Abstract
Testing vaccine efficacy against the highly lethal Ebola virus (EBOV) in humans is almost impossible due to obvious ethical reasons and the sporadic nature of outbreaks. For such situations, the “animal rule” was established, requiring the product be tested in animal models, expected to predict the response observed in humans. For vaccines, this testing aims to identify immune correlates of protection, such as antibody or cell-mediated responses. In the wake of the 2013 – 2016 EBOV epidemic, and despite advancement of promising candidates into clinical trials, protective correlates remain ambiguous. In the hope of identifying a reliable correlate by comparing preclinical and clinical trial data on immune responses to vaccination, we conclude that correlates are not universal for all EBOV vaccines.
Keywords: Ebola, vaccine, immune correlates, immunity, protection
Need for markers of vaccine-generated immunity for filoviruses.
Outbreaks of filoviruses Ebola (EBOV), the closely related Sudan (SUDV), Bundibugyo (BDBV) and more distantly related Marburg (MARV) viruses are historically sporadic, unpredictable and have a high case fatality rate making conventional vaccine efficacy testing in humans logistically difficult. In 2002, the United States Food and Drug Administration (FDA) established the “animal rule” for regulatory approval of drugs and biologics for which efficacy testing in humans is impossible, requiring relevant animal models which recapitulates human disease (https://www.ecfr.gov/cgi-bin/text-idx?SID=07ae7117f4af9184631f0ba5ab8e9bec&mc=true&node=sp21.5.314.i&rgn=div6). Among the EBOV research animal models, which include several small animal and non-human primate (NHP) species, macaques are preferred, expected to develop a response predictive for humans. Vaccine efficacy studies in animal models aim to identify the vaccine-induced markers such as the levels of antigen-specific binding and neutralizing antibodies, or cell-mediated responses, which correlate with protection against the targeted pathogen. This licensing pathway requires that immunogenicity results from clinical trials are consistent with previously identified immune correlates associated with protection; thus identifying reliable markers of vaccine-generated immunity becomes critically important for pathogens such as EBOV. Several EBOV vaccines currently in development have conferred protection against lethal infection in preclinical testing in NHP models. All protective EBOV vaccines use the viral glycoprotein (GP), the sole envelope protein capable of inducing protective responses, as an immunogen. These vaccines include recombinant replication-competent vectors vesicular stomatitis virus (VSV) [1], rhesus cytomegalovirus (RhCMV) [2], human parainfluenza type 3 (HPIV3) [3], and attenuated rabies virus [4]. They also include non-replicating vaccines based on replication-deficient adenoviral vectors [5, 6], Venezuelan equine encephalitis virus (VEEV)-based replicons [7], replication-deficient EBOV particles lacking VP30 (EBOVΔVP30) [8], as well as EBOV-like particles (VLP) [9] and some other forms such as GP subunit vaccines and adjuvanted GP nanoparticles (reviewed in references [10, 11]).
EBOV vaccine human clinical trials.
The unprecedented 2013 – 2016 EBOV epidemic which caused 28,616 suspected, probable and confirmed cases and 11,310 fatalities (http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html), resulted in fast tracking vaccines showing potential in preclinical studies into clinical trials through collaborative efforts involving the World Health Organization, several health agencies, biotech companies and research groups from around the globe, albeit catching the tail end of the epidemic when cases were on a decline. This revolutionized protocols and policies for vaccine trial implementation and for compassionate use of investigational new therapeutics and vaccines to counter epidemics. The lead candidate, VSV-EBOV vaccine, was accelerated into a ring vaccination phase III clinical trial in Guinea in April 2015 and demonstrated protective efficacy [12, 13]. Adenovirus (Ad) vectored vaccines based on chimpanzee Ad3 (ChAd3) [14], human Ad5 [15], and human Ad26 boosted with a modified vaccinia virus Ankara (MVA) [16, 17] also were assessed in Phase I and II studies. At present, there are 60 ongoing and/or completed trials according to clinicaltrials.gov (see review by Martins et al. for more details [10]). EBOV-specific immune responses analyzed in clinical trials were mostly the levels of GP-specific IgG binding in an ELISA format, due to the ease of such testing. Thus far, GP-specific IgG responses have been detected 2 and 1 years post vaccination for VSV-EBOV [18] and ChAd3 EBOV [19], respectively. The quest to identify immune correlates remains paramount as VSV-EBOV was deployed during the recent (April – June 2018) outbreak in the Democratic Republic of the Congo.
Immunological parameters established in animal models are not always reflected in clinical trials.
Immune responses elicited by a vaccine in humans are not always mirrored in animal models due to differences in dosage, preexisting vector-specific immunity, or divergent host genetics. In addition, cell-mediated responses demonstrated in NHP models are not often tested in clinical trials. As predicted from NHP models, VSV-vectored vaccines elicited humoral immune responses in humans [20-22]. T cell responses were not robust in NHPs during immunization [23, 24]. Cellular immunity was however, detected following post exposure treatment of a health care worker [25]. Only recently, data from a dose-escalation Phase I trial revealed a moderate to low magnitude of T cells, which were predominantly CD8+ T cells [26]. In another Phase I trial, the vaccine dose was lowered in an attempt to minimize adverse effects demonstrated in the higher dose cohorts, thus compromising the GP-specific IgG- and neutralizing antibody responses [20]. Dose reduction also negatively impacted the quality of the CD8+ T cell responses [26]. In a ChAd3 EBOV phase I clinical trial [27] a single dose primed CD4+ T cells more efficiently, whereas in NHPs, the vaccine had elicited stronger CD8+ T cell responses [6]. The polyfunctionality of T cells was however, associated with protection in preclinical studies [6]. Pre-existing adenovirus vector-specific immunity in humans potentially influenced this T cell skew [14, 28], which could be mitigated by heterologous boosting with MVA [27, 29] or potentially by a higher dose of ChAd3 EBOV [14]. These data, taken together with the sporadic nature of EBOV outbreaks that restricts the possibility of testing vaccine efficacy in humans, emphasize the need of in-depth investigation of immune correlates of protection for all EBOV vaccines.
Mechanistic immune correlates of protection: Distinct contribution of antibodies and cell-mediated immunity to protection conferred by different EBOV vaccines.
Immune correlates of protection can be divided into two categories: mechanistic, whereby the measured correlate is directly responsible for protection, and non-mechanistic, a surrogate indicator which may contribute to, but is not exclusively involved in protection [30]. Using immune depletion studies, the mechanism of vaccine protection against EBOV intramuscular (IM) challenge was elucidated for two filovirus vaccine platforms in the NHP model (Table 1). Preclinical NHP studies with the VSV-EBOV vaccine demonstrated that the levels of GP-specific antibodies correlated with protection, and survival was compromised upon ablation of the CD4+ T cell-dependent antibody response. In addition, cell-mediated immunity did not play a critical role in VSV-EBOV vaccine-mediated protection since survival from lethal challenge was not affected by depletion of CD8+ T cells at the time of vaccination or by depletion of CD4+ T cells at the time of infection [24]. Gene expression differences between the CD8+ depleted and non-depleted NHPs upon vaccination, however, indicate a potential minor regulatory role for CD8+ T cells in VSV-EBOV-mediated protection [31]. In contrast, depletion of CD8+ T cells at the time of infection and B cells at the time of vaccination in Ad5-vectored EBOV vaccinated macaques showed a requirement for CD8+ T cells and not antibodies for protection [32], although additional studies may be required to better elucidate the role of antibodies in protection by this vaccine.
Table 1:
Immune correlates of protection in preclinical EBOV vaccine studies
| Vaccine | Ani mal Mod el |
Immuniz a-tion route |
GP- specific binding antibodi es |
Neutrali zing antibodi es |
EBOV infect ion route |
Immune Correlate of Protection |
Mechan istic Correlat e? |
Refere nces |
|---|---|---|---|---|---|---|---|---|
|
VSV- EBOV |
NHP | IM | +++ | ++ | IM | EBOV GP- specific antibodies |
Yes | [24] |
| Mou se |
IP | +++ | +/− | IP | EBOV GP- specific antibodies |
Yes | [68] | |
| Ad5 | NHP | IM | ++/+++ | n.a. | IM | EBOV GP- specific CD8+ T cells |
Yes | [32] |
| NHP | IM | ++ | +/− | IM | EBOV GP- specific IgG |
No | [5] | |
|
HPIV3/Eb oGP |
NHP | Aerosol or liquid IN/IT |
+++ | +++ | IM | EBOV GP- specific mucosal and systemic IgG, IgA and neutralizing antibodies. Lung-resident polyfunctional T cells |
No | [64, 69] |
|
RhCMV/E BOV-GP |
NHP | SC | +++ | − | IM | EBOV GP- specific IgG |
No | [2] |
|
ChAd3 EBOV |
NHP | IM | ++/+++ | n.a. | IM | EBOV GP- specific IgG for short-term protection (challenge 5 weeks post vaccination) |
No | [6] |
|
ChAd3/M VA EBOV |
NHP | IM | +++ | n.a. | IM | EBOV GP- specific CD8+ T cell immunity for long term protection (challenge 10 months post vaccination) |
No | [6] |
|
RABV/EB OV |
NHP | IM | + | n.a. | IM | EBOV GP- specific IgG1:IgG2 ratio |
No | [4] |
| VLP | Mou se |
IM | ++/+++ | n.a. | IP | EBOV GP- specific CD8+ T cells and B cells |
Yes | [34, 35] |
|
VEEV- based replicon |
Mou se |
Subcutan eous |
n.a. | n.a. | IP | EBOV-specific CD8+ T cells |
Yes | [33] |
|
EBOV GP/VSV∆G pseudovir ions |
Mou se |
IM | ++/+++ | - | IP | EBOV GP- specific antibodies |
Yes | [36] |
n.a., not analyzed
Mechanistic correlates of protection for three additional vaccine platforms were determined in murine models where different components of the adaptive immune response were knocked-out or adoptively transferred (see Table 1). Protection conferred by VEEV-based replicon vaccines required only EBOV-specific CD8+ T cell immunity [33]. VLP-mediated protection required GP and VP40-specific CD8+ T cell responses [34]. However, the mounted antibody responses, but not B cells, were dispensable [34, 35]. These B cells may therefore possess unappreciated antibody-independent roles such as priming T cells, and only produce antibodies with T cell help following vaccination, which promote but are not predictive of survival. Protection by a replication-deficient vaccine based on VSV pseudotyped with EBOV GP was mediated by GP-specific binding antibodies (IgG and IgM) and not neutralizing antibodies [36]. Collectively, these studies raised the issue that EBOV vaccine platforms elicit distinct immune profiles and use different protection mechanisms [37] thus emphasizing that individual studies are required for each proposed vaccine platform to establish vaccine-specific mechanistic correlate(s) of protection.
Non-mechanistic immune correlates of protection.
While mechanistic correlates are most informative to predict protective efficacy of each vaccine, they may not be readily identifiable or are too difficult to quantitate. For such situations, non-mechanistic correlates may be used (Table 1). For example, Ad5 protection correlated with GP-specific antibody titers [5] yet the protection was mediated by CD8+ T cells and not antibodies [32]. Several surrogate markers may potentially be utilized for the VSV-EBOV vaccine in humans. First, the revelation that anti-GP IgM dominates the neutralizing response in humans indicates that IgG titers in fact represent a surrogate marker for efficacy of this vaccine [38]. Second, the frequency of a CD4+ T cell subpopulation, circulating follicular T helper (Tfh) cells and notably its Tfh17 subset, which supports the expansion and quality of B cell responses, may also serve as non-mechanistic correlates, as they were associated with GP-specific IgG titers in Phase I trial volunteers [22]. Third, systems vaccinology identified a signature of five vaccine-induced, key early innate immune mediators: the frequency of CD56bright NK cells, the expression of CXCR6 by CD56dim NK cells and the levels of cytokines IP-10, MIP-1β and MCP-1 in plasma, which correlated with antibody titers and may facilitate early protection before an adaptive immune response is mounted [39]. RhCMV-based vaccine platforms are typified as inducing biased effector memory T cell responses with minimal antibodies [40, 41]. Yet when used as an EBOV vaccine, GP-specific T cells were undetectable in NHPs. Rather, protection correlated with the levels of IgG antibodies, but not their neutralizing capacity [2] casting doubt on their direct mechanistic role and implicating the involvement of Fc-mediated protective mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) and complement-dependent cytotoxicity. The failure of some studies to protect following passive transfer of EBOV-specific immune serum also highlights the potential non-mechanistic role for GP-specific IgG titers, although this may also be explained by insufficient levels of transferred antibodies [32, 42]. Thus, for some EBOV vaccine platforms, regardless of the animal model, GP-specific IgG titers may be a prominent non-mechanistic correlate of protection while qualitative components of the antibody response that are yet to be determined, contribute to protection.
Different mechanisms of antibody-mediated protection.
The ability of vaccine-induced antibodies to confer protection through an array of mechanisms is supported by the successful preclinical use of monoclonal antibodies (mAb) with varying effector functions to treat lethal filovirus infections. ZMapp, a cocktail of three mAbs (2G4, 4G7 and 13F6), protected NHPs from death after EBOV challenge when administered up to five days post-infection [43]. ZMapp was also used in humans but its clinical testing at the tail end of the 2013-2016 EBOV epidemic suggested that the treatment did not meet the pre-specified statistical threshold for efficacy [44]. Post-exposure treatment of mice with exceptionally potent pan-ebolavirus neutralizing monoclonal antibodies ADI-15742 and ADI-15878 conferred significant levels of protection against death caused by EBOV, SUDV and BDBV challenges [45]. The combination of mAb100 and mAb114, two human monoclonal antibodies with both neutralizing and Fc domain-mediated protective functions, protected NHPs from death and disease caused by EBOV [46]. Furthermore, different strategies have been designed to enhance and broaden in vitro neutralization with the hope of translation into improved in vivo protection. Specific pairing of a weakly neutralizing mAb with a non-neutralizing mAb targeting the glycan cap created cooperative neutralization and protected mice from death and disease caused by EBOV when delivered at high dose and from death by EBOV and SUDV when delivered at a lower dose [47]. Two distinct antibody specificities, against EBOV and SUDV, were combined in a single engineered bi-specific mAb which protected mice from death caused by EBOV and SUDV [48]. The bispecific mAb method was further refined to confer neutralization by combining two non-neutralizing mAbs targeting the receptor recognition region and an exposed, conserved epitope of GP. The resulting mAb was demonstrated to neutralize recombinant VSV bearing GP from all filovirus species and protected mice from death caused by EBOV and SUDV [49].
Antibody-mediated protection does not necessarily correlate with in vitro neutralizing capacity. VSV-MARV vaccine elicited GP-specific binding but low-level neutralizing antibodies and no detectable cellular immune responses in NHPs; nonetheless, one year post vaccination, little to no neutralizing activity was detected, yet 100% of animals were protected from the disease and death caused by MARV challenge [50]. NHPs succumbed to EBOV infection despite post-exposure treatment with high concentrations of KZ52, a monoclonal antibody with EBOV neutralizing capacity at the post-entry step of infection [51, 52]. Therefore, since antibodies are functionally multifaceted, focus is shifting toward synergistic antibody combinations with various effector capabilities including neutralization and Fc-mediated protective mechanisms such as ADCC and ADCP. Comparison of replication-competent, replication-incompetent and chemically inactivated rhabdovirus-vectored bivalent EBOV/rabies virus vaccines suggested that the protection of NHPs did not correlate with GP-specific IgG titers, neutralizing antibodies, antibody avidity or their recognition of GP’s mucin-like domain. Instead, it correlated with lower GP-specific IgG2:IgG1 isotype ratios (only two IgG subclasses were analyzed) [4]; a prominent IgG1 response implies the involvement of ADCC in protection. In another study, protection of mice from death by EBOV was achieved by nonneutralizing monoclonal antibodies with Fc-mediated protective functions [53]. MR191, a MARV-specific weakly neutralizing mAb isolated from a human survivor, protected NHPs against lethal infection with MARV [54]. The antibody used in the study lacks fucosylation (due to production in tobacco plants); as afucosylated antibodies have enhanced binding to FcγRIII receptors [55], protection was likely enhanced by its Fc-mediated mechanisms. Taken together, these studies highlight the importance of the quality of the antibody effector functions including both Fc-mediated and neutralizing capacities and suggest that different vaccines may induce qualitatively distinct antibodies, which orchestrate different protective mechanisms.
Different protective mechanisms against EBOV infection caused by different exposure routes.
While it is believed that most human-to-human EBOV infections in outbreaks involve direct contact with infected tissues and biological fluids, transmission of the virus through aerosols or droplets of respiratory secretions and other biological fluids from infected individuals could also be a possibility [56]. EBOV can infect mucosal surfaces including the respiratory tract. Laboratory generated EBOV aerosols are infectious [57], and experiments with NHP and rodent models demonstrated that EBOV may possibly be transmitted without direct contact [58, 59]. EBOV infection of the respiratory mucosa is pathophysiologically distinct from IM or intraperitoneal (IP) infection [60-62], so immune correlates inferred from current NHP IM challenge models may be only partially relevant in establishing requirements for protection of humans in a natural exposure setting.
A single dose of VSV-EBOV, or VSV-MARV protected NHPs against exposure to the respective aerosolized virus [63]. Aerosolized SUDV however, may necessitate higher or broader immune responses, as protection required booster vaccinations of the VEEV-based replicon vaccine, shown to be protective at one dose against IM challenge [7], or the bivalent Ad5-vectored vaccine targeting EBOV and SUDV [62]. This may indicate the need for elevated antibody titers and/or an immune marker exclusive to the protection against mucosal filovirus infections. Hence, the threshold of binding or neutralizing antibodies used to gauge protection against IM challenge may not necessarily be predictive against mucosal exposure. However, the successful implementation of VSV-EBOV in the Guinea ring vaccination trial [12, 13], where spread from infected persons conceivably included transmission via the mucosa, may be attributed to the very high immunogenicity of this vaccine. Indeed, the vaccine was tested in the stringent preclinical IM challenge models: macaques, which are very susceptible to EBOV and a challenge dose which far exceeds natural exposure doses.
Vaccine-induced protective mechanisms may also depend on the administration route. The respiratory HPIV3/EboGP vaccine elicited both systemic and mucosal neutralizing antibodies and a cell-mediated response most prominent in the lungs of NHPs [64]. Therefore, in case of respiratory mucosal EBOV infection, protection may be mediated by both antibody and cell-mediated responses in the respiratory tract, while in case of IM infection, protection is likely mediated by systemic antibodies. Delivery of VSV-EBOV to the respiratory tract induced stronger humoral and T lymphocyte responses, and potentially primed different memory T cell populations compared to vaccination via the IM route [23]. Thus, respiratory administration of an EBOV vaccine to broaden the scope and quality of the response and elicit mucosal immunity could be highly advantageous against mucosal exposure and would most likely utilize different immune mechanisms of protection. Moreover, respiratory vaccination would be more feasible and practical in countries with limited infrastructure, requiring the use of disposable nebulizers and eliminating the need for medical personnel.
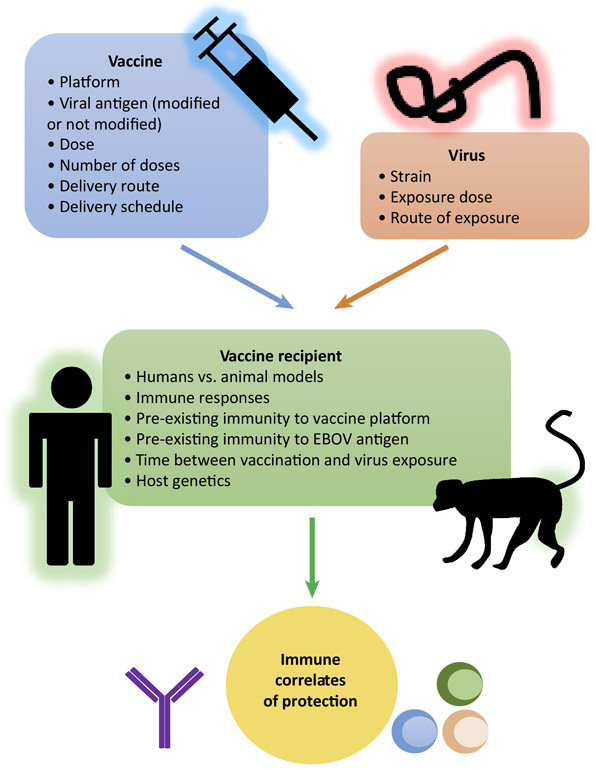
Concluding remarks.
This Opinion article offers a survey of preclinical and clinical studies which suggest that immune correlates of protection for EBOV vaccines are not universal but are defined by parameters, including: vaccine platform, viral antigen, vaccine dose, number of doses, delivery route, immunization schedule, time between the last dose and exposure to EBOV, route of exposure (IM or inhaled aerosol), EBOV challenge dose, host genetics, as well as pre-existing immunity to vaccine components (EBOV antigen and the vaccine vector) (Figure 1). The EBOV vaccines tested in recent clinical trials elicited immune responses, which did not always replicate the responses observed in animal models. Moreover, the mechanisms involved in the induction of innate immunity [65] and molecular determinants of EBOV virulence are different between murine models and humans [66]. From the available data, the most reliable immune correlates of protection for an EBOV vaccine can only be obtained from testing in NHPs, though NHP data should also be interpreted cautiously, as the close to 100% lethality of EBOV infections in NHPs [67] greatly exceeds that in humans http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Currently, characterization of immune responses is limited by the use of classical serological and cell-mediated assays, and because these EBOV vaccines induce different responses, identification of a universal correlate is unlikely. With different immune mechanisms at work among the platforms, we need to reassess the apparent conformity for using IgG titers as the universal protective gauge (see Outstanding Questions). New strategies and advanced technologies are needed to comprehensively profile each vaccine’s immune signature to improve our knowledge on the distinct mechanisms of protection thereby revealing key mechanistic and non-mechanistic correlates.
Figure 1. Immune correlates of protection of EBOV vaccines are not universal.
Immune correlates of protection for EBOV vaccines are limited to a specific vaccine and vaccination regimen tested in each study, including: vaccine platform, viral antigen, vaccine dose, number of doses, delivery route, immunization schedule, time between the last dose and exposure to EBOV, route of exposure and EBOV challenge dose, as well as pre-existing immunity to vaccine components and host genetics.
Outstanding Questions Box.
How to best standardize parameter testing for EBOV vaccine platforms so that comparisons between vaccines and studies are more robust?
Should the role of IgG titers as an obligatory immune correlate for all EBOV vaccines be revised?
Are samples from clinical trials and survivors properly utilized by mining all possible analytical information and data sets on innate and adaptive immune responses?
Should new high throughput, cutting-edge technologies be employed in addition to the classical serological and cell-mediated response assays in an attempt to broaden testing protocols and establish comprehensive immune signatures of each vaccine?
Are immune mechanisms and the levels of correlates required to achieve protection in NHP models of EBOV infections applicable to humans in a natural exposure setting?
How durable is protection elicited by EBOV vaccines?
Will it be possible one day to define a universal correlate of protection for all EBOV vaccines?
Highlights.
Correlates of protection are crucial predictive tools for EBOV vaccine efficacy in humans.
Different EBOV vaccines elicit distinct immune profiles defined by many parameters including vaccine design and delivery.
Immunological parameters identified in EBOV vaccine studies in animal models are not always reflected in clinical trials.
Antibody and cell-mediated immune responses have different contributions to protection by different EBOV vaccines.
Qualitative features of the antibody response, in addition to magnitude, can affect protection.
Different vaccination routes can elicit qualitatively different immune responses. EBOV infection through different routes will likely require distinct vaccine-mediated protective mechanisms.
There is currently no universal correlate of protection for EBOV vaccines and it is unlikely one will be identified in the near future.
ACKNOWLEDGEMENTS
This work was supported by the NIH grant U19 AI109945-01 Project 2 Molecular Basis for Ebola Virus Immune Paralysis (A.B.) and the NIH grant 1R01AI102887-01A1 (A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jones SM et al. (2005) Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 11 (7), 786–790. [DOI] [PubMed] [Google Scholar]
- 2.Marzi A. et al. (2016) Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci Rep 6, 21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukreyev AA et al. (2010) Mucosal parainfluenza virus-vectored vaccine against Ebola virus replicates in the respiratory tract of vector-immune monkeys and is immunogenic. Virology 399 (2), 290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaney JE et al. (2013) Antibody Quality and Protection from Lethal Ebola Virus Challenge in Nonhuman Primates Immunized with Rabies Virus Based Bivalent Vaccine. PLoS pathogens 9 (5), e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan NJ et al. (2006) Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med 3 (6), e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley DA et al. (2014) Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 20 (10), 1126–9. [DOI] [PubMed] [Google Scholar]
- 7.Herbert AS et al. (2013) Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol 87 (9), 4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marzi A. et al. (2015) Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science 348 (6233), 439–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warfield KL et al. (2007) Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis 196 Suppl 2, S430–7. [DOI] [PubMed] [Google Scholar]
- 10.Martins KA et al. (2016) Ebola virus disease candidate vaccines under evaluation in clinical trials. Expert Rev Vaccines 10.1080/14760584.2016.1187566, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatraman N. et al. (2017) Vaccines against Ebola virus. Vaccine 10.1016/j.vaccine.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Henao-Restrepo AM et al. (2017) Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 389 (10068), 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henao-Restrepo AM et al. (2015) Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386 (9996), 857–66. [DOI] [PubMed] [Google Scholar]
- 14.Ledgerwood JE et al. (2017) Chimpanzee Adenovirus Vector Ebola Vaccine. N Engl J Med 376 (10), 928–938. [DOI] [PubMed] [Google Scholar]
- 15.Zhu FC et al. (2017) Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 389 (10069), 621–628. [DOI] [PubMed] [Google Scholar]
- 16.Milligan ID et al. (2016) Safety and Immunogenicity of Novel Adenovirus Type 26- and Modified Vaccinia Ankara-Vectored Ebola Vaccines: A Randomized Clinical Trial. JAMA 315 (15), 1610–23. [DOI] [PubMed] [Google Scholar]
- 17.Shukarev G. et al. (2017) A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum Vaccin Immunother 13 (2), 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huttner A. et al. (2018) Determinants of antibody persistence across doses and continents after singledose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect Dis 18 (7), 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy SB et al. (2017) Phase 2 Placebo-Controlled Trial of Two Vaccines to Prevent Ebola in Liberia. N Engl J Med 377 (15), 1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huttner A. et al. (2015) The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 15 (10), 1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regules JA et al. (2015) A Recombinant Vesicular Stomatitis Virus Ebola Vaccine - Preliminary Report. N Engl J Med Published online on April 1, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooq F. et al. (2016) Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Sci Rep 6, 27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu X. et al. (2009) Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One 4 (5), e5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzi A. et al. (2013) Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America 110 (5), 1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai L. et al. (2015) Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. JAMA 313 (12), 1249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahlke C. et al. (2017) Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine 19, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewer K. et al. (2016) A Monovalent Chimpanzee Adenovirus Ebola Vaccine Boosted with MVA. N Engl J Med 374 (17), 1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Santis O. et al. (2016) Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 16 (3), 311–20. [DOI] [PubMed] [Google Scholar]
- 29.Tapia MD et al. (2016) Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 16 (1), 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plotkin SA and Gilbert PB (2012) Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 54 (11), 1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menicucci AR et al. (2017) Transcriptomic analysis reveals a previously unknown role for CD8(+) T-cells in rVSV-EBOV mediated protection. Sci Rep 7 (1), 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan NJ et al. (2011) CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat Med 17 (9), 1128–31. [DOI] [PubMed] [Google Scholar]
- 33.Olinger GG et al. (2005) Protective cytotoxic T-cell responses induced by venezuelan equine encephalitis virus replicons expressing Ebola virus proteins. J Virol 79 (22), 14189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warfield KL et al. (2005) Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol 175 (2), 1184–91. [DOI] [PubMed] [Google Scholar]
- 35.Cooper CL et al. (2017) T-cell-dependent mechanisms promote Ebola VLP-induced antibody responses, but are dispensable for vaccine-mediated protection. Emerg Microbes Infect 6 (6), e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennemann NJ et al. (2017) Vesicular stomatitis virus pseudotyped with Ebola virus glycoprotein serves as a protective, non-infectious vaccine against Ebola virus challenge in mice. J Virol 10.1128/JVI.00479-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradfute SB et al. (2011) Filovirus vaccines. Hum Vaccin 7 (6), 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khurana S. et al. (2016) Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med 22 (12), 1439–1447. [DOI] [PubMed] [Google Scholar]
- 39.Rechtien A. et al. (2017) Systems Vaccinology Identifies an Early Innate Immune Signature as a Correlate of Antibody Responses to the Ebola Vaccine rVSV-ZEBOV. Cell Rep 20 (9), 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen SG et al. (2011) Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473 (7348), 523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen SG et al. (2009) Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15 (3), 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jahrling PB et al. (1996) Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch Virol Suppl 11, 135–40. [DOI] [PubMed] [Google Scholar]
- 43.Qiu X. et al. (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514 (7520), 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Group PIW et al. (2016) A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. N Engl J Med 375 (15), 1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wec AZ et al. (2017) Antibodies from a Human Survivor Define Sites of Vulnerability for Broad Protection against Ebolaviruses. Cell 169 (5), 878–890 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corti D. et al. (2016) Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 351 (6279), 1339–42. [DOI] [PubMed] [Google Scholar]
- 47.Howell KA et al. (2017) Cooperativity Enables Non-neutralizing Antibodies to Neutralize Ebolavirus. Cell Rep 19 (2), 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frei JC et al. (2016) Bispecific Antibody Affords Complete Post-Exposure Protection of Mice from Both Ebola (Zaire) and Sudan Viruses. Sci Rep 6, 19193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wec AZ et al. (2016) A "Trojan horse" bispecific-antibody strategy for broad protection against ebolaviruses. Science 354 (6310), 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mire CE et al. (2014) Durability of a vesicular stomatitis virus-based Marburg virus vaccine in nonhuman primates. PLoS One 9 (4), e94355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oswald WB et al. (2007) Neutralizing Antibody Fails to Impact the Course of Ebola Virus Infection in Monkeys. PLoS Pathog 3 (1), e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dias JM et al. (2011) A shared structural solution for neutralizing ebolaviruses. Nature structural & molecular biology 18 (12), 1424–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duehr J. et al. (2017) Novel Cross-Reactive Monoclonal Antibodies against Ebolavirus Glycoproteins Show Protection in a Murine Challenge Model. J Virol 91 (16), e00652–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mire CE et al. (2017) Therapeutic treatment of Marburg and Ravn virus infection in nonhuman primates with a human monoclonal antibody. Sci Transl Med 9 (384), eaai8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmaljohn AL (2013) Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts. Curr HIV Res 11 (5), 345–53. [DOI] [PubMed] [Google Scholar]
- 56.Osterholm MT et al. (2015) Transmission of Ebola viruses: what we know and what we do not know. MBio 6 (2), e00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson E. et al. (1995) Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus. Int J Exp Pathol 76 (4), 227–36. [PMC free article] [PubMed] [Google Scholar]
- 58.Jaax N. et al. (1995) Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet 346 (8991–8992), 1669–71. [DOI] [PubMed] [Google Scholar]
- 59.Weingartl HM et al. (2012) Transmission of Ebola virus from pigs to non-human primates. Sci Rep 2, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong G. et al. (2015) Ebola virus transmission in Guinea pigs. J Virol 89 (2), 1314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Twenhafel NA et al. (2013) Pathology of experimental aerosol Zaire ebolavirus infection in rhesus macaques. Vet Pathol 50 (3), 514–29. [DOI] [PubMed] [Google Scholar]
- 62.Pratt WD et al. (2010) Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol 17 (4), 572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geisbert TW et al. (2008) Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 26 (52), 6894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyer M. et al. (2015) Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J Clin Invest 125 (8), 3241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farrar JD et al. (2000) Selective loss of type I interferon-induced STAT4 activation caused by a minisatellite insertion in mouse Stat2. Nat Immunol 1 (1), 65–9. [DOI] [PubMed] [Google Scholar]
- 66.Ebihara H. et al. (2006) Molecular determinants of Ebola virus virulence in mice. PLoS Pathog 2 (7), e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldmann H. et al. (2013) Filoviridae: Marburg and Ebola Viruses. In Virology Fields (6 edn) (Knipe DM and Howley PM eds), pp. 923–956, Lippincott Williams & Wilkins. [Google Scholar]
- 68.Jones SM et al. (2007) Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis 196 Suppl 2, S404–12. [DOI] [PubMed] [Google Scholar]
- 69.Bukreyev A. et al. (2007) Successful topical respiratory tract immunization of primates against Ebola virus. J Virol 81 (12), 6379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]