Abstract
This study was aimed to evaluate the effects of near-infrared (NIR) photobiomodulation (PBM) combined with coenzyme Q10 (CoQ10) on depressive-like behavior, cerebral oxidative stress, inflammation, and apoptosis markers in mice. To induce a depressive-like model, mice were subjected to sub-chronic restraint stress for 5 consecutive days. NIR PBM (810 nm laser, 33.3 J/cm2) and/or CoQ10 (500 mg/kg/day, gavage) were administered for five days concomitantly with immobilization. Behavior was evaluated by the forced swim test (FST), tail suspension test (TST), and open field test (OFT). Mitochondrial membrane potential as well as oxidative stress, neuroinflammatory, and markers of apoptosis were evaluated in the prefrontal cortex (PFC) and hippocampus (HIP). The serum levels of pro-inflammatory cytokines, cortisol, and corticosterone were also measured. PBM or CoQ10, or the combination, ameliorated depressive-like behaviors induced by restraint stress as indicated by decreased immobility time in both the FST and TST. PBM and/or CoQ10 treatments decreased lipid peroxidation and enhanced total antioxidant capacity (TAC), GSH levels, GPx and SOD activities in both brain areas. The neuroinflammatory response in the HIP and PFC was suppressed, as indicated by decreased NF-kB, p38, and JNK levels in PBM and/or CoQ10 groups. Intrinsic apoptosis biomarkers, BAX, Bcl-2, cytochrome c release, and caspase-3 and −9, were also significantly down-regulated by both treatments. Furthermore, both treatments decreased the elevated serum levels of cortisol, corticosterone, TNF-, and IL-6 induced by restraint stress. Transcranial NIR PBM and CoQ10 therapies may be effective antidepressant strategies for the prevention of psychopathological and behavioral symptoms induced by stress.
Keywords: Photobiomodulation, Coenzyme Q10, Restraint Stress, Oxidative Stress, Neuroinflammation, Apoptosis, Cortisol
1. Introduction
Major depressive disorder (MDD) is a debilitating condition affecting mood, drive, cognitive, neurovegetative, and psychomotor functions (Fava and Kendler, 2000). In addition, feelings of guilt and frustration, anxiety states, repeated thoughts of suicide, and actual suicide attempts are very common among MDD patients.
Many studies have demonstrated that pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-03B1) are involved in the pathophysiology of MDD (Liu et al., 2012). These cytokines not only boost neuroinflammation (Kim and Maes, 2003) but also affect synaptic plasticity, neurotransmitter metabolism, and eventually mood regulation (Du et al., 2008; Villanueva, 2013). Likewise, animal studies suggest that external stressors increase the expression of nuclear factor-kB (NF-kB), IL-6, and TNF- α in the brain (Kubera et al., 2011), and consequently lead to adverse behavioral changes in rodents such as appetite loss and anhedonia (Dunn et al., 2005; Krishnan and Nestler, 2008; Maes et al., 2009). The mitogen-activated protein kinase (MAPK) signaling cascade can disrupt the glucocorticoid receptor pathway and might eventually change glucocorticoid-mediated inhibitory feedback of pro-inflammatory cytokine production (Wang et al.,2004. Among the various subgroups of MAPKs, c-Jun amino-terminal kinases (JNK) and p38 are the most responsive to stress stimuli (Pearson et al., 2001; Roux and Blenis, 2004). In addition, increased inflammatory cytokines are associated with a compensatory response of the hypothalamic-pituitary-adrenal (HPA) axis, and with corticosterone secretion from the adrenal glands (Gądek-Michalska et al., 2013; Gold et al., 1995).
Furthermore, accumulating evidence suggests that oxidative stress plays a crucial role in the pathology of stress-related disorders such as MDD (Pandya et al., 2013). Clinical studies evaluating indices of oxidative stress in MDD patients have shown deficits in blood levels of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), and increased levels of a marker of lipid peroxidation, malondialdehyde (MDA) (Bilici et al., 2001; Khanzode et al., 2003; Ozcan et al., 2004). Persistent oxidative stress can potentially activate a cascade of events resulting in neurodegeneration caused by an apoptotic process (Beckman and Ames, 1998). In this respect, gene expression profiling of brain tissue samples from the prefrontal cortex (PFC) area has shown that MDD is characterized by increased apoptosis (Shelton et al., 2011). In animals, restraint stress also induces depression-like behaviors via the BAX/Bcl-2 apoptotic pathway in the PFC and hippocampus (HIP) (Wang et al., 2016). Given the high societal burden of MDD and given that a substantial proportion of depressed patients dislike current antidepressant intervention, researchers have been attempting to find effective and safe complementary-alternative strategies to treat depression, without the side effects produced by anti-depressant medications.
Recently, transcranial photobiomodulation (PBM) therapy has been suggested as an innovative, promising treatment modality for a wide range of neurological and psychological disorders (Salehpour et al., 2018c). Transcranial PBM involves exposing the head/scalp to laser or light emitting diode (LED) light in the red to near-infrared (NIR) regions (600 to 1200 nm), for stimulation of neuronal processes in the brain (Chung et al., 2012; Morries et al., 2015). The mitochondrial electron transfer chain -primarily the cytochrome c oxidase (COX) enzyme- is the most likely site for the primary absorption of red (630–670 nm) and NIR (800–880 nm) photons (Karu and Kolyakov, 2005); COX excitation increases the synthesis of neuronal ATP and improves bioenergetic function (de Freitas and Hamblin, 2016; Mintzopoulos et al., 2017). Low levels of light at these wavelengths modulates the release of nitric oxide (NO) (Uozumi et al., 2010) and increases both cerebral oxygenation (Wang et al., 2017) and blood flow (Salgado et al., 2015). Besides these effects, transcranial PBM can stimulate neurogenesis (Tanaka et al., 2011) and provide neuroprotection via antioxidant, anti-neuroinflammatory, and anti-apoptotic activities (De Taboada et al., 2011; Hamblin, 2017; Lu et al., 2017; Salehpour et al., 2017; Salehpour et al., 2018a). In particular, it has been demonstrated that transcranial PBM is able to increase regional cerebral blood flow in MDD patients (Schiffer et al., 2009). Furthermore, the potential antidepressant effect of transcranial NIR PBM in animal models of depression and anxiety has recently been addressed (Salehpour and Rasta, 2017).
Coenzyme Q10 (CoQ10) also known as ubiquinone, is an essential cofactor for the activity of complexes I-III of the mitochondrial electron transfer chain acting either as a donor or acceptor of electrons(Dallner and Sindelar, 2000). CoQ10 is found in its highest concentrations in tissues with high energy consumption such as the brain (Flint Beal, 2002). CoQ10 plays a pivotal role in several cellular processes such as regulation of cellular metabolism and redox state, H2O2 formation, bioenergetic functions, and gene regulation (Crane, 2001). CoQ10 supplementation improves neuronal mitochondrial function by increasing ATP synthesis (Hargreaves, 2014). Moreover, its neuroprotective properties have been demonstrated in multiple neuropsychiatric disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and depression (Morris et al., 2013). In neuronal cells, the powerful antioxidant action of CoQ10 is credited to its electron donating properties that tends to neutralize free radicals and preserve mitochondria and lipid membranes (Somayajulu et al., 2005). CoQ10 has also been shown to protect cultured hippocampal neurons against oxidative stress (Won et al., 2011). Moreover, chronic CoQ10 administration attenuates both hippocampal oxidative stress (MDA and nitrite) and pro-inflammatory (TNF-α) markers in a rat model of AD (Singh and Kumar, 2015). The antidepressant-like properties of CoQ10 have also been shown in a chronic stress model of depression (Aboul-Fotouh, 2013).
The current study explores the effects of transcranial NIR PBM combined with CoQ10 on depression-like behaviors in a mouse restraint stress model, as well as the putative, underlying neurobiological mechanisms. In particular, we examined the possible impacts of NIR light and CoQ10 supplementation on oxidative, neuroinflammatory, and apoptotic markers in the brain areas associated with depression, the PFC and HIP.
2. Materials and methods
2.1. Animals and experimental design
Seventy-five adult male BALB/c mice, 8–10-weeks-old, weighing 30–32 g, were provided by the animal house of Tabriz University of Medical Sciences, Iran. All experimental procedures were carried out under the guidelines of the National Institutes of Health (NIH; Publication No. 85–23, revised 1985) and approved by the Higher Academic Education Institute of Rab-Rashid ethics committee (protocol number: 97/0637/14). Mice were socially housed in standard cages (5 in each cage), kept under controlled conditions at 25±1°C on a 12/12 hour light/dark cycle, and fed standard pellet food with tap water ad libitum. After a week of acclimatization, animals were randomly divided into five groups (n=15): (I) control, (II) restraint stress + sham-PBM + normal saline gavage (RS), (III) restraint stress + real-PBM + normal saline gavage (RS+PBM), (IV) restraint stress + sham-PBM + CoQ10 gavage (RS+CoQ10), and (V) restraint stress + real-PBM + CoQ10 gavage (RS+PBM+CoQ10) groups.
2.2. Sub-chronic restraint stress
Stress groups underwent sub-chronic restraint stress for 150 min/day for 5 consecutive days. Mice were placed in a horizontal position inside a well-ventilated (with 12 holes) 50 ml falcon tube. The mice in the control group were kept in the animal room and only subjected to daily handling stress (10 min.) for 5 days (Das et al., 2000).
2.3. PBM & CoQ10 therapies
Transcranial PBM therapy was performed with a NIR GaAlAs laser (Thor Photomedicine, Chesham, UK) at 810 nm wavelength, 200 mW maximum output power, a beam area of 0.03 cm2 with 6.66 W/cm2 irradiance. The laser operated at 10-Hz pulsed wave frequency on a 88% duty cycle. Each mouse was held firmly and the tip of the laser fiber was placed over the midline of the dorsal surface of the head in the region between the eyes and ears. Mice were treated once a day (at 11:00 am) for a duration of 5 seconds to provide a dose of 33.3 J/cm2 (total energy of 1 J) at scalp level (Salehpour, et al., 2018a). The CoQ10 was purchased from Sigma-Aldrich (USA). Mice in CoQ10 group received the treatment (500 mg/kg/day) via gavage (Schilling et al., 2001). Mice in the RS+PBM+CoQ10 group received the same daily dose of CoQ10 and were treated with PBM one hour before the gavage. Both PBM and/or CoQ10 treatments were applied once a day (two hours after the end of immobilization) for 5 consecutive days. The RS group received oral normal saline (in a volume of 8 ml/kg body weight) and underwent identical NIR PBM therapy procedures except that the laser device was kept off.
2.4. Behavioral Tests
2.4.1. Open field test
The open field test (OFT) procedure was similar to what already described by our group (Salehpour et al., 2018b). Mice were gently placed individually in the center of the open field arena (33×33×33 cm). The activity of mice during the 5 min of the test was videotaped and behavioral parameters were extracted using a video tracking program Etho Vision™ (Noldus, The Netherlands) (Salehpour, et al., 2017). After each trial, the apparatus was cleaned with a 70% ethanol solution in order to remove any scent clues. The total distance moved (cm) was the parameter of interest.
2.4.2. Forced swim test
The forced swim test (FST) procedure was similar to what previously described by Porsolt et al. (Porsolt et al., 1977). Briefly, each mouse was individually forced to swim in a vertical transparent cylinder (diameter 14 cm, height 20 cm), containing tap water at 25 ± 2 °C and 10 cm in depth. The test duration was 6 min. Periods of immobility ware digitally recorded during the last 4 min and then analyzed by the Etho Vision™ (Noldus, The Netherlands) video tracking software (Juszczak et al., 2008).
2.4.3. Tail suspension test
The tail suspension test (TST) is a behavioral model and one of the most frequently used tasks to evaluate depression-like behavior in mice (Mahmoudi et al., 2015). The apparatus used in the present work consisted of a wooden box (60 ×30 ×40 cm) with a metal hook positioned at the center of the top panel. Each mouse was suspended about 40 cm above the floor by means of adhesive tape affixed to the tail. The immobility time during the last 4 min of the 6-min test was digitally recorded and scored using a video tracking program Etho Vision™ (Noldus, The Netherlands) (Juszczak et al., 2006).
2.5. Biochemical assessment
2.5.1. Sampling
For biochemical analysis, mice were deeply anesthetized with ketamine/xylazine (90/10 mg/kg, intraperitoneal, respectively) and blood samples were collected from the heart and centrifuged at 1500 × g for 10 min at 4 °C to obtain serum. Then, the animals were sacrificed and the brain tissues were removed from the skull. Then, PFC and HIP were rapidly isolated on an ice-cold platform and stored at −70 °C.
2.5.2. Mitochondrial isolation
The PFC and HIP tissue samples were homogenized in ice-cold isolation buffer with a tissue homogenizer and immediately centrifuged at 2000 g for 3 min at 4°C. The supernatant was centrifuged again at 2000 g for 3 min at 4°C, and the second supernatant was transferred into a new tube and then centrifuged once more at 12,000 g for 10 min at 4°C. The supernatant was removed, and the precipitated pellet was resuspended in isolation buffer (Salehpour, et al., 2017). Subsequently, the suspension was centrifuged at 12,000 g for 10 min. The obtained dark brown mitochondrial pellets were then suspended in storage buffer (Salehpour, et al., 2017). Both resulting cytosolic and mitochondrial fractions were kept at −20 °C until future assessment.
2.5.3. Antioxidant and lipid peroxidation assays
To determine oxidative stress status, PFC and HIP samples were homogenized in 1.15 % KCl solution and subsequently centrifuged at 112 × g for 10 min at 4 °C to obtain the supernatant (Pourmemar et al., 2017).
The MDA levels, an index of lipid peroxidation, were measured using the thiobarbituric acid reactive substances (TBARS) assay (Farajpour et al., 2017; Khorrami et al., 2014).
The activity of GPx in the PFC and HIP was assessed according to the method of Paglia and Valentine using a spectrophotometric method by RANSEL (RANDOX Laboratories Ltd., UK) diagnostic kit. Results were expressed as units (U) per mg protein.
SOD activity was determined using a RANSOD kit (Randox Laboratories Ltd, Crumlin, United Kingdom) based on the manufacturer’s instructions and the absorbance was measured at 03BB=505 nm at 37 °C (Pourmemar, et al., 2017) and results were expressed as U per mg protein.
Total antioxidant capacity (TAC) in the PFC and HIP homogenates was measured colorimetrically according to the assay kit protocol (Randox Laboratories Ltd, Crumlin, United Kingdom) and the results were expressed as nmol/l.
2.5.4. Reduced glutathione (GSH) levels
Reduced GSH levels in the HIP and PFC lysates were also measured using the method of Ellman with 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) (Ellman, 1959). Briefly, 100μl. of brain homogenates were mixed with 12.5% trichloroacetic acid, vortexed for 10 min and centrifuged at 900 × g for 15 min to remove protein. Then, 0.1 ml of the acquired supernatant, 2 ml of phosphate buffer, 0.5 ml of DNTB, and 0.4 ml of double distilled water was added and mixed. Absorbance of the yielding yellow color was determined using spectrophotometry at 412 nm within 10 min with a microplate reader and expressed as μmol/mg protein.
2.5.5. Western Blotting
Cytosolic and mitochondrial protein expressions were assessed by Western blot method as previously described (Sadigh-Eteghad et al., 2015). Briefly, PFC and HIP frozen tissue samples were lysed and homogenized in 100 μl RIPA lysis buffer supplemented with a protease inhibitor cocktail. The homogenate was centrifuged at 12000 × g for 15 min at 4 °C. Total protein concentration in the supernatant was assessed by the Bradford assay method using Bio-Rad reagent. Equal amount of protein samples (20 μg) were separated using 12.5% SDS-polyacrylamide gel electrophoresis and transferred onto a polyvinylidenedifluoride (PVDF) membrane (Roche, UK). In order to block non-specific binding reactions, membranes were incubated with blocking solution consisting of bovine serum albumin (BSA) 3% in Tris-buffered saline (pH 7.5) for 2 h with rotation at room temperature. Subsequently, the membranes were incubated overnight with rabbit primary antibodies against anti-BAX (sc-70405), anti-Bcl-2 (sc-7382), anti-caspase-3 (sc-136219), anti-caspase-9 (sc-81663), anti-cytochrome c (sc-13156), anti-VDAC (sc-390996), anti-β-actin (sc-47778), anti-JNK (sc-7345), anti-p-JNK (sc-6254), anti-p38 (sc-535), anti-p-p38 (sc-17852), all purchased from Santa Cruz, and anti-NF-kB (ab16502), obtained from Abcam, in 1:500 concentration. After washing three times with PBS, the membrane was incubated with horseradish peroxidase-conjugated (HRP) goat anti-rabbit IgG secondary antibody for 2 h at room temperature. Finally, an enhanced chemiluminescence (ECL) detection kit (Pierce, Rockford, IL) was used to visualize the protein bands (Amersham, UK). Anti β-Actin and anti-VDAC antibodies were used for internal control of cytosolic and mitochondrial proteins, respectively. Images of the protein bands were acquired and the relative optical density of each band was quantified using Image J 1.62 software.
2.5.6. Serum pro-inflammatory cytokines and neuroendocrine hormones assessments
The enzyme-linked immunosorbent assay (ELISA) method was used for measurement of serum concentrations of IL-6, TNF-α, corticosterone, and cortisol, based on the manufacturers’ protocols.
2.5.7. Mitochondrial membrane potential assay
To determine mitochondrial membrane potential (MMP), samples were incubated with 2.5 μg/ml JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolyl-carbocyanine iodide) fluorescent probe (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 15 min. Fluorimetry was used to measure fluorescence intensity of the samples. The MMP was calculated by the ratio of red (aggregated) (λex=490 nm, λem=590 nm) to green (monomer) (λex=488 nm, λem=530 nm) fluorescence intensity in the mitochondrial suspension and normalized to mg protein of the samples and presented as percentage of control.
2.6. Statistical analysis
Comparisons between experimental and control groups were performed by one-way ANOVA followed by the Tukey post hoc test. All analyses were conducted using Graph Pad Prism 6.01 (Graph Pad Software Inc., La Jolla, CA, USA). A p-value <0.05 was considered statistically significant and descriptive data were expressed as mean ± SEM.
3. Results
3.1. Behavioral assessments
3.1.1. Open field test
The results of OFT test showed no significant differences for total traveled distance between groups (Fig. 1A).
Fig 1:
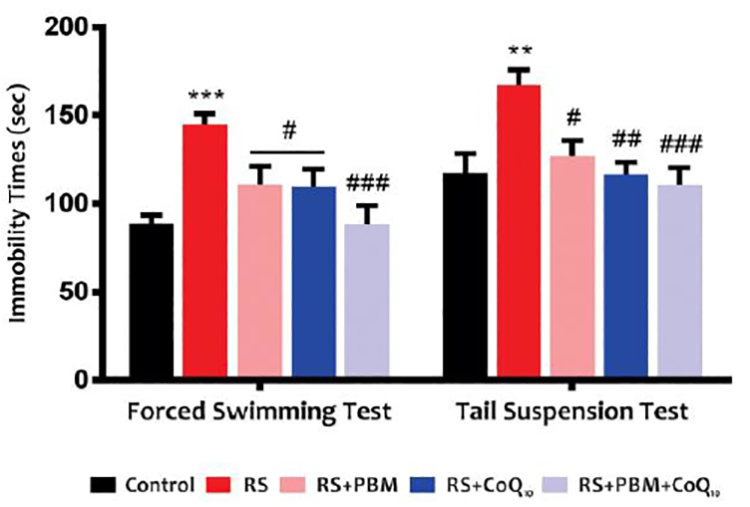
A) Effects of PBM and/or CoQ10 therapies on the traveled distance in OFT and B) immobility time in the FST (left) and TST (right) after the restraint stress paradigm. n = 13–15. Data represent mean ± S.E.M. **p < 0.01, ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. RS group. (CoQ10, coenzyme Q10; FST, forced swimming test; OFT, open field test; PBM, photobiomodulation; RS, restraint stress; TST, tail suspension test).
3.1.2. TST and FST
The results of the TST and FST are shown in Figure 1B. Restraint stress induced a marked increase in immobility time during the TST (Fig. 1B, right) and FST (Fig. 1B, left) (p<0.05, p<0.01, respectively). However, PBM alone or CoQ10 alone significantly decreased the immobility time in FST (for both treatments: p<0.05) compared to the stress group. In addition, the combination therapy completely reversed the increase in immobility time in FST (p<0.001) induced by restraint stress. For TST, both PBM and CoQ10 treatments, alone or in combination, alleviated the restraint stress-induced increase in immobility time (PBM: p<0.05; CoQ10: p<0.01; PBM+Co10™: p<0.001).
3.2. Biochemical assessments
3.2.1. Brain MDA levels
As shown in Fig. 2A, sub-chronic restraint stress significantly increased MDA levels in the PFC (p<0.001) and HIP (p<0.01). However, MDA levels in the PFC and HIP were significantly decreased following PBM (for both areas: p<0.05), CoQ10 (p<0.01 and p<0.05, respectively), and combination treatments (for both areas: p<0.01).
Fig 2:
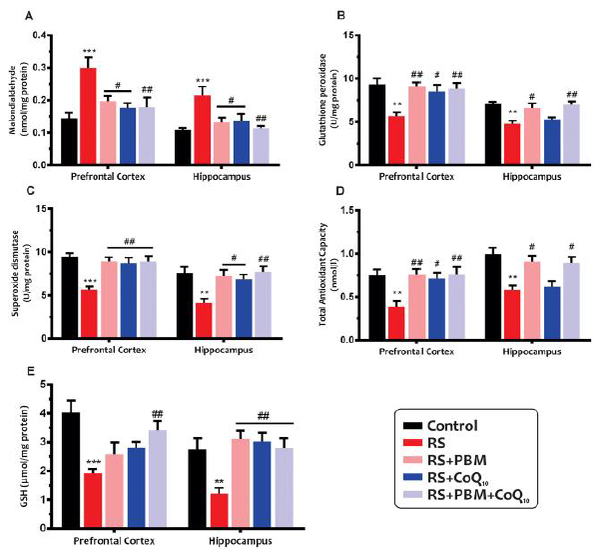
Effects of PBM and/or CoQ10 therapies on the A) MDA, B) GPx, C) SOD, D) TAC, and E) GSH levels in the PFC and HIP areas after the restraint stress paradigm. n = 6. Data represent mean ± S.E.M. **p < 0.01, ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. RS group. (CoQ10, coenzyme Q10; GPx, glutathione peroxidase; GSH, glutathione; HIP, hippocampus; MDA, malondialdehyde; PBM, photobiomodulation; PFC, prefrontal cortex; RS, restraint stress; SOD, superoxide dismutase; TAC, total antioxidant capacity).
3.2.2. Brain antioxidant enzyme activities
Our results (Fig. 2B) showed that the stress group exhibited a decline in GPx activity in the PFC and HIP (for both areas: p<0.01). The GPx activity in the PFC was markedly elevated by five days of PBM (p<0.01), CoQ10 (p<0.05), and the combination (p<0.01) compared to the untreated stress group. Although hippocampal GPx activity in the PBM alone (p<0.05) and combination (p<0.01) treatment groups were similar to those in the control group, CoQ10 alone treatment did not exhibit a significant improvement in this index (p>0.05).
As shown in Fig. 2C, following sub-chronic restraint stress, a significant decrease in SOD enzyme activity was also observed in the PFC and HIP of animals when compared with control animals (for both areas: p<0.01). The SOD activity in the PFC was remarkably elevated by five days of PBM (p<0.01), CoQ10 (p<0.05), and combination treatment (p<0.01) compared to untreated stress group. Although both PBM alone (p<0.05) and combination (p<0.01) treatment significantly increased SOD activity in the HIP, CoQ10 alone treatment did not exhibit improvement (p>0.05).
3.2.3. Brain TAC levels
As shown in Fig. 2D, the TAC levels were decreased in the PFC and HIP of mice subjected to restraint stress as compared to the control mice (for both areas: p<0.01). The TAC levels in the PFC was noticeably increased by PBM (p<0.01), CoQ10 (p<0.05), and combination treatment (p<0.01) compared to untreated stress group. Although PBM alone and combination treatment significantly increased TAC levels in the HIP (for both treatments: p<0.05), this increase was not significant for the CoQ10 alone group (p>0.05).
3.2.4. Brain GSH levels
As shown in Fig. 2E, restraint stress significantly increased GSH levels in the PFC (p<0.001) and HIP (p<0.01) samples when compared to the control animals. Nevertheless, GSH levels were significantly increased in the PFC following combination treatment (p<0.01). In addition, both PBM and CoQ10 treatments, alone or in combination, markedly increased GSH levels in the HIP (for all treatments, p <0.01).
3.2.5. Brain NK-Kb, p38, and JNK levels
Figure 3A shows that the restraint stress significantly up-regulated NF-kB protein levels in the PFC (p<0.001) and HIP (p<0.01). Although NF-kB levels in the PFC in the PBM alone treatment group were significantly decreased (p<0.05), CoQ10 alone and combination treatment did not exhibit any significant change compared to the stress animals (for both treatments: p>0.05). Moreover, data from the HIP area showed a significant decrease in NF-kB levels in the PBM and CoQ10 alone (for both treatments: p<0.01) and combination (p<0.001) treatments groups.
Fig 3:
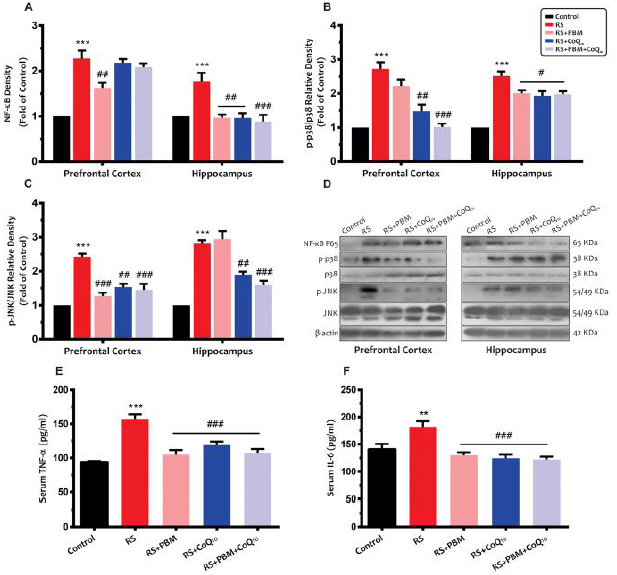
Effects of PBM and/or CoQ10 therapies on the A) NF-kB, B) p38, and C) JNK protein expressions in the PFC and HIP areas after the restraint stress paradigm. D) Representative images of corresponding protein levels detected by Western blot (n = 3). Serum E) TNF-α and F) IL-6 levels in different experimental groups (n = 8). Data represent mean ± S.E.M. **p < 0.01, ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. RS group. (CoQ10, coenzyme Q10; HIP, hippocampus; PBM, photobiomodulation; PFC, prefrontal cortex; RS, restraint stress).
As shown in Fig. 3B, a significant increase in p38 protein expression was observed in the PFC and HIP of the stress group when compared to the control animals (for both areas: p<0.001). The CoQ10 alone (p<0.01) and combination (p<0.001) treatment noticeably restored p38 levels in the PFC, whereas no significant difference was observed between the PBM and stress groups (p>0.05). In addition, both PBM and CoQ10 treatments, alone or in combination, significantly reduced p38 levels in the HIP (for all treatments, p<0.05).
As shown in Fig. 3C, sub-chronic restraint stress caused a significant increase in JNK levels in the PFC and HIP (for both areas: p<0.001). On the other hand, JNK levels were significantly reduced in the PFC following PBM alone, CoQ10 alone, and combination treatments (p<0.001, p<0.01, and p<0.001, respectively). Although hippocampal JNK levels were significantly decreased following CoQ10 alone (p<0.01) and combination treatment (p<0.001), PBM alone treatment did not change JNK levels compared to the stress group (p>0.05).
3.2.6. Serum TNF-α and IL-6 levels
Our results (Fig. 3E) showed that sub-chronic restraint stress resulted in a significant increase in serum TNF-α levels (p<0.001). However, all treatments significantly returned TNF-α levels to the control values (for all treatments: p<0.001).
As shown in Fig. 3F, restraint stress group also exhibited a significant increase in serum IL-6 levels as compared to the control animals (p<0.01). On the other hand, serum IL-6 levels were significantly reduced following 5 days of PBM alone, CoQ10 alone, and combination treatments (for all treatments: p<0.001).
3.2.7. Brain MMP levels
As shown in Fig. 4, significant decreases were observed in MMP levels in the PFC and HIP of stress group when compared to the control group (for both areas: p<0.001). However, for both brain areas, PBM and CoQ10 treatments, alone (for both treatments: p<0.05) or in combination (p<0.01), remarkably increased MMP levels.
Fig 4:
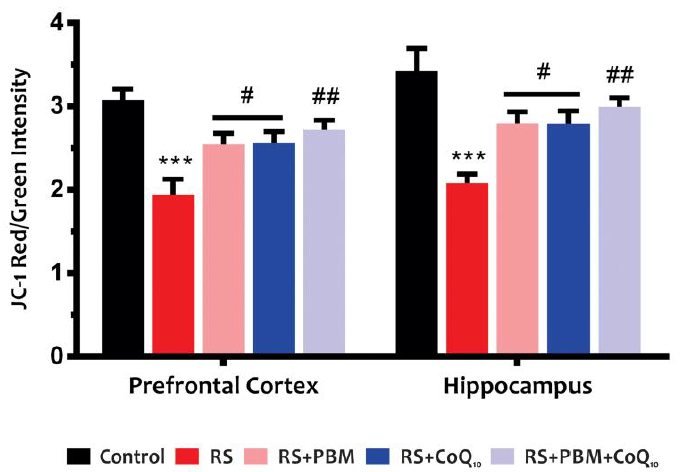
Effects of PBM and/or CoQ10 therapies on the JC-1, as a mitochondrial membrane potential index, in the PFC and HIP areas. n = 6. Data represent mean ± S.E.M. ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01 vs. RS group. (CoQ10, coenzyme Q10; HIP, hippocampus; PBM, photobiomodulation; PFC, prefrontal cortex; RS, restraint stress).
3.2.8. BAX/Bcl-2 ratio
Our results (Fig. 5A) also showed that sub-chronic restraint stress considerably increased the BAX/Bcl-2 ratio in the PFC and HIP (for both areas: p<0.001). Data from the HIP showed a significant decrease in the BAX/Bcl-2 ratio in the PBM (p<0.001) and CoQ10 (p<0.01) alone and combination (p<0.01) treatment groups. Although BAX/Bcl-2 ratio in the HIP was partially reversed following PBM or CoQ10 alone treatments (for both treatments: p<0.05), this decrease was much more pronounced in the combination treatment group (p<0.01).
Fig 5:
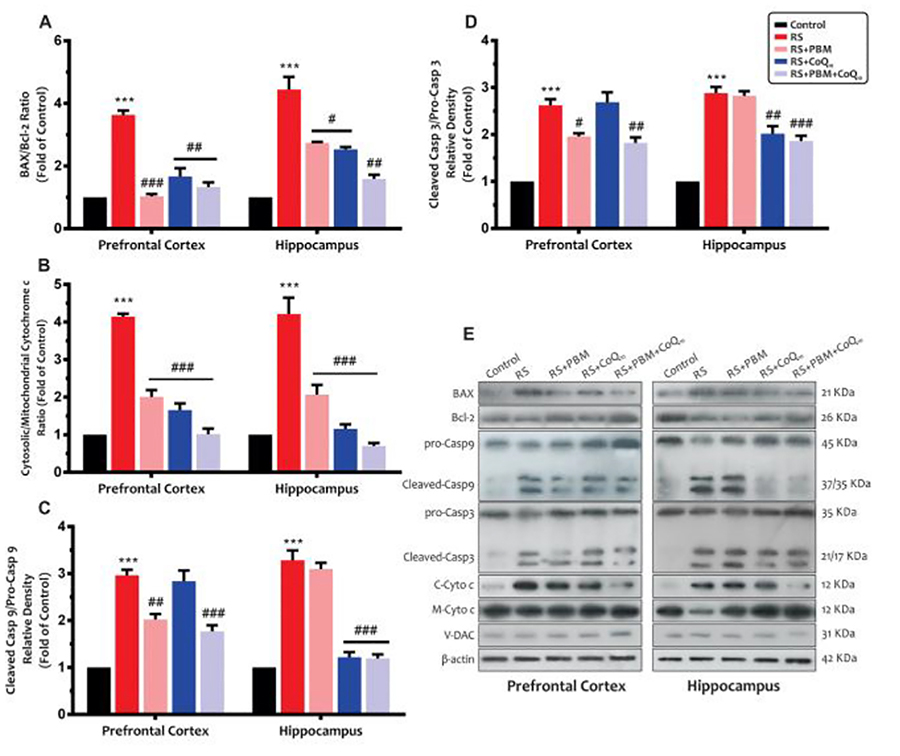
Effects of PBM and/or CoQ10 therapies on the A) BAX/Bcl-2 ratio, B) cytosolic/mitochondrial cytochrome c ratio, C) caspase-9, and D) caspase-3 expressions in the PFC and HIP areas after the restraint stress paradigm. E) Representative images of corresponding protein levels detected by Western blot. The β-Actin, VDAC, and Cyt. c were used as cytosolic and mitochondrial internal controls, respectively. n = 3. Data represent mean ± S.E.M. ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs. RS group. (CoQ10, coenzyme Q10; HIP, hippocampus; PBM, photobiomodulation; PFC, prefrontal cortex; RS, restraint stress).
3.2.9. Cytosolic/mitochondrial cytochrome c ratio
Figure 5B shows that the restraint stress significantly increased the cytosolic/mitochondrial cytochrome c ratio in the PFC and HIP (for both areas: p<0.001), which indicates higher release of cytochrome c from the membrane in this group. On the other hand, all treatments significantly decreased the cytosolic/mitochondrial cytochrome c ratio to control values (for both areas and all treatments: p<0.001).
3.2.10. Caspase-9 and caspase-3 levels
Our results (Fig. 5C) also showed that sub-chronic restraint stress resulted in a significant increase in caspase-9 levels in the PFC and HIP (for both areas: p<0.001). The PBM alone (p<0.01) and combination (p<0.001) treatment partially restored caspase-9 levels in the PFC, whereas no significant difference was observed between the CoQ10 and stress groups (p>0.05). In addition, data from the HIP area showed that, although caspase-9 levels significantly decreased following CoQ10 alone and combination treatment (for both treatments: p<0.001), PBM alone treatment did not change the levels compared to the stress group (p>0.05).
As shown in Fig. 5D, restraint stress animals also exhibited a significant increase in caspase-3 levels in the PFC and HIP when compared to the control animals (for both areas: p<0.001). Although caspase-3 levels in the PFC of PBM alone (p<0.05) and combination (p<0.01) treatment groups were significantly decreased, CoQ10 alone treatment did not exhibit any significant improvement in this index (p>0.05). Moreover, caspase-3 levels in the HIP were somewhat decreased in CoQ10 alone (p<0.01) and combination treatment (p<0.001) groups. However, PBM alone treatment did not show any improvement compared to the restraint stress group (p>0.05).
3.2.11. Serum corticosterone and cortisol levels
Sub-chronic restraint stress caused a significant increase in serum cortisol (Fig. 6A) and corticosterone (Fig. 6B) levels (for both indexes: p<0.001). On the other hand, all treatments significantly restored cortisol and corticosterone levels to the control values (for both indexes and all treatments: p<0.001).
Fig 6:
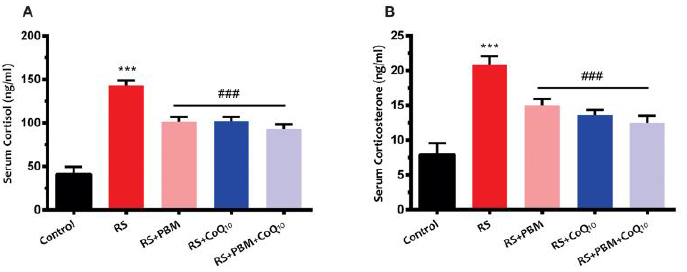
Effects of PBM and/or CoQ10 therapies on the serum A) cortisol and B) corticosterone levels after the restraint stress paradigm. n = 8. Data represent mean ± S.E.M. ***p < 0.001 vs. control group. ###p < 0.001 vs. RS group. (CoQ10, coenzyme Q10; PBM, photobiomodulation; RS, restraint stress).
4. Discussion
A large body of evidence indicates that stress plays a decisive role in the onset of many neuropsychiatric disorders, including major depression (Manji et al., 2001). Restraint stress in mice has been proposed as a model of depression (induced by immobilization), which includes both physical and mental aspects of stress. Restraint stress likely causes emotional disturbances, as evidenced by depressive behaviors and by changes in locomotion (Buynitsky and Mostofsky, 2009). As a result of immobilization, the incidence of some neurochemical alterations in the brain can lead to neurobehavioral deficits such as immobility in the FST and TST (Wang, et al., 2016; Xu et al., 2017). The latter behavioral tests are widely employed to screen the efficiency of antidepressant drugs (Bettio et al., 2014; Cryan et al., 2005; Porsolt, et al., 1977). In agreement with other reports (Bettio, et al., 2014; Freitas et al., 2014), five days of sub-chronic restraint stress in our study resulted in an increase in the immobility time as compared to the control animals. Recently, transcranial NIR PBM therapy has been suggested as a non-invasive neurostimulation modality in the central nervous system and is emerging as a novel promising treatment to defeat depression (Caldieraro et al., 2018; Cassano et al., 2015; Cassano et al., 2016; Salehpour and Rasta, 2017). Previous reports from our laboratory and others have shown that repeated delivery of NIR laser therapy produces an antidepressant-like effect in the FST (Mohammed, 2016; Salehpour et al., 2016; Wu et al., 2012; Xu, et al., 2017) and TST (Xu, et al., 2017). Nevertheless, in the current study, we showed that transcranial NIR PBM therapy for 5 days produced a notable antidepressant-like effect, as indicated by shorter immobility time in the FST and TST. Moreover, sub-chronic administration of CoQ10 (500 mg/kg/day), alone or in combination with PBM therapy markedly inhibited the prolonged immobility time. In agreement with our results, Aboul-Fotouh found that intraperitoneal administration of 100 or 150 mg/kg/day CoQ10 for three-weeks significantly reduced restraint stress-induced immobility in rats (Aboul-Fotouh, 2013).
The exposure to restraint stress and the subsequent increase in glucocorticoids levels impair neuronal function in the HIP and PFC areas (Lowy et al., 1995; Musazzi et al., 2010). In this respect, due to high levels of glucocorticoid receptors, the HIP is particularly sensitive to stressful conditions, and hippocampal dysfunction has been reported to be deeply involved in the pathophysiology of MDD (McEwen, 1999). In addition, the HIP is connected to the PFC and amygdala, which their dysfunctions potentially are associated with depressive disorders (Goshen et al., 2008; Shelton, et al., 2011). Given these connections, the HIP dysfunction, induced by the restraint stress, could result in abnormal or decreased neuroplasticity in the corticolimbic networks (Godsil et al., 2013). Precisely to assess the impact on corticolimbic networks, we evaluated the biochemical changes in the HIP and PFC areas, in addition to the neurobehavioral assessments. Our goal was also to explore the mechanisms underlying the neuroprotective and anti-depressant effects of NIR PBM and CoQ10 therapies.
From a neurobiological perspective, there is evidence that the restraint stress provokes depression-like behavior through activation of oxidative stress, neuroinflammatory, and apoptotic pathways (Freitas, et al., 2014; Kubera, et al., 2011). Stressful events trigger an imbalance in oxidant/antioxidant levels in the brain and it is well established that increased production of free radical is implicated in the pathogenesis of MDD (Bilici, et al., 2001). Indeed, oxidative stress can damage neuronal components including lipids, proteins, and nucleic acids, affecting several neuronal functions, which in turn can result in psychopathological conditions (Moretti et al., 2012; Wang and Michaelis, 2010). It is believed that such effects induced by external stress are in part mediated through an imbalance between pro-oxidant (MDA content) and antioxidant (i.e., TAC and levels of antioxidant enzymes including SOD, and GPx) parameters in the PFC (Li et al., 2016) and HIP (Fontella et al., 2005). Our results showed that overall, transcranial NIR PBM therapy mitigated oxidative damage-induced by restraint stress as evidenced by alterations in the levels of brain oxidative stress parameters including MDA, TAC, SOD, and GPx, as well as GSH levels. This result is consistent with previous studies on acute (Dong et al., 2015; Salehpour, et al., 2018a) and chronic (Lu, et al., 2017) 810-nm transcranial PBM therapy showing antioxidant effects in the HIP of mice with cognitive impairment. In addition, with respect to CoQ10, it is believed that its effects hinges on its electron donating ability, which neutralizes free radicals and preserves mitochondrial activity as well as the integrity of the lipid membranes (Somayajulu, et al., 2005). CoQ10 administration has been demonstrated to protect hippocampal cultured neurons against oxidative stress (Won, et al., 2011). Moreover, a protective effect of CoQ10 against hippocampal oxidative stress and lipid peroxidation has been shown in a rat restraint stress depression model (Aboul-Fotouh, 2013). In accordance with these findings, in the present study CoQ10 supplementation ‒alone or in combination with PBM therapy‒ markedly increased reduced GSH levels in the PFC and HIP, the major endogenous antioxidant, and increased cerebral antioxidant capacity.
It is accepted that oxidative stress accompanies inflammatory reactions; both neuroinflammation and its associated oxidative stress are considered important determinants of MDD (Maes, et al., 2009; Zhang et al., 2015). As a result of free radical production and oxidative damage, the inhibitor of NF-κB is degraded, and active NF-κB undergoes translocation into the nucleus, which in turn triggers expression of further pro-inflammatory cytokines (Gloire et al., 2006). An excessive inflammatory response causes damage to endothelial cells and microglia undergoes activation that can be detrimental to HIP and PFC function. Due to the high expression of pro-inflammatory cytokine receptors in the HIP, this limbic structure is more vulnerable to pro-inflammatory mediators (Rothwell and Hopkins, 1995). Previous studies showed that restraint stress up-regulates NF-κB in the cortex (Madrigal et al., 2001) and increases JNK and p38MAPK in the PFC and HIP (Liu et al., 2004). Likewise, reports have demonstrated that depression-like behavior induced by immobilization stress is accompanied by increased blood levels of TNF-α and IL-6 (Kubera, et al., 2011; Takaki et al., 1994). In the present study, we observed that serum levels of TNF-α and IL-6 were markedly increased after restraint stress, while a 5-day application of NIR laser effectively attenuated the elevation of these pro-inflammatory cytokines induced by immobilization. However, data from Moreira et al. (Moreira et al., 2009) in a model of rat traumatic brain injury (TBI) showed increased blood TNF-α and IL-6 levels at 24 hour post-780 nm laser irradiation. It could be speculated that the beneficial anti-inflammatory effects of PBM on the blood cytokines might be time-dependent. Moreover, in our study, PBM suppressed restraint stress-induced activation of microglia and secretion of pro-inflammatory signaling mediators, namely NF-kB, JNK, and p38, in the HIP and PFC areas. Pre-clinical investigations also demonstrated that transcranial PBM exhibited strong anti-neuroinflammatory actions via inhibition of NF-kB signaling pathways in stroke models (Lee et al., 2017; Lee et al., 2016). Furthermore, in agreement with our results, these studies reported that PBM therapy diminished neuroinflammation in the ischemic cortex of mice via down-regulation of the expression of JNK and p38 (Lee, et al., 2017; Lee, et al., 2016). With regard to CoQ10, our results also showed an anti-inflammatory effect produced by this agent, which is in line with previous reports showing that CoQ10 suppressed neuroinflammation in Parkinsonian mice via decrease of IL-6, TNF-α, and NF-kB expressions (Ebadi et al., 2004; Sharma et al., 2006).
Furthermore, increased pro-inflammatory cytokine expression following stress stimuli has been linked with the activation of the HPA axis, which can stimulate the secretion of corticosterone from the adrenal glands (Gądek-Michalska, et al., 2013). Evidence has also shown that MDD is characterized by hyperactivity of the HPA axis accompanied by elevated serum cortisol levels (Stokes, 1995). In the current study, we demonstrated that restraint stress provoked HPA axis dysfunction, as reflected in the markedly elevated serum levels of corticosterone and cortisol, and that PBM and/or CoQ10 reversed these alterations. Taken together, we propose that the depression-like behavioral alterations in the stressed animals were partly dependent on the induction of cerebral pro-inflammatory cytokines, mediated by oxidative stress, and that inhibition of these inflammatory processes by PBM and/or CoQ10 and the consequent attenuation of the hyperactivity of the HPA axis could ameliorate the depressive symptoms.
NF-kB is an important transcriptional regulator of apoptosis in neuronal cells (Panet et al., 2001), and is responsible for elevated levels of the pro-inflammatory cytokine, TNF-α, which is also considered an important factor for promotion of proapoptotic signals (Mogi et al., 2000). In addition, it is well established that the mitochondrial apoptotic pathway is linked to neuropsychological stress and depression, and boosting of mitochondrial function has been suggested as a strategy for treating MDD (Shelton, et al., 2011). In response to stress condition, pro-apoptotic (BAX) and anti-apoptotic (Bcl-2) proteins regulate the release of cytochrome c from the outer mitochondrial membrane, which activates caspases and thus triggers apoptosis (Kubera, et al., 2011). It has been shown that restraint stress causes cellular apoptosis through activation of caspases-9 and −3 and increase in the BAX/Bcl-2 ratio (Zhu et al., 2014). In our study, sub-chronic restraint stress for 5 days produced a pro-apoptotic response, as evidenced by increased BAX/Bcl-2 ratio and elevated caspase-9 and caspase-3 activities in the PFC and HIP. Interestingly, PBM therapy prevented stress-induced alteration of the BAX/Bcl-2 ratio and avoided an increase in caspases activity most likely through the preservation of MMP and a reduction of cytochrome c leakage into the cytoplasm. Our results are consistent with other studies showing anti-apoptotic effects of red/NIR PBM therapy via inhibition of the intrinsic mitochondrial apoptosis pathway in animal models of aging (Salehpour, et al., 2017), AD (Lu, et al., 2017), stroke (Lee, et al., 2017), and TBI (Xuan et al., 2014). Although our findings suggest that the anti-apoptotic activity may also contribute to the antidepressant-like effects of PBM therapy, the ability of NIR light to inhibit stress-mediated cell apoptosis needs further investigation. Furthermore, it has been suggested that CoQ10 supplementation could inhibit apoptosis by blocking Bax translocation and by preventing activation of the mitochondrial permeability transition pore (Naderi et al., 2006; Papucci et al., 2003). In the present study, we also showed that CoQ10 is able to ameliorate stress-induced apoptosis via mitochondrial signaling pathways.
5. Conclusion
Taken together, our data suggest that reversing oxidative stress, neuroinflammation, and neuronal apoptosis in the PFC and HIP might be a possible mechanism for the observed antidepressant-like effects of PBM and CoQ10 therapies in this study. Our data also showed that the combined-modality therapy led to better behavioral and biochemical results than either singlemodality treatment used alone. These findings underline the potential of the combination of the NIR PBM therapy with CoQ10 as a treatment for stress-induced depression.
Highlights:
Photobiomodulation and CoQ10 ameliorate depressive-like behaviors in a mouse restraint stress model
Photobiomodulation and CoQ10 improve brain antioxidant defense capacity in a mouse restraint stress model
Photobiomodulation and CoQ10 suppress neuroinflammation via reduction of NF-kB, p38, and JNK levels in brain
Photobiomodulation and CoQ10 reduce neuronal apoptosis via mitochondrial apoptotic signaling pathways
Photobiomodulation and CoQ10 decrease elevated serum levels of cortisol, corticosterone, TNF-, and IL-6 induced by restraint stress
Acknowledgment
We would like to express our deepest gratitude to the director of Neurosciences Research Center (NSRC) Prof. Mehdi Farhoudi for providing support and encouragement for the work. Michael R Hamblin was funded by US NIH Grants R01AI050875 and R21AI121700.
Conflicts of interests
Dr. Cassano’s salary was supported by the Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award), by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and by the Photothera Inc. unrestricted grant. Drug donation from TEVA. Travel reimbursement from Pharmacia-Upjohn. Dr. Cassano has received consultation fees from Janssen Research and Development. Dr. Cassano has filed a provisional patent related to the use of near-infrared light in psychiatry. PhotoMedex, Inc. supplied four devices for a clinical study. Dr. Cassano is/has: 1. Received unrestricted funding from Litecure Inc. to conduct a study on transcranial photobiomodulation for the treatment of major depressive disorder; 2. Received unrestricted funding from Cerebral Sciences to conduct a study on transcranial photobiomodulation for the treatment of generalized anxiety disorder; 3. Co-founded, member of the board of directors and consultant of Niraxx Light Therapeutics Inc., a company focused on the development of new modalities of treatment based on near-infrared light. The other authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aboul-Fotouh S (2013). Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol Biochem Behav 104, 105–112. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN (1998). The free radical theory of aging matures. Physiol Rev 78, 547–581. [DOI] [PubMed] [Google Scholar]
- Bettio LE, Freitas AE, Neis VB, Santos DB, Ribeiro CM, Rosa PB, Farina M, Rodrigues ALS (2014). Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol Biochem Behav 127, 7–14. [DOI] [PubMed] [Google Scholar]
- Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O (2001). Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J. Affect. Disord 64, 43–51. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI (2009). Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev 33, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Caldieraro MA, Sani G, Bui E, Cassano P (2018). Long-Term Near-Infrared Photobiomodulation for Anxious Depression Complicated by Takotsubo Cardiomyopathy. J Clin Psychopharmacol 38, 268–270. [DOI] [PubMed] [Google Scholar]
- Cassano P, Cusin C, Mischoulon D, Hamblin MR, De Taboada L, Pisoni A, Chang T, Yeung A, Ionescu DF, Petrie SR (2015). Near-infrared transcranial radiation for major depressive disorder: proof of concept study. Psychiatr J 2015, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano P, Petrie SR, Hamblin MR, Henderson TA, Iosifescu DV (2016). Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and neurogenesis. Neurophotonics 3, 031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR (2012). The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40, 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane FL (2001). Biochemical functions of coenzyme Q10. J Am Coll Nutr 20, 591–598. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A (2005). The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29, 571–625. [DOI] [PubMed] [Google Scholar]
- Dallner G, Sindelar PJ (2000). Regulation of ubiquinone metabolism. Free Radic. Biol. Med 29, 285–294. [DOI] [PubMed] [Google Scholar]
- Das A, Kapoor K, Sayeepriyadarshini A, Dikshit M, Palit G, Nath C (2000). Immobilization stress-induced changes in brain acetylcholinesterase activity and cognitive function in mice. Pharmacol Res 42, 213–217. [DOI] [PubMed] [Google Scholar]
- de Freitas LF, Hamblin MR (2016). Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22, 348–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, Streeter J, Kindy MS (2011). Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. Int J Alzheimers Dis 23, 521–535. [DOI] [PubMed] [Google Scholar]
- Dong T, Zhang Q, Hamblin MR, Wu MX (2015). Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metab 35, 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Creson TK, Wu L-J, Ren M, Gray NA, Falke C, Wei Y, Wang Y, Blumenthal R, Machado-Vieira R (2008). The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci 28, 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R (2005). Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev 29, 891–909. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Sharma S, Wanpen S, Amornpan A (2004). Coenzyme Q10 inhibits mitochondrial complex-1 down-regulation and nuclear factor-kappa B activation. J Cell Mol Med 8, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82, 70–77. [DOI] [PubMed] [Google Scholar]
- Farajpour R, Sadigh-Eteghad S, Ahmadian N, Farzipour M, Mahmoudi J, Majdi A (2017). Chronic Administration of Rosa canina Hydro-Alcoholic Extract Attenuates Depressive-Like Behavior and Recognition Memory Impairment in Diabetic Mice: A Possible Role of Oxidative Stress. Med. Princ. Pract 26, 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Kendler KS (2000). Major depressive disorder. Neuron 28, 335–341. [DOI] [PubMed] [Google Scholar]
- Flint Beal M (2002). Coenzyme Q 10 as a possible treatment for neurodegenerative diseases. Free Radic Res 36, 455–460. [DOI] [PubMed] [Google Scholar]
- Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C (2005). Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res 30, 105–111. [DOI] [PubMed] [Google Scholar]
- Freitas AE, Bettio LE, Neis VB, Santos DB, Ribeiro CM, Rosa PB, Farina M, Rodrigues ALS (2014). Agmatine abolishes restraint stress-induced depressive-like behavior and hippocampal antioxidant imbalance in mice. Prog Neuropsychopharmacol Biol Psychiatry 50, 143–150. [DOI] [PubMed] [Google Scholar]
- Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J (2013). Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep 65, 1655–1662. [DOI] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J (2006). NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72, 1493–1505. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM (2013). The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol 23, 1165–1181. [DOI] [PubMed] [Google Scholar]
- Gold PW, Licinio J, WONG ML, Chrousos GP (1995). Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann N Y Acad Sci 771, 716–729. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13, 717. [DOI] [PubMed] [Google Scholar]
- Hamblin MR (2017). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys 4, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves I (2014). Coenzyme Q 10 as a therapy for mitochondrial disease. Int. J. Biochem. Cell. Biol 49, 105–111. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Lisowski P, Sliwa AT, Swiergiel AH (2008). Computer assisted video analysis of swimming performance in a forced swim test: simultaneous assessment of duration of immobility and swimming style in mice selected for high and low swim-stress induced analgesia. Physiol Behav 95, 400–407. [DOI] [PubMed] [Google Scholar]
- Juszczak GR, Sliwa AT, Wolak P, Tymosiak-Zielinska A, Lisowski P, Swiergiel AH (2006). The usage of video analysis system for detection of immobility in the tail suspension test in mice. Pharmacol Biochem Behav 85, 332–338. [DOI] [PubMed] [Google Scholar]
- Karu T, Kolyakov S (2005). Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 23, 355–361. [DOI] [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R (2003). Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep 8, 365–370. [DOI] [PubMed] [Google Scholar]
- Khorrami A, Ghanbarzadeh S, Mahmoudi J, Nayebi A, Maleki-Dizaji N, Garjani A (2014). Investigation of the memory impairment in rats fed with oxidized-cholesterol-rich diet employing passive avoidance test. Drug Res. [DOI] [PubMed] [Google Scholar]
- Kim YK, Maes M (2003). The role of the cytokine network in psychological stress. Acta Neuropsychiatr 15, 148–155. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2008). The molecular neurobiology of depression. Nature 455, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M (2011). In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35, 744–759. [DOI] [PubMed] [Google Scholar]
- Lee HI, Lee SW, Kim NG, Park KJ, Choi BT, Shin YI, Shin HK (2017). Low-level light emitting diode (LED) therapy suppresses inflammasome-mediated brain damage in experimental ischemic stroke. J Biophotonics 10, 1502–1513. [DOI] [PubMed] [Google Scholar]
- Lee HI., Park JH, Park MY, Kim NG, Park K-J, Choi BT, Shin Y-I, Shin HK (2016). Pre-conditioning with transcranial low-level light therapy reduces neuroinflammation and protects blood-brain barrier after focal cerebral ischemia in mice. Restor Neurol Neurosci 34, 201–214. [DOI] [PubMed] [Google Scholar]
- Li R, Wang X, Qin T, Qu R, Ma S (2016). Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav Brain Res 296, 318–325. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RC-M, Mak A (2012). Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 139, 230–239. [DOI] [PubMed] [Google Scholar]
- Liu YF, Bertram K, Perides G, McEwen BS, Wang D (2004). Stress induces activation of stress-activated kinases in the mouse brain. J Neurochem 89, 1034–1043. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Wittenberg L, Yamamoto BK (1995). Effect of acute stress on hippocampal glutamate levels and spectrin proteolysis in young and aged rats. J Neurochem 65, 268–274. [DOI] [PubMed] [Google Scholar]
- Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC-Y, Cohen RM, Zhang Q (2017). Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging 49, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC (2001). Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor KB-mediated mechanisms. J Neurochem 76, 532–538. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24, 27–53. [DOI] [PubMed] [Google Scholar]
- Mahmoudi J, Farhoudi M, Talebi M, Sabermarouf B, Sadigh-Eteghad S (2015). Antidepressant-like effect of modafinil in mice: evidence for the involvement of the dopaminergic neurotransmission. Pharmacol Rep 67, 478–484. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS (2001). The cellular neurobiology of depression. Nat Med 7, 541. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1999). Stress and hippocampal plasticity. Annu Rev Neurosci 22, 105–122. [DOI] [PubMed] [Google Scholar]
- Mintzopoulos D, Gillis TE, Tedford CE, Kaufman MJ (2017). Effects of near-infrared light on cerebral bioenergetics measured with phosphorus magnetic resonance spectroscopy. Photomed Laser Surg 35, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T (2000). Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from parkinsonian brain. J Neural Transm 107, 335–341. [DOI] [PubMed] [Google Scholar]
- Mohammed HS (2016). Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers Med Sci 31, 1651–1656. [DOI] [PubMed] [Google Scholar]
- Moreira MS, Velasco IT, Ferreira LS, Ariga SKK, Barbeiro DF, Meneguzzo DT, Abatepaulo F, Marques MM (2009). Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J Photochem Photobiol B 97, 145–151. [DOI] [PubMed] [Google Scholar]
- Moretti M, Colla A, de Oliveira Balen G, dos Santos DB, Budni J, de Freitas AE, Farina M, Severo Rodrigues AL (2012). Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J Psychiatr Res 46, 331–340. [DOI] [PubMed] [Google Scholar]
- Morries LD, Cassano P, Henderson TA (2015). Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr Dis Treat 11, 2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Anderson G, Berk M, Maes M (2013). Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol Neurobiol 48, 883–903. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G (2010). Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PloS One 5, e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi J, Somayajulu-Nitu M, Mukerji A, Sharda P, Sikorska M, Borowy-Borowski H, Antonsson B, Pandey S (2006). Water-soluble formulation of Coenzyme Q10 inhibits Bax-induced destabilization of mitochondria in mammalian cells. Apoptosis 11, 1359–1369. [DOI] [PubMed] [Google Scholar]
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O (2004). Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol 19, 89–95. [DOI] [PubMed] [Google Scholar]
- Pandya CD, Howell KR, Pillai A (2013). Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 46, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet H, Barzilai A, Daily D, Melamed E, Offen D (2001). Activation of nuclear transcription factor kappa B (NF-kB) is essential for dopamine-induced apoptosis in PC12 cells. J Neurochem 77, 391–398. [DOI] [PubMed] [Google Scholar]
- Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A, Formigli L, Zecchi-Orlandini S, Orlandini G, Carella G (2003). Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem 278, 28220–28228. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu B. e., Karandikar M, Berman K, Cobb MH (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22, 153–183. [DOI] [PubMed] [Google Scholar]
- Porsolt R, Bertin A, Jalfre M (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229, 327–336. [PubMed] [Google Scholar]
- Pourmemar E, Majdi A, Haramshahi M, Talebi M, Karimi P, Sadigh-Eteghad S (2017). Intranasal cerebrolysin attenuates learning and memory impairments in D-galactose-induced senescence in mice. Exp. Gerontol 87, 16–22. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Hopkins SJ (1995). Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci 18, 130–136. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J (2004). ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68, 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Talebi M, Mahmoudi J, Babri S, Shanehbandi D (2015). Selective activation of α7 nicotinic acetylcholine receptor by PHA-543613 improves Aβ25–35-mediated cognitive deficits in mice. Neuroscience 298, 81–93. [DOI] [PubMed] [Google Scholar]
- Salehpour F, Ahmadian N, Rasta SH, Farhoudi M, Karimi P, Sadigh-Eteghad S (2017). Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiol Aging 58, 140–150. [DOI] [PubMed] [Google Scholar]
- Salehpour F, Farajdokht F, Erfani M, Sadigh-Eteghad S, Shotorbani SS, Hamblin MR, Karimi P, Rasta SH, Mahmoudi J (2018a). Transcranial near-infrared photobiomodulation attenuates memory impairment and hippocampal oxidative stress in sleep-deprived mice. Brain Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour F, Mahmoudi J, Eyvazzadeh N (2018b). Effects of Acute and Chronic Noise Stress on Depressive-and Anxiety-like Behaviors in Mice. J Exp Clin Neurosci 5, 1–6. [Google Scholar]
- Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR (2018c). Brain Photobiomodulation Therapy: a Narrative Review. Mol Neurobiol 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour F, Rasta SH (2017). The potential of transcranial photobiomodulation therapy for treatment of major depressive disorder. Rev Neurosci 28, 441–453. [DOI] [PubMed] [Google Scholar]
- Salehpour F, Rasta SH, Mohaddes G, Sadigh-Eteghad S, Salarirad S (2016). Therapeutic effects of 10-HzPulsed wave lasers in rat depression model: A comparison between near-infrared and red wavelengths. Lasers Surg Med 48, 695–705. [DOI] [PubMed] [Google Scholar]
- Salgado AS, Zângaro RA, Parreira RB, Kerppers II (2015). The effects of transcranial LED therapy (TCLT) on cerebral blood flow in the elderly women. Lasers Med Sci 30, 339–346. [DOI] [PubMed] [Google Scholar]
- Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR (2009). Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 5, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling G, Coonfield ML, Ross CA, Borchelt DR (2001). Coenzyme Q10 and remacemide hydrochloride ameliorate motor deficits in a Huntington’s disease transgenic mouse model. Neurosci Lett 315, 149–153. [DOI] [PubMed] [Google Scholar]
- Sharma SK, El ReFaey H, Ebadi M (2006). Complex-1 activity and 18F-DOPA uptake in genetically engineered mouse model of Parkinson’s disease and the neuroprotective role of coenzyme Q10. Brain Res Bull 70, 22–32. [DOI] [PubMed] [Google Scholar]
- Shelton R, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis D, Mirnics K (2011). Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kumar A (2015). Microglial inhibitory mechanism of coenzyme Q10 against Aβ (1–42) induced cognitive dysfunctions: possible behavioral, biochemical, cellular, and histopathological alterations. Front Pharmacol 6, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somayajulu M, McCarthy S, Hung M, Sikorska M, Borowy-Borowski H, Pandey S (2005). Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q 10. Neurobiol. Dis 18, 618–627. [DOI] [PubMed] [Google Scholar]
- Stokes PE (1995). The potential role of excessive cortisol induced by HPA hyperfunction in the pathogenesis of depression. Eur Neuropsychopharmacol 5, 77–82. [DOI] [PubMed] [Google Scholar]
- Takaki A, Huang Q-H, Somogyvári-Vigh A, Arimura A (1994). Immobilization Stress May Increase Plasma lnterleukin-6 via Central and Peripheral Catecholamines. Neuroimmunomodulation 1, 335–342. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Akiyoshi J, Kawahara Y, Ishitobi Y, Hatano K, Hoaki N, Mori A, Goto S, Tsuru J, Matsushita H (2011). Infrared radiation has potential antidepressant and anxiolytic effects in animal model of depression and anxiety. Brain Stimul 4, 71–76. [DOI] [PubMed] [Google Scholar]
- Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M (2010). Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med 42, 566–576. [DOI] [PubMed] [Google Scholar]
- Villanueva R (2013). Neurobiology of major depressive disorder. Neural Plast 2013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK (2010). Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tian F, Reddy DD, Nalawade SS, Barrett DW, Gonzalez-Lima F, Liu H (2017). Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: a broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab 37, 3789–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu H, Miller A (2004). Interleukin 1α (IL-1α) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry 9, 65. [DOI] [PubMed] [Google Scholar]
- Wang X, Xie Y, Zhang T, Bo S, Bai X, Liu H, Li T, Liu S, Zhou Y, Cong X (2016). Resveratrol reverses chronic restraint stress-induced depression-like behaviour: involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res Bull 125, 134–143. [DOI] [PubMed] [Google Scholar]
- Won R, Lee KH, Lee BH (2011). Coenzyme Q10 protects neurons against neurotoxicity in hippocampal slice culture. Neuroreport 22, 721–726. [DOI] [PubMed] [Google Scholar]
- Wu X, Alberico SL, Moges H, De Taboada L, Tedford CE, Anders JJ (2012). Pulsed light irradiation improves behavioral outcome in a rat model of chronic mild stress. Lasers Surg Med 44, 227–232. [DOI] [PubMed] [Google Scholar]
- Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, Zhang G, Yang L, Li J, Du X (2017). Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol 54, 4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Vatansever F, Huang L, Hamblin MR (2014). Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J Biomed Opt 19, 108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang J-H, Chen X-Y, Hu Q-H, Wang M-X, Jin R, Zhang Q-Y, Wang W, Wang R, Kang L-L (2015). Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal 22, 848–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Gu L, Wang Y, Jia L, Zhao Z, Peng S, Lei L (2014). The role of alpha-1 and alpha-2 adrenoceptors in restraint stress-induced liver injury in mice. PLoS One 9, e92125. [DOI] [PMC free article] [PubMed] [Google Scholar]


