Abstract
Cerebral edema (CE) and resultant intracranial hypertension are associated with unfavorable prognosis in traumatic brain injury (TBI). CE is a leading cause of in-hospital mortality, occurring in >60% of patients with mass lesions, and ~15% of those with normal initial computed tomography scans. After treatment of mass lesions in severe TBI, an important focus of acute neurocritical care is evaluating and managing the secondary injury process of CE and resultant intracranial hypertension. This review focuses on a contemporary understanding of various pathophysiologic pathways contributing to CE, with a subsequent description of potential targeted therapies. There is a discussion of identified cellular/cytotoxic contributors to CE, as well as mechanisms that influence blood-brain-barrier (BBB) disruption/vasogenic edema, with the caveat that this distinction may be somewhat artificial since molecular processes contributing to these pathways are interrelated. While an exhaustive discussion of all pathways with putative contributions to CE is beyond the scope of this review, the roles of some key contributors are highlighted, and references are provided for further details. Potential future molecular targets for treating CE are presented based on pathophysiologic mechanisms. We thus aim to provide a translational synopsis of present and future strategies targeting CE after TBI in the context of a paradigm shift towards precision medicine.
Keywords: traumatic brain injury, cerebral edema, cytotoxic edema, ionic edema, vasogenic edema
1. Introduction
Cerebral edema (CE) can be defined as an increase in brain tissue water, including in individual cells and their surrounding interstitial space. The generation of CE after TBI is a complex heterogeneous process. Underlying mechanisms of CE may differ based on the non-modifiable nature of primary injury (etiology, velocity, force, severity, hemorrhage pattern), patient characteristics (age, sex, genetics, comorbidities), and additional clinical insults (hypoxia, hypotension, hyperthermia, seizure). A comprehensive molecular understanding of the complex networks underlying CE in TBI, although rapidly evolving, remains in its infancy. Research advances have implicated several fundamental pathophysiologic processes that contribute to edema development, including disruption of BBB integrity, cellular volume regulation by various ionic pumps, oncotic gradients and inflammatory responses (Hadass et al., 2013; Jayakumar et al., 2011; Kiening et al., 2002; Kimbler et al., 2012; Kochanek et al., 2015; Laird et al., 2014; Liang et al., 2015; Lopez-Rodriguez et al., 2015; Marmarou et al., 2006; Okuma et al., 2012; Shigemori et al., 2006; Walcott et al., 2011; Yao et al., 2015; Zweckberger et al., 2014) (Figure 1). Classically, CE after central nervous system (CNS) injury has been categorized as ‘vasogenic’ or ‘cytotoxic’, but it is increasingly recognized that these processes may be interrelated (Marmarou, 2007; Simard et al., 2007; Winkler et al., 2016). Unchecked, CE can lead to intracranial hypertension and fatal brainstem herniation. Treatment strategies aimed at removing CE after it has formed may be less beneficial than those aimed at modulating the degree and timing of activating/inhibiting the various pathways that contribute to edema formation.
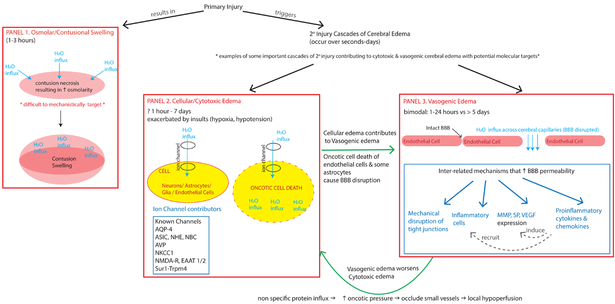
Figure 1. Schema of some key contributors to intracranial swelling after TBI.
This diagram highlights different pathways that contribute to intracranial swelling and cerebral edema (CE). Panel 1: Immediately after primary injury, there may be osmolar/contusional swelling. As described by Katayama et al (1992), the necrotic core of the contusion has high osmolarity that drives water movement along this gradient (direction of water movement shown by light blue arrows). This results in acute swelling of the contusion. Primary injury simultaneously triggers many cascades of secondary injury including cellular/cytotoxic edema (CytE, Panel 2) and vasogenic edema (VasE, Panel 3). Some important examples of CytE (Panel 2) include the activation/upregulation of various ion channels (some known channels include AQP4, ASIC, NHE, NBC, AVP, NKCC1, NMDA-R, Sur1-Trpm4). These channels allow water influx into different cell types depending on the cell’s expression of the respective channel. Excessive ion-channel related water influx (light blue arrow) into a cell (yellow oval) may result in oncotic cell death. This occurs in neurons/astrocytes/glia and can occur within 1 hour −7 days. When this process occurs in cells contributing to blood-brain-barrier (BBB) integrity like endothelial cells/some astrocytes, it contributes to VasE (straight green arrow showing relationship between CytE in Panel 2 and VasE in Panel 3). Secondary injury cascades also include additional processes that disrupt the BBB, result in water movement across the disrupted BBB capillaries (light blue arrows) and cause VasE (Panel 3). These cascades include mechanical disruption, release of proinflammatory cytokines and chemokines that recruit migration/activation of inflammatory cells, increased expression and release of factors (such as MMP, SP, VEGF) that disrupt tight junctions and basement membrane proteins. VasE, with its proteinaceous fluid influx, may further raise oncotic pressure in the interstitium, occluding small vessels causing local hypoperfusion, which in turn may further exacerbate CytE development and upregulation of ion channels like Sur1-Trpm4. This relationship is shown by the curved green arrow between Panels 2 & 3).
AQP-4 = aquaporin 4, ASIC =Acid sensing ion channel, NHE = Na+/H+ exchanger, NBC = Na+/HCO3− transporter family channel, AVP = arginine vasopressin, NKCC1 = Na+-K+-2Cl− cotransporter, Sur1 = sulfonylurea receptor 1, Trpm4 = transient receptor potential cation channel subfamily M member 4, MMP = matrix metalloproteinase, SP = substance P, VEGF = vascular endothelial growth factor
2. Cerebral edema and intracranial hypertension in TBI
An important relationship between cerebral edema, intracranial hypertension, and functional outcome in TBI has been recognized for centuries. The Edwin-Smith Papyrus from the 17th century BC describes the use of neurological examinations to classify injury severity, identify intracranial hypertension and prognosticate outcome as early as 3000-2500 BC (Helgason, 1987). The use of external ventricular drains for therapeutic diversion of cerebrospinal fluid, first reported in 1744, was expanded to TBI in the 1980s, two centuries after the development of the Monro-Kellie doctrine from 1783 (Srinivasan et al., 2014). The Monro-Kellie doctrine correlates the development of CE to the practical measure of intracranial pressure (ICP) – it states that pressure within the cranial vault is a function of the volume of individual intracranial compartments (blood, brain, CSF) contained within a rigid skull/ dura matter (Macintyre, 2014). ICP elevation after TBI thus can be a product of volume increase from hematoma/contusions as well as secondary injury processes such as CE. Although hematoma expansion is a primary concern in the first few hours after TBI, after this has been managed, CE is a major contributor to ICP elevation (Stocchetti and Maas, 2014). Intracranial hypertension can compromise cerebral perfusion pressure (CPP) and cerebral blood flow (CBF), which can eventually cause irreversible brain injury, herniation and death (Stocchetti and Maas, 2014; Winkler et al., 2016). Patients with disrupted cerebral autoregulation may be particularly vulnerable, as is often the case in TBI (Stocchetti and Maas, 2014).
Clinically, intracranial hypertension, measured by ICP monitor, has served as an evaluation and treatment proxy for CE and been a focus of guideline-based TBI care (Carney et al., 2017). Both ICP elevation and radiographic measures of CE in TBI have long been associated with unfavorable outcome in multiple cohorts (Chesnut et al., 1993; Eisenberg et al., 1990; Feickert et al., 1999; Feldmann et al., 1979; Hudak et al., 2014; Iaccarino et al., 2014; Marmarou et al., 1991; Marshall et al., 1979; Miller et al., 1977; Saul and Ducker, 1982; Stocchetti et al., 2008; Tucker et al., 2017; Vik et al., 2008). ICP elevation above the accepted threshold of 20–22 mmHg occurs in 45%–80% of patients with TBI (Marmarou et al., 1991; Narayan et al., 1982; Vik et al., 2008). There appears to be a dose-dependent effect between ICP>20 mmHg and clinical outcome after TBI (Vik et al., 2008). Preclinical studies support this strong association between CE and unfavorable outcome in TBI. Preclinical research also demonstrates that treatment of targeted pathways can reduce CE and/or improve outcome (Cao et al., 2016; Donkin et al., 2011; Fukuda et al., 2013; Gao et al., 2016; Hadass et al., 2013; Hou et al., 2018; Ruchira M Jha et al., 2018; Liu et al., 2018; McBride et al., 2014; Patel et al., 2010; Rauen et al., 2013; Shamsi Meymandi et al., 2018; Xu et al., 2016; Yang et al., 2018; Yao et al., 2015; M. Zhang et al., 2016; Zweckberger et al., 2014). In humans, no targeted therapies are clinically available. Reactive non-specific treatments such as craniectomy are invasive and carry morbidities (Stiver, 2009; Stocchetti and Maas, 2014). While decompressive craniectomy has been effective at reducing ICP and mortality in recent large randomized-controlled trials, it has an unclear benefit on functional outcome (Cooper et al., 2011; Hutchinson et al., 2016). There are similar concerns about clinical benefit despite ICP reduction with other non-specific treatments like hypothermia and hyperosmolar therapies (Andrews et al., 2015; Gottlieb and Bailitz, 2016). ICP is likely an incomplete reflection of CE, thus generalized treatment of ICP alone may not influence key pathophysiological mechanisms (Jha et al., 2018a). This underscores the importance of developing targeted therapies to expand the treatment armamentarium for CE by focusing on important molecular contributors and underlying pathways.
ICP and CPP are clinically available measures in TBI, with guidelines available to inform their management (Carney et al., 2017). Future strategies also may incorporate individual measures of intracranial compliance, pressure reactivity indices (PRx), pulse amplitude index (PAx) and optimal-cerebral perfusion pressure (CPPOPT) that are currently of research interest. While a focus on these measures is beyond the scope of this review, a brief summary is provided below with references available for further review.
Not explicitly factored into the Monro-Kellie doctrine is the concept of an individual’s intracranial compliance and elastance which affect the degree of ICP elevation. While difficult to measure clinically, quantifying individual cerebral compliance (ΔV/ΔP) or its inverse, i.e., elastance (ΔP/ΔV), could be useful to predict impending ICP crises and herniation (Czosnyka and Citerio, 2012; Howells et al., 2012). Originally described by Marmarou et al. in a cat model, the pressure volume index (PVI) was one of the first measures of intracranial elastance (Czosnyka et al., 2012; Marmarou et al., 1975). PVI is the volume (mL) required to raise ICP 10-fold and is normally 20-25 mL (PVI (mL) = V/log (Pp/Po) where V= volume in mL, Pp= peak ICP, and Po= ICP prior to volume change) (Marmarou et al., 1978; Shapiro et al., 1980). Despite its potential utility, PVI is technically challenging, carries an infection risk, and may precipitate ICP crises (Hawthorne and Piper, 2014). Research efforts have largely been redirected towards indirect measures of cerebral compliance such as ICP waveform/amplitude analysis and MRI based quantification (Alperin et al., 2000; Atsumi et al., 2014; Czosnyka and Citerio, 2012; Howells et al., 2012; Raksin et al., 2003). Similarly, measures of cerebrovascular reactivity such as the PRx, PAx and CPPOPT are ongoing research interests with the goal of eventually directing precision-medicine based treatment for ICP and/or CPP targets and optimal blood flow (Dias et al., 2015; Zeiler et al., 2018, 2017). PRx, a dynamic correlation coefficient (−1 to +1), is obtained from a 5-second average of 40 consecutive measurements of mean arterial pressure (MAP) and ICP. With intact autoregulation, an increase in MAP results in vasoconstriction, thus reducing ICP (negative PRx); conversely, absent pressure reactivity results in ICP passively following MAP (positive PRx) (Zweifel et al., 2008). CPPOPT, has been defined as the point of lowest PRx – a value that may differ not only between individual patients, but also within the same patient at different times (Steiner et al., 2002). PAx is the ICP pulse amplitude index given by the Pearson correlation coefficient between MAP and ICP pulse amplitude (Zeiler et al., 2017). As intracranial elastance increases (compliance decreases), ICP pulse amplitude increases (Hawthorne and Piper, 2014). In research studies, these measures are predictive of outcome and may be manipulable; however they are yet to be validated for clinical/guideline-based care (Dias et al., 2015; Zeiler et al., 2017; Zweifel et al., 2008).
The measures described above are all focal reflections of a diffuse, multifaceted, heterogeneous and dynamic process. In an evolving world of precision-medicine, it may be prudent to complement these measures with other forms of multimodal monitoring, imaging studies, phenotypic and genetic biomarkers to provide a nuanced and mechanistic approach to understanding and ultimately treating CE in TBI (Jha and Kochanek, 2017).
3. Pathways involved in ionic and cellular/cytotoxic edema (CytE)
Cytotoxic edema (CytE) results from ionic pump failure or activation of select ion channels, with ensuing loss of homeostatic ionic gradients. This causes cellular swelling wherein water moves from the interstitial to intracellular space (Hudak et al., 2014; Winkler et al., 2016). It has been reported as early as 1 hour post-TBI in humans (Ito et al., 1996). Cellular edema can occur in all CNS cell types, including astrocytes, endothelial cells and neurons; it has been carefully characterized in astrocytes (Stokum et al., 2016). Isolated cellular swelling does not increase total brain water, since it is a redistribution of water from the interstitial to the intracellular space. A true increase in brain water content requires perfusion from an external fluid source, which is debated to be the cerebral vasculature (driven by osmotic forces), and/or the glymphatic system (due to enhanced cerebrospinal-fluid (CSF) influx or impaired efflux); importantly, these hypotheses are not mutually exclusive (Iliff et al., 2014; Stokum et al., 2016; Thrane et al., 2014). If unrestrained, ionic failure and resultant cellular edema becomes cytotoxic and leads to cell death (Hudak et al., 2014; Simard et al., 2007). Ionic edema is analogous to CytE of endothelial cells, but there is a polarity to transcapillary ion and water fluxes. A continuum of CytE, ionic edema, vasogenic edema (VasE), and progressive secondary hemorrhage (PSH) is illustrated in Figures 2-5 (Simard et al., 2007). As described below, many of these pathways and their components are inter-connected.
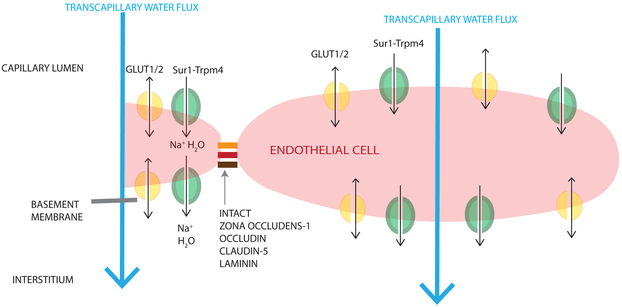
Figure 2. Mechanisms contributing to ionic edema after TBI.
The diagram is oriented such that the capillary lumen is superior to the endothelial cell, and the interstitium and neuronal/glial tissue is inferior. Ionic edema involves the transcapillary net water flux (large light blue arrow) from the capillary lumen to the brain interstitium without violation of the BBB or basement membrane. Like cellular/cytotoxic edema (CytE), water is transported through luminal ion channels (e.g. GLUT1/2 (shown in yellow), Sur1-Trpm4 (shown in green), NKCC1, NHE1/2, ASIC, NBC, EAAT1/2, SGLT1) into the endothelial cell; water is subsequently transported out of the endothelial cells by channels expressed abluminally (GLUT1/2 (yellow), Sur1-Trpm4 (green)) primarily due to an osmotic gradient created by CytE. Single arrows (black) are used for water co-transport, double headed arrows are used for passive water movement through a channel pore.
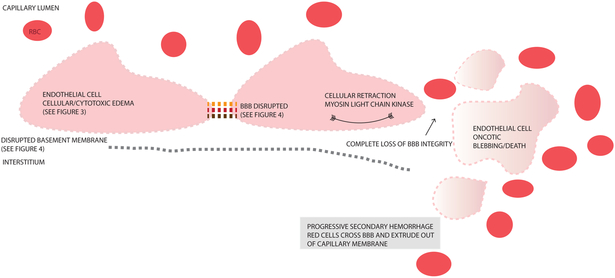
Figure 5. Progressive Secondary Hemorrhage.
This figure depicts consequences of blood brain barrier (BBB) breakdown with loss of tight junctions (interrupted orange/red/brown links between endothelial cells), disrupted basement membrane, retraction of endothelial cells, and oncotic cell blebbing/death of endothelial cells. This ultimately destroys all BBB integrity, allowing extravasation of blood components across the capillary membrane eventually resulting in progressive secondary hemorrhage (PSH). Details of the precursor CytE and VasE mechanisms that lead up to these processes and PSH are outlined in Figures 3 and 4.
3.1. Na+-K+-2Cl− cotransporter (NKCC1)
NKCC1 is a Na+-K+-2Cl− cotransporter expressed constitutively in neurons, glia, capillary and choroid-plexus endothelial cells (J. Zhang et al., 2017). Normally, NKCC1 uses the electrochemical gradient generated by the Na+/K+/ATPase to mediate transport of Na+, K+, 2X Cl− ions (with water co-transport) across the plasmalemma, thus regulating cell volume and ion homeostasis (J. Zhang et al., 2017). In preclinical TBI models, NKCC1 is upregulated as early as 1 hour post-injury and is activated by a variety of mechanisms including increased extracellular K+ (after Na+/K+/ATPase failure, particularly in astrocytes), IL-1β, and glutamate (see Sections 2.5 and 3.1) (Jayakumar et al., 2011; Lu et al., 2006, 2008, 2017; Simard et al., 2010; J. Zhang et al., 2017; M. Zhang et al., 2016). Transporter activation after TBI results in elevated intracellular ionic content and water, i.e., CytE. In CNS endothelial cells, NKCC1 is expressed primarily on the luminal side, thus contributing to cellular edema loading Na+ into cells (Simard et al., 2010). Some in vivo data suggest a link between this pathway and AQP4 upregulation after controlled cortical impact (CCI) (see Section 2.2), as well as matrix-metalloproteinase (MMP)-9 production (see Section 3.5) and BBB integrity (J. Zhang et al., 2017; M. Zhang et al., 2016). NKCC1 activation also may trigger a mitogen-activated protein kinase cascade, further increasing CE and neuronal damage after TBI (Lu et al., 2017, 2008).
3.2. Aquaporins (AQP)
AQPs likely influence both cellular/CytE and VasE in TBI. In the CNS, AQP1 and AQP4 are most prevalent (Papadopoulos and Verkman, 2013). AQP1, involved in CSF secretion, is primarily expressed in the ventricular-facing plasmalemma of choroid plexus epithelium; it is absent from cerebrovascular endothelium (except in circumventricular locations lacking a BBB) (Papadopoulos and Verkman, 2013). AQP4 localizes to brain-fluid interfaces including perivascular astrocyte endfeet, glia limitans, basolateral membrane of ependymal cells and subependymal astrocyte processes. In CytE, water enters the CNS through AQP4 on perivascular astrocyte foot-processes. In VasE, water is eliminated via AQP4 via different routes: astrocyte foot-processes into the blood stream, subpial astrocyte processes and pial cells into subarachnoid CSF, and across subependymal astrocyte processes and ependymal endothelium into the ventricle, the glymphatic system (Filippidis et al., 2016; Hubbard et al., 2018; Iliff et al., 2014). The M23 AQP4 isoform associates in membranes as a tetramer, and can aggregate into orthogonal arrays of particles (supramolecular assemblies) (Papadopoulos and Verkman, 2013).
In TBI, since there are contributions from both CytE and VasE, determining the overall contribution of AQP4 to edema formation versus elimination has been challenging and may be related to spatial/temporal expression patterns. TBI studies have shown AQP4 downregulation for up to 48 hours after TBI (potentially coinciding with VasE), some of which occurs specifically in regions with BBB disruption (Cartagena et al., 2014; Ke et al., 2001; Kiening et al., 2002; Liu et al., 2015; Zhang et al., 2015). Other studies have shown AQP4 upregulation coinciding with CytE development within 72 hours (Lopez-Rodriguez et al., 2015; Lu et al., 2013; Taya et al., 2010). A study in murine closed head injury demonstrated that, while there was a global increase in cortical and striatal AQP4 expression (peak at 7 days), perivascular AQP4 expression was markedly reduced by day 3 (persisting until day 28) (Ren et al., 2013). While not clearly noted, this suggests that changes in AQP4 localization/loss of polarization, while potentially worsening VasE (by limiting water clearance), may be a compensatory mechanism to counteract/decrease CytE (Ren et al., 2013). A subsequent study in mild-CCI demonstrated that astrocytic foot-process edema was reduced in AQP4−/−mice (from decreased CytE); however, the effects were smaller than AQP4 deletion in models of pure CytE, likely a consequence of the decreased AQP4-dependent clearance of VasE (Yao et al., 2015). Increased AQP4 expression has been reported in human TBI tissues, and CSF levels are significantly higher in patients with severe TBI versus controls. Further studies are warranted to evaluate the impact on edema (Hu et al., 2005; Lo Pizzo et al., 2013).
3.3. Sulfonylurea-Receptor 1 – Transient Receptor Potential member 4 (Sur1- Trpm4)
Sulfonylurea receptor 1 (Sur1) co-assembles with various pore-forming subunits (e.g., Kir6.2, Trpm4) to form hetero-octameric channels. The association of Sur1 with the non-selective cation channel Trpm4, and the relationship of this channel to CytE was first described in ischemic stroke, and has since been characterized in other CNS diseases including TBI (Patel et al., 2010; Simard et al., 2006, 2009, 2012b). Unique to this pathway, the Sur1-Trpm4 channel is not normally expressed in the CNS but is transcriptionally upregulated by injury, thus rendering it an ideal target for therapeutic intervention. Unlike NKCC1, intracellular ATP depletion activates Sur1-Trpm4, promoting channel opening, sodium influx, cell depolarization, blebbing, and cellular/cytotoxic death. In TBI, Sur1 is upregulated in endothelial capillaries, astrocytes and neurons (Patel et al., 2010; Simard et al., 2009). Neuronal or astrocytic increases in Sur1-Trpm4 result in CytE and eventually oncotic death of these cells. Luminal Sur1-Trpm4 expression in endothelial cells contributes to ionic edema, and eventually cytotoxic/cellular edema of the endothelial cells. This further contributes to VasE, as the endothelial cells continue to swell and tight junctions between them are degraded, thereby transforming capillaries into ‘fenestrated’ capillaries that allow proteinaceous fluid extravasation (Kurland et al., 2012; Stokum et al., 2016). In its extreme form, capillary/BBB integrity is lost, resulting in PSH (Figure 5) (Kurland et al., 2012; Simard et al., 2007). Thus, CytE, VasE and PSH may represent a continuum of CE in TBI.
Human CSF Sur1 is elevated in TBI versus controls, and correlates with CE (Jha et al., 2017a). Sur1 overexpression has also been demonstrated in post-traumatic human contusions, particularly in neurons and endothelial cells (Martínez-Valverde et al., 2015). Polymorphisms in the Sur1 gene (ABCC8) have been associated with increased risk of CE (Jha et al., 2017b; Ruchira Menka Jha et al., 2018b), which may have important implications for precision medicine patient risk-stratification, therapeutic response, and selection for clinical trials. A recent study demonstrated a tripartite-complex association between Sur1-Trpm4-AQP4 (Stokum et al., 2018). While this has not been demonstrated in TBI, it has important implications for the pathophysiology of edema generation and interconnections between contributory pathways.
3.4. Acid sensing ion channel (ASICs), Na+/H+ exchanger (NHE), Na+/HCO3− transporter family channel (NBC)
ASICs are upregulated and activated by extracellular H+, and open to inwardly conduct Na+ (some subunits, e.g., ASIC1a, also conduct Ca2+) (Gu et al., 2010; Xiong et al., 2004; Yermolaieva et al., 2004; Yin et al., 2013; Zhao et al., 2008). In the CNS, ASIC1a and ASIC2 heterotrimers are the most widely expressed. In human cortical neurons, the pH required for half maximal activations is 6.60 (Li et al., 2010). Channel opening and Na+ influx causes cell depolarization and an increase in water content resulting in cell swelling. Glutamate receptor-independent Ca2+ entry via these channels also contributes to excitotoxicity and apoptosis. Other constitutively expressed pH-regulated channels (NHE, NBC) activated by an interstitial pH ≤6.8, also mediate astrocytic swelling in vitro; however their contribution to CytE in TBI in vivo has not been established (Stokum et al., 2016).
3.5. Glutamate
After TBI, glutamate excitotoxicity has both deleterious effects on secondary injury cascades (including CE) as well as a later beneficial impact on neuronal survival (Chodobski et al., 2011). Early after injury, extracellular glutamate concentrations may rise beyond 200 μM (synaptic release, cellular/neuronal lysis, spreading depolarization) (Stokum et al., 2016); even 5–50 μM can induce astrocytic edema via the constitutively expressed EAAT1/2 channels. In normal brain, EAAT2 forms a multiprotein-complex with AQP4, and contributes to glutamate uptake and homeostasis. Elevated glutamate levels after injury mediate the influx of glutamate via EAAT1/2 expressed on astrocytes, with co-transport of Na+ and water, thus inducing astrocytic CytE (Stokum et al., 2016). Glutamate can also contribute to CytE through its ionotropic NMDA-receptor, leading to intracellular influx of Na+ and Ca2+ (Winkler et al., 2016). In vitro experiments suggest that glutamate acting through metabotropic receptors also may increase BBB permeability (Chodobski et al., 2011; Collard et al., 2002). Glutamate may induce brain endothelial apoptosis through increased production of nitric oxide and reactive oxygen species; however, this is controversial (Domoki et al., 2008; Parfenova et al., 2006, 2003).
3.6. Arginine Vasopressin
Arginine vasopressin (AVP), a nonapeptide produced by the paraventricular and supraoptic hypothalamic nuclei, is upregulated after TBI (Krieg et al., 2017; Winkler et al., 2016). The V1a receptor, widely distributed in neurons, astrocytes and endothelial cells, is also upregulated perilesionally after injury (Krieg et al., 2017; Marmarou et al., 2014; Pascale et al., 2006). Both genetic and pharmacologic inhibition in TBI models has successfully reduced CytE (Kleindienst et al., 2013; Krieg et al., 2017, 2015; Pascale et al., 2006; Rauen et al., 2013). The mechanism of V1a-mediated CytE is unknown but is hypothesized to be related to modulation of parenchymal vasopressin regulation of AQP4 expression and function (Filippidis et al., 2014). Modulation of V1a in rats has shown an impact on brain extracellular Na+ concentrations and ICP; however, this has not been demonstrated in humans (Allen et al., 2018; Filippidis et al., 2014).
3.7. Commentary on molecular candidates contributing to CytE
There has been an exponential growth in literature identifying various pathways contributing to different cerebral edema subtypes. The candidates discussed in this subsection on ionic and CytE were selected for inclusion based on research and evidence specifically in TBI, with the most promising candidates being presented based on multiple studies performed by more than one laboratory group. Given the connective network between many of these molecular targets such as NKCCl, glutamate, AQP4, and Sur1-Trpm4 (Stokum et al., 2018; J. Zhang et al., 2017; M. Zhang et al., 2016), it is difficult to isolate a selective hierarchy of importance among these with the information available at this stage. There is also overlap between some of these molecular components and those involved in VasE (e.g., MMP-9; see Subsection-4). Identification of targets that are inter-related (or even inter-dependent), suggests their relative importance in the underlying pathophysiology of CE versus more apparently isolated targets such as vasopressin, NHE, NBC. Indeed, it is possible that a multi-targeted approach may eventually be most beneficial and should be evaluated in preclinical and clinical studies. While the authors’ biases towards NKCCl, AQP4, Sur1-Trpm4 and MMP-9 are based on available evidence both in terms of individual studies as well as their potential inter-relatedness, it is important to remain cautious since connections between pathways and other molecular targets may exist but are currently unknown. As discussed in Subsection-5, of the targets outlined, the only one that to date has shown promise in a prospective human randomized trial of TBI is Sur1-Trpm4, where glibenclamide decreased contusion expansion (Khalili et al., 2017); results from another clinical trial evaluating glibenclamide (NCT01454154) are pending. Strategies against AQP-4 and NKCC1 while promising have not yet been assessed for clinical relevance in human TBI studies.
4. Pathways involved in BBB disruption and vasogenic edema
One of the first contributors to BBB breakdown in TBI is immediate mechanical disruption after primary injury (Figure 1). Contusional edema is generated from an osmotic potential across central necrotic tissue (high osmolality) and surrounding brain (Katayama et al., 1998; Katayama and Kawamata, 2003). Katayama et al. initially characterized this pattern of edema in animals by measuring contusion and peri-contusional osmolality and water content (Katayama et al., 1998). They confirmed this in patients using apparent diffusion coefficient imaging (Ito et al., 1996; Katayama et al., 1998; Katayama and Kawamata, 2003). Mechanical disruption, although the most immediate cause, is not the sole etiology of BBB breakdown; second messenger cascades including proinflammatory cytokines/neuroinflammation, angiogenic factors, tight junction degradation, adhesion molecules/factors promoting protein extravasation and cytoskeletal rearrangements are some of the additional factors contributing to a leaky endothelium/VasE that may peak between 6–24 hours (Figure 2) (Winkler et al., 2016). Proteinaceous fluid leaking into the interstitial space may further increase oncotic pressure and occlude small vessels, causing local hypoperfusion/ischemia that can exacerbate ionic failure/CytE (Stocchetti and Maas, 2014; Winkler et al., 2016). Maximal BBB permeability has been noted within the first few hours post-injury in different TBI models, and can persist for 3–4 days (Readnower et al., 2010; Shetty et al., 2014; Tanno et al., 1992; Whalen et al., 2000b; Yeoh et al., 2013). A second peak in BBB breakdown may occur after 5 days, which is thought to be mediated by microglial activation (Readnower et al., 2010; Winkler et al., 2016). Historically, CE in TBI was thought to be primarily vasogenic, but cytotoxic edema has now been shown to be a significant contributor (Barzó et al., 1997; Hudak et al., 2014; Marmarou et al., 2006; Winkler et al., 2016). While an earlier study (44 subjects) found a predominance of cellular edema post-TBI, a recent study (97 subjects) showed a more even split with 46% of patients demonstrating predominantly CytE and 54% with predominantly VasE (Hudak et al., 2014; Marmarou et al., 2006). The predominant edema type may indeed be highly dependent on the individual patient based on the injury phenotype, genetics, as well as secondary insults and treatments provided.
4.1. Inflammatory cytokines
Neuroinflammation after TBI is a multifaceted process implicated in both secondary injury and repair/ recovery (Jassam et al., 2017; Simon et al., 2017). Both primary and secondary injury result in the release of damage associated molecular patterns (DAMPS) that, in turn, activate networks of cells (glia, neurons, endothelial cells, leukocytes) to induce inflammatory gene expression, guiding the ensuing immune response (Jassam et al., 2017). This includes upregulation and release of cytokines such as tumor necrosis factor (TNF) and interleukins (IL) 6 and 1β, which are early mediators of post-traumatic inflammation (Simon et al., 2017). The mechanisms by which pro-inflammatory cytokines induce BBB dysfunction are many, and are still being elucidated. Reported pathways include increased MMP expression (Alluri et al., 2016; Guilfoyle et al., 2015; Hadass et al., 2013; Rosenberg and Yang, 2007), leukocyte/neutrophil recruitment with subsequent loss of tight junctions (Bolton et al., 1998), production of permeability-increasing molecules (bradykinin, substance P) (Walker et al., 1995), increased chemokine synthesis, expression of inflammatory cell adhesion molecules (intracellular adhesion molecule (ICAM)/vascular cell adhesion molecule) and recruitment of other systemic inflammatory cells (Chodobski et al., 2011; Winkler et al., 2016). One reported cascade involves HMGB1 binding to TLR4, which upregulates IL-6; this in turn leads to astrocytic upregulation of AQP4 and VasE (Laird et al., 2014). While there is some support for this pathway influencing outcome in humans, its role in the development of CE remains to be characterized (Au et al., 2012). Another key pro-inflammatory cytokine, TNF, has been shown to promote actin stress fiber formation followed by endothelial cell retraction (and formation of intracellular gaps mediated by myosin light chain kinase MLCK), as well as downregulating the tight junction protein, occludin (Bolton et al., 1998; Chodobski et al., 2011; Mankertz et al., 2000; Wójciak-Stothard et al., 1998); however, these action have yet to be demonstrated in TBI models.
While early pro-inflammatory cytokines are themselves associated with BBB disruption, they also induce additional cytokines such as tumor growth factor (TGF) β, which further compromise BBB integrity (Morganti-Kossmann et al., 1999). In vitro studies demonstrated a dose-dependent effect of increasing TGF-β levels on paracellular permeability of brain endothelial monolayers, thought to be due to decreased expression of VE-cadherin and the tight junction protein, claudin-5 (Shen et al., 2011). In 22 patients, elevated CSF TGF-β levels were associated with increased BBB permeability (Morganti-Kossmann et al., 1999). IL-8, another upregulated cytokine in glia and endothelial cells, increased BBB permeability in animal and human studies. In children, high CSF IL-8 levels were associated with mortality; in adults, this was associated with BBB permeability but not mortality (Buttram et al., 2007; Helmy et al., 2011; Maier et al., 2001; Whalen et al., 2000a). Elevated CSF complement levels (C3 and factor B), peaking 24 hours post-injury and declining on days 2–7, also have been associated with BBB dysfunction after TBI (Kossmann et al., 1997; Stahel et al., 2001).
4.2. Chemokines
Elevated chemokine levels have been demonstrated in many TBI models (Woodcock et al., 2017). Early after TBI, chemokines appear to be synthesized by astrocytes and the cerebrovascular endothelium, with later contributions from invading neutrophils and monocytes (Chodobski et al., 2011). Commonly studied chemokines in TBI include chemokine (C-X-C motif) ligands CXCL1 and CXCL2 (neutrophil attractants), and chemokine (C-C motif) ligand CCL2, which induces a chemotactic response in macrophages, monocytes and microglia (Woodcock et al., 2017). In addition to recruiting leukocytes after TBI, chemokines also may increase BBB permeability via mechanisms similar to cytokines. For example, CCL2 induces the formation of actin stress fibers and causes redistribution of claudin5, occludin, and zona-occludens (ZO) 1 and 2 (Chodobski et al., 2011; Stamatovic et al., 2003; Woodcock et al., 2017). As with the balance in neuroinflammation between pro/anti-inflammatory cytokines, atypical chemokines (e.g., ACKR2) also serve as scavengers that internalize and promote degradation of proinflammatory chemokines (Woodcock et al., 2017).
4.3. Inflammatory cells
Migration of peripheral and brain-resident inflammatory cells to the site of injury in TBI can be both beneficial and maladaptive (Corrigan et al., 2016; Simon et al., 2017). In focal injury, there is an early microglial response and neutrophilic infiltration within the injury site (Jassam et al., 2017; Simon et al., 2017). Migration of monocytes, lymphocytes and astrocytes occurs later (Corrigan et al., 2016). In diffuse injury, there is minimal neutrophil infiltration, and the early cellular response (seen primarily in white-matter tracts) consists of microglia and astrocytes (Corrigan et al., 2016). In animal models, cerebrovascular endothelium in the injured hemisphere increases expression of neutrophilic vascular adhesion molecules (E-selectin, ICAM-1) within 4 hours, thus facilitating migration of these peripheral immune cells beyond the BBB (Simon et al., 2017). In stroke, neutrophilic release of elastase and MMPs has been shown to compromise BBB integrity and result in CE (Ikegame et al., 2010; Kenne et al., 2012; Morancho et al., 2010). While this effect of neutrophils in TBI remains controversial, there are recent data in CCI to suggest that neutrophil depletion reduces CE, microglial activation, and caspase-3 (Jassam et al., 2017; Kenne et al., 2012). Antagonism of neutrophil elastase was beneficial in attenuating VasE as well as cell death in CCI (Semple et al., 2015b). Activation of M1-like microglia by proinflammatory cytokines and chemokines is thought to increase BBB permeability by activating MMPs and modulating proteins essential to tight junction formation (Corrigan et al., 2016; da Fonseca et al., 2014).
4.4. Vascular endothelial growth factor A (VEGF-A)
VEGF-A, a secreted glycoprotein, is critical for angiogenesis and also increases microvascular permeability (Chodobski et al., 2011). It is normally expressed in neurons, astrocytes, ependymal cells, and the pial/glial lining. VEGF-A binds to two major receptors: VEGF-receptor (R)-1 (a tyrosine kinase), and VEGFR-2 (kinase domain insert receptor). VEGF-A is upregulated in astrocytes and the cerebrovascular endothelium rapidly after TBI in preclinical models and in human tissues (Chodobski et al., 2003; Suzuki et al., 2003). In vitro (brain and endothelial cells), a VEGF-A-dependent increase in brain endothelial cell permeability is mediated by VEGFR1; mechanisms reported include downregulation and/or ubiquination of the tight junction proteins, occludin and claudin-5 (Argaw et al., 2009; Murakami et al., 2009; Wang et al., 2001). Like MMP-9 (see Section 3.5) and many other mediators of BBB permeability, VEGF also plays a role in neurogenesis and repair; post-TBI treatment with exogenous VEGF had been shown to decrease lesion volume and improve functional outcomes (Thau-Zuchman et al., 2010). These effects may differ temporally, whereby early VEGF expression disrupts the BBB but later is required for neural repair. Of note, MMP-9 processes and activates pro-VEGF and matrix-bound VEGF, one of many examples showing that pathways of CE are often highly interconnected networks, not stand-alone targets (Zhao et al., 2006).
4.5. Matrix metalloproteinase (MMP)
MMPs, a family of zymogens, have been extensively implicated in BBB pathophysiology due to their role in extracellular matrix degradation, as well as regulation of (and induction by) cytokines and chemokines. In TBI, MMPs can be activated by oxidative stress, cytokines, chemokines, infiltrating or resident inflammatory cells, neurons and endothelial cells (Winkler et al., 2016). They are regulated by endogenous inhibitors (tissue-inhibitors of metalloproteinases) (Chodobski et al., 2011). MMP-2 and MMP-9, the most abundantly expressed CNS MMPs, have been the most studied regarding their impact on BBB integrity. A small prospective study in severe human TBI revealed the earliest appearance of MMPs 8 and 9 was at 12–18 hours, followed by MMP-2 (detection 30–35 hours, peak 42–48 hours) (Roberts et al., 2013).
MMP-2 is a gelatinase (type-IV collagenase), predominantly expressed in normal astrocytes (Sharma et al., 2017). It is tethered to the cell surface, thus restricted in its proteolytic reach (S. Zhang et al., 2016). MMP-2 upregulation post-TBI has been noted within 72 hours in animal and human studies, and is associated with ultrastructural changes in endothelial cells and perivascular hemorrhage reflecting BBB disruption (Guilfoyle et al., 2015; Vajtr et al., 2009; Vilalta et al., 2008; S. Zhang et al., 2016).
MMP-9 is released into the extra-cellular space and can have effects distant from the site of release (S. Zhang et al., 2016). Neutrophils are a primary source of MMP-9, but it is expressed in other invading leukocytes, endothelial cells, and also weakly in astrocytes and neurons (Chodobski et al., 2011; Zhao et al., 2006). A study in 12 patients demonstrated elevated MMP-9 microdialysate levels in peri-contusional tissue at ≤ 72 hours. No differences in any of the other MMPs studied (1, 2, 7, 10) were noted between injured versus “normal”/contralateral brain. This, combined with prior reports of CSF MMP-9 levels, suggests its potential utility as a biomarker (Guilfoyle et al., 2015; Nwachuku et al., 2016; Roberts et al., 2013). In human studies of stroke, serum MMP-9 levels correlate with BBB disruption and malignant edema (Jha et al., 2014; Kimberly et al., 2014; Serena et al., 2005; Sheth et al., 2016; Simard et al., 2012a). In a recent clinical stroke trial, MMP-9 levels were lower in glibenclamide-treated subjects, who also had less radiographic midline shift, a measure of CE (Sheth et al., 2016). Glibenclamide inhibits Sur1-Trpm4, and is also an indirect MMP-9 inhibitor (Simard et al., 2012a).
4.6. Substance P (SP)
SP, a tachykinin, is a potent contributor to neurogenic inflammation due to its association with increased vascular permeability, promotion of leukocyte chemotaxis, and activation of astrocytes and microglia to produce proinflammatory cytokines via pathways involving nuclear factor κB (NF-κB), all of which increase BBB disruption (Corrigan et al., 2016; Donkin et al., 2009; Lorente et al., 2015). In the CNS, SP is contained in sensory nerve fibers (densely surrounding cerebral arteries) (Corrigan et al., 2016). SP effects are predominantly mediated by tachykinin (neurokinin; NK) receptors (preferentially NK1 expressed on endothelial cells, astrocytes, microglia, and other immune cells). These are G-protein receptors that regulate ion channel and enzyme activity as well as gene expression (Corrigan et al., 2016). In preclinical and clinical TBI studies, SP levels are elevated early, and are detectable in plasma (Donkin et al., 2009; Lorente et al., 2015; Zacest et al., 2010). One human TBI study (23 subjects) demonstrated increased SP immunoreactivity peri-vascularly and in neurons and astrocytes (Zacest et al., 2010). A second study (100 subjects), demonstrated correlations between plasma SP elevation and unfavorable outcome and mortality, but not with ICP (Lorente et al., 2015).
4.7. Commentary on molecular candidates contributing to VasE
Akin to the discussion in subsection 3.7 regarding candidates contributing to CytE, it is challenging to identify the relative importance of individual VasE targets/pathways, particularly given the dearth of evidence in TBI. Nonetheless, it is an important area for future research. One may speculate that candidates with evidence of inter-relatedness with other targets and across different pathways are likely to have a more significant impact on CE generation. Many of the candidates presented here meet this criterion. For example, Sur1-Trpm4 and AQP-4 have a reported inter-connectedness; both of these contribute to CytE and VasE, and appear to overlap with pathways involving MMP-9 (Amtul et al., 2018; Gerzanich et al., 2018; Lee et al., 2014; Sheth et al., 2016; Stokum et al., 2018). Proinflammatory cytokines and chemokines activate MMP-9, which in turn modulates VEGF (Corrigan et al., 2016; da Fonseca et al., 2014; Zhao et al., 2006). All of these also are linked independently to BBB breakdown and VasE. It is thus possible that analogous to various chemotherapeutic regimens, a multi-pronged treatment approach targeting Sur1-Trpm4, AQP4, VEGF and MMP-9 may be more potent than a single-targeted approach. Such questions may be beneficial to explore in different TBI models, especially in terms of optimizing timing and dose of the various therapies, and evaluating combined toxicity profiles.
5. Targeting cerebral edema in TBI
Current clinical management of CE is targeted towards reducing ICP and maintaining cerebral perfusion pressure, as outlined in the Brain Trauma Foundation guidelines (Carney et al., 2017). These therapies (such as hyperosmolar treatment, sedation, neuromuscular blockade, hypothermia, and craniectomy) are non-targeted, reactive, and have significant side effects. This section discusses some promising molecular candidates targeting the underlying pathophysiology of CE presented earlier that, if successful, may allow clinicians to limit the use of non-targeted therapies.
5.1. Pharmacologic agents targeting cellular swelling
Whether cellular swelling is cytotoxic versus protective in TBI remains unclear, and the two may lie on the same continuum depending on the degree, timing, and location. What is clear, is that this process plays a significant, possibly dominant, role in many TBI patients (Hudak et al., 2014; Marmarou et al., 2006). This section summarizes many promising strategies targeting pathways discussed in Section 2, that are at various stages of development (Table 1). Many of the same molecules influence both cellular swelling and BBB disruption.
Table 1:
Pharmacologic agents targeting cellular swelling
| Agent | Target | Preclinical TBI models | Human Studies |
|---|---|---|---|
|
Bumetanide |
NKCC1 inhibitor |
● ↓ cellular swelling ● ↓ BBB disruption (? via MMP-9, AQP-4 upregulation) |
NCT00830531 (neonatal seizures) |
| AER-271 | AQP-4 inhibitor | ● ↓ ICP in CCI + HS ● No effect on brain water |
- |
| Aquaporumab | AQP-4 monoclonal antibody |
- | - |
| Glibenclamide | Sur1-Trpm4 inhibitor |
● ↓ regional edema ● ↓ ICP ● ↓ PSH ● ↓ BBB disruption ● improve functional outcome |
NCT01454154 (TBI) GAMES-RP (Ischemic stroke, Lancet Neurol, 2016) |
| Amiloride | NHE-1 ASIC1a |
● ↓ brain water (weight drop) | - |
| SR 49059 | V1a receptor antagonist |
● ↓ ICP ● ↓ brain water ● ↓ contusion volume |
- |
| V1880 | V1 receptor antagonist |
● ↓ ICP ● ↓ contusion volume |
- |
5.1.1. Bumetanide
Bumetanide inhibits NKCC1 and reduces astrocytic swelling in vitro after fluid percussion injury (FPI) (Jayakumar et al., 2018, 2011). In vivo TBI models have shown reductions in cellular swelling and BBB disruption (Lu et al., 2006, 2008, 2017; Simard et al., 2010; J. Zhang et al., 2017; M. Zhang et al., 2016). Given the mechanistic and temporal differences in NKCC1 and Sur1-Trpm4 contributions to CE (see Sections 2.1 and 2.3), therapeutic synergy between bumetanide and glibenclamide has been proposed (Simard et al., 2010). Currently, there are no studies of this drug in human TBI but it is being evaluated in neonatal seizures (ClinicalTrials.gov NCT00830531).
5.1.2. AER-271 and Aquaporumab
AQP-4 inhibition in vivo (knockout, RNA-interference) has shown mixed results, with many studies showing edema amelioration and a few showing no-effect/potential worsening (Chen et al., 2016; Fukuda et al., 2013; Higashida et al., 2011; Kiening et al., 2002; Ren et al., 2013; Yao et al., 2015). AQP4−/− mice have reduced edema in models of relatively pure CytE (cerebral ischemia, water intoxication), suggesting a role for AQP4 in CytE generation, but worse edema in models with predominantly VasE (tumors, subarachnoid-hemorrhage, abscesses) (Manley et al., 2000; Papadopoulos et al., 2004; Papadopoulos and Verkman, 2013), indicating the potential importance of AQP4 in elimination of VasE (Filippidis et al., 2016; Hubbard et al., 2018; Iliff et al., 2014). Uninjured AQP−/− mice have normal ICP and minimally increased brain water content, suggesting that slow water fluxes can occur independently of AQP4 (Papadopoulos and Verkman, 2013). AER-271 is a selective AQP-4 antagonist that showed a trend towards decreased ICP in a combined model of CCI and hemorrhagic shock; surprisingly, however, it did not reduce brain water (Wallisch et al., 2015). Aquaporumab, a monoclonal AQP-4 specific antibody, has been utilized to reduce neuromyelitis optica pathology in vivo, but has not been evaluated in TBI (Papadopoulos and Verkman, 2013). No human studies have been reported.
5.1.3. Glibenclamide (glyburide)
Glibenclamide binds to Sur1 (EC50 48 nM at pH 7.4) and blocks the Sur1-Trpm4 channel by prolonging and increasing the probability of the channel’s long-closed state without affecting conductance (Chen et al., 2003; Simard et al., 2014). Its potency is increased eight-fold in acidic environments (pH 6.8), which may be encountered in injury states due to lactic acidosis. In an acidic milieu, glibenclamide’s ability to cross the BBB is also enhanced; thus, it may be selectively taken up in distressed CNS microenvironments (Simard et al., 2014). In preclinical TBI models, glibenclamide (at various doses/durations) reduced regional edema, ICP, PSH, BBB disruption, and neurologic dysfunction (R. Jha et al., 2015; R. M. Jha et al., 2015; Patel et al., 2010; Simard et al., 2009; Xu et al., 2016; Zweckberger et al., 2014). Glibenclamide is being evaluated in a clinical TBI trial (ClinicalTrials.gov NCT01454154, results pending). A Phase-II trial in stroke has shown promising results, decreasing edema measured by midline-shift. In a recent RCT (40 subjects), glibenclamide improved outcomes after moderate-to-severe diffuse axonal injury; however, an effect on edema was not evaluated (Zafardoost et al., 2016). Another RCT (66 subjects) demonstrated that glibenclamide reduced contusion expansion but did not influence clinical outcome in moderate-to-severe TBI (Khalili et al., 2017).
5.1.4. Amiloride
Amiloride inhibits NHE-1 and ASIC1a and was neuroprotective in a preclinical TBI study, as well as other neurologic diseases (Arun et al., 2013; Durham-Lee et al., 2011; Stankowska et al., 2018; Tai and Truong, 2013; Zhao et al., 2008). Its effect on edema has not been well explored but one report in a weight-drop model suggested an ameliorative effect (Vaz et al., 1998). No human studies have been reported.
5.1.5. SR 49059, V1880, Conivaptan and vasopressin
Genetic and pharmacologic inhibition of V1a receptors reduces CytE in vivo (Kleindienst et al., 2013; Krieg et al., 2017, 2015; Pascale et al., 2006; Rauen et al., 2013). SR 49059 is a small molecule selective V1a receptor antagonist, and V1880 is a peptide selective V1 receptor antagonist. Both agents reduce CytE and ICP in preclinical TBI models (Krieg et al., 2017, 2015; Marmarou et al., 2014; Rauen et al., 2013). In a pilot RCT (10 subjects) with randomization to Conivaptan (V1a and V2 antagonist) versus standard-of-care, ICP was lower in the treatment arm (p=0.046) with a concomitant increase in serum Na+ (p=0.02) (Galton et al., 2011). Conversely, human studies evaluating vasopressin (an AVP agonist) in severe TBI have not shown ICP increases or CE exacerbation (Allen et al., 2018; Van Haren et al., 2013).
5.2. Pharmacologic agents targeting BBB disruption
Maintaining and/or restoring BBB integrity after TBI is likely important for protection against both VasE and PSH. However, evolutionarily, BBB disruption (and VasE) may be important, indeed necessary, for facilitating the process of neural repair and regeneration. The key to appropriate management may lie in maintaining a delicate balance of enabling neuroprotection/repair while preventing the process from becoming detrimental; the challenge is in determining where that switch lies and how to control it. While not approved for clinical use, several targeted strategies are being evaluated (Table 2).
Table 2:
Pharmacologic agents targeting blood brain barrier disruption
| Agent | Target | Preclinical TBI models | Human Studies |
|---|---|---|---|
|
ML-7 |
MLCK inhibitor |
● ↓ BBB disruption ● Improved motor/cognitive function |
- |
| Fenofibrate | PPAR-α agonist | ● ↓ BBB permeability (FPI) | - |
| Pioglitazone/Rosiglitazone | PPAR-γ agonist | ● ↓ contusion volume ● ↓ pro-inflammatory cytokine expression ● ↓ neuronal apoptosis |
- |
| SB-3CT | MMP-2/9 inhibitor |
● ↓ BBB disruption ● ↓ lesion volume ● ↓ microglial activation & astrogliosis ● ↓ cortical & hippocampal damage |
- |
| VEGI | VEGI | ● ↓ tissue loss ● ↓ claudin-5, ZO-1, occludin |
- |
| Bevacizumab | Anti-VEGF antibody |
● No effect on ICP/brain water ● Worse functional outcome |
Beneficial in human glioblastoma multiforme |
| Curcumin | Unk (multiple potential) |
● ↓ inflammation ● ↓ brain water content |
|
| NAT | NK1 receptor antagonist |
● ↓ BBB permeability ● ↓ brain water ● ↓ ICP |
5.2.1. ML-7
ML-7 is a MLCK inhibitor that decreases myosin-mediated contraction of endothelial cells, thus decreasing BBB permeability. In preclinical CCI, ML-7 reduced CE and also improved motor and cognitive function (Luh et al., 2010; Rossi et al., 2013). This strategy has not been attempted in humans. Given the ubiquitous distribution of MLCK, there may be substantial side effects, thus necessitating further preclinical studies on dose/timing of administration prior to translation.
5.2.2. Fenofibrate, pioglitazone and rosiglitazone
Peroxisome proliferator-activated receptor (PPAR) agonists have anti-inflammatory properties, including downregulation of cytokines (TNF, IL-1β), NF-κB, expression of ICAM-1 and MMP-9 from PPAR dimerization with retinoid X-receptors (Thal and Neuhaus, 2014; Winkler et al., 2016). Fenofibrate (PPAR-α agonist) stabilized BBB integrity in vitro and decreased BBB permeability and CE in an in vivo FPI model (Besson et al., 2005; Chen et al., 2007; Thal and Neuhaus, 2014). Mechanistically, this has been reported to occur by reducing ICAM-1, iNOS (NOS2), MMP-9, and markers of oxidative stress (e.g., loss of glutathione, glutathione oxidation ratio) (Besson et al., 2005; Chen et al., 2007; Winkler et al., 2016). Pioglitazone and rosiglitazone (PPAR-γ agonists) also have shown promising preliminary results in CCI with reductions in lesion size, proinflammatory cytokine expression and neuronal apoptosis, but have not been evaluated with regards to CE/BBB disruption (Sauerbeck et al., 2011; Yi et al., 2008). One study in CCI suggested that pioglitazone also may have PPAR-γ independent effects, since PPAR- γ inhibition did not reverse its beneficial effect on contusion volume (Thal et al., 2011). Further data are needed to determine the effects of these agents on BBB permeability and outcome in TBI.
5.2.3. SB-3CT and glibenclamide
MMP-9 inhibition remains an active area of research for reducing BBB disruption. MMP-9−/− mice have shown improved BBB integrity, decreased vascular permeability, limited ZO-1 degradation, decreased lesion volume, and a controversial effect on outcome after TBI (Muradashvili et al., 2015; Semple et al., 2015a; Wang et al., 2000). SB-3CT is a highly selective inhibitor of MMP-2 and MMP-9. In stroke, SB-3CT reduces laminin degradation and further attenuates BBB damage by reducing occludin loss and claudin-5 redistribution (Cui et al., 2012; Liu et al., 2012). SB-3CT also has shown promising results in preclinical models of TBI (FPI, CCI), with reductions in MMP-9 activity, lesion volume, microglial activation and astrogliosis as well as long term (30-day) protection from cortical and hippocampal damage (Hadass et al., 2013; Jia et al., 2014). There are no current human studies of targeted inhibition in TBI. Glibenclamide, an indirect MMP-9 inhibitor, has shown decreased MMP-9 levels and reduced midline shift in clinical stroke trials (Kimberly et al., 2014; Sheth et al., 2016). While targeted MMP-9 inhibition appears to be a promising avenue for future research, it is also important to emphasize that MMPs, including MMP-9, significantly contribute to neurovascular remodeling and repair, thus rendering the time and degree of MMP-9 modulation critical in determining beneficial versus adverse effects (Chodobski et al., 2011; Zhao et al., 2006).
5.2.4. Vascular endothelial growth inhibitor and Bevacizumab
Vascular endothelial growth inhibitor (VEGI), also known as tumor necrosis factor superfamily-15, is a cytokine that modulates anti-angiogenesis and anti-inflammation, and is balanced with VEGF to maintain homeostasis. In one study of FPI, treatment with exogenous VEGI reduced tissue loss, microgliosis and upregulated tight junction proteins including claudin-5, ZO-1 and occludin, thus protecting the BBB (Gao et al., 2015). However, exogenous VEGF has also been shown to improve neurogenesis and outcome in TBI, while VEGF inhibition can be harmful (Lee and Agoston, 2010; Sköld et al., 2006; Thau-Zuchman et al., 2010). Thus, like many mediators of CE and BBB integrity, there is an important balance between benefit and deleterious effects of shifting homeostasis towards one particular pathway (i.e., VEGI versus VEGF). Timing of modulation may be key: in a human biomarker study (40 subjects, 30 controls), increasing VEGI levels were associated with improved outcomes, whereas VEGF levels were initially associated with adverse effects early post-TBI (days 4–14) but subsequently were associated with recovery (days 14–21); a Day 7 VEGF/VEGI ratio>2.366 was associated with higher hospital mortality (M. Li et al., 2016).
Bevacizumab, an anti-VEGF antibody with anti-angiogenic effects, has been studied for its beneficial effects on BBB disruption, peri-tumoral edema, and progression-free survival in glioblastoma multiforme (Khasraw et al., 2014). There are no human TBI studies evaluating bevacizumab. One preclinical study of bevacizumab in CCI demonstrated that a single intravenous injection of 10 mg/kg (previously reported to persist for 17±4.7 days in rats), did not influence BBB permeability or water content, but significantly worsened neurologic deficits and contusion volume. While the authors concluded that VEGF expression does not contribute to edema after CCI but may be neuroprotective, it is possible (even likely) that the balance between detrimental versus protective effects is dose- and time-dependent. Further preclinical work is needed to inform potential future human studies of exogenous VEGI or VEGF modulation/activation/inhibition.
5.2.5. Curcumin
Curcumin is an active ingredient of turmeric, and has been found to have anti-inflammatory, anti-carcinogenic, anti-oxidant, and neuroprotective effects in many neurological diseases (W. Li et al., 2016; Qureshi et al., 2018; Reddy et al., 2018; Rodriguez et al., 2016; Yuan et al., 2017; Z.-Y. Zhang et al., 2017). In stroke and subarachnoid hemorrhage, the anti-edema/BBB protective effects of curcumin are proposed to be mediated through various mechanisms including preventing disruption of tight junction proteins (ZO-1, occludin, claudin-5), upregulating glutamate-transporter-1, inhibition of ICAM-1/VCAM-1 and inflammatory cytokines (IL-1β, IL-6, TNF, NF-κB), AQP-4 downregulation, reduced MMP-9 expression and inhibition of microglial activation (W. Li et al., 2016; Wang et al., 2013; Yu et al., 2012; Yuan et al., 2017; Z.-Y. Zhang et al., 2017). Preliminary preclinical TBI studies have focused on potential anti-inflammatory and neuroprotective effects (Ashbaugh and McGrew, 2016; Gao et al., 2017, 2016; Samini et al., 2013; Wu et al., 2011; Zhu et al., 2014). One study in CCI found that curcumin significantly reduced brain water content, attenuated IL-1β and AQP-4 expression, but did not evaluate the BBB; additional studies are needed to further evaluate these findings (Laird et al., 2010).
5.2.6. N-acetyl-L-tryptophan
N-acetyl-L-tryptophan (NAT) is an NK1 tachykinin receptor antagonist with encouraging results in attenuating BBB permeability, CE and functional deficits in TBI (Ameliorate et al., 2017; Donkin et al., 2011, 2009; Gabrielian et al., 2013; Vink et al., 2017). In rodent models, these favorable effects were seen with NAT treatment 30 minutes after TBI, but the therapeutic window extended to 12 hours with evidence of dose dependence (Corrigan et al., 2012; Donkin et al., 2011, 2009). These findings have been replicated in a large-animal (sheep) model of diffuse injury; NAT administration 30 minutes post-injury was associated with a significant and sustained ICP decrease with near-normalization by 4 hours (versus 36% increase in vehicle-treated animals) (Gabrielian et al., 2013). This appears to be a promising therapeutic approach and warrants further investigation.
6. Conclusions
The complexity of CE after TBI is humbling. Over the past few decades, the focus in severe TBI has been on neuronal death, axonal injury and other secondary mechanisms rather than CE. However, a specific focus on CE is emerging as a critical need, given the significant clinical resources dedicated to this condition. This review discusses some known pathophysiologic contributors and targeted treatment prospects, but there are likely many more yet to be discovered. An important step moving forward may be to define quantitative contributions of these various molecular participants in various TBI phenotypes and/or brain regions; a combination of physiological molecular and imaging tools may be essential to such a goal. Current treatments like hypertonic saline, mannitol, or decompressive craniectomy, while effective at reducing intracranial hypertension, have an unclear impact on outcome, highlighting the elusive but essential balance of adaptive versus pathologic swelling. This balance, as well as the underlying mechanisms contributing to CE and clinical ICP targets, may differ between patients based on a variety of characteristics including age, gender, injury characteristics, and genetics. Much like the field of oncology, which has pioneered advances in therapy based on molecular signaling and precision medicine, the future of CE treatment after TBI may require pathophysiology-based targeted treatments. Ongoing discoveries continue to expose exciting new targets, yielding further research in potential therapeutics as well as essential phenotyping of radiographic markers (distinguishing cellular swelling versus BBB disruption), biomarkers (identifying major pathways activated/inhibited) and analysis of multimodal monitoring data (e.g., ICP waveforms, compliance, autoregulation). Although not focused specifically on CE, it is imperative to complement multicenter initiatives such as Transforming Research and Clinical Knowledge in TBI and Collaborative European NeuroTrauma Effectiveness Research in TBI with rigorous preclinical consortiums like Operation Brain Trauma Therapy (Kochanek et al., 2016; Maas et al., 2015; Yue et al., 2013). The tools for implementing a precision-medicine approach to CE after TBI are still in early stages and require significant financial and collaborative support. Nonetheless, the paradigm-shift has begun.
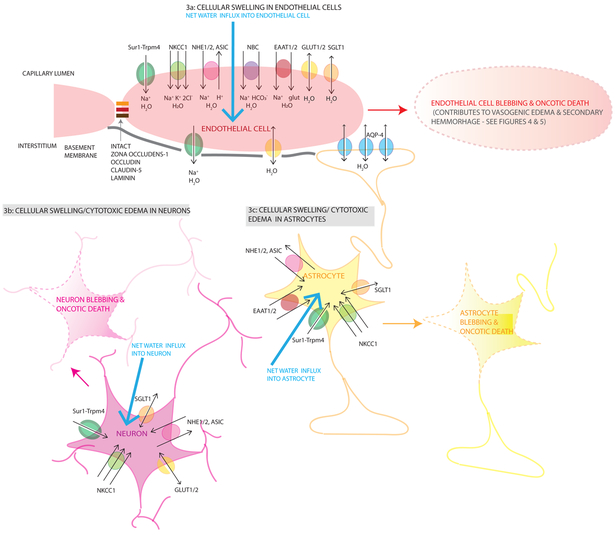
Figure 3. Mechanisms contributing to cellular/cytotoxic edema after TBI.
3a Endothelial (light pink cells) cellular swelling results from net water influx (large light blue arrow) into an endothelial cell driven by ionic contributors including GLUT1/2 (yellow channel), Sur1-Trpm4 (green channel), NKCC1 (light green channel), NHE1/2 (pink channel), ASIC (pink channel), NBC (purple channel), EAAT1/2 (red channel), SGLT1 (orange channel)). Sur1-Trpm4 cotransports Na+ with water into the cell, NKCC1 transports 1 Na+, 1 K+, 2 Cl− with 1 molecule of water into the cell, NHE 1/2 transports Na+ into the cell (with 1 molecule of water) and H+ out of the cell. NBC transports Na++ and HCO3− into the cell. EAAT1/2 transport 1 Na+ and 1 glutamate into the cell with 1 molecule of water. GLUT1/2 and SGLT1 transport glucose and Na+ into the cell respectively with water passively following. At this stage, the tight junctions are intact (solid orange/red/brown links between endothelial cells) as is the basement membrane (solid gray line under the endothelial layer). Cellular swelling/CytE in neurons (dark pink cells, 3b) and astrocytes (yellow cells, 3c) occurs by the same mechanisms with the same ion pumps and channels resulting in Na+ and water entering the cells causing cellular swelling (large light blue arrows). Eventually, this cellular swelling becomes cytotoxic and causes cell blebbing and oncotic death illustrated using lightened colors and dashed membrane outlines. Astrocytic endfeet also express AQP-4 at the blood-brain and blood-CSF junctions that allow passive water transport driven by an osmotic or pressure gradient. During cellular swelling, this gradient may direct water inwards. However, during vasogenic edema, these channels may be important in water egress from the cells as shown in Figure 4. Single arrows (black) are used for water co-transport, double headed arrows are used for passive water movement through a channel pore.
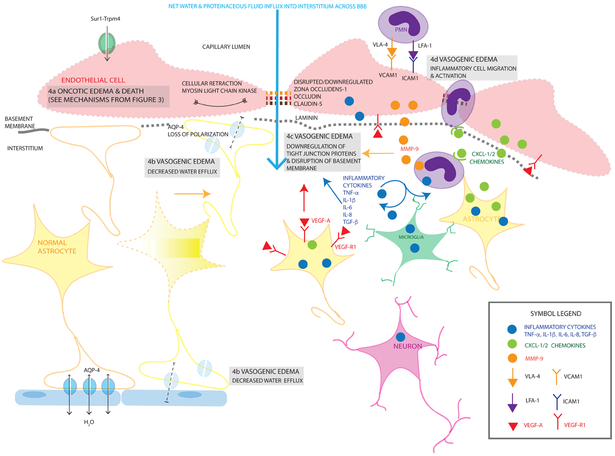
Figure 4. Mechanisms contributing to vasogenic edema after TBI.
Vasogenic edema (VasE) results from BBB compromise resulting in net water and proteinaceous fluid influx into the interstitium (large light blue arrow). There are multiple contributors to this process including cellular retraction (via actin and myosin light chain kinase contraction of the cytoskeleton), cytotoxic edema in endothelial cells resulting in membrane disruption and eventual oncotic death (4a), decreased water efflux (4b), degradation of tight junction proteins (4c) and activation of inflammatory cells (4d).
Mechanistic details of oncotic edema in endothelial cells (4a) are depicted earlier in Figure 3. Decreased water efflux (4b) also contributes to VasE; one potential mechanism illustrated here is by a loss of polarization of AQP-4 channels in the perivascular and peri-ependymal endfeet (depicted with a faded blue AQP4 channel). Additionally, as astrocytes undergo cell blebbing/oncotic cell death, their endfeet are no longer able to contribute towards sealing the BBB, furthering VasE formation. Another contributor to VasE is due to downregulation of tight junction proteins and disruption of the basement membrane (4c). Tight junction degradation involves proteins such as zona occludens, occludin, laminin shown by the interrupted links (orange/red/brown) between endothelial cells. Disrupted laminin/basement membrane is shown by the dashed-gray line under the endothelial cells. This occurs via multiple proposed mechanisms including MMP-9 (orange filled circles) produced primarily by neutrophils (purple cells) and endothelial cells, inflammatory cytokines (blue filled circles, TNF, IL-1β, IL-6, IL-8, TGF-β) produced by multiple cell types activated by injury including neurons, microglia, astrocytes, endothelial cells and migrating leukocytes, and VEGF-A (red filled triangle) and VEGF-R1 (red-Y shaped receptor) upregulation by multiple cell types including endothelial cells and astrocytes. Activation of and migration of inflammatory cells further exacerbates VasE (4d). PMNs adhere to the luminal endothelial cell surface via upregulated receptors/ligands such as VCAM-1 (orange Y-shaped receptor) and VLA-4 (orange filled arrowhead), ICAM-1 (purple Y-shaped receptor) and LFA-1 (purple filled arrowhead). Their migration into the interstitium is facilitated by chemokines (CXCL-1/2 for PMNs, green filled circles) secreted by astrocytes and other activated cells, and upregulated chemokine receptors. Each channel/cytokine/cell-type/chemokine/receptor is labelled once, and the same color and shape scheme is maintained for each item throughout the diagram with a legend provided in the inset panel. Cell types are labelled and color-coded in the figure: endothelial cells (light red), astrocytes (yellow), neurons (blue), polymorphonuclear leukocytes (PMN, purple), microglia (green). Single arrows (black) are used for water co-transport, double headed arrows are used for passive water movement through a channel pore.
Highlights.
Brain swelling is a major contributor to adverse outcome in TBI
Brain swelling in TBI is due to the combined mass effects of extravasated blood, cellular (cytotoxic) edema, vasogenic edema and osmolyte-driven swelling
Our understanding of molecular mechanisms of edema formation is in its infancy
Here, we review 12 pathways implicated in edema formation and 11 drugs with potential benefit for targeting edema in TBI
Acknowledgments
Funding
RMJ is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (K23NS101036) and a UPP foundation award. PMK is supported by grants from the NINDS (R01NS087978), the U.S. Department of Defense grant WH81XWH-14-2-0018, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (T32HD040686). JMS is supported by grants from the Department of Veterans Affairs (I01BX002889), the Department of Defense (SCI170199), the National Heart, Lung and Blood Institute (R01HL082517) and the NINDS (R01NS060801; R01NS102589; R01NS105633).
Abbreviations:
- AQP
aquaporin
- ASIC
acid sensing ion channel
- ATP
adenosine triphosphate
- AVP
arginine vasopressin
- BBB
blood-brain barrier
- CCI
controlled cortical impact
- CCL
chemokine (C-C motif) ligand
- CNS
central nervous system
- CPP
cerebral perfusion pressure
- CSF
cerebrospinal fluid
- CXCL
chemokine (C-X-C motif) ligand
- CytE
cytotoxic edema
- EAAT
excitatory amino acid transporter
- FPI
fluid percussion injury
- Glu
glutamate
- H+
hydrogen ion
- K+
potassium ion
- HMGB1
high mobility group box-1
- ICAM
intracellular adhesion molecule
- ICP
intracranial pressure
- IL
interleukin
- MLCK
myosin light chain kinase
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- Na+
sodium ion
- NAT
N-acetyl-L-tryptophan
- NBC
Na+/HCO3− transporter family channel
- NF-κB
nuclear factor κB
- NHE
Na+/H+ exchanger
- NK
neurokinin
- NKCC1
Na+-K+-2Cl− cotransporter
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- PMN
polymorphonuclear leukocyte/ neutrophil
- PPAR
peroxisome proliferator-activated receptor
- PSH
progressive secondary hemorrhage
- RCT
randomized controlled trial
- SP
substance P
- Sur1
sulfonylurea receptor 1
- TBI
traumatic brain injury
- TGF-β
tumor growth factor β
- TLR4
toll like receptor 4
- TNF
tumor necrosis factor
- Trpm4
transient receptor potential cation channel subfamily M member 4
- VasE
vasogenic edema
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- VEGI
vascular endothelial growth inhibitor
- ZO
zona occludens
Footnotes
Competing interests: all authors declare no competing interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Allen CJ, Subhawong TK, Hanna MM, Chelala L, Bullock MR, Schulman CI, Proctor KG, 2018. Does Vasopressin Exacerbate Cerebral Edema in Patients with Severe Traumatic Brain Injury? Am. Surg 84, 43–50. [PubMed] [Google Scholar]
- Alluri H, Wilson RL, Anasooya Shaji C, Wiggins-Dohlvik K, Patel S, Liu Y, Peng X, Beeram MR, Davis ML, Huang JH, Tharakan B, 2016. Melatonin Preserves Blood-Brain Barrier Integrity and Permeability via Matrix Metalloproteinase-9 Inhibition. PLoS One 11, e0154427. doi: 10.1371/journal.pone.0154427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperin NJ, Lee SH, Loth F, Raksin PB, Lichtor T, 2000. MR-Intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology 217, 877–885. doi: 10.1148/radiology.217.3.r00dc42877 [DOI] [PubMed] [Google Scholar]
- Ameliorate JL, Ghabriel MN, Vink R, 2017. Magnesium enhances the beneficial effects of NK1 antagonist administration on blood-brain barrier permeability and motor outcome after traumatic brain injury. Magnes. Res 30, 88–97. doi: 10.1684/mrh.2017.0427 [DOI] [PubMed] [Google Scholar]
- Amtul Z, Yang J, Nikolova S, Lee T-Y, Bartha R, Cechetto DF, 2018. The Dynamics of Impaired Blood-Brain Barrier Restoration in a Rat Model of Co-morbid Injury. Mol. Neurobiol doi: 10.1007/s12035-018-0904-4 [DOI] [PubMed] [Google Scholar]
- Andrews PJD, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JKJ, Murray GD, Eurotherm3235 Trial Collaborators, 2015. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N. Engl. J. Med 373, 2403–2412. doi: 10.1056/NEJMoa1507581 [DOI] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR, 2009. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 106, 1977–1982. doi: 10.1073/pnas.0808698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun T, Tomassini V, Sbardella E, de Ruiter MB, Matthews L, Leite MI, Gelineau-Morel R, Cavey A, Vergo S, Craner M, Fugger L, Rovira A, Jenkinson M, Palace J, 2013. Targeting ASIC1 in primary progressive multiple sclerosis: evidence of neuroprotection with amiloride. Brain 136, 106–115. doi: 10.1093/brain/aws325 [DOI] [PubMed] [Google Scholar]
- Ashbaugh A, McGrew C, 2016. The role of nutritional supplements in sports concussion treatment. Curr. Sports Med. Rep 15, 16–19. doi: 10.1249/JSR.0000000000000219 [DOI] [PubMed] [Google Scholar]
- Atsumi H, Matsumae M, Hirayama A, Kuroda K, 2014. Measurements of intracranial pressure and compliance index using 1.5-T clinical MRI machine. Tokai J Exp Clin Med 39, 34–43. [PubMed] [Google Scholar]
- Au AK, Aneja RK, Bell MJ, Bayir H, Feldman K, Adelson PD, Fink EL, Kochanek PM, Clark RSB, 2012. Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J. Neurotrauma 29, 2013–2021. doi: 10.1089/neu.2011.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F, 1997. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J. Neurosurg 87, 900–907. doi: 10.3171/jns.1997.87.6.0900 [DOI] [PubMed] [Google Scholar]
- Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C, 2005. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci. Lett 388, 7–12. doi: 10.1016/j.neulet.2005.06.019 [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH, 1998. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience 86, 1245–1257. doi: 10.1016/S0306-4522(98)00058-X [DOI] [PubMed] [Google Scholar]
- Buttram SDW, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RSB, Kochanek PM, 2007. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J. Neurotrauma 24, 1707–1717. doi: 10.1089/neu.2007.0349 [DOI] [PubMed] [Google Scholar]
- Cao F, Jiang Y, Wu Y, Zhong J, Liu J, Qin X, Chen L, Vitek MP, Li F, Xu L, Sun X, 2016. Apolipoprotein E-Mimetic COG1410 Reduces Acute Vasogenic Edema following Traumatic Brain Injury. J. Neurotrauma 33, 175–182. doi: 10.1089/neu.2015.3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J, 2017. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 80, 6–15. doi: 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- Cartagena CM, Phillips KL, Tortella FC, Dave JR, Schmid KE, 2014. Temporal alterations in aquaporin and transcription factor HIF1α expression following penetrating ballistic-like brain injury (PBBI). Mol. Cell. Neurosci 60, 81–87. doi: 10.1016/j.mcn.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Chen J-Q, Zhang C-C, Jiang S-N, Lu H, Wang W, 2016. Effects of aquaporin 4 knockdown on brain edema of the uninjured side after traumatic brain injury in rats. Med. Sci. Monit 22, 4809–4819. doi: 10.12659/MSM.898190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Dong Y, Simard JM, 2003. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J. Neurosci 23, 8568–8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C, 2007. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J. Neurotrauma 24, 1119–1131. doi: 10.1089/neu.2006.0216 [DOI] [PubMed] [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA, 1993. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 34, 216–222. [DOI] [PubMed] [Google Scholar]
- Chodobski A, Chung I, Kozniewska E, Ivanenko T, Chang W, Harrington JF, Duncan JA, Szmydynger-Chodobska J, 2003. Early neutrophilic expression of vascular endothelial growth factor after traumatic brain injury. Neuroscience 122, 853–867. doi: 10.1016/j.neuroscience.2003.08.055 [DOI] [PubMed] [Google Scholar]
- Chodobski A, Zink BJ, Szmydynger-Chodobska J, 2011. Blood-brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res 2, 492–516. doi: 10.1007/s12975-011-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP, 2002. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J. Biol. Chem 277, 14801–14811. doi: 10.1074/jbc.M110557200 [DOI] [PubMed] [Google Scholar]
- Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, Wolfe R, DECRA Trial Investigators, Australian and New Zealand Intensive Care Society Clinical Trials Group, 2011. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med 364, 1493–1502. doi: 10.1056/NEJMoa1102077 [DOI] [PubMed] [Google Scholar]
- Corrigan F, Leonard A, Ghabriel M, Van Den Heuvel C, Vink R, 2012. A substance P antagonist improves outcome in female Sprague Dawley rats following diffuse traumatic brain injury. CNS Neurosci Ther 18, 513–515. doi: 10.1111/j.1755-5949.2012.00332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan F, Mander KA, Leonard AV, Vink R, 2016. Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflammation 13, 264. doi: 10.1186/s12974-016-0738-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R, Hadass O, Lehmidi T, Blair GJ, Lee M, Chang M, Mobashery S, Sun GY, Gu Z, 2012. Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol. Neurodegener 7, 21. doi: 10.1186/1750-1326-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnyka M, Citerio G, 2012. Brain compliance: the old story with a new “et cetera”. Intensive Care Med 38, 925–927. doi: 10.1007/s00134-012-2572-6 [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Czosnyka Z, Agarwal-Harding KJ, Pickard JD, 2012. Modeling of CSF dynamics: legacy of Professor Anthony Marmarou. Acta Neurochir. Suppl 113, 9–14. doi: 10.1007/978-3-7091-0923-6_2 [DOI] [PubMed] [Google Scholar]
- da Fonseca ACC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FRS, 2014. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell Neurosci 8, 362. doi: 10.3389/fncel.2014.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias C, Silva MJ, Pereira E, Monteiro E, Maia I, Barbosa S, Silva S, Honrado T, Cerejo A, Aries MJH, Smielewski P, Paiva J-A, Czosnyka M, 2015. Optimal Cerebral Perfusion Pressure Management at Bedside: A Single-Center Pilot Study. Neurocrit. Care 23, 92–102. doi: 10.1007/s12028-014-0103-8 [DOI] [PubMed] [Google Scholar]
- Domoki F, Kis B, Gáspár T, Bari F, Busija DW, 2008. Cerebromicrovascular endothelial cells are resistant to L-glutamate. Am. J. Physiol. Regul. Integr. Comp. Physiol 295, R1099–108. doi: 10.1152/ajpregu.90430.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin JJ, Cernak I, Blumbergs PC, Vink R, 2011. A substance P antagonist reduces axonal injury and improves neurologic outcome when administered up to 12 hours after traumatic brain injury. J. Neurotrauma 28, 217–224. doi: 10.1089/neu.2010.1632 [DOI] [PubMed] [Google Scholar]
- Donkin JJ, Nimmo AJ, Cernak I, Blumbergs PC, Vink R, 2009. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab 29, 1388–1398. doi: 10.1038/jcbfm.2009.63 [DOI] [PubMed] [Google Scholar]
- Durham-Lee JC, Mokkapati VUL, Johnson KM, Nesic O, 2011. Amiloride improves locomotor recovery after spinal cord injury. J. Neurotrauma 28, 1319–1326. doi: 10.1089/neu.2011.1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg HM, Gary HE, Aldrich EF, Saydjari C, Turner B, Foulkes MA, Jane JA, Marmarou A, Marshall LF, Young HF, 1990. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J. Neurosurg 73, 688–698. doi: 10.3171/jns.1990.73.5.0688 [DOI] [PubMed] [Google Scholar]
- Feickert HJ, Drommer S, Heyer R, 1999. Severe head injury in children: impact of risk factors on outcome. The Journal of trauma 47, 33–38. [DOI] [PubMed] [Google Scholar]
- Feldmann H, Klages G, Gartner F, Scharfenberg J, 1979. The prognostic value of intracranial pressure monitoring after severe head injuries. Acta Neurochir. Suppl. (Wien) 28, 74–77. [DOI] [PubMed] [Google Scholar]
- Filippidis AS, Carozza RB, Rekate HL, 2016. Aquaporins in brain edema and neuropathological conditions. Int. J. Mol. Sci 18. doi: 10.3390/ijms18010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippidis AS, Liang X, Wang W, Parveen S, Baumgarten CM, Marmarou CR, 2014. Real-time monitoring of changes in brain extracellular sodium and potassium concentrations and intracranial pressure after selective vasopressin-1a receptor inhibition following focal traumatic brain injury in rats. J. Neurotrauma 31, 1258–1267. doi: 10.1089/neu.2013.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda AM, Adami A, Pop V, Bellone JA, Coats JS, Hartman RE, Ashwal S, Obenaus A, Badaut J, 2013. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J. Cereb. Blood Flow Metab 33, 1621–1632. doi: 10.1038/jcbfm.2013.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielian L, Helps SC, Thornton E, Turner RJ, Leonard AV, Vink R, 2013. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir. Suppl 118, 201–204. doi: 10.1007/978-3-7091-1434-6_37 [DOI] [PubMed] [Google Scholar]
- Galton C, Deem S, Yanez ND, Souter M, Chesnut R, Dagal A, Treggiari M, 2011. Open-label randomized trial of the safety and efficacy of a single dose conivaptan to raise serum sodium in patients with traumatic brain injury. Neurocrit. Care 14, 354–360. doi: 10.1007/s12028-011-9525-8 [DOI] [PubMed] [Google Scholar]
- Gao W, Zhao Z, Yu G, Zhou Z, Zhou Y, Hu T, Jiang R, Zhang J, 2015. VEGI attenuates the inflammatory injury and disruption of blood-brain barrier partly by suppressing the TLR4/NF-κB signaling pathway in experimental traumatic brain injury. Brain Res 1622, 230–239. doi: 10.1016/j.brainres.2015.04.035 [DOI] [PubMed] [Google Scholar]
- Gao Y, Li J, Wu L, Zhou C, Wang Q, Li X, Zhou M, Wang H, 2016. Tetrahydrocurcumin provides neuroprotection in rats after traumatic brain injury: autophagy and the PI3K/AKT pathways as a potential mechanism. J. Surg. Res 206, 67–76. doi: 10.1016/j.jss.2016.07.014 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhuang Z, Gao S, Li X, Zhang Z, Ye Z, Li L, Tang C, Zhou M, Han X, Li J, 2017. Tetrahydrocurcumin reduces oxidative stress-induced apoptosis via the mitochondrial apoptotic pathway by modulating autophagy in rats after traumatic brain injury. Am J Transl Res 9, 887–899. [PMC free article] [PubMed] [Google Scholar]
- Gerzanich V, Kwon MS, Woo SK, Ivanov A, Simard JM, 2018. SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS One 13, e0195526. doi: 10.1371/journal.pone.0195526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Bailitz J, 2016. Does mannitol reduce mortality from traumatic brain injury? Ann. Emerg. Med 67, 83–85. doi: 10.1016/j.annemergmed.2015.06.027 [DOI] [PubMed] [Google Scholar]
- Gu L, Liu X, Yang Y, Luo D, Zheng X, 2010. ASICs aggravate acidosis-induced injuries during ischemic reperfusion. Neurosci. Lett 479, 63–68. doi: 10.1016/j.neulet.2010.05.029 [DOI] [PubMed] [Google Scholar]
- Guilfoyle MR, Carpenter KLH, Helmy A, Pickard JD, Menon DK, Hutchinson PJA, 2015. Matrix metalloproteinase expression in contusional traumatic brain injury: A paired microdialysis study. J. Neurotrauma 32, 1553–1559. doi: 10.1089/neu.2014.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM, Zhang C, Giritharan AB, Purnell W, Robinson CR, Shin D, Schroeder VA, Suckow MA, Simonyi A, Sun GY, Mobashery S, Cui J, Chang M, Gu Z, 2013. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One 8, e76904. doi: 10.1371/journal.pone.0076904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne C, Piper I, 2014. Monitoring of intracranial pressure in patients with traumatic brain injury. Front. Neurol 5, 121. doi: 10.3389/fneur.2014.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason CM, 1987. Commentary on the significance for modern neurology of the 17th century B.C. Surgical Papyrus. Can J Neurol Sci 14, 560–563. [PubMed] [Google Scholar]
- Helmy A, Carpenter KLH, Menon DK, Pickard JD, Hutchinson PJA, 2011. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J. Cereb. Blood Flow Metab 31, 658–670. doi: 10.1038/jcbfm.2010.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, Ding JY, Dornbos D, Li X, Guthikonda M, Rossi NF, Ding Y, 2011. The role of hypoxia-inducible factor-1α, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J. Neurosurg 114, 92–101. doi: 10.3171/2010.6.JNS10207 [DOI] [PubMed] [Google Scholar]
- Hou Z, Tian R, Han F, Hao S, Wu W, Mao X, Tao X, Lu T, Dong J, Zhen Y, Liu B, 2018. Decompressive craniectomy protects against hippocampal edema and behavioral deficits at an early stage of a moderately controlled cortical impact brain injury model in adult male rats. Behav. Brain Res 345, 1–8. doi: 10.1016/j.bbr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- Howells T, Lewén A, Sköld MK, Ronne-Engström E, Enblad P, 2012. An evaluation of three measures of intracranial compliance in traumatic brain injury patients. Intensive Care Med 38, 1061–1068. doi: 10.1007/s00134-012-2571-7 [DOI] [PubMed] [Google Scholar]
- Hu H, Yao H, Zhang W, Zhang L, Ding W, Zhang S, Chen Z, Wei E, 2005. Increased expression of aquaporin-4 in human traumatic brain injury and brain tumors. J Zhejiang Univ Sci B 6, 33–37. doi: 10.1631/jzus.2005.B0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JA, Szu JI, Binder DK, 2018. The role of aquaporin-4 in synaptic plasticity, memory and disease. Brain Res. Bull 136, 118–129. doi: 10.1016/j.brainresbull.2017.02.011 [DOI] [PubMed] [Google Scholar]
- Hudak AM, Peng L, Marquez de la Plata C, Thottakara J, Moore C, Harper C, McColl R, Babcock E, Diaz-Arrastia R, 2014. Cytotoxic and vasogenic cerebral oedema in traumatic brain injury: assessment with FLAIR and DWI imaging. Brain Inj 28, 1602–1609. doi: 10.3109/02699052.2014.936039 [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, Wadley J, Mendelow AD, Mitchell PM, Wilson MH, Critchley G, Sahuquillo J, Unterberg A, Servadei F, Teasdale GM, Pickard JD, Menon DK, Murray GD, Kirkpatrick PJ, RESCUEicp Trial Collaborators, 2016. Trial of decompressive craniectomy for traumatic intracranial hypertension. N. Engl. J. Med 375, 1119–1130. doi: 10.1056/NEJMoa1605215 [DOI] [PubMed] [Google Scholar]
- Iaccarino C, Schiavi P, Picetti E, Goldoni M, Cerasti D, Caspani M, Servadei F, 2014. Patients with brain contusions: predictors of outcome and relationship between radiological and clinical evolution. J. Neurosurg 120, 908–918. doi: 10.3171/2013.12.JNS131090 [DOI] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi S, Yoshimura S, Nakashima S, Iwama T, 2010. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertens Res 33, 703–707. doi: 10.1038/hr.2010.58 [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M, 2014. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci 34, 16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Marmarou A, Barzó P, Fatouros P, Corwin F, 1996. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J. Neurosurg 84, 97–103. doi: 10.3171/jns.1996.84.1.0097 [DOI] [PubMed] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J, 2017. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95, 1246–1265. doi: 10.1016/j.neuron.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Curtis KM, Tong XY, Moriyama M, Norenberg MD, 2011. Na-K-Cl cotransporter-1 in the mechanism of cell swelling in cultured astrocytes after fluid percussion injury. J. Neurochem 117, 437–448. doi: 10.1111/j.1471-4159.2011.07211.x [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Taherian M, Panickar KS, Shamaladevi N, Rodriguez ME, Price BG, Norenberg MD, 2018. Differential Response of Neural Cells to Trauma-Induced Swelling In Vitro. Neurochem. Res 43, 397–406. doi: 10.1007/s11064-017-2434-2 [DOI] [PubMed] [Google Scholar]
- Jha R, Battey TWK, Pham L, Lorenzano S, Furie KL, Sheth KN, Kimberly WT, 2014. Fluid-attenuated inversion recovery hyperintensity correlates with matrix metalloproteinase-9 level and hemorrhagic transformation in acute ischemic stroke. Stroke 45, 1040–1045. doi: 10.1161/STROKEAHA.113.004627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R, Molyneaux B, Jackson T, Wallisch J, Vagni V, Poloyac S, Dixon CE, Kochanek P, 2015. Glibenclamide Reduces Diffuse Cerebral Edema In A Combined Model of Traumatic Brain Injury And Shock. Crit. Care Med 43, 124. [Google Scholar]
- Jha Ruchira Menka, Elmer J, Zusman B, Puccio A, Okonkwo D, Shutter L, Wallisch J, Conley Y, Kochanek P, 2018a. Intracranial Pressure Trajectories: a novel tool to inform severe TBI phenotypes. Crit. Care Med In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha RM, Kochanek PM, 2017. Adding insight to injury: a new era in neurotrauma. Lancet Neurol 16, 578–580. doi: 10.1016/S1474-4422(17)30225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha Ruchira Menka, Koleck TA, Puccio AM, Okonkwo DO, Park S-Y, Zusman BE, Clark RSB, Shutter LA, Wallisch JS, Empey PE, Kochanek PM, Conley YP, 2018b. Regionally clustered ABCC8 polymorphisms in a prospective cohort predict cerebral oedema and outcome in severe traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. doi: 10.1136/jnnp-2017-317741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, Ruchira M, Molyneaux BJ, Jackson TC, Wallisch JS, Park S-Y, Poloyac S, Vagni VA, Janesko-Feldman KL, Hoshitsuki K, Minnigh MB, Kochanek PM, 2018. Glibenclamide Produces Region-Dependent Effects on Cerebral Edema in a Combined Injury Model of Traumatic Brain Injury and Hemorrhagic Shock in Mice. J. Neurotrauma. doi: 10.1089/neu.2016.4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha RM, Puccio AM, Chou SH-Y, Chang C-CH, Wallisch JS, Molyneaux BJ, Zusman BE, Shutter LA, Poloyac SM, Janesko-Feldman KL, Okonkwo DO, Kochanek PM, 2017a. Sulfonylurea Receptor-1: A Novel Biomarker for Cerebral Edema in Severe Traumatic Brain Injury. Crit. Care Med 45, e255–e264. doi: 10.1097/CCM.0000000000002079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha RM, Puccio AM, Okonkwo DO, Zusman BE, Park S-Y, Wallisch J, Empey PE, Shutter LA, Clark RSB, Kochanek PM, Conley YP, 2017b. ABCC8 Single Nucleotide Polymorphisms are Associated with Cerebral Edema in Severe TBI. Neurocrit. Care 26, 213–224. doi: 10.1007/s12028-016-0309-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha RM, Yan H, Dixon CE, Poloyac S, Jackson T, Hoshitsuki K, Ma X, Henchir J, Kochanek PM, 2015. Evaluation of Glibenclamide in the Pittsburgh Controlled Cortical Impact Model of Traumatic Brain Injury: An OBTT Consortium Study. Journal of neurotrauma 32, 119-A–152. [Google Scholar]
- Jia F, Yin YH, Gao GY, Wang Y, Cen L, Jiang J-Y, 2014. MMP-9 inhibitor SB-3CT attenuates behavioral impairments and hippocampal loss after traumatic brain injury in rat. J. Neurotrauma 31, 1225–1234. doi: 10.1089/neu.2013.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Kawamata T, 2003. Edema fluid accumulation within necrotic brain tissue as a cause of the mass effect of cerebral contusion in head trauma patients. Acta Neurochir. Suppl. (Wien) 86, 323–327. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Mori T, Maeda T, Kawamata T, 1998. Pathogenesis of the mass effect of cerebral contusions: rapid increase in osmolality within the contusion necrosis. Acta Neurochir. Suppl 71, 289–292. [DOI] [PubMed] [Google Scholar]
- Ke C, Poon WS, Ng HK, Pang JC, Chan Y, 2001. Heterogeneous responses of aquaporin-4 in oedema formation in a replicated severe traumatic brain injury model in rats. Neurosci. Lett 301, 21–24. [DOI] [PubMed] [Google Scholar]
- Kenne E, Erlandsson A, Lindbom L, Hillered L, Clausen F, 2012. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J. Neuroinflammation 9, 17. doi: 10.1186/1742-2094-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili H, Derakhshan N, Niakan A, Ghaffarpasand F, Salehi M, Eshraghian H, Shakibafard A, Zahabi B, 2017. Effects of Oral Glibenclamide on Brain Contusion Volume and Functional Outcome of Patients with Moderate and Severe Traumatic Brain Injuries: A Randomized Double-Blind Placebo-Controlled Clinical Trial. World Neurosurg 101, 130–136. doi: 10.1016/j.wneu.2017.01.103 [DOI] [PubMed] [Google Scholar]
- Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N, 2014. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst. Rev. CD008218. doi: 10.1002/14651858.CD008218.pub3 [DOI] [PubMed] [Google Scholar]
- Kiening KL, van Landeghem FKH, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, Stover JF, 2002. Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci. Lett 324, 105–108. doi: 10.1016/S0304-3940(02)00180-5 [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Battey TWK, Pham L, Wu O, Yoo AJ, Furie KL, Singhal AB, Elm JJ, Stern BJ, Sheth KN, 2014. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit. Care 20, 193–201. doi: 10.1007/s12028-013-9917-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM, 2012. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One 7, e41229. doi: 10.1371/journal.pone.0041229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst A, Dunbar JG, Glisson R, Marmarou A, 2013. The role of vasopressin V1A receptors in cytotoxic brain edema formation following brain injury. Acta Neurochir. (Wien) 155, 151–164. doi: 10.1007/s00701-012-1558-z [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Bramlett HM, Dixon CE, Shear DA, Dietrich WD, Schmid KE, Mondello S, Wang KKW, Hayes RL, Povlishock JT, Tortella FC, 2016. Approach to Modeling, Therapy Evaluation, Drug Selection, and Biomarker Assessments for a Multicenter Pre-Clinical Drug Screening Consortium for Acute Therapies in Severe Traumatic Brain Injury: Operation Brain Trauma Therapy. J. Neurotrauma 33, 513–522. doi: 10.1089/neu.2015.4113 [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Jackson TC, Ferguson NM, Carlson SW, Simon DW, Brockman EC, Ji J, Bayir H, Poloyac SM, Wagner AK, Kline AE, Empey PE, Clark RSB, Jackson EK, Dixon CE, 2015. Emerging therapies in traumatic brain injury. Semin Neurol 35, 83–100. doi: 10.1055/s-0035-1544237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann T, Stahel PF, Morganti-Kossmann MC, Jones JL, Barnum SR, 1997. Elevated levels of the complement components C3 and factor B in ventricular cerebrospinal fluid of patients with traumatic brain injury. J. Neuroimmunol 73, 63–69. [DOI] [PubMed] [Google Scholar]
- Krieg SM, Sonanini S, Plesnila N, Trabold R, 2015. Effect of small molecule vasopressin V1a and V2 receptor antagonists on brain edema formation and secondary brain damage following traumatic brain injury in mice. J. Neurotrauma 32, 221–227. doi: 10.1089/neu.2013.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg SM, Trabold R, Plesnila N, 2017. Time-Dependent Effects of Arginine-Vasopressin V1 Receptor Inhibition on Secondary Brain Damage after Traumatic Brain Injury. J. Neurotrauma 34, 1329–1336. doi: 10.1089/neu.2016.4514 [DOI] [PubMed] [Google Scholar]
- Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM, 2012. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J. Neurotrauma 29, 19–31. doi: 10.1089/neu.2011.2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B, Youssef P, Yanasak N, Vender JR, Dhandapani KM, 2014. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 62, 26–38. doi: 10.1002/glia.22581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Sukumari-Ramesh S, Swift AEB, Meiler SE, Vender JR, Dhandapani KM, 2010. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4? J. Neurochem 113, 637–648. doi: 10.1111/j.1471-4159.2010.06630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Agoston DV, 2010. Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J. Neurotrauma 27, 541–553. doi: 10.1089/neu.2009.0905 [DOI] [PubMed] [Google Scholar]
- Lee JY, Choi HY, Na WH, Ju BG, Yune TY, 2014. Ghrelin inhibits BSCB disruption/hemorrhage by attenuating MMP-9 and SUR1/TrpM4 expression and activation after spinal cord injury. Biochim. Biophys. Acta 1842, 2403–2412. doi: 10.1016/j.bbadis.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Li M, Inoue K, Branigan D, Kratzer E, Hansen JC, Chen JW, Simon RP, Xiong Z-G, 2010. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J. Cereb. Blood Flow Metab 30, 1247–1260. doi: 10.1038/jcbfm.2010.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jia Q, Chen T, Zhao Z, Chen J, Zhang J, 2016. The role of vascular endothelial growth factor and vascular endothelial growth inhibitor in clinical outcome of traumatic brain injury. Clin. Neurol. Neurosurg 144, 7–13. doi: 10.1016/j.clineuro.2016.02.032 [DOI] [PubMed] [Google Scholar]
- Li W, Suwanwela NC, Patumraj S, 2016. Curcumin by down-regulating NF-κB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res 106, 117–127. doi: 10.1016/j.mvr.2015.12.008 [DOI] [PubMed] [Google Scholar]
- Liang F, Luo C, Xu G, Su F, He X, Long S, Ren H, Liu Y, Feng Y, Pei Z, 2015. Deletion of aquaporin-4 is neuroprotective during the acute stage of micro traumatic brain injury in mice. Neurosci. Lett 598, 29–35. doi: 10.1016/j.neulet.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Liu H, Qiu G. ping, Zhuo F, Yu W. hua, Sun S. quan, Li F. hong, Yang M, 2015. Lost Polarization of Aquaporin4 and Dystroglycan in the Core Lesion after Traumatic Brain Injury Suggests Functional Divergence in Evolution. Biomed Res. Int 2015, 471631. doi: 10.1155/2015/471631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jin X, Liu KJ, Liu W, 2012. Matrix metalloproteinase-2-mediated occluding degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci 32, 3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-L, Yuan F, Yang D, Xu Z-M, Jing Y, Yang G-Y, Geng Z, Xia W-L, Tian H-L, 2018. Adjudin attenuates cerebral edema and improves neurological function in mice with experimental traumatic brain injury. J. Neurotrauma. doi: 10.1089/neu.2017.5397 [DOI] [PubMed] [Google Scholar]
- Lo Pizzo M, Schiera G, Di Liegro I, Di Liegro CM, Pál J, Czeiter E, Sulyok E, Doczi T, 2013. Aquaporin-4 distribution in control and stressed astrocytes in culture and in the cerebrospinal fluid of patients with traumatic brain injuries. Neurol. Sci 34, 1309–1314. doi: 10.1007/s10072-012-1233-4 [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez AB, Acaz-Fonseca E, Viveros M-P, Garcia-Segura LM, 2015. Changes in cannabinoid receptors, aquaporin 4 and vimentin expression after traumatic brain injury in adolescent male mice. Association with edema and neurological deficit. PLoS One 10, e0128782. doi: 10.1371/journal.pone.0128782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, Martín MM, Almeida T, Hernández M, Ramos L, Argueso M, Cáceres JJ, Solé-Violán J, Jiménez A, 2015. Serum substance P levels are associated with severity and mortality in patients with severe traumatic brain injury. Crit. Care 19, 192. doi: 10.1186/s13054-015-0911-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Lei X-Y, Hu H, He Z-P, 2013. Relationship between AQP4 expression and structural damage to the blood-brain barrier at early stages of traumatic brain injury in rats. Chin. Med. J 126, 4316–4321. [PubMed] [Google Scholar]
- Lu K-T, Cheng N-C, Wu C-Y, Yang Y-L, 2008. NKCC1-mediated traumatic brain injury-induced brain edema and neuron death via Raf/MEK/MAPK cascade. Crit. Care Med 36, 917–922. doi: 10.1097/CCM.0B013E31816590C4 [DOI] [PubMed] [Google Scholar]
- Lu K-T, Huang T-C, Tsai Y-H, Yang Y-L, 2017. Transient receptor potential vanilloid type 4 channels mediate Na-K-Cl-co-transporter-induced brain edema after traumatic brain injury. J. Neurochem 140, 718–727. doi: 10.1111/jnc.13920 [DOI] [PubMed] [Google Scholar]
- Lu K-T, Wu C-Y, Cheng N-C, Wo Y-YP, Yang J-T, Yen H-H, Yang Y-L, 2006. Inhibition of the Na+ -K+ -2Cl− -cotransporter in choroid plexus attenuates traumatic brain injury-induced brain edema and neuronal damage. Eur. J. Pharmacol 548, 99–105. doi: 10.1016/j.ejphar.2006.07.048 [DOI] [PubMed] [Google Scholar]
- Luh C, Kuhlmann CR, Ackermann B, Timaru-Kast R, Luhmann HJ, Behl C, Werner C, Engelhard K, Thal SC, 2010. Inhibition of myosin light chain kinase reduces brain edema formation after traumatic brain injury. J. Neurochem 112, 1015–1025. doi: 10.1111/j.1471-4159.2009.06514.x [DOI] [PubMed] [Google Scholar]
- Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, Hill S, Legrand V, Sorgner A, CENTER-TBI Participants and Investigators, 2015. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76, 67–80. doi: 10.1227/NEU.0000000000000575 [DOI] [PubMed] [Google Scholar]
- Macintyre I, 2014. A hotbed of medical innovation: George Kellie (1770-1829), his colleagues at Leith and the Monro-Kellie doctrine. J Med Biogr 22, 93–100. doi: 10.1177/0967772013479271 [DOI] [PubMed] [Google Scholar]
- Maier B, Schwerdtfeger K, Mautes A, Holanda M, Muller M, Steudel WI, Marzi I, 2001. Differential release of interleukines 6, 8, and 10 in cerebrospinal fluid and plasma after traumatic brain injury. Shock 15, 421–426. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD, 2000. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J. Cell Sci 113 (Pt 11), 2085–2090. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS, 2000. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat. Med 6, 159–163. doi: 10.1038/72256 [DOI] [PubMed] [Google Scholar]
- Marmarou A, 2007. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg. Focus 22, E1. [DOI] [PubMed] [Google Scholar]
- Marmarou A, L Anderson R, D Ward J, C Choi S, F Young H, M Eisenberg H, A Foulkes M, F Marshall L, A Jane J, 1991. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J. Neurosurg 75, S59–S66. [Google Scholar]
- Marmarou A, Shulman K, LaMorgese J, 1975. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J. Neurosurg 43, 523–534. doi: 10.3171/jns.1975.43.5.0523 [DOI] [PubMed] [Google Scholar]
- Marmarou A, Shulman K, Rosende RM, 1978. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J. Neurosurg 48, 332–344. doi: 10.3171/jns.1978.48.3.0332 [DOI] [PubMed] [Google Scholar]
- Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR, 2006. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J. Neurosurg 104, 720–730. doi: 10.3171/jns.2006.104.5.720 [DOI] [PubMed] [Google Scholar]
- Marmarou CR, Liang X, Abidi NH, Parveen S, Taya K, Henderson SC, Young HF, Filippidis AS, Baumgarten CM, 2014. Selective vasopressin-1a receptor antagonist prevents brain edema, reduces astrocytic cell swelling and GFAP, V1aR and AQP4 expression after focal traumatic brain injury. Brain Res 1581, 89–102. doi: 10.1016/j.brainres.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall LF, Smith RW, Shapiro HM, 1979. The outcome with aggressive treatment in severe head injuries. Part I: the significance of intracranial pressure monitoring. J. Neurosurg 50, 20–25. doi: 10.3171/jns.1979.50.1.0020 [DOI] [PubMed] [Google Scholar]
- Martínez-Valverde T, Vidal-Jorge M, Martínez-Saez E, Castro L, Arikan F, Cordero E, Rădoi A, Poca M-A, Simard JM, Sahuquillo J, 2015. Sulfonylurea Receptor 1 in Humans with Post-Traumatic Brain Contusions. J. Neurotrauma 32, 1478–1487. doi: 10.1089/neu.2014.3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DW, Szu JI, Hale C, Hsu MS, Rodgers VGJ, Binder DK, 2014. Reduction of cerebral edema after traumatic brain injury using an osmotic transport device. J. Neurotrauma 31, 1948–1954. doi: 10.1089/neu.2014.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ, 1977. Significance of intracranial hypertension in severe head injury. J. Neurosurg 47, 503–516. doi: 10.3171/jns.1977.47.4.0503 [DOI] [PubMed] [Google Scholar]
- Morancho A, Rosell A, Garcia-Bonilla L, Montaner J, 2010. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann. N. Y. Acad. Sci 1207, 123–133. doi: 10.1111/j.1749-6632.2010.05734.x [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Hans VH, Lenzlinger PM, Dubs R, Ludwig E, Trentz O, Kossmann T, 1999. TGF-beta is elevated in the CSF of patients with severe traumatic brain injuries and parallels blood-brain barrier function. J. Neurotrauma 16, 617–628. doi: 10.1089/neu.1999.16.617 [DOI] [PubMed] [Google Scholar]
- Muradashvili N, Benton RL, Saatman KE, Tyagi SC, Lominadze D, 2015. Ablation of matrix metalloproteinase-9 gene decreases cerebrovascular permeability and fibrinogen deposition post traumatic brain injury in mice. Metab. Brain Dis 30, 411–426. doi: 10.1007/s11011-014-9550-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Felinski EA, Antonetti DA, 2009. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem 284, 21036–21046. doi: 10.1074/jbc.M109.016766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan RK, Kishore PR, Becker DP, Ward JD, Enas GG, Greenberg RP, Domingues Da Silva A, Lipper MH, Choi SC, Mayhall CG, Lutz HA, Young HF, 1982. Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J. Neurosurg 56, 650–659. doi: 10.3171/jns.1982.56.5.0650 [DOI] [PubMed] [Google Scholar]
- Nwachuku EL, Puccio AM, Adeboye A, Chang Y-F, Kim J, Okonkwo DO, 2016. Time course of cerebrospinal fluid inflammatory biomarkers and relationship to 6-month neurologic outcome in adult severe traumatic brain injury. Clin. Neurol. Neurosurg 149, 1–5. doi: 10.1016/j.clineuro.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, Yoshino T, Ohtsuka A, Otani N, Tomura S, Shima K, Yamamoto Y, Yamamoto H, Takahashi HK, Mori S, Nishibori M, 2012. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol 72, 373–384. doi: 10.1002/ana.23602 [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS, 2004. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 18, 1291–1293. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS, 2013. Aquaporin water channels in the nervous system.Nat. Rev. Neurosci 14, 265–277. doi: 10.1038/nrn3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, 2006. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am. J. Physiol. Cell Physiol 290, C1399–410. doi: 10.1152/ajpcell.00386.2005 [DOI] [PubMed] [Google Scholar]
- Parfenova H, Fedinec A, Leffler CW, 2003. Ionotropic glutamate receptors in cerebral microvascular endothelium are functionally linked to heme oxygenase. J. Cereb. Blood Flow Metab 23, 190–197. doi: 10.1097/01.WCB.000004823561824.C4 [DOI] [PubMed] [Google Scholar]
- Pascale CL, Szmydynger-Chodobska J, Sarri JE, Chodobski A, 2006. Traumatic brain injury results in a concomitant increase in neocortical expression of vasopressin and its V1a receptor. J Physiol Pharmacol 57 Suppl 11, 161–167. [PubMed] [Google Scholar]
- Patel AD, Gerzanich V, Geng Z, Simard JM, 2010. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J. Neuropathol. Exp. Neurol 69, 1177–1190. doi: 10.1097/NEN.0b013e3181fbf6d6 [DOI] [PubMed] [Google Scholar]
- Qureshi M, Al-Suhaimi EA, Wahid F, Shehzad O, Shehzad A, 2018. Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci 39, 207–214. doi: 10.1007/s10072-017-3149-5 [DOI] [PubMed] [Google Scholar]
- Raksin PB, Alperin N, Sivaramakrishnan A, Surapaneni S, Lichtor T, 2003. Noninvasive intracranial compliance and pressure based on dynamic magnetic resonance imaging of blood flow and cerebrospinal fluid flow: review of principles, implementation, and other noninvasive approaches. Neurosurg. Focus 14, e4. [DOI] [PubMed] [Google Scholar]
- Rauen K, Trabold R, Brem C, Terpolilli NA, Plesnila N, 2013. Arginine vasopressin V1a receptor-deficient mice have reduced brain edema and secondary brain damage following traumatic brain injury. J. Neurotrauma 30, 1442–1448. doi: 10.1089/neu.2012.2807 [DOI] [PubMed] [Google Scholar]
- Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG, 2010. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res 88, 3530–3539. doi: 10.1002/jnr.22510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, Kumar S, Wang R, Pradeepkiran JA, Ogunmokun G, Thamarai K, Quesada K, Boles A, Reddy AP, 2018. Protective Effects of Indian Spice Curcumin Against Amyloid-β in Alzheimer’s Disease. J. Alzheimers Dis 61, 843–866. doi: 10.3233/JAD-170512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, Giese RN, Wang B, Shi X, Nedergaard M, 2013. “Hit & Run” model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab 33, 834–845. doi: 10.1038/jcbfm.2013.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Jenne CN, Léger C, Kramer AH, Gallagher CN, Todd S, Parney IF, Doig CJ, Yong VW, Kubes P, Zygun DA, 2013. A prospective evaluation of the temporal matrix metalloproteinase response after severe traumatic brain injury in humans. J. Neurotrauma 30, 1717–1726. doi: 10.1089/neu.2012.2841 [DOI] [PubMed] [Google Scholar]
- Rodriguez GA, Shah AH, Gersey ZC, Shah SS, Bregy A, Komotar RJ, Graham RM, 2016. Investigating the therapeutic role and molecular biology of curcumin as a treatment for glioblastoma. Ther Adv Med Oncol 8, 248–260. doi: 10.1177/1758834016643518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y, 2007. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus 22, E4. [DOI] [PubMed] [Google Scholar]
- Rossi JL, Todd T, Bazan NG, Belayev L, 2013. Inhibition of Myosin light-chain kinase attenuates cerebral edema after traumatic brain injury in postnatal mice. J. Neurotrauma 30, 1672–1679. doi: 10.1089/neu.2013.2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M, 2013. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol. Biochem. Behav 110, 238–244. doi: 10.1016/j.pbb.2013.07.019 [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG, 2011. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Exp. Neurol 227, 128–135. doi: 10.1016/j.expneurol.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul TG, Ducker TB, 1982. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury. J. Neurosurg 56, 498–503. doi: 10.3171/jns.1982.56.4.0498 [DOI] [PubMed] [Google Scholar]
- Semple BD, Noble-Haeusslein LJ, Gooyit M, Tercovich KG, Peng Z, Nguyen TT, Schroeder VA, Suckow MA, Chang M, Raber J, Trivedi A, 2015a. Early Gelatinase Activity Is Not a Determinant of Long-Term Recovery after Traumatic Brain Injury in the Immature Mouse. PLoS One 10, e0143386. doi: 10.1371/journal.pone.0143386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Trivedi A, Gimlin K, Noble-Haeusslein LJ, 2015b. Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain. Neurobiol. Dis 74, 263–280. doi: 10.1016/j.nbd.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena J, Blanco M, Castellanos M, Silva Y, Vivancos J, Moro MA, Leira R, Lizasoain I, Castillo J, Dávalos A, 2005. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke 36, 1921–1926. doi: 10.1161/01.STR.0000177870.14967.94 [DOI] [PubMed] [Google Scholar]
- Shamsi Meymandi M, Soltani Z, Sepehri G, Amiresmaili S, Farahani F, Moeini Aghtaei M, 2018. Effects of pregabalin on brain edema, neurologic and histologic outcomes in experimental traumatic brain injury. Brain Res. Bull 140, 169–175. doi: 10.1016/j.brainresbull.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Shapiro K, Marmarou A, Shulman K, 1980. Characterization of clinical CSF dynamics and neural axis compliance using the pressure-volume index: I. The normal pressure-volume index. Ann. Neurol 7, 508–514. doi: 10.1002/ana.410070603 [DOI] [PubMed] [Google Scholar]
- Sharma R, Rosenberg A, Bennett ER, Laskowitz DT, Acheson SK, 2017. A blood-based biomarker panel to risk-stratify mild traumatic brain injury. PLoS One 12, e0173798. doi: 10.1371/journal.pone.0173798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud P-O, Romero IA, Weksler B, Gillies MC, 2011. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-β1-induced permeability of centrally derived vascular endothelium. Eur. J. Cell Biol 90, 323–332. doi: 10.1016/j.ejcb.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, Ostwaldt A-C, Del Zoppo GJ, Simard JM, Jacobson S, Kimberly WT, 2016. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 15, 1160–1169. doi: 10.1016/S1474-4422(16)30196-X [DOI] [PubMed] [Google Scholar]
- Shetty AK, Mishra V, Kodali M, Hattiangady B, 2014. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front. Cell Neurosci 8, 232. doi: 10.3389/fncel.2014.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemori Y, Katayama Y, Mori T, Maeda T, Kawamata T, 2006. Matrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in rats. Acta neurochirurgica. Supplement 96, 130–133. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V, 2006. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med 12, 433–440. doi: 10.1038/nm1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, Colucci M, Gerzanich V, 2012a. Does inhibiting Sur1 complement rt-PA in cerebral ischemia? Ann. N. Y. Acad. Sci 1268, 95–107. doi: 10.1111/j.1749-6632.2012.06705.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kahle KT, Gerzanich V, 2010. Molecular mechanisms of microvascular failure in central nervous system injury--synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg 113, 622–629. doi: 10.3171/2009.11.JNS081052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V, 2007. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 6, 258–268. doi: 10.1016/S1474-4422(07)70055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V, 2009. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma 26, 2257–2267. doi: 10.1089/neu.2009.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Sheth KN, Kimberly WT, Stern BJ, del Zoppo GJ, Jacobson S, Gerzanich V, 2014. Glibenclamide in cerebral ischemia and stroke. Neurocrit. Care 20, 319–333. doi: 10.1007/s12028-013-9923-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V, 2012b. Sulfonylurea receptor 1 in central nervous system injury: a focused review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 32, 1699–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayir H, Clark RSB, Loane DJ, Kochanek PM, 2017. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol 13, 171–191. doi: 10.1038/nrneurol.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld MK, Risling M, Holmin S, 2006. Inhibition of vascular endothelial growth factor receptor 2 activity in experimental brain contusions aggravates injury outcome and leads to early increased neuronal and glial degeneration. Eur. J. Neurosci 23, 21–34. doi: 10.1111/j.1460-9568.2005.04527.x [DOI] [PubMed] [Google Scholar]
- Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY, 2014. The history of external ventricular drainage. J. Neurosurg 120, 228–236. doi: 10.3171/2013.6.JNS121577 [DOI] [PubMed] [Google Scholar]
- Stahel PF, Morganti-Kossmann MC, Perez D, Redaelli C, Gloor B, Trentz O, Kossmann T, 2001. Intrathecal levels of complement-derived soluble membrane attack complex (sC5b-9) correlate with blood-brain barrier dysfunction in patients with traumatic brain injury. J. Neurotrauma 18, 773–781. doi: 10.1089/089771501316919139 [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV, 2003. Potential role of MCP-1 in endothelial cell tight junction “opening”: signaling via Rho and Rho kinase. J. Cell Sci 116, 4615–4628. doi: 10.1242/jcs.00755 [DOI] [PubMed] [Google Scholar]
- Stankowska DL, Mueller BH, Oku H, Ikeda T, Dibas A, 2018. Neuroprotective effects of inhibitors of Acid-Sensing ion channels (ASICs) in optic nerve crush model in rodents. Curr Eye Res 43, 84–95. doi: 10.1080/02713683.2017.1383442 [DOI] [PubMed] [Google Scholar]
- Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD, 2002. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med 30, 733–738. [DOI] [PubMed] [Google Scholar]
- Stiver SI, 2009. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg. Focus 26, E7. doi: 10.3171/2009.4.FOCUS0965 [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Maas AIR, 2014. Traumatic intracranial hypertension. N. Engl. J. Med 370, 2121–2130. doi: 10.1056/NEJMra1208708 [DOI] [PubMed] [Google Scholar]
- Stocchetti N, Zanaboni C, Colombo A, Citerio G, Beretta L, Ghisoni L, Zanier ER, Canavesi K, 2008. Refractory intracranial hypertension and “second-tier” therapies in traumatic brain injury. Intensive Care Med 34, 461–467. doi: 10.1007/s00134-007-0948-9 [DOI] [PubMed] [Google Scholar]
- Stokum JA, Gerzanich V, Simard JM, 2016. Molecular pathophysiology of cerebral edema. J. Cereb. Blood Flow Metab 36, 513–538. doi: 10.1177/0271678X15617172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum JA, Kwon MS, Woo SK, Tsymbalyuk O, Vennekens R, Gerzanich V, Simard JM, 2018. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 66, 108–125. doi: 10.1002/glia.23231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Fukai N, Nagashijma G, Asai JI, Itokawa H, Nagai M, Suzuki T, Fujimoto T, 2003. Very early expression of vascular endothelial growth factor in brain oedema tissue associated with brain contusion. Acta Neurochir. Suppl 86, 277–279. [DOI] [PubMed] [Google Scholar]
- Tai KK, Truong DD, 2013. Amiloride but not memantine reduces neurodegeneration, seizures and myoclonic jerks in rats with cardiac arrest-induced global cerebral hypoxia and reperfusion. PLoS One 8, e60309. doi: 10.1371/journal.pone.0060309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno H, Nockels RP, Pitts LH, Noble LJ, 1992. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: Distribution and time course of protein extravasation. J. Neurotrauma 9, 21–32. doi: 10.1089/neu.1992.9.21 [DOI] [PubMed] [Google Scholar]
- Taya K, Marmarou CR, Okuno K, Prieto R, Marmarou A, 2010. Effect of secondary insults upon aquaporin-4 water channels following experimental cortical contusion in rats. J. Neurotrauma 27, 229–239. doi: 10.1089/neu.2009.0933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal SC, Heinemann M, Luh C, Pieter D, Werner C, Engelhard K, 2011. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-γ-independent mechanisms. J. Neurotrauma 28, 983–993. doi: 10.1089/neu.2010.1685 [DOI] [PubMed] [Google Scholar]
- Thal SC, Neuhaus W, 2014. The blood-brain barrier as a target in traumatic brain injury treatment. Arch Med Res 45, 698–710. doi: 10.1016/j.arcmed.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR, 2010. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J. Cereb. Blood Flow Metab 30, 1008–1016. doi: 10.1038/jcbfm.2009.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, Nedergaard M, 2014. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci 37, 620–628. doi: 10.1016/j.tins.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B, Aston J, Dines M, Caraman E, Yacyshyn M, McCarthy M, Olson JE, 2017. Early Brain Edema is a Predictor of In-Hospital Mortality in Traumatic Brain Injury. J. Emerg. Med 53, 18–29. doi: 10.1016/j.jemermed.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Vajtr D, Benada O, Kukacka J, Průsa R, Houstava L, Toupalík P, Kizek R, 2009. Correlation of ultrastructural changes of endothelial cells and astrocytes occurring during blood brain barrier damage after traumatic brain injury with biochemical markers of BBB leakage and inflammatory response. Physiol. Res 58, 263–268. [DOI] [PubMed] [Google Scholar]
- Van Haren RM, Thorson CM, Ogilvie MP, Valle EJ, Guarch GA, Jouria JA, Busko AM, Harris LT, Bullock MR, Jagid JR, Livingstone AS, Proctor KG, 2013. Vasopressin for cerebral perfusion pressure management in patients with severe traumatic brain injury: preliminary results of a randomized controlled trial. J. Trauma Acute Care Surg 75, 1024–30; discussion 1030. doi: 10.1097/TA.0b013e3182a99d48 [DOI] [PubMed] [Google Scholar]
- Vaz R, Sarmento A, Borges N, Cruz C, Azevedo I, 1998. Effect of mechanogated membrane ion channel blockers on experimental traumatic brain oedema. Acta Neurochir (Wien) 140, 371–4; discussion 375. [DOI] [PubMed] [Google Scholar]
- Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, Manley GT, 2008. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J. Neurosurg 109, 678–684. doi: 10.3171/JNS/2008/109/10/0678 [DOI] [PubMed] [Google Scholar]
- Vilalta A, Sahuquillo J, Rosell A, Poca MA, Riveiro M, Montaner J, 2008. Moderate and severe traumatic brain injury induce early overexpression of systemic and brain gelatinases. Intensive Care Med 34, 1384–1392. doi: 10.1007/s00134-008-1056-1 [DOI] [PubMed] [Google Scholar]
- Vink R, Gabrielian L, Thornton E, 2017. The Role of Substance P in Secondary Pathophysiology after Traumatic Brain Injury. Front. Neurol 8, 304. doi: 10.3389/fneur.2017.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott BP, Kahle KT, Simard JM, 2011. Novel Treatment Targets for Cerebral Edema. Neurotherapeutics 9, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Perkins M, Dray A, 1995. Kinins and kinin receptors in the nervous system. Neurochem. Int 26, 1–16; discussion 17. doi: 10.1016/0197-0186(94)00114-A [DOI] [PubMed] [Google Scholar]
- Wallisch J, Jha R, Vagni V, Feldman K, Dixon C, Farr G, Kochanek P, 2015. Effect of the novel aquaporin-4 antagonist AER-271 in combined TBI plus hemorrhagic shock in mice. Crit. Care Med 43, 6–7. doi: 10.1097/01.ccm.0000473851.20338.3b [DOI] [Google Scholar]
- Wang W, Dentler WL, Borchardt RT, 2001. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am. J. Physiol. Heart Circ. Physiol 280, H434–40. doi: 10.1152/ajpheart.2001.280.1.H434 [DOI] [PubMed] [Google Scholar]
- Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH, 2000. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J. Neurosci 20, 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gu Y, Qin G, Zhong L, Meng Y, 2013. Curcumin ameliorates the permeability of the blood-brain barrier during hypoxia by upregulating heme oxygenase-1 expression in brain microvascular endothelial cells. J. Mol. Neurosci 51, 344–351. doi: 10.1007/s12031-013-9989-4 [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Kochanek PM, Wisniewski SR, Bell MJ, Clark RS, DeKosky ST, Marion DW, Adelson PD, 2000a. Interleukin-8 is increased in cerebrospinal fluid of children with severe head injury. Crit. Care Med 28, 929–934. [DOI] [PubMed] [Google Scholar]
- Whalen MJ, Carlos TM, Wisniewski SR, Clark RS, Mellick JA, Marion DW, Kochanek PM, 2000b. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit. Care Med 28, 3710–3717. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Minter D, Yue JK, Manley GT, 2016. Cerebral edema in traumatic brain injury: pathophysiology and prospective therapeutic targets. Neurosurg. Clin. N. Am 27, 473–488. doi: 10.1016/j.nec.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Wójciak-Stothard B, Entwistle A, Garg R, Ridley AJ, 1998. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J. Cell Physiol 176, 150–165. doi: [DOI] [PubMed] [Google Scholar]
- Woodcock TM, Frugier T, Nguyen TT, Semple BD, Bye N, Massara M, Savino B, Besio R, Sobacchi C, Locati M, Morganti-Kossmann MC, 2017. The scavenging chemokine receptor ACKR2 has a significant impact on acute mortality rate and early lesion development after traumatic brain injury. PLoS One 12, e0188305. doi: 10.1371/journal.pone.0188305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Schubert D, Gomez-Pinilla F, 2011. Brain and spinal cord interaction: a dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabil. Neural Repair 25, 332–342. doi: 10.1177/1545968310397706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z-G, Zhu X-M, Chu X-P, Minami M, Hey J, Wei W-L, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP, 2004. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698. doi: 10.1016/j.cell.2004.08.026 [DOI] [PubMed] [Google Scholar]
- Xu Z-M, Yuan F, Liu Y-L, Ding J, Tian H-L, 2016. Glibenclamide Attenuates Blood-Brain Barrier Disruption in Adult Mice after Traumatic Brain Injury. J. Neurotrauma 34, 925–933. doi: 10.1089/neu.2016.4491 [DOI] [PubMed] [Google Scholar]
- Yang Lijun, Wang F, Yang Liang, Yuan Y, Chen Y, Zhang G, Fan Z, 2018. HMGB1 a-Box Reverses Brain Edema and Deterioration of Neurological Function in a Traumatic Brain Injury Mouse Model. Cell Physiol. Biochem 46, 2532–2542. doi: 10.1159/000489659 [DOI] [PubMed] [Google Scholar]
- Yao X, Uchida K, Papadopoulos MC, Zador Z, Manley GT, Verkman AS, 2015. Mildly Reduced Brain Swelling and Improved Neurological Outcome in Aquaporin-4 Knockout Mice following Controlled Cortical Impact Brain Injury. J. Neurotrauma 32, 1458–1464. doi: 10.1089/neu.2014.3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S, Bell ED, Monson KL, 2013. Distribution of blood-brain barrier disruption in primary blast injury. Ann. Biomed. Eng 41, 2206–2214. doi: 10.1007/s10439-013-0805-7 [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ, 2004. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 101, 6752–6757. doi: 10.1073/pnas.0308636100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J-H, Park S-W, Brooks N, Lang BT, Vemuganti R, 2008. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res 1244, 164–172. doi: 10.1016/j.brainres.2008.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Lindley TE, Albert GW, Ahmed R, Schmeiser PB, Grady MS, Howard MA, Welsh MJ, 2013. Loss of Acid sensing ion channel-1a and bicarbonate administration attenuate the severity of traumatic brain injury. PLoS One 8, e72379. doi : 10.1371/journal.pone.0072379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Yi J, Ye G, Zheng Y, Song Z, Yang Y, Song Y, Wang Z, Bao Q, 2012. Effects of curcumin on levels of nitric oxide synthase and AQP-4 in a rat model of hypoxia-ischemic brain damage. Brain Res 1475, 88–95. doi: 10.1016/j.brainres.2012.07.055 [DOI] [PubMed] [Google Scholar]
- Yuan J, Liu W, Zhu H, Zhang X, Feng Y, Chen Y, Feng H, Lin J, 2017. Curcumin attenuates blood-brain barrier disruption after subarachnoid hemorrhage in mice. J. Surg. Res 207, 85–91. doi: 10.1016/j.jss.2016.08.090 [DOI] [PubMed] [Google Scholar]
- Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, Gordon WA, Maas AIR, Mukherjee P, Yuh EL, Puccio AM, Schnyer DM, Manley GT, TRACK-TBI Investigators, 2013. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 30, 1831–1844. doi: 10.1089/neu.2013.2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacest AC, Vink R, Manavis J, Sarvestani GT, Blumbergs PC, 2010. Substance P immunoreactivity increases following human traumatic brain injury. Acta Neurochir. Suppl 106, 211–216. doi: 10.1007/978-3-211-98811-4_39 [DOI] [PubMed] [Google Scholar]
- Zafardoost P, Ghasemi AA, Salehpour F, Piroti C, Ziaeii E, 2016. Evaluation of the effect of glibenclamide in patients with diffuse axonal injury due to moderate to severe head trauma. Trauma Mon 21, e25113. doi: 10.5812/traumamon.25113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler FA, Donnelly J, Menon DK, Smielewski P, Hutchinson PJA, Czosnyka M, 2018. A Description of a New Continuous Physiological Index in Traumatic Brain Injury Using the Correlation between Pulse Amplitude of Intracranial Pressure and Cerebral Perfusion Pressure. J. Neurotrauma. doi: 10.1089/neu.2017.5241 [DOI] [PubMed] [Google Scholar]
- Zeiler FA, Donnelly J, Smieleweski P, Menon D, Hutchinson PJ, Czosnyka M, 2017. Critical Thresholds of ICP Derived Continuous Cerebrovascular Reactivity Indices for outcome prediction in Non-Craniectomized TBI Patients: PRx, PAx and RAC. J. Neurotrauma. doi: 10.1089/neu.2017.5472 [DOI] [PubMed] [Google Scholar]
- Zhang C, Chen J, Lu H, 2015. Expression of aquaporin-4 and pathological characteristics of brain injury in a rat model of traumatic brain injury. Mol. Med. Rep 12, 7351–7357. doi: 10.3892/mmr.2015.4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Pu H, Zhang H, Wei Z, Jiang X, Xu M, Zhang L, Zhang W, Liu J, Meng H, Stetler RA, Sun D, Chen J, Gao Y, Chen L, 2017. Inhibition of Na+-K+-2Cl−cotransporter attenuates blood-brain-barrier disruption in a mouse model of traumatic brain injury. Neurochem. Int 111, 23–31. doi: 10.1016/j.neuint.2017.05.020 [DOI] [PubMed] [Google Scholar]
- Zhang M, Cui Z, Cui H, Cao Y, Zhong C, Wang Y, 2016. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci 17, 60. doi: 10.1186/s12868-016-0295-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kojic L, Tsang M, Grewal P, Liu J, Namjoshi D, Wellington CL, Tetzlaff W, Cynader MS, Jia W, 2016. Distinct roles for metalloproteinases during traumatic brain injury. Neurochem. Int 96, 46–55. doi: 10.1016/j.neuint.2016.02.013 [DOI] [PubMed] [Google Scholar]
- Zhang Z-Y, Jiang M, Fang J, Yang M-F, Zhang S, Yin Y-X, Li D-W, Mao L-L, Fu X-Y, Hou Y-J, Fu X-T, Fan C-D, Sun B-L, 2017. Enhanced Therapeutic Potential of Nano-Curcumin Against Subarachnoid Hemorrhage-Induced Blood-Brain Barrier Disruption Through Inhibition of Inflammatory Response and Oxidative Stress. Mol. Neurobiol 54, 1–14. doi: 10.1007/s12035-015-9635-y [DOI] [PubMed] [Google Scholar]
- Zhao B-Q, Wang S, Kim H-Y, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH, 2006. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med 12, 441–445. doi: 10.1038/nm1387 [DOI] [PubMed] [Google Scholar]
- Zhao X, Gorin FA, Berman RF, Lyeth BG, 2008. Differential hippocampal protection when blocking intracellular sodium and calcium entry during traumatic brain injury in rats. J. Neurotrauma 25, 1195–1205. doi: 10.1089/neu.2008.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Bian C, Yuan J, Chu W, Xiang X, Chen F, Wang C, Feng H, Lin J-K, 2014. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-KB signaling pathway in experimental traumatic brain injury. J. Neuroinflammation 11, 59. doi: 10.1186/1742-2094-11-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweckberger K, Hackenberg K, Jung CS, Hertle DN, Kiening KL, Unterberg AW, Sakowitz OW, 2014. Glibenclamide reduces secondary brain damage after experimental traumatic brain injury. Neuroscience 272, 199–206. doi: 10.1016/j.neuroscience.2014.04.040 [DOI] [PubMed] [Google Scholar]
- Zweifel C, Lavinio A, Steiner LA, Radolovich D, Smielewski P, Timofeev I, Hiler M, Balestreri M, Kirkpatrick PJ, Pickard JD, Hutchinson P, Czosnyka M, 2008. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg. Focus 25, E2. doi: 10.3171/FOC.2008.25.10.E2 [DOI] [PubMed] [Google Scholar]