Abstract
During pregnancy, programming of the fetal central nervous system (CNS) establishes vulnerabilities for emergence of neuropsychiatric phenotypes later in life. Psychosocial influences during pregnancy, such as stressful life events or chronic stress, correlate with offspring neuropsychiatric disorders and with inflammation, respectively. Stress promotes inflammation, but the role of inflammation as a mediator between maternal psychosocial stress and offspring neuropsychiatric outcomes has not been extensively studied in humans. This review summarizes clinical evidence linking specific types of stress to maternal inflammatory load during pregnancy. We propose that inflammation is a mediator in the relationship between psychosocial stress and offspring neuropsychiatric outcomes, potentially influenced by poor maternal glucocorticoid-immune coordination. We present relevant experimental animal research supporting this hypothesis. We conclude that clinical and preclinical research support the premise that stress-induced maternal immune activation (MIA) contributes in part to prenatal programming of risk. Programming of risk is likely due to a combination of vulnerabilities, including multiple or repeated inflammatory events, timing of such events, poor maternal regulation of inflammation, genetic vulnerability, and lifestyle contributors.
Keywords: pregnancy, cytokines, stress, hypothalamic pituitary adrenal, transgenerational, cytokine-glucocorticoid feedback
INTRODUCTION
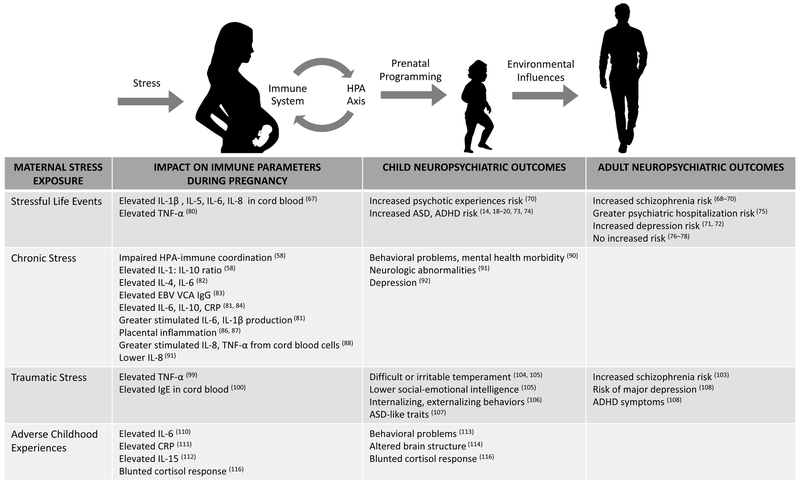
Pregnancy represents a key developmental window when prenatal programming of the offspring central nervous system (CNS) occurs (1). Perturbations to the prenatal environment, such as stress or immune activation, are associated with increased risk for offspring neuropsychiatric disorders in the epidemiologic literature (2–20). Numerous reviews have addressed sickness-induced immune activation and offspring neuropsychiatric risk, but few have addressed stress-induced immune activation and offspring neuropsychiatric risk in the context of human pregnancy. In this review, we propose that inflammation is a potential mediator between prenatal stress and offspring neuropsychiatric outcomes (Figure 1). We describe the clinical evidence linking specific forms of stress (stressful life events (SLEs), chronic stress, acute stress, traumatic stress, adverse childhood experiences (ACEs)) to maternal inflammation during pregnancy, and to neuropsychiatric risk in the offspring. Finally, we provide preclinical data to explore potential mechanisms by which inflammation may modulate the relationship between maternal psychosocial stress exposure and offspring neuropsychiatric outcomes.
Figure 1. Impact of Stressors on Maternal Inflammation During Pregnancy.
We propose that inflammation is an intermediary in the relationship between maternal psychosocial stress and offspring neuropsychiatric outcomes, potentially influenced by poor maternal glucocorticoid-immune coordination. In this model, stress-induced maternal immune activation (MIA) may induce CNS vulnerabilities in the offspring, potentially manifesting as subtle neurocognitive changes. Interactions with the environment through childhood and adolescent development may eventually result in emergence of neuropsychiatric disorder.
STRESS AND INFLAMMATION DURING PREGNANCY
Stress and the immune milieu
Decades of research have established that psychosocial stress dysregulates aspects of immune function in healthy, nonpregnant adults (21–28). Elevated circulating inflammatory markers and impaired immune function have been associated with numerous types of stress including SLEs such as death of a spouse (29–31), daily hassles (32, 33), chronic stress such as caregiving or unemployment (34–37), traumatic stress (38, 39), ACEs (40–43), as well as acute laboratory stressors (44–47). At the root of the relationship between stress and inflammation is coordination between the hypothalamic pituitary adrenal (HPA) axis and immune system. Glucocorticoids impact both innate immunity (e.g. inflammation) and adaptive immunity (specific, T/B-cell mediated), modulating activity of numerous immune cell types including monocytes and macrophages, and mediators such as cytokines and chemokines (48). Although historically considered anti-inflammatory, glucocorticoids can suppress or potentiate immune function, in a biphasic manner (49). This reciprocal relationship maintains an appropriate allostatic load, however, simultaneous elevation of proinflammatory cytokines and cortisol indicate dysregulated glucocorticoid-immune feedback, often resulting from stress (50). A meta-analysis of over 300 studies found that different types of stress impact different aspects of immune function: acute laboratory stressors upregulated innate immunity and downregulated adaptive immunity; brief naturalistic stressors such as academic examinations shifted function away from cellular immunity (T-helper type 1; Th1) and toward humoral immunity (Th2); and chronic stressors, which are pervasive and unrelenting, suppressed both innate and adaptive immunity (28). A well-regulated, flexible glucocorticoid-immune system responds appropriately to stimulation, e.g., a brief spike in proinflammatory cytokines in response to acute stress is physiologically appropriate, but an exaggerated or prolonged immune response is maladaptive, and chronic stress may result in glucocorticoid resistance (34).
During pregnancy, the immune and glucocorticoid milieu shift. The first and third trimesters are often considered broadly proinflammatory with increased activation of peripheral leukocytes (51), while the second trimester is conceptualized as anti-inflammatory (52). The immune system is tightly regulated during pregnancy, as it is involved in cervical ripening, rupture of membranes, and myometrial contractivity (53). It is possible that perturbations to the normal trajectory of immune function across pregnancy (54–57), impaired coordination of glucocorticoid-immune function, or compromise to immune system flexibility, could contribute to increased offspring risk (28). As inflammatory response to acute stress is dampened during pregnancy (45), an exaggerated inflammatory response to stressors, or inability to dampen this response in the face of routine stressors, could contribute to inflammatory burden over the course of pregnancy. Appropriate coordination between the glucocorticoid and immune systems are important for materno-fetal health (58, 59). While studies have assessed the potential moderating role of glucocorticoids in the association between prenatal stress and offspring outcomes (60–63), there has been little focus on inflammation as a component of this relationship. It may not be glucocorticoids alone, but poor regulation of cytokine-glucocorticoid negative feedback (64), that influences the relationship between maternal stress during pregnancy and offspring outcomes.
Stressful life events
Stressful life events (SLEs), such as death of a loved one or divorce, range from mild to severe (65). Healthy adults who recently experienced an SLE exhibit immune dysregulation (29, 66). In particular, meta-analysis found a decline in natural killer (NK) cell cytotoxicity among those who had experienced recent death of a spouse (28). There is less research on SLEs and immune function specifically in pregnancy. Offspring of women who experienced an interpersonal SLE during the prior trimester of pregnancy had higher levels of interleukin (IL)-1β in umbilical cord blood at delivery compared with women who did not experience an SLE, and those who had a health-related SLE in the first trimester had higher cord blood levels of IL-5, IL-6, and IL-8 than women who had not experienced SLEs (67). However, elevated cord blood cytokine levels among those with a health-related SLE may have been confounded with the health issue itself.
While offspring neurocognitive outcomes were not assessed in the aforementioned studies, epidemiologic studies suggest that SLEs during pregnancy increase risk of poor offspring neuropsychiatric outcomes. Death of a close relative during pregnancy was associated with greater risk for offspring schizophrenia in some studies (68, 69). Women in the Avon Longitudinal Study of Parents and Children (ALSPAC) who experienced more SLEs during pregnancy were more likely to have a child with psychotic experiences at age 12 than women with fewer SLEs, although this was not significant after adjusting for maternal anxiety and depression (70). SLEs similarly increase risk of autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD) and depression among offspring in multiple studies (18–20, 71, 72), with some suggesting a male risk bias (14, 73–75). While some studies found that SLEs during pregnancy were associated with increased risk for offspring schizophrenia or psychotic experiences (68–70), ASD (18), or ADHD symptoms (14, 18–20, 73), not all did (76–78). The mixed findings may result from differences in severity of stress, maternal psychological reaction to the stress, maternal inflammatory response to the stressor, or mitigating factors such as social support. For instance, impact of SLEs on offspring neuropsychiatric outcomes in ALSPAC was driven by maternal anxiety and depression (70). Similarly, perceived stress (79) and exposure to interpersonal violence (80) predicted higher levels of proinflammatory cytokines during pregnancy particularly in the context of low social support (81). Thus, it is important to consider maternal SLE exposure in the context of moderating influences that could potentiate or dampen impact of the SLE on immune function.
Chronic stress
Stressors that persist for an extended duration -- caregiving for an individual with severe chronic illness, extended work stress or unemployment, racial discrimination, poverty -- are considered chronic. A meta-analysis revealed that chronic stress impacted nearly all aspects of functional immunity studied, including T cell proliferative response and antibody response to vaccine (28). This was generally associated with duration of the stressor; longer duration was associated with decrements in functional immunity, versus shorter term stressors associated with declines in cell counts consistent with redistribution (28). Of the few studies on chronic stress and immune function during pregnancy, minority status and low income, both chronic stressors, were associated with higher IL-1:IL-10 ratio, as well as poor coordination of glucocorticoid-immune response, at weeks 32 - 36 of pregnancy (58). African-American women reporting greater racial discrimination had elevated levels of circulating IL-6 and IL-4 in the second trimester (82), and greater Epstein-Barr viral capsid antigen (EBV VCA) IgG antibody titers across pregnancy and postpartum (83), suggesting immune dysregulation. Greater exposure to chronic stressors was associated with elevated peripheral inflammatory markers in the first and third trimesters (81, 84), which partially mediated the impact of stress on gestational age at delivery (85). Low income during pregnancy was associated with chronic inflammation in placental tissue at delivery (86) and a transcriptional profile suggesting higher immune activation (87). In a study of 560 newborns, maternal exposure to chronic stressors during pregnancy, including financial hardship, community violence, and poor housing conditions, was associated with increased stimulated chemoattractant cytokine IL-8 and pro-inflammatory cytokine tumor necrosis factor (TNF)-α production in cells isolated from cord blood (88). The authors propose that this is evidence for prenatal psychosocial stress contributing to programming of the infant immune response.
Forms of chronic stress such as racial discrimination or poverty may be distinct from other types of chronic stress (89). Further, poverty is often associated with confounding factors such as exposure to environmental pollutants or poor diet (87). As these factors also directly affect the health of the pregnancy and fetal development, it is a challenge to isolate the specific effects of prenatal stress on offspring neurodevelopment. Regardless, studies focusing on the chronic stress of discrimination or socioeconomic disadvantage consistently report adverse impacts on offspring neuropsychiatric risk (90). Maternal socioeconomic disadvantage during pregnancy was associated with 4.6-fold greater odds of offspring neurologic abnormalities at four months of age, an effect that was modulated by peripheral levels of IL-8 (91). Further, maternal daily stress compounded the effect of second trimester infection on offspring depression scores; offspring who were exposed to prenatal infection alone had significantly lower levels of depression than offspring who were exposed to both infection and stress in utero (92). This supports the notion that stress potentiates the inflammatory effects of infection or illness during pregnancy.
Acute laboratory stress
Acute laboratory stressors, meant to evoke physiologic responses similar to what one would experience in the face of routine daily stressors, reliably increase cortisol and proinflammatory cytokines (93). The magnitude and duration of the inflammatory response is regulated by glucocorticoids (94), and thus laboratory stressors provide useful information about individual regulation of inflammation and immune-glucocorticoid feedback. While acute stressors such as the Trier Social Stress Test (TSST) have been extensively studied in nonpregnant adults, inflammatory response to acute laboratory stress is not well characterized in pregnant women. One study found that inflammatory response to TSST is blunted in pregnant women relative to nonpregnant women overall, but pregnant African-American women’s IL-6 response was still greater than that of nonpregnant Caucasian women (45). Poor ability to regulate glucocorticoid-immune response to routine stressors may contribute to persistent inflammation over time, thus potentially increasing inflammatory load across pregnancy.
Traumatic stress
Individuals with PTSD have elevated cerebrospinal fluid (CSF) IL-6 (95), elevated peripheral inflammatory markers (96, 97), and impaired immune cell glucocorticoid sensitivity (98). In pregnant women, history of trauma exposure was associated with elevated circulating TNF-α (99) and elevated IgE levels in cord blood at delivery (100).
Prenatal traumatic stress, such as exposure to war, is associated with deficits in child neurodevelopment and mental health in the epidemiologic literature (101–103). Natural disasters, such as floods or hurricanes, are variably stressful depending upon individual factors such as access to resources or ability to escape. Children of women pregnant during the 2011 Queensland flood (QF2011) or 1988 Quebec ice storm had more difficult or irritable temperaments (104, 105). QF2011 offspring had lower social-emotional intelligence scores the later in pregnancy they were exposed and the more severely their mothers rated their exposure (105). Higher-rated subjective exposure to the Quebec ice storm among mothers was associated with increased maternal report of internalizing and externalizing problems and severity of autistic-like traits in children (106, 107). Among children prenatally exposed to heightened maternal anxiety during the Chernobyl disaster, there was a 2.32-fold risk of lifetime depression symptoms, an increased risk of meeting criteria for MDD, and a two-fold risk of ADHD symptoms (108), noteworthy in those exposed from the second trimester onwards. In war or natural disaster, it is difficult to disentangle whether the effects on the developing child are due to physical deprivation, emotional strain or ongoing difficulties after birth. Further, none of these studies assessed maternal markers of inflammation resulting from trauma exposure during pregnancy, failing to establish relationships between maternal stress, inflammation, and offspring outcomes.
Maternal adverse childhood experiences
Adverse childhood experiences (ACEs), such as abuse or chronic stress, are associated with elevated markers of inflammation in non-pregnant adults (42, 109), including at baseline (41), in response to acute stress (40), in response to daily stressors (42), and in the context of chronic stress (36). Few studies have assessed the impact of ACEs on inflammation during human pregnancy. Pregnant teenagers who reported a history of abuse had elevated IL-6 levels in the second trimester, particularly among those with current major depression (110). Childhood physical abuse, emotional abuse and emotional neglect were associated with elevated serum CRP across pregnancy (111). Women with gestational diabetes who had a history of childhood maltreatment had elevated IL-15 in pregnancy (112).
Epidemiologic studies suggest possible links between maternal ACEs and offspring neuropsychiatric outcomes. Offspring of high ACE mothers had higher rates of behavioral problems in childhood (113), and as newborns, had altered brain structure (114). Strikingly, among pregnant women, it was not depression nor anxiety that was associated with elevated inflammatory markers, but history of trauma (99). Maternal childhood economic hardship, a proxy for adversity, was associated with adverse pregnancy outcomes, and this relationship was partially mediated by elevated maternal IL-6 (115). Finally, our group found that postpartum women with a history of childhood adversity exhibited a dampened glucocorticoid response to a mild stressor compared to women without such history, a finding that was mirrored by their 6-month old infants in response to stress (116). Dysregulated glucocorticoid function allows exaggerated inflammatory response (58), leading us to hypothesize that poor glucocorticoid regulation of inflammation during pregnancy may impact prenatal programming.
Summary of Clinical Literature and Proposed Mechanism
Epidemiologic and clinical data indicate that offspring neuropsychiatric outcomes are associated independently with maternal stress experience and with MIA. The few studies that have assessed all three factors in conjunction -- maternal stress, MIA, and offspring outcomes -- suggest a mediating effect of inflammation on pregnancy outcomes or offspring neurodevelopment (85, 91, 115). IL-8 modulated the association between maternal chronic socioeconomic stress during pregnancy and increased risk of offspring neurologic abnormalities (91). Although not a neuropsychiatric outcome, maternal IL-6 partially mediated the relationship between maternal childhood adversity and poor pregnancy outcomes (115). Similarly, maternal stress predicted peripheral IL-6 and TNF- α, which in turn predicted gestational age at delivery (85). These findings are also consistent with recent studies demonstrating that maternal IL-6 across pregnancy is associated with greater amygdala volume and connectivity in infants and poor impulse control at age two (117), functional brain connectivity in infants and poorer working memory function at age two (118), and reduced frontolimbic white matter integrity in newborns and poorer cognitive function at twelve months (119).
A potential mechanism for the proposed relationship between maternal stress, inflammation, and offspring neuropsychiatric outcomes is poor HPA regulation of inflammatory response. Numerous studies have assessed the potential moderating role of glucocorticoids in the association between prenatal stress and offspring outcomes (60), finding associations between placental CRH, glucocorticoid exposure, and maternal cortisol levels with offspring internalizing symptoms, cortical thinning, and increased amygdala volume, respectively (61–63). However, there has been little focus on inflammation as a component of this relationship. It may not be glucocorticoids per se, but poor regulation of cytokine-glucocorticoid negative feedback (64), that influences the relationship between maternal stress during pregnancy and offspring outcomes. To test the hypothesis that stress induces impairments in glucocorticoid-immune function that then affects prenatal programming of offspring neuropsychiatric risk in humans, at minimum one would need to collect data on clearly characterized forms of maternal stress exposure, maternal markers of glucocorticoid function and inflammation at multiple points in pregnancy, and offspring neuropsychiatric outcomes. This requires careful, well-integrated study of both maternal and offspring factors. Maternal glucocorticoid-immune coordination could be studied via glucocorticoid and cytokine response to acute stress, examining correlations among cytokines and cortisol (59), or ex vivo measures of immune cell responsiveness to glucocorticoids. Dysregulated control of placental inflammation is also a potential factor, and could be examined via placental tissue at delivery. Offspring outcomes would benefit from both biological and clinical measures. Maternal IL-6 across pregnancy has been associated with altered offspring brain structure and function as well as neurocognitive deficits (117–119), suggesting that MIA during pregnancy confers risk by inducing potentially subtle neurocognitive patterning that may or may not be unmasked later in life depending on environmental factors. While no studies of this nature have been conducted in humans, such work has been done in animals, providing information on potential mechanisms.
THE ANIMAL LITERATURE: POTENTIAL MECHANISMS
Prenatal inflammation influences offspring brain and behavior
Directly inducing inflammation during the perinatal period, using pathogens or chemicals that mimic pathogenic infection, or direct injection of IL-6, produces altered brain structure and behavior in offspring (120–126). Administration of the viral mimic polyinosinic:polycytidylic acid (poly(I:C)) results in the production of pro-inflammatory cytokines IL-1β, TNF-α, IL-17a, and IL-6 in the plasma and placenta, which are correlated with fetal brain damage (127, 128). When pregnant mice were administered poly(I:C) at 13-15 days post-conception (dpc), poly(I:C)-exposed offspring had altered cerebellum structures and increased numbers of Purkinje cells, and both male and female animals had reduced performance on motor function tests and perturbed social behavior (120). Co-administration of anti-IL-6 antibody with poly(I:C) prevented the neurobehavioral deficits (125). In mouse MIA models, CD4+T helper 17 (TH17) cells, that produce IL-17a, are required for ASD-like phenotypes (121). Antibody-mediated blocking of IL-17a protected against the development of MIA-induced behavior changes and also rescued fetal cortical development. This indicates that pathological activation of TH17 cells and the IL-17a pathway during gestation alters fetal brain development and results in ASD-like behavioral phenotypes, but future work to determine downstream pathways of maternal IL-17a producing T cells is necessary to understand the mechanisms of ASD development resulting from in utero inflammation.
Prenatal stress induces inflammation and alters behavior in a sex-specific way
In rodents, prenatal stress causes extended inflammation in the fetal brain, with elevated ex vivo microglial production of pro-inflammatory cytokines IL-1β, IL-18, TNF-α, and IL-6 and chemokines CCL1 and CXCL12 (129), which are mediators of local immune responses during neuro-inflammation. Prenatal stress induced increased expression of immune response genes in the placenta, including IL-6 and IL-1β, resulting in male-specific locomotor hyperactivity and increased HPA axis response (130, 131); pretreatment with nonsteroidal anti-inflammatory drugs (NSAIDS) prevented stress-induced immune gene expression changes and ameliorated the behavior defects (130). Maternal coordination of glucocorticoid-immune function was not assessed. Prenatal stress increased serum glucocorticoid levels in male offspring but not females, and exposure to air pollutants exacerbated male-specific inflammation in the brain, with elevated IL-1β levels and increased expression of innate immune genes Tlr4 and caspase-1 (132). Interestingly, the female brains had increased levels of the anti-inflammatory cytokine IL-10 after exposure to air pollutants, suggesting that the female brain may mount a more effective anti-inflammatory response. Similarly, a restraint stress model of pregnant dams increased IL-1β expression in placentas and female fetal brain (male animals were not examined), and female adult amygdalae from stress-exposed mothers and controls had similar levels of IL-1β expression, suggesting that female immune system may tolerate or remediate prenatal-induced inflammation better than males (133). When female rats were exposed to early life stress (ELS), a proxy for ACE, their offspring exhibited increased repetitive behavior and less time in social interactions, suggestive of an ASD-like phenotype (134). While the female offspring of ELS-exposed mothers had double the baseline interferon (IFN)γ levels of control offspring, the researchers did not include male rats in their study, which could have exhibited more pronounced deficits with social interactions. Indeed, some studies suggest sexually dimorphic effects of MIA, with males more susceptible to brain development perturbations resulting from inflammation (135). LPS treatment of pregnant rats reduced juvenile social play behavior exclusively in males (136). TNF-α injections in neonatal mice increased anxiety and despair-like behaviors later in life exclusively in male animals (137). MIA can also induce sex-specific changes with astrocyte markers and morphology, where male cells are more affected (138). However, sufficiently powered research investigating sex differences resulting from immune challenges is necessary to elucidate sex-specific responses to early-life inflammation.
Together, these studies support the model that prenatal stress may negatively impact offspring behavioral outcomes via inflammation. While the majority of animal research suggests males are more vulnerable to prenatal insults, more work is needed to elucidate the sex differences with immune responses and behavioral outcomes, including glucocorticoid-immune coordination and the identification of immune cell types responsible for increased pro-inflammatory cytokine production.
CONCLUSIONS
Clinical research demonstrates clear connections between maternal stress and elevated inflammation, and between maternal inflammation and offspring CNS development, although human studies have not assessed these factors simultaneously. In rodents, stress increases inflammation peripherally and at the placenta, and induces behavioral dysregulation in offspring, establishing causal links (130, 131). Preclinical research suggests that MIA, particularly the cytokines IL-6 and IL-17a, are key in the link between maternal stress and offspring outcomes (121). In humans, maternal stress was associated with elevated peripheral IL-6, IL-8 and TNF-α during pregnancy (67, 82, 85, 88, 91, 99, 110). Intriguingly, these three proinflammatory cytokines were also found to mediate relationships between maternal stress and offspring outcomes in clinical studies (85, 115, 117–119). Peripheral cytokines may cross the placenta and fetal blood-brain barrier to access the fetal CNS, thus influencing prenatal patterning (139). Prolonged elevation of peripheral cytokines as a consequence of stress are likely due to poor maternal glucocorticoid-immune coordination (58, 116). Placental inflammation was also associated with fetal brain damage and altered behavioral phenotypes in offspring among rodents (130, 131), and in humans, placental tissue at delivery showed evidence of inflammation (86) and a transcriptional profile consistent with elevated immune activation (87) among women experiencing chronic stress. In rodents, NSAID treatment prior to maternal stress prevented placental stress-induced immune gene expression changes and ameliorated the behavior defects (130). Similarly in women who were ill during pregnancy, antipyretic medications attenuated offspring neuropsychiatric risk (140). In sum, the clinical and preclinical research support the premise that stress-induced MIA contributes in part to prenatal programming of risk, potentially due to poor coordination or plasticity of the glucocorticoid-immune axes.
However, the role of inflammation in prenatal programming is multifaceted, with diverse contributors ranging from diet to infection that should be considered in conjunction with psychosocial stress. It is likely that a combination of vulnerabilities, including multiple or repeated inflammatory events, timing and duration of such events, poor maternal regulation of inflammation, genetic vulnerability, and lifestyle contributors are synergistic factors in increased risk for neuropsychiatric disorder in offspring. Factors such as type, number and chronicity of stressors and their associated context and consequences, timing of events in relationship to the mother’s development or gestational age of the fetus, and the mother’s ongoing physiological response to additional stressors during pregnancy should be considered for future studies (92). For instance, low-grade inflammation induced by psychosocial stress is typically more chronic than the transient inflammation induced by acute infection or injury (22). Hence, psychosocial or other chronic stressors that affect women across multiple trimesters may have a greater impact than an isolated infection (92, 108, 141), and this can be modeled in animals to determine how persistent inflammation during pregnancy impacts adult behavior. Animal research suggests that inflammation during different stages of pregnancy exhibits variable vulnerability to the developing CNS (142), which is supported by observations in humans where elevated cortisol in early pregnancy had deleterious effects on offspring neurocognitive outcomes, yet elevated cortisol later in pregnancy was beneficial (143). Finally, while a full exploration of genetic contributors is beyond the scope of this review, it is possible that only offspring who are genetically vulnerable and experience MIA are at risk, in a gene x environment interaction. MIA may induce CNS vulnerabilities, perhaps manifesting as subtle neurocognitive changes in the offspring (104–107, 118, 141) that do not develop into frank psychiatric disorder. However, interactions with the environment through childhood and adolescent development may elicit or perpetuate stressors in a gene-environment correlation (144), eventually resulting in emergence of neuropsychiatric disorder.
ACKNOWLEDGMENTS
The authors are supported by the National Institute of Mental Health (NIMH) (K23MH107831 Hantsoo; K23MH102360 Kornfield; P50 MH099910 Epperson, Hantsoo), The National Institute of Allergy and Infectious Diseases (NIAID) (AI124084 Anguera), Office of Research on Women’s Health (ORWH) (P50 MH099919 Epperson, Hantsoo; K12HD085848 Epperson, Anguera), and Brain & Behavior Research Foundation NARSAD Young Investigator Award (Hantsoo).
Footnotes
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kim DR, Bale TL, Epperson CN (2015): Prenatal Programming of Mental Illness: Current Understanding of Relationship and Mechanisms. Curr Psychiatry Rep. 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET (2010): Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 40: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 3.Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET (2012): Autism After Infection, Febrile Episodes, and Antibiotic Use During Pregnancy: An Exploratory Study. Pediatrics. 130: e1447–e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES (2000): Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 26: 287–295. [DOI] [PubMed] [Google Scholar]
- 5.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J (2005): Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 159: 151–157. [DOI] [PubMed] [Google Scholar]
- 6.Flinkkilä E, Keski-Rahkonen A, Marttunen M, Raevuori A (2016): Prenatal Inflammation, Infections and Mental Disorders. Psychopathology. 49: 317–333. [DOI] [PubMed] [Google Scholar]
- 7.Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, et al. (2017): Prenatal fever and autism risk. Mol Psychiatry. . doi: 10.1038/mp.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang H, Xu L, Shao L, Xia R, Yu Z, Ling Z, et al. (2016): Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain, Behavior, and Immunity. 58: 165–172. [DOI] [PubMed] [Google Scholar]
- 9.Khandaker GM, Zimbron J, Lewis G, Jones PB (2013): Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 43: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I (2014): Maternal Immune-Mediated Conditions, Autism Spectrum Disorders, and Developmental Delay. J Autism Dev Disord. 44: 1546–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen PR, Laursen TM, Mortensen PB (2013): Association Between Parental Hospital-Treated Infection and the Risk of Schizophrenia in Adolescence and Early Adulthood. Schizophr Bull. 39: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sørensen HJ, Mortensen EL, Reinisch JM, Mednick SA (2009): Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 35: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA (2015): Maternal Infection during Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord. 45: 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Olsen J, Vestergaard M, Obel C (2010): Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiatry. 19: 747–753. [DOI] [PubMed] [Google Scholar]
- 15.Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD (2009): Cognitive Functioning Prior to the Onset of Psychosis: The Role of Fetal Exposure to Serologically Determined Influenza Infection. Biol Psychiatry. 65: 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selten JP, Brown AS, Moons KG, Slaets JP, Susser ES, Kahn RS (1999): Prenatal exposure to the 1957 influenza pandemic and non-affective psychosis in The Netherlands. Schizophr Res. 38: 85–91. [DOI] [PubMed] [Google Scholar]
- 17.Selten J-P, Frissen A, Lensvelt-Mulders G, Morgan VA (2010): Schizophrenia and 1957 Pandemic of Influenza: Meta-analysis. Schizophr Bull. 36: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, et al. (2014): Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol Med. 44: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grizenko N, Fortier M-E, Zadorozny C, Thakur G, Schmitz N, Duval R, Joober R (2012): Maternal Stress during Pregnancy, ADHD Symptomatology in Children and Genotype: Gene-Environment Interaction. J Can Acad Child Adolesc Psychiatry. 21: 9–15. [PMC free article] [PubMed] [Google Scholar]
- 20.MacKinnon N, Kingsbury M, Mahedy L, Evans J, Colman I (2018): The Association Between Prenatal Stress and Externalizing Symptoms in Childhood: Evidence From the Avon Longitudinal Study of Parents and Children. Biological Psychiatry, Early Life Environments and Later Vulnerabilities. 83: 100–108. [DOI] [PubMed] [Google Scholar]
- 21.Kiecolt-Glaser JK, Gouin J-P, Hantsoo L (2010): Close relationships, inflammation, and health. Neurosci Biobehav Rev. 35: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohleder N (2014): Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 76: 181–189. [DOI] [PubMed] [Google Scholar]
- 23.Berens AE, Jensen SKG, Nelson CA (2017): Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 15. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danese A Lewis SJ (2017): Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology. 42: 99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK, Garner W, Speicher C, Penn GM, Holliday J, Glaser R (1984): Psychosocial modifiers of immunocompetence in medical students. Psychosom Med. 46: 7–14. [DOI] [PubMed] [Google Scholar]
- 26.Glaser R, Kiecolt-Glaser JK, Stout JC, Tarr KL, Speicher CE, Holliday JE (1985): Stress-related impairments in cellular immunity. Psychiatry Res. 16: 233–239. [DOI] [PubMed] [Google Scholar]
- 27.Herbert TB, Cohen S (1993): Stress and immunity in humans: a meta-analytic review. Psychosom Med. 55: 364–379. [DOI] [PubMed] [Google Scholar]
- 28.Segerstrom SC, Miller GE (2004): Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychol Bull. 130: 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fagundes CP, Murdock KW, LeRoy A, Baameur F, Thayer JF, Heijnen C (2018): Spousal bereavement is associated with more pronounced ex vivo cytokine production and lower heart rate variability: Mechanisms underlying cardiovascular risk? Psychoneuroendocrinology. 93: 65–71. [DOI] [PubMed] [Google Scholar]
- 30.Lopizzo N, Tosato S, Begni V, Tomassi S, Cattane N, Barcella M, et al. (2017): Transcriptomic analyses and leukocyte telomere length measurement in subjects exposed to severe recent stressful life events. Transl Psychiatry. 7: e1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones SM, Weitlauf J, Danhauer SC, Qi L, Zaslavsky O, Wassertheil-Smoller S, et al. (2017): Prospective data from the Women’s Health Initiative on depressive symptoms, stress, and inflammation. J Health Psychol. 22: 457–464. [DOI] [PubMed] [Google Scholar]
- 32.Peters ML, Godaert GLR, Ballieux RE, Heijnen CJ (2003): Moderation of physiological stress responses by personality traits and daily hassles: less flexibility of immune system responses. Biol Psychol. 65: 21–48. [DOI] [PubMed] [Google Scholar]
- 33.Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J (2012): Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 31: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB (2012): Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 109: 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM (1998): Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol. 17: 214–223. [DOI] [PubMed] [Google Scholar]
- 36.Kiecolt-Glaser JK, Gouin J-P, Weng N-P, Malarkey WB, Beversdorf DQ, Glaser R (2011): Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 73: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R (2003): Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 100: 9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teche SP, Rovaris DL, Aguiar BW, Hauck S, Vitola ES, Bau CHD, et al. (2017): Resilience to traumatic events related to urban violence and increased IL10 serum levels. Psychiatry Res. 250: 136–140. [DOI] [PubMed] [Google Scholar]
- 39.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. (2015): Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2: 1002–1012. [DOI] [PubMed] [Google Scholar]
- 40.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH (2010): Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 35: 2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R (2007): Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 104: 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK (2012): Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med. 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagundes CP, Glaser R, Kiecolt-Glaser JK (2013): Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 27: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treadway MT, Admon R, Arulpragasam AR, Mehta M, Douglas S, Vitaliano G, et al. (2017): Association Between Interleukin-6 and Striatal Prediction-Error Signals Following Acute Stress in Healthy Female Participants. Biol Psychiatry. 82: 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christian LM, Glaser R, Porter K, Iams JD (2013): Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom Med. 75: 658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK (2013): Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 38: 2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steptoe A, Hamer M, Chida Y (2007): The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 21: 901–912. [DOI] [PubMed] [Google Scholar]
- 48.Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, et al. (2011): The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev. 10: 305–310. [DOI] [PubMed] [Google Scholar]
- 49.Yeager MP, Pioli PA, Wardwell K, Beach ML, Martel P, Lee HK, et al. (2008): In vivo exposure to high or low cortisol has biphasic effects on inflammatory response pathways of human monocytes. Anesth Analg. 107: 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoffman CL, Higham JP, Heistermann M, Coe CL, Prendergast BJ, Maestripieri D (2011): Immune function and HPA axis activity in free-ranging rhesus macaques. Physiol Behav. 104: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacks GP, Studena K, Sargent K, Redman CW (1998): Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 179: 80–86. [DOI] [PubMed] [Google Scholar]
- 52.Mor G, Cardenas I (2010): The Immune System in Pregnancy: A Unique Complexity. Am J Reprod Immunol. 63: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F (2009): Inflammation and pregnancy. Reprod Sci. 16: 206–215. [DOI] [PubMed] [Google Scholar]
- 54.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, et al. (2008): Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 77: 152–160. [DOI] [PubMed] [Google Scholar]
- 55.Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, et al. (2011): Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 53: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD (2014): Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am J Reprod Immunol. 72: 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gillespie SL, Porter K, Christian LM (2016): Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. J Reprod Immunol. 114: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, et al. (2013): Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 38: 1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO (2015): Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun. 49: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Donnell K, O’Connor TG, Glover V (2009): Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci. 31: 285–292. [DOI] [PubMed] [Google Scholar]
- 61.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA (2012): Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. PNAS. . doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howland MA, Sandman CA, Glynn LM, Crippen C, Davis EP (2016): Fetal exposure to placental corticotropin-releasing hormone is associated with child self-reported internalizing symptoms. Psychoneuroendocrinology. 67: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis EP, Sandman CA, Buss C, Wing DA, Head K (2013): Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry. 74: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP (2005): Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 12: 255–269. [DOI] [PubMed] [Google Scholar]
- 65.Holmes TH, Rahe RH (1967): The Social Readjustment Rating Scale. J Psychosom Res. 11: 213–218. [DOI] [PubMed] [Google Scholar]
- 66.Phillips AC, Carroll D, Evans P, Bosch JA, Clow A, Hucklebridge F, Der G (2006): Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle aged and elderly. Brain Behav Immun. 20: 191–197. [DOI] [PubMed] [Google Scholar]
- 67.Andersson NW, Li Q, Mills CW, Ly J, Nomura Y, Chen J (2016): Influence of prenatal maternal stress on umbilical cord blood cytokine levels. Arch Womens Ment Health. 19: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huttunen MO, Niskanen P (1978): Prenatal Loss of Father and Psychiatric Disorders. Arch Gen Psychiatry. 35: 429–431. [DOI] [PubMed] [Google Scholar]
- 69.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, et al. (2008): Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 65: 146–152. [DOI] [PubMed] [Google Scholar]
- 70.Dorrington S, Zammit S, Asher L, Evans J, Heron J, Lewis G (2014): Perinatal maternal life events and psychotic experiences in children at twelve years in a birth cohort study. Schizophr Res. 152: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herbison CE, Allen K, Robinson M, Newnham J, Pennell C (2017): The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev Psychopathol. 29: 1443–1454. [DOI] [PubMed] [Google Scholar]
- 72.Kingsbury M, Weeks M, MacKinnon N, Evans J, Mahedy L, Dykxhoorn J, Colman I (2016): Stressful Life Events During Pregnancy and Offspring Depression: Evidence From a Prospective Cohort Study. J Am Acad Child Adolesc Psychiatry. 55: 709–716.e2. [DOI] [PubMed] [Google Scholar]
- 73.Ronald A, Pennell CE, Whitehouse AJO (2010): Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol. 1: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu P, Hao J-H, Tao R-X, Huang K, Jiang X-M, Zhu Y-D, Tao F-B (2015): Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: a longitudinal study in China. Eur Child Adolesc Psychiatry. 24: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 75.Santavirta T, Santavirta N, Gilman SE (2017): Association of the World War II Finnish Evacuation of Children With Psychiatric Hospitalization in the Next Generation. JAMA Psychiatry. . doi: 10.1001/jamapsychiatry.2017.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J (2009): A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 123: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 77.Rai D, Golding J, Magnusson C, Steer C, Lewis G, Dalman C (2012): Prenatal and Early Life Exposure to Stressful Life Events and Risk of Autism Spectrum Disorders: Population-Based Studies in Sweden and England. PLoS One. 7. doi: 10.1371/journal.pone.0038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abel KM, Heuvelman HP, Jörgensen L, Magnusson C, Wicks S, Susser E, et al. (2014): Severe bereavement stress during the prenatal and childhood periods and risk of psychosis in later life: population based cohort study. BMJ. 348: f7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng CY, Pickler RH (2014): Perinatal Stress, Fatigue, Depressive Symptoms, and Immune Modulation in Late Pregnancy and One Month Postpartum. Scientific World Journal. 2014. doi: 10.1155/2014/652630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robertson Blackmore E, Mittal M, Cai X, Moynihan JA, Matthieu MM, O’Connor TG (2016): Lifetime Exposure to Intimate Partner Violence and Proinflammatory Cytokine Levels Across the Perinatal Period. J Womens Health (Larchmt). 25: 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coussons-Read ME, Okun ML, Nettles CD (2007): Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 21: 343–350. [DOI] [PubMed] [Google Scholar]
- 82.Giurgescu C, Engeland CG, Templin TN, Zenk SN, Koenig MD, Garfield L (2016): Racial discrimination predicts greater systemic inflammation in pregnant African American women. Appl Nurs Res. 32: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christian LM (2012): Psychoneuroimmunology in Pregnancy: Immune Pathways Linking Stress with Maternal Health, Adverse Birth Outcomes, and Fetal Development. Neurosci Biobehav Rev. 36: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coussons-Read ME, Okun ML, Schmitt MP, Giese S (2005): Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 67: 625–631. [DOI] [PubMed] [Google Scholar]
- 85.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, et al. (2012): The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 26: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keenan-Devlin LS, Ernst LM, Ross KM, Qadir S, Grobman WA, Holl JL, et al. (2017): Maternal Income during Pregnancy is Associated with Chronic Placental Inflammation at Birth. Am J Perinatol. 34: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 87.Miller GE, Borders AE, Crockett AH, Ross KM, Qadir S, Keenan-Devlin L, et al. (2017): Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav Immun. 64: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. (2010): Prenatal Maternal Stress and Cord Blood Innate and Adaptive Cytokine Responses in an Inner-City Cohort. Am J Respir Crit Care Med. 182: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, Granger DA (2017): Perceived Discrimination, Racial Identity, and Multisystem Stress Response to Social Evaluative Threat Among African American Men and Women. Psychosom Med. 79: 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson M, Mattes E, Oddy WH, Pennell CE, van Eekelen A, McLean NJ, et al. (2011): Prenatal stress and risk of behavioral morbidity from age 2 to 14 years: the influence of the number, type, and timing of stressful life events. Dev Psychopathol. 23: 507–520. [DOI] [PubMed] [Google Scholar]
- 91.Gilman SE, Hornig M, Ghassabian A, Hahn J, Cherkerzian S, Albert PS, et al. (2017): Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proc Natl Acad Sci U S A. 114: 6728–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murphy SK, Fineberg AM, Maxwell SD, Alloy LB, Zimmermann L, Krigbaum NY, et al. (2017): Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res. 257: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marsland AL, Walsh C, Lockwood K, John-Henderson NA (2017): The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. . doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parker VJ, Douglas AJ (2010): Stress in early pregnancy: maternal neuro-endocrine-immune responses and effects. Journal of Reproductive Immunology, 7th European Congress on Reproductive Immunology. 85: 86–92. [DOI] [PubMed] [Google Scholar]
- 95.Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. (2001): Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. 9: 209–217. [DOI] [PubMed] [Google Scholar]
- 96.von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U (2007): Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 41: 744–752. [DOI] [PubMed] [Google Scholar]
- 97.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM (2009): Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 26: 447–455. [DOI] [PubMed] [Google Scholar]
- 98.Pace TWW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM (2012): Increased peripheral NF-κB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 26: 13–17. [DOI] [PubMed] [Google Scholar]
- 99.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG (2011): Psychiatric Symptoms and Proinflammatory Cytokines in Pregnancy. Psychosom Med. 73: 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sternthal MJ, Enlow MB, Cohen S, Canner MJ, Staudenmayer J, Tsang K, Wright RJ (2009): Maternal interpersonal trauma and cord blood IgE levels in an inner-city cohort: A life-course perspective. J Allergy Clin Immunol. 124: 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. (2005): Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 294: 557–562. [DOI] [PubMed] [Google Scholar]
- 102.Susser ES, Lin SP (1992): Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch Gen Psychiatry. 49: 983–988. [DOI] [PubMed] [Google Scholar]
- 103.van Os J, Selten JP (1998): Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 172: 324–326. [DOI] [PubMed] [Google Scholar]
- 104.Laplante DP, Brunet A, King S (2016): The effects of maternal stress and illness during pregnancy on infant temperament: Project Ice Storm. Pediatr Res. 79: 107–113. [DOI] [PubMed] [Google Scholar]
- 105.Simcock G, Elgbeili G, Laplante DP, Kildea S, Cobham V, Stapleton H, et al. (2017): The Effects of Prenatal Maternal Stress on Early Temperament: The 2011 Queensland Flood Study. J Dev Behav Pediatr. 38: 310–321. [DOI] [PubMed] [Google Scholar]
- 106.King S, Dancause K, Turcotte-Tremblay A-M, Veru F, Laplante DP (2012): Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res C Embryo Today. 96: 273–288. [DOI] [PubMed] [Google Scholar]
- 107.Walder DJ, Laplante DP, Sousa-Pires A, Veru F, Brunet A, King S (2014): Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res. 219: 353–360. [DOI] [PubMed] [Google Scholar]
- 108.Huizink AC, Dick DM, Sihvola E, Pulkkinen L, Rose RJ, Kaprio J (2007): Chernobyl exposure as stressor during pregnancy and behaviour in adolescent offspring. Acta Psychiatr Scand. 116: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998): Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 14: 245–258. [DOI] [PubMed] [Google Scholar]
- 110.Walsh K, Basu A, Werner E, Lee S, Feng T, Osborne LM, et al. (2016): Associations Among Child Abuse, Depression, and Interleukin-6 in Pregnant Adolescents: Paradoxical Findings. Psychosom Med. 78: 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitchell AM, Kowalsky JM, Epel ES, Lin J, Christian LM (2018): Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology. 87: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bublitz M, De La Monte S, Martin S, Larson L, Bourjeily G (2017): Childhood maltreatment and inflammation among pregnant women with gestational diabetes mellitus: A pilot study. Obstet Med. 10: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collishaw S, Dunn J, O’Connor TG, Golding J, Avon Longitudinal Study of Parents and Children Study Team (2007): Maternal childhood abuse and offspring adjustment over time. Dev Psychopathol. 19: 367–383. [DOI] [PubMed] [Google Scholar]
- 114.Moog NK, Entringer S, Rasmussen JM, Styner M, Gilmore JH, Kathmann N, et al. (2018): Intergenerational Effect of Maternal Exposure to Childhood Maltreatment on Newborn Brain Anatomy. Biol Psychiatry. 83: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller GE, Culhane J, Grobman W, Simhan H, Williamson DE, Adam EK, et al. (2017): Mothers’ childhood hardship forecasts adverse pregnancy outcomes: Role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav Immun. 65: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, et al. (2017): Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biol Psychiatry. 81: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, et al. (2018): Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol Psychiatry. 83: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, et al. (2018): Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 21: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, et al. (2018): Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage. . doi: 10.1016/j.neuroimage.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aavani T, Rana SA, Hawkes R, Pittman QJ (2015): Maternal immune activation produces cerebellar hyperplasia and alterations in motor and social behaviors in male and female mice. Cerebellum. 14: 491–505. [DOI] [PubMed] [Google Scholar]
- 121.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. (2016): The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 351: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin Y-L, Lin S-Y, Wang S (2012): Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain Behav Immun. 26: 459–468. [DOI] [PubMed] [Google Scholar]
- 123.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J (2008): Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 22: 469–486. [DOI] [PubMed] [Google Scholar]
- 124.Ratnayake U, Quinn T, LaRosa DA, Dickinson H, Walker DW (2014): Prenatal exposure to the viral mimetic poly I:C alters fetal brain cytokine expression and postnatal behaviour. Dev Neurosci. 36: 83–94. [DOI] [PubMed] [Google Scholar]
- 125.Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH (2007): Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 27: 10695–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van den Eynde K, Missault S, Fransen E, Raeymaekers L, Willems R, Drinkenburg W, et al. (2014): Hypolocomotive behaviour associated with increased microglia in a prenatal immune activation model with relevance to schizophrenia. Behav Brain Res. 258: 179–186. [DOI] [PubMed] [Google Scholar]
- 127.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG (2000): Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 47: 64–72. [DOI] [PubMed] [Google Scholar]
- 128.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO (1997): Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 177: 19–26. [DOI] [PubMed] [Google Scholar]
- 129.Ślusarczyk J, Trojan E, Głombik K, Budziszewska B, Kubera M, Lasoń W, et al. (2015): Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front Cell Neurosci. 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bronson SL, Bale TL (2014): Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology. 155: 2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mueller BR, Bale TL (2008): Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 28: 9055–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bolton JL, Huff NC, Smith SH, Mason SN, Foster WM, Auten RL, Bilbo SD (2013): Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect. 121: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gur TL, Shay L, Palkar AV, Fisher S, Varaljay VA, Dowd S, Bailey MT (2017): Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav Immun. 64: 50–58. [DOI] [PubMed] [Google Scholar]
- 134.Murgatroyd CA, Hicks-Nelson A, Fink A, Beamer G, Gurel K, Elnady F, et al. (2016): Effects of Chronic Social Stress and Maternal Intranasal Oxytocin and Vasopressin on Offspring Interferon-γ and Behavior. Front Endocrinol (Lausanne). 7. doi: 10.3389/fendo.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nelson LH, Lenz KM (2017): The immune system as a novel regulator of sex differences in brain and behavioral development. J Neurosci Res. 95: 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ (2012): Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Babri S, Doosti M-H, Salari A-A (2014): Tumor necrosis factor-alpha during neonatal brain development affects anxiety- and depression-related behaviors in adult male and female mice. Behav Brain Res. 261: 305–314. [DOI] [PubMed] [Google Scholar]
- 138.de Souza DF, Wartchow KM, Lunardi PS, Brolese G, Tortorelli LS, Batassini C, et al. (2015): Changes in Astroglial Markers in a Maternal Immune Activation Model of Schizophrenia in Wistar Rats are Dependent on Sex. Front Cell Neurosci. 9: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE (2004): Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 103: 546–550. [DOI] [PubMed] [Google Scholar]
- 140.Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I (2013): Is Maternal Influenza or Fever During Pregnancy Associated with Autism or Developmental Delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) Study. J Autism Dev Disord. 43: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghassabian A, Albert PS, Hornig M, Yeung E, Cherkerzian S, Goldstein RB, et al. (2018): Gestational cytokine concentrations and neurocognitive development at 7 years. Transl Psychiatry. 8: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. (2006): The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 26: 4752–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davis EP, Sandman CA (2010): The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 81: 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jaffee SR, Price TS (2008): Genotype-environment correlations: implications for determining the relationship between environmental exposures and psychiatric illness. Psychiatry. 7: 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]