9031–9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourbeti IS, Papadakis JA, Neophytou C, Filippou M, Ioannou A, Karabetsos DA, Bertsias G, Anastasaki M, Vakis AF, 2011. Infections in patients with traumatic brain injury who undergo neurosurgery. Br J Neurosurg 25, 9–15. [DOI] [PubMed] [Google Scholar]
- Krainz T, Gaschler MM, Lim C, Sacher JR, Stockwell BR, Wipf P, 2016. A Mitochondrial-Targeted Nitroxide Is a Potent Inhibitor of Ferroptosis. ACS Central Science 2, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Chen Y, Watkins SC, Nathaniel PD, Guo F, Kochanek PM, Jenkins LW, Szabo C, Clark RS, 2008. Identification of poly-ADP-ribosylated mitochondrial proteins after traumatic brain injury. J Neurochem 104, 1700–1711. [DOI] [PubMed] [Google Scholar]
- Li H, Rao A, Hogan PG, 2011. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol 21, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP, 1969. Mechanism of Coupling of Oxidative Phosphorylation and the Membrane Potential of Mitochondria. Nature 222, 1076. [DOI] [PubMed] [Google Scholar]
- Loson OC, Song Z, Chen H, Chan DC, 2013. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24, 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AIR, Stocchetti N, Bullock R, 2008. Moderate and severe traumatic brain injury in adults. The Lancet Neurology 7, 728–741. [DOI] [PubMed] [Google Scholar]
- Marrache S, Dhar S, 2012. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A 109, 16288–16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache S, Pathak RK, Darley KL, Choi JH, Zaver D, Kolishetti N, Dhar S, 2013. Nanocarriers for tracking and treating diseases. Curr Med Chem 20, 3500–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi A, Islam F, Mandel P, 2006. ADP‐Ribosylation of Highly Purified Rat Brain Mitochondria. J Neurochem 51, 188–193. [DOI] [PubMed] [Google Scholar]
- Masmoudi A, Mandel P, 1987. ADP-ribosyl transferase and NAD glycohydrolase activities in rat liver mitochondria. Biochemistry 26, 1965–1969. [DOI] [PubMed] [Google Scholar]
- Mazzeo AT, Alves OL, Gilman CB, Hayes RL, Tolias C, Niki Kunene K, Ross Bullock M, 2008. Brain metabolic and hemodynamic effects of cyclosporin A after human severe traumatic brain injury: a microdialysis study. Acta Neurochir (Wien) 150, 1019–1031; discussion 1031. [DOI] [PubMed] [Google Scholar]
- Mazzeo AT, Brophy GM, Gilman CB, Alves OL, Robles JR, Hayes RL, Povlishock JT, Bullock MR, 2009. Safety and tolerability of cyclosporin a in severe traumatic brain injury patients: results from a prospective randomized trial. J Neurotrauma 26, 2195–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo AT, Kunene NK, Gilman CB, Hamm RJ, Hafez N, Bullock MR, 2006. Severe human traumatic brain injury, but not cyclosporin a treatment, depresses activated T lymphocytes early after injury. J Neurotrauma 23, 962–975. [DOI] [PubMed] [Google Scholar]
- Mbye LH, Singh IN, Carrico KM, Saatman KE, Hall ED, 2009. Comparative neuroprotective effects of cyclosporin A and NIM811, a nonimmunosuppressive cyclosporin A analog, following traumatic brain injury. J Cereb Blood Flow Metab 29, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbye LH, Singh IN, Sullivan PG, Springer JE, Hall ED, 2008. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a nonimmunosuppressive cyclosporin A analog. Exp Neurol 209, 243–253. [DOI] [PubMed] [Google Scholar]
- McManus MJ, Murphy MP, Franklin JL, 2011. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci 31, 15703–15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McStay GP, Clarke SJ, Halestrap AP, 2002. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochemical Journal 367, 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum B, Garthwaite J, 1990. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends in Pharmacological Sciences 11, 379–387. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Weiss MJ, Chen LB, Aprille JR, 1984. Rhodamine 123 inhibits bioenergetic function in isolated rat liver mitochondria. Biochem Biophys Res Commun 118, 717–723. [DOI] [PubMed] [Google Scholar]
- Mrakovcic-Sutic I, Tokmadzic VS, Laskarin G, Mahmutefendic H, Lucin P, Zupan Z, Sustic A, 2010. Early changes in frequency of peripheral blood lymphocyte subpopulations in severe traumatic brain-injured patients. Scand J Immunol 72, 57–65. [DOI] [PubMed] [Google Scholar]
- Murphy MP, 2008. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 1777, 1028–1031. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Smith RA, 2000. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev 41, 235–250. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Melon DE, Pellicane AJ, Mutlu LK, Rubin DG, Stone JR, Helm GA, 2003. Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport 14, 463–466. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Povlishock JT, 1999. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J Cereb Blood Flow Metab 19, 443–451. [DOI] [PubMed] [Google Scholar]
- Orsucci D, Mancuso M, Ienco EC, LoGerfo A, Siciliano G, 2011. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr Med Chem 18, 4053–4064. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG, 2007. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma 24, 798–811. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Readnower RD, Patel SP, Yonutas HM, Pauly JR, Goldstein GA, Rabchevsky AG, Sullivan PG, 2014. N-acetylcysteineamide confers neuroprotection, improves bioenergetics and behavioral outcome following TBI. Experimental neurology 257, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Goldstein GA, VanRooyen JL, Yonutas HM, Eldahan KC, Morehouse J, Magnuson DSK, Rabchevsky AG, 2014. N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Experimental neurology 257, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak RK, Kolishetti N, Dhar S, 2015. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7, 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner EJ, Lewis RN, McElhaney RN, 1999. The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes. Biochim Biophys Acta 1462, 201–221. [DOI] [PubMed] [Google Scholar]
- Prins M, Greco T, Alexander D, Giza CC, 2013. The pathophysiology of traumatic brain injury at a glance. Disease Models & Mechanisms 6, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundir S, Martin MJ, O’Donovan C, 2017. UniProt Protein Knowledgebase In: Wu CH, Arighi CN, Ross KE, (Eds), Protein Bioinformatics: From Protein Modifications and Networks to Proteomics. Springer New York, New York, NY, pp. 41–55. [Google Scholar]
- Ransom BR, Sontheimer H, 1992. The neurophysiology of glial cells. J Clin Neurophysiol 9, 224–251. [DOI] [PubMed] [Google Scholar]
- Raturi A, Simmen T, 2013. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim Biophys Acta 1833, 213–224. [DOI] [PubMed] [Google Scholar]
- Razmkon A, Sadidi A, Sherafat-Kazemzadeh E, Mehrafshan A, Jamali M, Malekpour B, Saghafinia M, 2011. Administration of vitamin C and vitamin E in severe head injury: a randomized double-blind controlled trial. Clin Neurosurg 58, 133–137. [DOI] [PubMed] [Google Scholar]
- Readnower RD, Pandya JD, McEwen ML, Pauly JR, Springer JE, Sullivan PG, 2011. Post-injury administration of the mitochondrial permeability transition pore inhibitor, NIM811, is neuroprotective and improves cognition after traumatic brain injury in rats. J Neurotrauma 28, 1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reily C, Mitchell T, Chacko BK, Benavides GA, Murphy MP, Darley-Usmar VM, 2013. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biology 1, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess P, Bareyre FM, Saatman KE, Cheney JA, Lifshitz J, Raghupathi R, Grady MS, Neugebauer E, McIntosh TK, 2001. Effects of chronic, post-injury Cyclosporin A administration on motor and sensorimotor function following severe, experimental traumatic brain injury. Restor Neurol Neurosci 18, 1–8. [PubMed] [Google Scholar]
- Saatman KE, Creed J, Raghupathi R, 2010. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics 7, 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell MA, Zhang X, Kochanek PM, Dixon CE, Jenkins LW, Melick J, Szabo C, Clark RS, 2003. A dual role for poly-ADP-ribosylation in spatial memory acquisition after traumatic brain injury in mice involving NAD+ depletion and ribosylation of 14–3-3gamma. J Neurochem 85, 697–708. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG, 1999. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma 16, 783–792. [DOI] [PubMed] [Google Scholar]
- Schirmer-Mikalsen K, Moen KG, Skandsen T, Vik A, Klepstad P, 2013. Intensive care and traumatic brain injury after the introduction of a treatment protocol: a prospective study. Acta Anaesthesiol Scand 57, 46–55. [DOI] [PubMed] [Google Scholar]
- Schmidt O, Pfanner N, Meisinger C, 2010. Mitochondrial protein import: from proteomics to functional mechanisms. Nature Reviews Molecular Cell Biology 11, 655. [DOI] [PubMed] [Google Scholar]
- Scholpa NE, Schnellmann RG, 2017. Mitochondrial-Based Therapeutics for the Treatment of Spinal Cord Injury: Mitochondrial Biogenesis as a Potential Pharmacological Target. J Pharmacol Exp Ther 363, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Gottlieb E, 2009. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta 1788, 2022–2031. [DOI] [PubMed] [Google Scholar]
- Sheu S-S, Nauduri D, Anders MW, 2006. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1762, 256–265. [DOI] [PubMed] [Google Scholar]
- Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, Mossman BT, 1991. Differential regulation of antioxidant enzymes in response to oxidants. Journal of Biological Chemistry 266, 24398–24403. [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED, 2006. Time course of posttraumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab 26, 1407–1418. [DOI] [PubMed] [Google Scholar]
- Smith RA, Murphy MP, 2010. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci 1201, 96–103. [DOI] [PubMed] [Google Scholar]
- Smith RAJ, Porteous CM, Gane AM, Murphy MP, 2003. Delivery of bioactive molecules to mitochondria in vivo. Proceedings of the National Academy of Sciences of the United States of America 100, 5407–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM, Protect Study G, 2010. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord 25, 1670–1674. [DOI] [PubMed] [Google Scholar]
- Song Z, Ghochani M, McCaffery JM, Frey TG, Chan DC, 2009. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell 20, 3525–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, Imai S, 2012. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab 23, 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica BA, Loane DJ, Zhao Z, Kabadi SV, Hanscom M, Byrnes KR, Faden AI, 2014. PARP-1 Inhibition Attenuates Neuronal Loss, Microglia Activation and Neurological Deficits after Traumatic Brain Injury. J Neurotrauma 31, 758–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D, Bohnert M, Pfanner N, van der Laan M, 2012. Mechanisms of protein sorting in mitochondria. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Rabchevsky AG, Hicks RR, Gibson TR, Fletcher-Turner A, Scheff SW, 2000. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 101, 289–295. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Sebastian AH, Hall ED, 2011. Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury. J Neurotrauma 28, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny B, Brunyanszki A, Olah G, Mitra S, Szabo C, 2014. Opposing roles of mitochondrial and nuclear PARP1 in the regulation of mitochondrial and nuclear DNA integrity: implications for the regulation of mitochondrial function. Nucleic Acids Research 42, 1316113173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH, 2008. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10, 601–619. [DOI] [PubMed] [Google Scholar]
- Szeto HH, 2014. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. British Journal of Pharmacology 171, 2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Borgeld H-J, Zhang J, Muramatsu S. i., Gong J-S, Yoneda M, Maruyama W, Naoi M, Ibi T, Sahashi K, 2002. Gene therapy for mitochondrial disease by delivering restriction endonucleaseSmaI into mitochondria. Journal of biomedical science 9, 534–541. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S, 2007. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12, 835–840. [DOI] [PubMed] [Google Scholar]
- Tyurina YY, Poloyac SM, Tyurin VA, Kapralov AA, Jiang J, Anthonymuthu TS, Kapralova VI, Vikulina AS, Jung MY, Epperly MW, Mohammadyani D, Klein-Seetharaman J, Jackson TC, Kochanek PM, Pitt BR, Greenberger JS, Vladimirov YA, Bayir H, Kagan VE, 2014. A mitochondrial pathway for biosynthesis of lipid mediators. Nat Chem 6, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina YY, Tungekar MA, Jung MY, Tyurin VA, Greenberger JS, Stoyanovsky DA, Kagan VE, 2012. Mitochondria targeting of non-peroxidizable triphenylphosphonium conjugated oleic acid protects mouse embryonic cells against apoptosis: role of cardiolipin remodeling. FEBS Lett 586, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B, Hunter SE, Meyer JN, 2016. Mitochondrial DNA damage induced autophagy, cell death, and disease. Frontiers in bioscience (Landmark edition) 21, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Duan X, Naghdi S, Khan MJ, Trenker M, Malli R, Graier WF, 2010. Uncoupling protein 3 adjusts mitochondrial Ca(2+) uptake to high and low Ca(2+) signals. Cell calcium 48, 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Liu WG, Muharram A, Wu ZY, Lin JH, 2014. Neuroprotective effects of autophagy induced by rapamycin in rat acute spinal cord injury model. Neuroimmunomodulation 21, 257–267. [DOI] [PubMed] [Google Scholar]
- Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J, 1998. DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm Res 15, 334337. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K, 2007. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia 99, 4–9. [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Pfanner N, 2017. Mitochondrial Machineries for Protein Import and Assembly. Annual Review of Biochemistry 86, 685–714. [DOI] [PubMed] [Google Scholar]
- Williams GSB, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ, 2013. Mitochondrial calcium uptake. Proceedings of the National Academy of Sciences 110, 10479–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, Kagan VE, 2005. Mitochondrial Targeting of Selective Electron Scavengers: Synthesis and Biological Analysis of Hemigramicidin−TEMPO Conjugates. Journal of the American Chemical Society 127, 12460–12461. [DOI] [PubMed] [Google Scholar]
- Wongrakpanich A, Geary SM, Joiner ML, Anderson ME, Salem AK, 2014. Mitochondria-targeting particles. Nanomedicine (Lond) 9, 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M, 2009. Emerging treatments for traumatic brain injury. Expert opinion on emerging drugs 14, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, Yamashita K, Kobayashi H, Kikuchi H, Harashima H, 2008. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta 1778, 423–432. [DOI] [PubMed] [Google Scholar]
- Yonutas HM, Vekaria HJ, Sullivan PG, 2016. Mitochondrial specific therapeutic targets following brain injury. Brain Res 1640, 77–93. [DOI] [PubMed] [Google Scholar]
- Yu H, Koilkonda RD, Chou T-H, Porciatti V, Ozdemir SS, Chiodo V, Boye SL, Boye SE, Hauswirth WW, Lewin AS, 2012. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proceedings of the National Academy of Sciences 109, E1238–E1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Sriratana A, Minamikawa T, Nagley P, 1998. Photosensitisation Properties of Mitochondrially Localised Green Fluorescent Protein. Biochemical and Biophysical Research Communications 242, 390–395. [DOI] [PubMed] [Google Scholar]
- Zhao K, Luo G, Zhao GM, Schiller PW, Szeto HH, 2003. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J Pharmacol Exp Ther 304, 425–432. [DOI] [PubMed] [Google Scholar]
- Zhou HZ, Swanson RA, Simonis U, Ma X, Cecchini G, Gray MO, 2006. Poly(ADP-ribose) polymerase-1 hyperactivation and impairment of mitochondrial respiratory chain complex I function in reperfused mouse hearts. Am J Physiol Heart Circ Physiol 291, H714–723. [DOI] [PubMed] [Google Scholar]