Abstract
During the course of pregnancy, the maternal-fetal interface is tightly regulated and undergoes dynamic changes that promote the successful development of the semi-allogeneic fetus. In response to embryo implantation, the uterus remodels with maternal immune cells occupying the maternal-fetal interface and uterine natural killer (uNK) cells becoming the most prominent leukocyte. Recently, uNK cells have been discovered to be heterogeneous, including conventional NK (cNK) and tissue-resident NK (trNK) cells. Here, we will review the recent advances in uNK cell biology and discuss their functional mechanisms which protect and nurture the growing fetus.
Keywords: pregnancy, uterine NK cells, tissue-resident NK cells, conventional NK
Introduction
The uterus is an organ under constant re-construction. In a nonpregnant female, it undergoes cyclic tissue breakdown and remodeling, requiring regeneration and maintenance of barrier immunity. The pregnant uterus is even more complex with the development of the placenta, a transient structure and an environment that must nurture the fetus.
Pregnancy is an immunological paradox, where the allogeneic fetus does not provoke a maternal immune response to expel the foreign entity (Billingham, Brent, & Medawar, 1953). Fetal, maternal and placental units must work in concert to induce tolerance mechanisms that protect the fetus from rejection. Pregnancy must induce essential changes to the uterus making it a safe and nurturing microenvironment for the growing conceptus. The modification of the endometrium and placentation occurs during early pregnancy and is complete by mid-gestation (K. Y. Lee & DeMayo, 2004). A successful pregnancy includes complex interactions with the fetal invading trophoblasts and maternal immune cells, which permit the developing embryo to take residence. Uterine natural killer (uNK) cells are found in abundant numbers at the maternal-fetal interface in early pregnancy and decline by mid-gestation (B. A. Croy et al., 2012; Hofmann, Gerber, & Croy, 2014). Curiously, the rise and decline of uNK cells coincides with major uterine adaptations that take place in early pregnancy.
Uterine adaptations to pregnancy
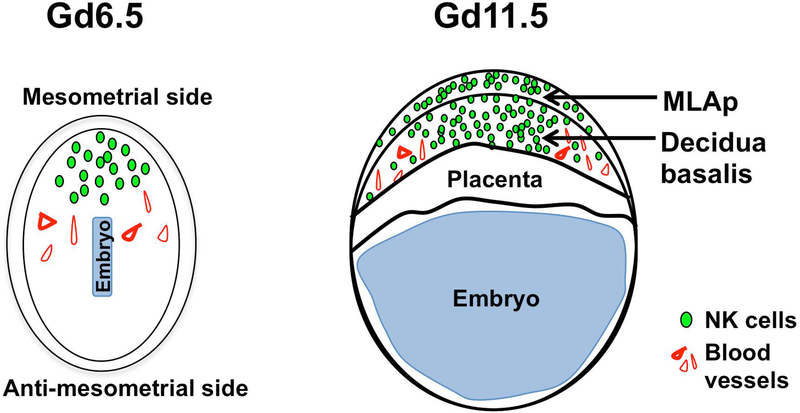
The uterine wall is divided into two major domains, a mesometrial and antimesometrial region (Figure 1). The embryo implants on the antimesometrial side and triggers the decidualization process and trophoectoderm differentiation. The uterine endometrium is remodeled during decidualization whereby the uterine stromal cells are transformed into large decidual cells, which proliferate rapidly, along with local immune cells (Erlebacher, 2013). The embryo is completely embedded within the decidualized endometrium. The decidualization of the antimesometrial region of the uterus gives support to the conceptus via the primitive choriovitelline placenta during early embryo organogenesis. The definitive chorioallantoic placenta, as a consequence of the rapid growth and differentiation of trophoblasts and the decidua basalis, replaces the primitive placenta for subsequent full fetal development.
Figure 1:
Schematic of a murine implantation site at gestational day (gd) 6.5 and 11.5. At gd6.5 the decidualization process occurs at the mesometrial side where uNK cells and decidual cells expand and give rise to the decidua basalis. At gd11.5 the mesometrial aggregate of pregnancy (MLAp), decidua basalis, and placenta are all fully formed. NK cells occupy the MLAp and decidua basalis regions, and not the placenta, of the implantation site.
The development of the definitive chorioallantoic placenta provides gas exchange, nutritional support, and waste elimination for the growing fetus during the second half of pregnancy. Several specialized cells and regions contribute to the development of the placenta that participates in the modulation of the immune and vascular systems. Fetal extravillous trophoblasts (EVTs) penetrate and invade the decidua basalis, engage uterine natural killer (uNK) cells that extensively remodel the uterine arteries. The spiral arteries are transformed into thin walled vessels with large lumens to enhance maternal-fetal exchange. In genetically altered mouse models lacking uNK cells, analysis of the placentas showed defects in spiral artery remodeling and growth restricted pups (Ashkar, Di Santo, & Croy, 2000; Boulenouar et al., 2016). Thus, uNK cells are required for normal placentation in mice.
Complications of pregnancy
In humans, the great obstetrical syndromes (GOS) include intrauterine fetal growth restriction (FGR), preterm labor, late spontaneous abortion and preeclampsia all of which are characterized by abnormal placentation (Brosens, Pijnenborg, Vercruysse, & Romero, 2011). Of particular relevance to uNK cells is preeclampsia, a disorder that affects 2–7% of pregnancies, diagnosed by onset of high blood pressure in mid-gestation, and is associated with placental perfusion defects and FGR. The only treatment is to deliver the baby but this option is life threatening for both mother and baby if undiagnosed or preterm. Epidemiological studies have linked preeclampsia with the genotypes of maternal NK cell receptors and their fetal ligands (HLA class I antigens) expressed by EVTs (detailed below). Hence, uNK cells appear to be critical to maintenance of normal pregnancies in humans as well as mice.
Natural Killer (NK) Cells
Uterine NK (uNK) cells
The terms uterine NK (uNK) cells and decidual NK (dNK) cells are often used interchangeably. Here we will use uNK cells to include all NK cells in the uterus, regardless of origin or location. Initially described as granulated metrial gland cells, uNK cells constitute the vast majority of maternal lymphocytes at sites of implantation. Early in mouse and human gestations, 70% of all lymphocytes are uNK cells with a decline in frequency by mid-pregnancy (Erlebacher, 2013; Pang et al., 1993). Although uNK cells appear to contribute to pregnancy success, they are phenotypically and functionally distinct from conventional NK cells, and less well understood.
Conventional NK (cNK) cells
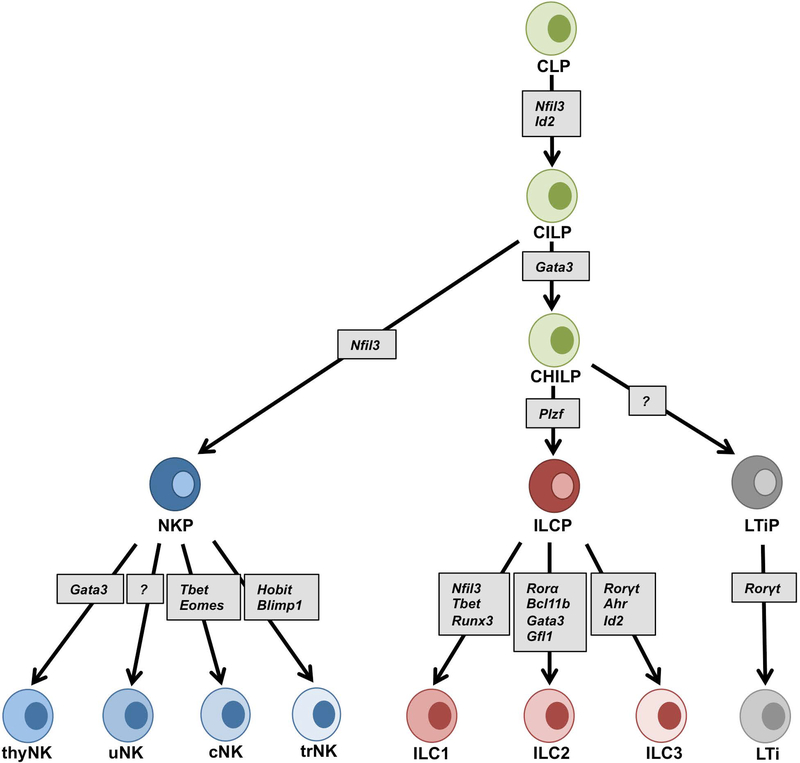
Most of our understanding of NK cell biology comes from studying NK cells in the mouse spleen and human peripheral blood. Conventional natural killer (cNK) cells are the foundational members of a large family of lymphocytes called innate lymphoid cells (ILCs) that are distinguished from B and T cells by the absence of antigen-specific B and T cell receptors (BCRs, TCRs), respectively (Serafini, Vosshenrich, & Di Santo, 2015; Yokoyama, 2013). Recently ILCs were reclassified to include five groups; group 1 (ILC1s), group 2 (ILC2s), group 3 (ILC3s), lymphoid tissue inducer (LTi) cells and NK cells (Spits et al., 2013; Vivier et al., 2018) on the basis of their cytokine production profiles that resemble T helper cell phenotypes and other unique properties. Although originally grouped with ILC1s because of their capacity to produce IFNγ, NK cells are functionally distinct from the other ILCs because of their cytotoxic capacity and have more recently been noted to have further distinctions because of ILC developmental studies (Figure 2). All ILC lineages are derived from early common lymphoid progenitors (CLPs) expressing CXCR6+, integrin α4β7 and can differentiate into NK cells, ILC1s, ILC2s, ILC3s and LTi cells (Diefenbach, Colonna, & Koyasu, 2014). The common progenitor to all helper-like innate lymphoid cell lineages (CHILP) gives rise to ILC1s, ILC2s and ILC3s but not the LTi cells or NK cells (Klose et al., 2014), separating the cNK cell developmental pathway from other ILC subsets at an earlier point (Klose & Diefenbach, 2014). Thus, cNK cells are distinct members of the ILCs.
Figure 2:
Development of ILCs. The common lymphoid progenitor (CLP) cells give rise to the common innate lymphoid progenitor (CILP) cells. The NFIL3 expressing cells mark the natural killer precursor (NKP) cells. The common helper innate progenitor (CHILP) cells are derived from the common lymphoid progenitor (CILP) cells and give rise to the PLZF+ lymphoid cell precursor (ILCP) cells and lymphoid tissue-inducer precursor (LTiP) cells. The NKP, ILCP, and LTiP cells generate five groups of ILCs; NK, ILC1, ILC2, ILC3 and LTi cells. The phenotype and the transcription factor requirement of each group of ILCs are dependent on the tissue microenvironment. Figure modified from (Vivier, et al., 2018). Nfil3 (nuclear factor IL-3 induced), Id2 (inhibitor of DNA binding 2), Gata3 (GATA binding protein 3), Plzf (promyelocytic leukemia zinc finger), Tbet (T box transcription factor), Eomes (eomesodermin), Runx3 (runt-related transcription factor 3), Rorγt (RAR related orphan receptor γt), Rorα (RAR-related orphan receptor α), Bcl11b (B cell lymphoma/leukemia11B), Gfi1 (growth factor independent 1), Ahr (Aryl hydrocarbon receptor), Blimp1 (PR domain zinc finger protein 1) and Hobit (homolog of Blimp1 in T cells).
Tissue-resident NK (trNK) cells
We recently identified distinct tissue-resident NK (trNK) cell lineages in liver, skin, kidney and virgin uterus (Peng et al., 2013; Sojka et al., 2014; Victorino et al., 2015) that phenotypically share many features with cNK cells, including the NK1.1+ NKp46+ CD3– CD19– profile in C57BL/6 mice. However, parabiosis studies indicated that trNK cells do not recirculate and remain resident in the tissue while the cNK cells circulate freely in the blood. Phenotypically trNK cells can be distinguished from circulating cNK cells because they express the integrin CD49a and lack expression of DX5 in a mutually exclusive manner: trNK cells are CD49a+DX5- and cNK cells are CD49a-DX5+. Both trNK and cNK cells depend on signaling via the IL15Rα chain as both are absent in IL15ra−/− mice. Nfil3 and Tbx21 (Tbet) are two transcription factors required for the development of normal cNK cells (Gordon et al., 2012). Nfil3−/− mice have no cNK cells but have trNK cells in the liver, skin, kidney and virgin uterus. The Tbx21−/− mice have no liver or skin trNK cells while cNK cells are less affected. In the kidney and virgin uterus, both the cNK and trNK cells were unaffected in the Nfil3−/− and Tbx21−/− mice. Some investigators term liver trNK cells as ILC1s because of their shared features but this has not been well examined in other tissues (Figure 2).
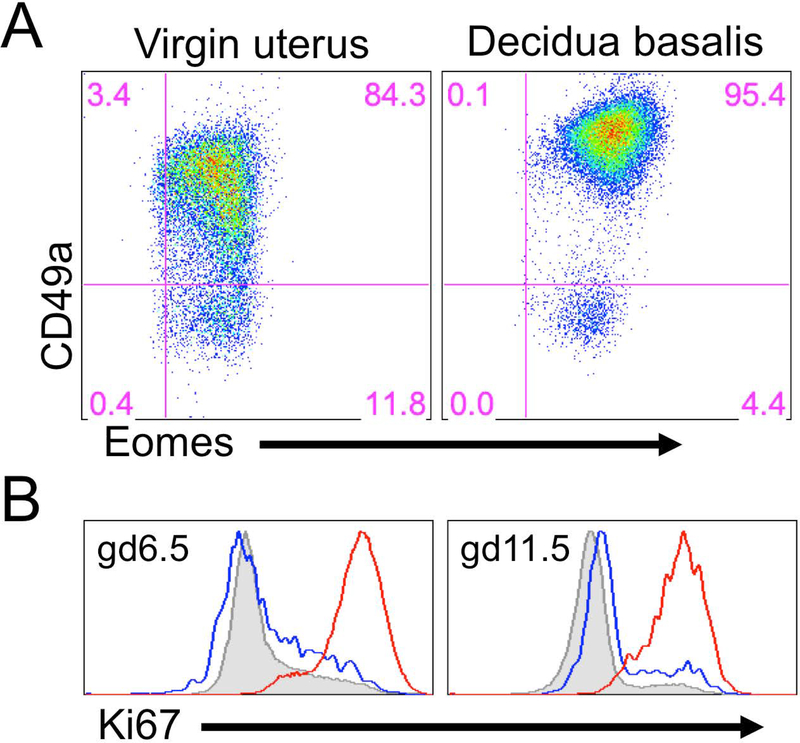
The T-box transcription factor Eomesodermin (Eomes) also has been used as a defining marker of cNK cells. The cNK cells require Eomes for their development, while liver trNK cells and ILC1s do not. However, uterine trNK cells express Eomes and can be distinguished from cNK by their tissue-residency in the virgin uterus, and by CD49a expression in the virgin and gd6.5 pregnant uterus (Figure 3A) (Boulenouar, et al., 2016; Doisne et al., 2015; Fu et al., 2017). Hence, uterine trNK cells share the residency characteristic of ILCs but are phenotypically distinct from cNK cells and other ILCs.
Figure 3:
Proliferation and phenotype of trNK cells during pregnancy. A) A virgin and pregnant uterus (gd6.5) of a B6 mouse was dissected and tissues prepared for flow cytometry. The percentages in the gates represent CD45+CD3-CD19-NK1.1+ that express either CD49a or Eomesodermin (Eomes). B) The pregnant uterus at gd6.5 (left panel) and gd11.5 (right panel) was dissected and the tissues prepared for flow cytometry. The histograms were gated on CD45+CD3-CD19-NK1.1+ and overlays are of the DX5+ (blue line) and CD49a+ (red line) from the decidua basalis and DX5+ spleen (shaded gray).
Origin of uNK cells
Uterine NK cells were originally identified histologically in the pregnant uterus. Classically recognized by periodic acid Schiff (PAS) and Dolichos biflorus agglutinin (DBA) stains (Chen et al., 2012; B.A. Croy, van den Heuvel, Borzychowski, & Tayade, 2006; Yadi et al., 2008; Zhang, Yamada, & Croy, 2009), uNK cells are localized to the decidua basalis, termed decidual NK (dNK) cells (Figure 1). They can also be found in the uterine wall, known as mesometrial lymphoid aggregate of pregnancy (MLAp).
There has been a long-standing debate regarding the origin of uNK cells during pregnancy. Whether uNK cells in the pregnant uterus develop in situ from precursors in the virgin uterus or home there from the periphery has been addressed previously using transplant and adoptive cell transfer experiments. Normal uterine horn transplanted into alymphoid mice that lacked NK cells indicated that the pregnant uterus was populated by progenitors from peripheral sources (Chantakru et al., 2002; B. A. Croy, Di Santo, Greenwood, Chantakru, & Ashkar, 2000). After adoptive transfer of bone marrow, thymus, lymph node, spleen or fetal liver cells from SCID mice into alymphoid recipients, donor-derived uNK cells could be detected in the pregnant uterus (Zhang, et al., 2009), providing further support for NK cell homing.
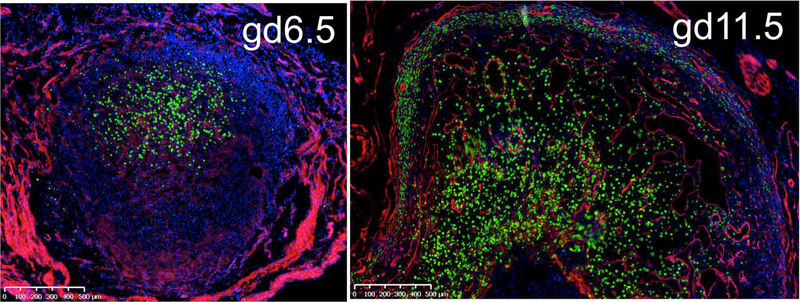
As we recently reported, however, virgin uteri contain few circulating CD49a- DX5+ cNK cells and a much higher percentage of CD49a+ DX5- NK cells that resembled trNK cells in other tissues (Cortez, Fuchs, Cella, Gilfillan, & Colonna, 2014; Fuchs et al., 2013; Sojka, et al., 2014). Indeed, this population was tissue-resident because they did not contribute to virgin uteri of the opposite parabiont in parabiosis experiments (Sojka, et al., 2014). Unlike trNK cells in the liver and skin, uterine trNK cells were present in mice deficient in either Nfil3 or Tbet, suggesting that they form a distinct lineage of NK cells. Moreover, the presence of a high percentage of trNK cells in the virgin uterus raised the possibility that they could contribute to the accumulation of uNK cells during pregnancy by local proliferation. Recently, we used a novel NK cell reporter mouse to better visualize the accumulation of NK cells during pregnancy (Sojka et al, in press). The NKp46 receptor, encoded by Ncr1, is selectively expressed on all NK cells. The Ncr1iCre mice permit improved Cre (iCre) recombinase to be restricted to NK cells (Narni-Mancinelli et al., 2011). RosamT/mG mice (Muzumdar, Tasic, Miyamichi, Li, & Luo, 2007) contain a double fluorescent reporter construct knocked into the Rosa26 locus. Membrane-bound Tomato (mT) is constitutively expressed in all tissues and upon Cre expression, the Tomato cassette and a stop codon are excised, allowing for expression of membrane-bound GFP (mG) instead of mT. We confirmed the faithful reporting of GFP in uNK cells in Ncr1iCre x RosamT/mG mice during pregnancy by evaluation of GFP+ and GFP– cells for NK cell specific markers (Sojka et al, in press). We found an accumulation of GFP+ uNK cells at gd6.5 and 11.5 implantation sites (Figure 4). As compared to histological analysis, we found uNK cells with greater sensitivity and could easily detect GFP+ cells in the myometrium, consistent with the MLAp. Moreover, flow cytometric analysis was greatly facilitated. Hence, Ncr1iCre x RosamT/mG mice allow detailed analysis of uNK cell heterogeneity during pregnancy.
Figure 4:
Accumulation of GFP+ cells in Ncr1iCre x RosamT/mG at the implantation site during pregnancy. Pregnant uteri of Ncr1iCre x RosamT/mG at gestational day (gd) 6.5 (left panel) and gd11.5 (right panel). Magnification is same in each panel; 500um bar is shown.
In the decidua basalis at gd6.5 and 11.5, trNK cells, but not cNK cells, highly expressed Ki67, an indication of local proliferation (Figure 3B). Although we were unsuccessful in using parabiosis to study otherwise normal pregnancy, we used it to study mice in which decidualization was artificially induced. Deciduomata showed NK cell accumulation and proliferation of trNK cells with minimal contribution from migrating cNK cells (Sojka et al, in press). Taken together, these findings suggest that accumulation of uNK cells in early pregnancy is due to local proliferation of trNK cells and not of cNK cells.
Our recent data does not exclude a contribution from non-proliferating cNK cells since they accumulated in number, though there was no evidence for their proliferation. In this way, our data are consistent with prior data suggesting a contribution from migrating NK cells (Chantakru, et al., 2002; B. A. Croy, et al., 2000). Taken together, these considerations support a new hypothesis to account for the accumulation of uNK cells during pregnancy.
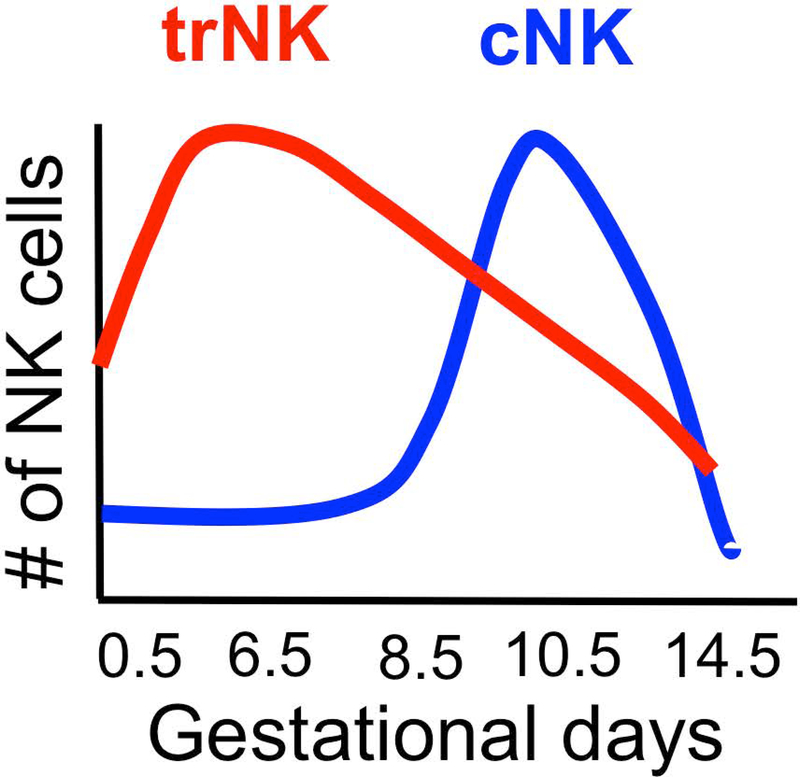
We propose a two-wave hypothesis for uNK cell accumulation in the pregnant uterus (Sojka et al, in press) (Figure 5). The first wave of uNK cells accumulates when the endometrium remodels during the decidualization process. Our data indicates that this wave is due to local proliferation of trNK cells while there is minimal contribution from circulating cNK cells to the expanding pool of uNK cells in early pregnancy. In the second wave, consistent with prior results and our data (Sojka et al, in press, (Chantakru, et al., 2002; B. A. Croy, et al., 2000; Zhang, et al., 2009), we propose that cNK cells are recruited from the periphery during placentation. Optimal placentation includes remodeling of the spiral arteries, which involves uNK cells. This is consistent with the reported deficiency in remodeling of the vasculature in the Nfil3−/− mice, which are deficient in cNK cells (Boulenouar, et al., 2016; Redhead et al., 2016). Hence, we suggest that pregnancy orchestrates the local proliferation as well as movement of NK cell subsets at different times during critical developmental changes to the pregnant uterus.
Figure 5:
Two-wave hypothesis. We propose that there are two waves of NK that occupy and accumulate at the implantation site during pregnancy. The first wave of NK cells is observed during the decidualization process. The primary cells are proliferating trNK cells with cNK cells appearing to play a negligible role. In a second wave, cNK cells are recruited from the periphery during remodeling of the spiral arteries critical for placentation (Sojka et al, in press).
NK Cell Function
To Protect
NK cells were first discovered because they were able to kill tumor cells without prior exposure and were responsible for a phenomenon termed natural cytotoxicity (Herberman, Nunn, & Lavrin, 1975; Kiessling, Klein, & Wigzell, 1975). In addition, NK cell cytolysis and cytokine production can combat viral infections. NK cell cytotoxicity requires cell contact with the target cells and is accomplished by engagement of NK cell receptors with their cognate ligands expressed on the surface of target cells.
NK cell receptors involved in target recognition are largely derived from work on cNK cells. These receptors utilize target recognition strategies in line with the missing-self hypothesis. The hypothesis states that NK cells survey tissues for normal, ubiquitous expression of major histocompatibility complex class I (MHC-I) as self that normally prevent NK cells attack. When MHC-I is down-regulated, NK cells attack and destroy their target (Karre, 2002a, 2002b; Kärre, Ljunggren, Piontek, & Kiessling, 1986). Missing-self recognition can be explained by NK cell inhibitory receptors that engage target MHC-I and deliver negative signals via cytoplasmic immunoreceptor Tyr-based inhibitory motifs (ITIMs) that recruit Tyr phosphatase, SHP1. These inhibitory receptors, lectin-like Ly49s in mice and killer immunoglobulin (Ig)-like receptors (KIRs) in human, prevent NK cell activation receptor functions. Ligand binding activation receptor chains couple to immunoreceptor Tyr-based activation motif (ITAM)-containing molecules, CD3ζ, FcεRIγ, or DAP12, that stabilize expression and transmit intracellular signals. In C57BL/6 (B6) mice, CD3– NK cells uniformly express NK1.1 (encoded by Nkrplc) and NKp46 (encoded by Ncr1). All NK cells express NKG2D, an activation receptor that recognizes host-encoded molecules, which in mice include members of RAE1 family, MULT1, and H60 whose expression is stress-induced, also leading to NK cell attack. Thus, NK cell triggering by their targets depends on integrating signals from inhibitory and activation receptors.
NK cells employ cytotoxicity to initiate anti-tumor and anti-viral immunity to eliminate the transformed or infected cells. NK cell mediated cytotoxicity initiated with activation receptor engagement can lead to direct killing of targets by granule exocytosis and the granule proteins, perforin and granzymes (Lieberman, 2010). Perforin, a pore-forming protein, punches holes in target cells by polymerizing in the membrane. Granzymes are serine proteases, which enter the target in a perforin-dependent process, and activate the caspase apoptosis pathway, which leads to death of the target cell. NK cell cytotoxicity can also be delivered in perforin-independent mechanisms such as Fas-Fas ligand interactions, tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL)-TRAIL receptor engagement, and soluble tumor necrosis factor-alpha (TNF-α) (R. K. Lee, Spielman, Zhao, Olsen, & Podack, 1996; Takeda et al., 2001). In viral infections, NK cell control is mediated by perforin-dependent mechanisms, suggesting it is more important in anti-viral defense. Hence, the method cNK cells use to induce cytotoxicity may be context-dependent but requires cell-cell contact.
Activation receptor triggering of cNK cells also leads to secretion of cytokines such as the NK cell signature cytokine IFN-γ, which can influence innate and adaptive immune responses. In tissues such as the liver, trNK cells have an expanded cytokine and chemokine profile to include TNF-α, GM-CSF, IL-2 (Sojka, et al., 2014) and CCL3 (Daussy et al., 2014) as well as IFN-γ. These cytokines are involved in many different immune responses to pathogens. Thus, the functional capabilities of NK cells are further diversified in the different tissue environments, implicating NK cells in many different anti-pathogen responses, though they have been less well studied in tissues.
These considerations lead to the possibility that NK cells primarily serve protective roles in pregnancy, i.e., by killing targets and producing cytokines that enhance immune responses. However, other studies have suggested that this notion may not be the entire story. Relevant to the function of uNK cells in pregnancy, it should be noted that MHC class I-specific inhibitory receptors are structurally distinct between species. Mouse NK cells express Ly49s, type II integral membrane proteins with lectin-like domains that have no carbohydrate interactions. By contrast, human NK cells express KIRs, type I integral membrane proteins belonging to the immunoglobulin superfamily. Despite these structural differences, Ly49s and KIRs share most other features, including functions. Thus, both species have derived different genetic solutions to solve important functions, critical to the survival of both species, i.e., they represent outstanding examples of convergent evolution (Kelley, Walter, & Trowsdale, 2005).
No more critical to species survival is the successful outcome of pregnancy. In support of this, epidemiological studies have clearly demonstrated increased risk from preeclampsia is linked to a sharing of genotypes for maternal KIRs and corresponding genotypes for their cognate HLA allele in the fetus. This link was first observed in European women and was replicated in another study involving African women who have different KIR and HLA alleles strongly suggesting an interaction between an inhibitory receptor on maternal uNK cells and its HLA ligand on fetal tissues leads to adverse pregnancy outcomes (Hiby et al., 2004; Moffett & Colucci, 2014). On the other hand, epidemiological studies have also examined the effects of genotypes for activation receptor on NK cells and their fetal HLA ligands. In this case, the converse finding was obtained. That is, genotype for a maternal KIR activation receptor recognizing a fetal HLA ligand is associated with protection from preeclampsia. Thus, these data suggest that uNK cells respond to fetal MHC class I molecules via their inhibitory and activation receptors to negatively and positively, respectively, control the development of preeclampsia.
To Nurture
The epidemiological studies demonstrating the link between NK cell receptors and fetal HLA ligands lead to the hypothesis that NK cells may serve another role in pregnancy, i.e., nurturing the fetus. Instead of, or in addition to their presumed protective role against pathogens, NK cells may directly respond to the fetus and support pregnancy. This function appears to require engagement of activation receptors, leading to NK cell activation. When stimulation through activation receptors is blocked by inhibitory receptors, then their nurturing function is abrogated and the prospect of a successful pregnancy diminishes.
Indeed, in a set of landmark studies in mice, Croy and colleagues proposed that during pregnancy the role of uNK cells was to aid vascular modification of the placenta. More specifically, uNK cells via their IFN-γ secretion promoted spiral arteries to transform into thin walled vessels with large lumens, a vital characteristic of pregnancy. In mice that completely lacked NK cells (Rag2−/−γc−/−) or lacked a subset of NK cells (Nfil3−/−), spiral artery remodeling was inefficient and growth-restricted pups were reported (Ashkar, et al., 2000; Boulenouar, et al., 2016; B. A. Croy & Xie, 2006; Redhead, et al., 2016). The defect in spiral artery remodeling was restored when the Rag2−/−γc−/− mice were reconstituted with wildtype but not IFN-γ−/− bone marrow (Ashkar, et al., 2000). In further support of a tissue building phenotype, uNK cells produce pro-angiogenic factors, including vascular endothelial growth factor (VEGF) and placental growth factor (PLGF) that both favor placenta growth in the decidua basalis. Studies of human uNK cells indicate this production can be triggered by the uNK-engagement of NKp30 and NKp44 with ligands expressed on the EVTs and maternal stromal cells (Hanna et al., 2006). These data provide evidence for uNK cells providing functions that are perhaps less cytotoxic and more involved in tissue building, working in cooperation with the invading trophoblasts and maternal stromal cells.
Interestingly, EVTs also express the non-classical HLA-G molecule whose expression is primarily restricted to EVTs invading the decidua basalis. The KIR-family member KIR2DL4 that is expressed on all NK cells recognizes HLA-G. This interaction in vitro results in the release of proangiogenic factors by the uNK cells (Le Bouteiller, 2015). Additionally soluble HLA-G is more potent than its membrane bound form in promoting proliferation and cytokine production by uNK cells (van der Meer et al., 2007). The engagement of HLA-G by the uNK cells in early pregnancy also resulted in the secretion of growth promoting factors essential for fetal development (Fu, et al., 2017). Collectively these data suggest that uNK receptor interactions with HLA-G endow uNK cells with unique functions to promote tissue remodeling.
Recently, in a provocative study Fu and colleagues reported that uNK cells stimulate fetal growth by producing growth-promoting factors, providing the developing embryo the nutritional support prior to the formation of the placenta (Fu, et al., 2017). They reported that mouse CD49a+ trNK cells produced growth-promoting factors such as pleiotrophin, osteoglycin, and osteopontin. Using a co-culture system they demonstrated that there needed to be cross-talk between HLA-G on fetal trophoblasts and the KIR2DL4/ILT2 inhibitory receptor on human trNK to promote the secretion of growth-promoting factors. To test if secretion of growth-promoting factors from maternal uNK cells affected fetal growth, Fu and colleagues analyzed fetal mice from the Nfil3−/− mouse that has no cNK cells, and other mice with defective trNK cells lacking growth-promoting factors. All of these mice had FGR pups and defects in bone development. When Nfil3−/− mice were reconstituted with in vitro expanded CD49a+ trNK cells that produced the growth-promoting factors, birth weights of the pups and bone development were restored. Hence, this recent study reveals a novel functional role of trNK cells during early fetal development involving growth-promoting factors.
Conclusion
It had been difficult to reconcile the classic cytotoxic functions of peripheral cNK cells, and their abundant presence at implantation sites during pregnancy. Although they could provide protective roles against pathogens, recent studies indicate that there is heterogeneity of uNK cells that can be distinguished from cNK cells. Moreover, uNK cells were known to promote vascular changes critical for normal pregnancy and new studies extend this concept by demonstrating that uNK cells also provide growth-promoting functions. Thus, uNK appear to provide both protective and nurturing roles in normal pregnancy.
Acknowledgments
Work in the Yokoyama lab on uterine NK cells is supported by grant R01-AI140397 from the National Institutes of Health.
References
- Ashkar AA, Di Santo JP, & Croy BA (2000). Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med, 192(2), 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham RE, Brent L, & Medawar PB (1953). Actively acquired tolerance of foreign cells. Nature, 172(4379), 603–606. [DOI] [PubMed] [Google Scholar]
- Boulenouar S, Doisne JM, Sferruzzi-Perri A, Gaynor LM, Kieckbusch J, Balmas E,…Colucci F (2016). The Residual Innate Lymphoid Cells in NFIL3-Deficient Mice Support Suboptimal Maternal Adaptations to Pregnancy. Frontiers in immunology, 7, 43. doi: 10.3389/fimmu.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- </>Brosens I, Pijnenborg R, Vercruysse L, & Romero R (2011). The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. [Research Support, N.I.H., Intramural [DOI] [PMC free article] [PubMed]
- Review]. American journal of obstetrics and gynecology, 204(3), 193–201. doi: 10.1016/j.ajog.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC,…Croy BA (2002). Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol, 168(1), 22–28. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang J, Hatta K, Lima PD, Yadi H, Colucci F,…Croy BA (2012). DBA-lectin reactivity defines mouse uterine natural killer cell subsets with biased gene expression. Biol Reprod, 87(4), 81. doi: 10.1095/biolreprod.112.102293biolreprod.112.[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez VS, Fuchs A, Cella M, Gilfillan S, & Colonna M (2014). Cutting edge: Salivary gland NK cells develop independently of Nfil3 in steady-state. [Research Support, N.I.H., Extramural]. J Immunol, 192(10), 4487–4491. doi: 10.4049/jimmunol.1303469 [DOI] [PubMed] [Google Scholar]
- Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, & Gerber SA (2012). Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Biology of reproduction, 87(5), 125. doi: 10.1095/biolreprod.112.102830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy BA, Di Santo JP, Greenwood JD, Chantakru S, & Ashkar AA (2000). Transplantation into genetically alymphoid mice as an approach to dissect the roles of uterine natural killer cells during pregnancy--a review. [Research Support, Non-U.S. Gov’t Review]. Placenta, 21 Suppl A, S77–80. [DOI] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, & Tayade C (2006). Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev, 214, 161–185. [DOI] [PubMed] [Google Scholar]
- Croy BA, & Xie X (2006). In vivo models for studying homing and function of murine uterine natural killer cells. [Research Support, Non-U.S. Gov’t]. Methods in molecular medicine, 122, 77–92. [DOI] [PubMed] [Google Scholar]
- Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, …Walzer T (2014). T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. [Research Support, Non-U.S. Gov’t]. J Exp Med, 211(3), 563–577. doi: 10.1084/jem.20131560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Colonna M, & Koyasu S (2014). Development, differentiation, and diversity of innate lymphoid cells. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. Immunity, 41(3), 354–365. doi: 10.1016/j.immuni.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, …Colucci F (2015). Composition, Development, and Function of Uterine Innate Lymphoid Cells. [Research Support, Non-U.S. Gov’t]. Journal of immunology, 195(8), 3937–3945. doi: 10.4049/jimmunol.1500689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A (2013). Immunology of the maternal-fetal interface. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. Annual review of immunology, 31, 387–411. doi: 10.1146/annurev-immunol-032712-100003 [DOI] [PubMed] [Google Scholar]
- Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z,… Wei H(2017). Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. [Research Support, Non-U.S. Gov’t]. Immunity, 47(6), 1100–1113 e1106. doi: 10.1016/j.immuni.2017.11.018 [DOI] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD,… Colonna M (2013). Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity, 38(4), 769–781. doi: 10.1016/j.immuni.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, …Reiner SL (2012). The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity, 36(1), 55–67. doi: 10.1016/j.immuni.2011.11.016S1074-7613(12)00005-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, …Mandelboim O (2006). Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med, 12(9), 1065–1074. [DOI] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, & Lavrin DH (1975). Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. International Journal of Cancer, 16, 216. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Walker JJ, O’Shaughnessy K M, Redman CW, Carrington M, Trowsdale J, & Moffett A (2004). Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med, 200(8), 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AP, Gerber SA, & Croy BA (2014). Uterine natural killer cells pace early development of mouse decidua basalis. [Research Support, Non-U.S. Gov’t]. Molecular human reproduction, 20(1), 66–76. doi: 10.1093/molehr/gat060 [DOI] [PubMed] [Google Scholar]
- Karre K (2002a). Immunology. A perfect mismatch. Science, 295(5562), 2029–2031. doi: 10.1126/science.1070538295/5562/2029 [pii] Licensing [DOI] [PubMed] [Google Scholar]
- Karre K (2002b). NK cells, MHC class I molecules and the missing self. Scand J Immunol, 55(3), 221–228. doi: 1053 [pii] Licensing [DOI] [PubMed] [Google Scholar]
- Kärre K, Ljunggren HG, Piontek G, & Kiessling R (1986). Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature, 319(6055), 675–678. [DOI] [PubMed] [Google Scholar]
- Kelley J, Walter L, & Trowsdale J (2005). Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet, 1(2), 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Klein E, & Wigzell H (1975). Natural killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells: Specificity and distribution according to genotype. European Journal of Immunology, 5, 112–117. [DOI] [PubMed] [Google Scholar]
- Klose CS, & Diefenbach A (2014). Transcription factors controlling innate lymphoid cell fate decisions. Curr Top Microbiol Immunol, 381, 215–255. doi: 10.1007/82_2014_381 [DOI] [PubMed] [Google Scholar]
- Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, …Diefenbach A (2014). Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. [Research Support, Non-U.S. Gov’t]. Cell, 157(2), 340–356. doi: 10.1016/j.cell.2014.03.030 [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P (2015). HLA-G in human early pregnancy: control of uterine immune cell activation and likely vascular remodeling. Biomedical journal, 38(1), 32–38. doi: 10.4103/2319-4170.131376 [DOI] [PubMed] [Google Scholar]
- Lee KY, & DeMayo FJ (2004). Animal models of implantation. [Research Support, U.S. Gov’t, P.H.S. Review]. Reproduction, 128(6), 679–695. doi: 10.1530/rep.1.00340 [DOI] [PubMed] [Google Scholar]
- Lee RK, Spielman J, Zhao DY, Olsen KJ, & Podack ER (1996). Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. Journal of Immunology, 157(5), 1919–1925. [PubMed] [Google Scholar]
- Lieberman J (2010). Anatomy of a murder: how cytotoxic T cells and NK cells are activated, develop, and eliminate their targets. Immunol Rev, 235(1), 5–9. doi: 10.1111/j.0105-2896.2010.00914.xIMR914 [pii] [DOI] [PubMed] [Google Scholar]
- Moffett A, & Colucci F (2014). Uterine NK cells: active regulators at the maternal-fetal interface. [Research Support, Non-U.S. Gov’t Review]. The Journal of clinical investigation, 124(5), 1872–1879. doi: 10.1172/JCI68107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, & Luo L (2007). A global double-fluorescent Cre reporter mouse. [Research Support, N.I.H., Extramural]. Genesis, 45(9), 593–605. doi: 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, …Vivier E (2011). Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc Natl Acad Sci U S A, 108(45), 18324–18329. doi: 1112064108 [pii] 10.1073/pnas.1112064108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang G, Buret A, Batey RT, Chen QY, Couch L, Cripps A, & Clancy R (1993). Morphological, phenotypic and functional characteristics of a pure population of CD56+ CD16- CD3- large granular lymphocytes generated from human duodenal mucosa. Immunology, 79(3), 498–505. [PMC free article] [PubMed] [Google Scholar]
- </>Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, …Tian Z (2013). Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Clin Invest, 123(4), 1444–1456. doi: 10.1172/JCI66381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhead ML, Portilho NA, Felker AM, Mohammad S, Mara DL, & Croy BA (2016). The Transcription Factor NFIL3 Is Essential for Normal Placental and Embryonic Development but Not for Uterine Natural Killer (UNK) Cell Differentiation in Mice. [Research Support, Non-U.S. Gov’t]. Biology of reproduction, 94(5), 101. doi: 10.1095/biolreprod.116.138495 [DOI] [PubMed] [Google Scholar]
- Serafini N, Vosshenrich CA, & Di Santo JP (2015). Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol, 15(7), 415–428. doi: 10.1038/nri3855nri3855 [pii] [DOI] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, …Yokoyama WM (2014). Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife, 3, e01659. doi: 10.7554/eLife.01659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, …Vivier E (2013). Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol, 13(2), 145–149. doi: 10.1038/nri3365nri3365 [pii] [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, …Okumura K (2001). Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med, 7(1), 94–100. [DOI] [PubMed] [Google Scholar]
- van der Meer A, Lukassen HG, van Cranenbroek B, Weiss EH, Braat DD, van Lierop MJ, & Joosten I (2007). Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Molecular human reproduction, 13(2), 123–133. doi: 10.1093/molehr/gal100 [DOI] [PubMed] [Google Scholar]
- </>Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, …Clambey ET (2015). Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. [Research Support, N.I.H., Extramural [DOI] [PMC free article] [PubMed]
- Research Support, Non-U.S. Gov’t]. Journal of immunology, 195(10), 4973–4985. doi: 10.4049/jimmunol.1500651 [DOI] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, …Spits H (2018). Innate Lymphoid Cells: 10 Years On. [Review]. Cell, 174(5), 1054–1066. doi: 10.1016/j.cell.2018.07.017 [DOI] [PubMed] [Google Scholar]
- Yadi H, Burke S, Madeja Z, Hemberger M, Moffett A, & Colucci F (2008). Unique receptor repertoire in mouse uterine NK cells. J Immunol, 181(9), 6140–6147. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM (2013). Chapter 17. Natural killer cells In Paul WE (Ed.), Fundamental immunology (7th ed., pp. 395–431). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Zhang JH, Yamada AT, & Croy BA (2009). DBA-lectin reactivity defines natural killer cells that have homed to mouse decidua. Placenta, 30(11), 968–973. doi: 10.1016/j.placenta.2009.08.011S0143-4004(09)00276-8 [pii] [DOI] [PubMed] [Google Scholar]