Abstract
Post-traumatic epilepsy (PTE) is diagnosed in 20% of individuals with acquired epilepsy, and can impact significantly the quality of life due to the seizures and other functional or cognitive and behavioral outcomes of the traumatic brain injury (TBI) and PTE. There is no available antiepileptogenic or disease modifying treatment for PTE. Animal models of TBI and PTE have been developed, offering useful insights on the value of inflammatory, neurodegenerative pathways, hemorrhages and iron accumulation, calcium channels and other target pathways that could be used for treatment development. Most of the existing preclinical studies test efficacy towards pathologies of functional recovery after TBI, while a few studies are emerging testing the effects towards induced or spontaneous seizures. Here we review the existing preclinical trials testing new candidate treatments for TBI sequelae and PTE, and discuss future directions for efforts aiming at developing antiepileptogenic and disease-modifying treatments.
Keywords: Inflammation, Tau, Neurodegeneration, Iron, Traumatic brain injury, Outcomes, Antiepileptogenesis, Disease modification, Preclinical trial
1. Introduction
Post-traumatic epilepsy (PTE) is diagnosed in 20% of individuals with acquired epilepsy (Piccenna et al., 2017) and may occur in 2–50% of individuals who have experienced traumatic brain injury (TBI), depending on the severity (Ding et al., 2016; Englander et al., 2009). PTE may negatively affect the quality of life of affected individuals due to direct and indirect impact of epileptic seizures (e.g., seizure-induced injuries or medical issues) and epilepsy-related comorbidities (e.g., behavioral, cognitive or other medical) or consequences (e.g., medication adverse effects, impact on quality of life, mortality) (Piccenna et al., 2017). PTE may further exacerbate or add to the effects of TBI on memory and cognition (Luo et al., 2014; Muccigrosso et al., 2016; Ogier et al., 2017), cerebral vasculature or blood brain barrier injury (Golding et al., 1999), sleep disorders (Castriotta et al., 2007), depression and post-traumatic stress disorder (Vos et al., 2018).
The risk of developing PTE depends on the severity of TBI. The most important risk factors to development of PTE are brain contusions, skull fracture, loss of consciousness for more than 30 minutes at initial TBI, subdural hemorrhage, acute symptomatic seizure, prolonged post-traumatic amnesia and the person’s age when the trauma occurred (Agrawal et al., 2006; D’Ambrosio and Perucca, 2004; Xu et al., 2017).
There is currently no treatment that prevents the development of PTE. The current clinical practice is to administer antiseizure drugs, such as phenytoin or levetiracetam, to prevent acute seizures after TBI, although these have limited or no effect on the development of PTE (Hung and Chen, 2012). Several promising drugs have been tested in preclinical and clinical trials, yet there is still a lack of anti-epileptogenic or disease-modifying therapies that could prevent epilepsy and the consequences of TBI and PTE (Marklund and Hillered, 2011). Here we will review the literature on preclinical studies investigating targets and candidate treatments in animal models of TBI and PTE, focusing on treatments targeting neuroinflammatory and antioxidant pathways, mechanistic target of rapamycin (mTOR), iron accumulation and hemorrhages, calcium channels and, to a lesser extent, neurodegenerative pathways, which will be reviewed in a separate manuscript in this special issue.
2. Animal models of PTE
2.1. Immature rodent models
Nearly half a million children under 14 years of age are treated for a TBI annually in US Emergency Departments (Langlois et al., 2005). Both acute and chronic seizures are not uncommon after TBI in this pediatric population, with several cohort studies identifying a higher incidence of PTE particularly in those under 3 years of age (Ates et al., 2006; Kieslich et al., 2001). The biological mechanisms underlying epileptogenesis and susceptibility to seizures after injury at a young age remain to be fully elucidated. Models used to study moderate to severe TBI in immature rodents include the controlled cortical impact (CCI) and lateral fluid percussion injury (LFPI) in postnatal day (PN) 17–21 rodents (Gurkoff et al., 2006; Nichols et al., 2015; Semple et al., 2017; Statler et al., 2008). Both models are characterized by considerable structural damage to the impacted cortex (left parietal or right frontoparietal) and underlying sub-cortical structures. Gross deformation of the ipsilateral hippocampus was visualized by magnetic resonance imaging (MRI) at 2–6 months post-injury in CCI-injured mice (Semple et al., 2017).
CCI injury in the right frontoparietal cortex in PN17 Sprague Dawley rats followed by a 2 week long video electroencephalography (vEEG) study immediately post-TBI, showed acute epileptiform discharges in 87.5% of the rats 24 h post-TBI, a quiescent period between day 3–7, while 44% (7/16) of the CCI rats manifested spontaneous seizures during the second week post-TBI (Nichols et al., 2015). Controls had no epileptiform activities. CCI to PN17 male Sprague-Dawley rats results in abnormal electroencephgraphy (EEG) epileptiform discharges between 4–11 months post-injury in the majority of animals (Statler et al., 2009). This model also results in a reduction in minimal clonic electroconvulsive seizure thresholds by adulthood (PN60–63), suggestive of a persistent increase in neuronal excitability (Statler et al., 2008). Only 1 in 8 rats were observed to exhibit spontaneous, recurrent convulsive seizures during the monitoring period (initiated 4–8 months post-TBI, average of 49 days, range 19–90 days per animal) while most of the CCI rats had epileptiform discharges. Sham animals had no epileptiform discharges or seizures. While such findings implicate a low seizure frequency and/or low incidence of PTE in this model, the observations may underestimate the true incidence due to the small sample size (Statler et al., 2009). More recently, PND21 mice were similarly exposed to CCI, and shown to display neuropathological characteristics consistent with PTE, such as mossy fiber sprouting and hippocampal sclerosis (Semple et al., 2017). CCI-injured mice exhibited a robust increase in susceptibility to provoked seizures in response to intraperitoneal (i.p.) pentylenetetrazole (PTZ) administration, as early as 2 weeks post-TBI and persisting until at least 6 months. In addition, video-EEG (vEEG) monitoring revealed at least one spontaneous convulsive seizure in the majority of CCI-injured mice (> 87% of CCI mice compared to < 10% of sham mice) during a 7 day recording period at 4–5 months post-injury, detected as abnormal epileptiform activity accompanied by behavioral manifestations.
Cognitive and histopathological injury has also been reported in the LFPI model, but no long-term vEEG studies have been reported yet (Gurkoff et al., 2006). Additional studies in immature animals are needed to validate and extend these findings, using rigorous methods for detection of both convulsive and non-convulsive seizures, as a function of TBI injury severities or impact locations.
2.2. Adult rodent models
As in immature animals, the LFPI and CCI models have most accurately recapitulated the epileptogenic mechanisms and changes that lead to the development of PTE after TBI in adult rodents (Bolkvadze and Pitkänen, 2012; Bragin et al., 2016; Liu et al., 2016; Thompson et al., 2005). For extensive review of the adult rodent models of TBI and PTE, we refer the reader to the relevant review in this special issue by (Brady et al., 2018).
Both CCI and LFPI models have shown to reproduce several aspects of human TBI, molecular, biochemical and pathology findings, including focal contusion, petechial, intraparenchymal and subarachnoid haemorrhages, tissue tears, and traumatic axonal injury (Bolkvadze and Pitkänen, 2012; Bragin et al., 2016; Golarai et al., 2001; Guo et al., 2013; Kharatishvili et al., 2006; Liu et al., 2016; Thompson et al., 2005). Moreover, MRI detected abnormalities such as enlarged ventricles, hippocampal atrophy, reduced fractional anisotropy in the corpus callosum, decreased N-acetyl aspartate/creatine ratio, and increased myoinositol/creatine ratio are seen both in this model and in patients (Liu et al., 2016; Shultz et al., 2015). The molecular and cellular changes can continue for weeks and months, and they associate with behavioural impairments and cognitive comorbidities (Guo et al., 2013; Kharatishvili et al., 2006; Liu et al., 2016; Shultz et al., 2015). Overall, a robust body of evidence supports the utility of these models in the identification and validation of biomarkers of PTE and the formulation of reasonable hypotheses regarding candidate mechanisms underlying epileptogenesis after TBI that would eventually result in the development of disease-modifying therapies for PTE.
A substantial proportion of animals had lowered seizure thresholds and increased seizure susceptibility to the chemoconvulsants PTZ, kainic acid or flurothyl (Bolkvadze and Pitkänen, 2012; Hunt et al., 2010; Kharatishvili et al., 2006; Thompson et al., 2005). Similar results have been shown in the weight drop (Chrzaszcz et al., 2010; Golarai et al., 2001) central fluid percussion injury (FPI) (Hamm et al., 1995) and blast injury models (Agoston, 2017; Williams et al., 2005), however further research is needed to fully characterize the occurrence of spontaneous recurrent seizures in these models.
2.3. Candidate target pathways and treatment in TBI animal models
TBI may cause molecular and structural alterations in the brain, some of which may lead to the emergence of PTE in susceptible individuals. TBI triggers local damage in the trauma adjacent region and mossy fiber sprouting in the dentate gyrus (Guo et al., 2013; Hunt et al., 2012). Diffuse axonal injury may cause a loss of organization in white matter tracts (Smith et al., 2013). Diffuse multifocal injury can rupture blood vessels (Sharp et al., 2014), which leads to blood-brain barrier (BBB) disruption, hemorrhage, and consequently neuronal death (Garton et al., 2016). Increase in extracellular glutamate in tissue adjacent to the trauma has also been reported (Faden et al., 1989). Neurodegeneration, e.g., hyperphosphorylated tau (p-tau) and neurofibrillary tangles, can be observed after TBI and has been linked to late TBI-consequences, such as cognitive decline, dementia, and possibly epilepsy (Sharp et al., 2014). In this section, we will review some of the treatment studies in TBI models that targeted these pathways and discuss their relevance to functional outcomes and PTE.
2.4. Hyperphosphorylation of tau
2.4.1. Relevance to epilepsy and TBI
Tau is a microtubule-associated protein that under normal physiological conditions maintains neuronal health, axonal transport, and microtubule stabilization by mediating a balance between binding and non-binding states to the microtubules, which in turn is regulated by partially phosphorylated tau (Morris et al., 2011). The aggregates of the insoluble p-tau are commonly associated with the pathology of neurodegenerative conditions including Alzheimer’s disease, frontotemporal dementia, and traumatic encephalopathy (Rostgaard et al., 2015). These neurological diseases have increased risk to also manifest epilepsy (Abou-Khalil, 2010; Friedman et al., 2012; Nicastro et al., 2016). In addition, there is evidence in both clinical and preclinical studies for the involvement of p-tau based neurofibrillary tangles in epilepsy (Thom et al., 2011), focal cortical dysplasia and drug resistant epilepsy (Sen et al., 2007) and post-TBI. Surgically resected epileptogenic brain regions from drug-resistant temporal lobe epilepsy (TLE) patients (Liu et al., 2017b; Puvenna et al., 2016) and animal models of epilepsy after chemoconvulsant-induced status epilepticus (SE) or kindling-induced seizures show increased p-tau based on Western blots (Liang et al., 2009; Liu et al., 2016; Tian et al., 2010). Likewise, genetic rodent models of tauopathies have reported alterations in the susceptibility to epileptic seizures (Liu et al., 2017a; Yan et al., 2012). Also, p-tau oligomers and increased p-tau by Western blots have been reported in experimental models (Hawkins et al., 2013; Shultz et al., 2015) and also in brain tissues from human patients acutely after TBI (Albayram et al., 2017; Seo et al., 2017).
2.4.2. Targeting p-tau in animal models of seizures and PTE
Studies using genetic modifications to reduce p-tau provided evidence that it reduces susceptibility to induced seizures (Holth et al., 2013; Li et al., 2014; Roberson et al., 2007). Sodium selenate is an oxidized, less toxic form of selenium that specifically activates protein phosphatase 2A (PP2A) containing the PR55 regulatory subunit and to decrease the level of p-tau (Corcoran et al., 2010b; van Eersel et al., 2010). PP2A dephosphorylates tau at several sites and particularly at Thr205, Thr212, Ser214 and Ser262 (Qian et al., 2010). Pharmacological interventions using sodium selenate to reduce p-tau showed reduced network hyperexcitability and susceptibility to seizure induction in the 6Hz corneal stimulation, PTZ and amygdala kindling models of seizures (Jones et al., 2012) as well as in a malin-deficiency mouse model of Lafora disease (Sanchez-Elexpuru et al., 2017). Moreover, sodium selenate treatment in the post-SE and TBI rat models administered acutely after the brain injury ameliorated MRI-detected abnormalities indicative of brain damage, reduced cognitive and motor deficits and retarded epileptogenesis (Liu et al., 2016; Shultz et al., 2015). Importantly, the effect on PTE was noted with a treatment protocol initiated after TBI and its effect lasted for at least 2 weeks after drug withdrawal. We refer the reader to the review on targeting neurodegeneration pathways in this special issue (Ali et al., 2018) for further details.
2.4.3. Relevance for PTE
Despite the promise of these findings, the underlying molecular mechanisms relating to changes in PP2A/PR55 following TBI and during post-TBI epileptogenesis are not entirely clear and are increasingly being investigated (Liu et al., 2016; Liu et al., 2017b). Similarly, a causative mechanism for the antiepileptogenesis or neuroprotective effect of inhibiting p-tau using sodium selenate is not established and off target effects are not ruled out. Such findings though provide insights on a common neurological link between the neurodegenerative diseases such as Alzheimer’s, brain trauma and acquired epilepsies (Wilson et al., 2017). Future studies identifying the mechanisms and validation of finding of protective effects of inhibiting p-tau in large multicenter preclinical trial will assist in translating the findings to human antiepileptogenesis trials. This possibility is further supported by the fact that sodium selenate treatment regimen has been demonstrated to be safe in phase I and phase II clinical trials in prostate cancer and Alzheimer’s disease patients (Corcoran et al., 2010a; Malpas et al., 2016).
2.5. Hemorrhages/Brain iron accumulation
2.5.1. Relevance to epilepsy and PTE
Severe TBI can cause subarachnoid, intracerebral, subdural or epidural hemorrhages. Beyond the direct structural lesions caused by intracranial bleeds, iron toxicity and/or accumulation from hemolysis or release of intracellular iron sources due to cell injury may further contribute to the TBI sequelae, including the increased risk for PTE. Excess iron can be toxic because it leads to generation of free radicals, mitochondrial fragmentation, may induce cell autophagy due to oxidative stress resulting in neuronal loss and peri-hematomal edema (Garton et al., 2016). Cell death may also occur as a result of heme induced apoptosis or iron-dependent cell death (ferroptosis) (Garton et al., 2016).
In an LFPI rat model, TBI causes BBB disruption, iron deposition in the ipsilateral hemisphere and upregulated heme oxygenase-1 (HO-1; catabolizes heme to biliverdin, carbon monoxide and Fe2+) (Okubo et al., 2013). Metals have been shown to contribute to epileptogenesis (Kendirli et al., 2014). Injection of ferric chloride into the sensorimotor cortex begets recurrent focal paroxysmal EEG discharges (Willmore et al., 1978). In the iron-induced PTE rat model, brains from rats with epilepsy had higher extracellular interictal levels of aspartate and glutamate than those that did not develop seizures, while serine levels were elevated in the cortex ipsilateral to iron deposits (Engstrom et al., 2001).
Intracellular iron accumulation increases amyloid precursor protein (APP), by inhibiting iron regulatory proteins (IRPs) that transcriptionally downregulate APP. APP has been proposed to have neuroprotective effects by preserving ferroportin in the plasma membrane and stabilizing iron export from cells. In support, APP knockout mice show less brain injury and iron accumulation after CCI (Ayton et al., 2014). However, iron may increase amyloid beta (Aβ) toxicity (Wang et al., 2012) and long-term HO-1 overexpression may increase iron accumulation and tau hyper-phosphorylation (Hui et al., 2011), suggesting that additional investigation is needed to further explore the interactions of APP signaling and iron-mediated brain injury in TBI.
2.5.2. Targeting iron accumulation in animal models of TBI and humans
In animal studies, deferoxamine (100 mg/kg intramuscular at 2 and 14 h post-TBI) reduced the TBI-induced lipocalin-2 (mediates transferrin-independent iron transport) increase and the ventricular enlargement and HO-1 upregulation in LFPI-injured adult male Sprague Dawley rats (Zhao et al., 2014; Zhao et al., 2016). In the weight drop model in adult male Sprague Dawley rats, deferoxamine treatment (100 mg/kg twice daily, starting 2 h post-TBI till day 28) reduced the volume of the lesion as well as iron load, ferritin, transferrin and transient receptor potential canonical channel 6 (TRPC6; involved in iron uptake) levels and improved performance in the Morris water maze test (Zhang et al., 2013). In rabbits subjected to TBI, deferoxamine (50 mg/kg) prevented the decrease in superoxide dismutase and partially of the glutathione peroxidase in the injured brain (Erkan Ustun et al., 2001). Deferoxamine pre-treatment (0.51 μmol/g i.p., 15 min prior to CCI) improved performance in Morris water maze, without affecting the size of the injury (Long et al., 1996).
Deferoxamine has been used in clinical studies for its potential to ameliorate hemorrhagic load and associated edema. The HI-DEF (high dose deferoxamine in intracerebral hemorrhage) phase I trial showed good tolerability of the intravenous (i.v.) infusions of 62 mg/kg/day deferoxamine mesylate dose (Yeatts et al., 2013). Due to concerns of possible association with acute respiratory distress a prospective multicenter double-blinded randomized, placebo-controlled phase II clinical trial of deferoxamine (32 mg/kg/day for 3 consecutive days) is ongoing (iDEF; NCT02175225). In a randomized blinded placebo controlled study of 94 patients with TBI, 5 day treatment with deferoxamine mesylate 20 mg/kg/day i.v., starting on the day of the TBI, accelerated hematoma absorption and reduced edema after traumatic intracerebral hemorrhage (Yu et al., 2017).
Minocycline, a drug with anti-inflammatory effects (see also below) can also act as an iron chelator. It has shown beneficial effects in models of iron toxicity, improving iron load, induced edema and blood brain disruption [reviewed in (Garton et al., 2016)]. In a small clinical trial with 15 patients with moderate or severe TBI, minocycline (100 mg orally, twice daily for 12 weeks vs vehicle) reduced microglial activation but increased neurofilament light levels, a finding that was also associated with faster rates of brain atrophy (Scott et al., 2018). These suggest that perhaps more targeted and selective treatment approaches could be beneficial to avoid disruption of pathways that would be important for regenerative processes. Further preclinical and clinical studies are needed to further characterize the effect of interventions that prevent iron accumulation and toxicity on disease modification and antiepileptogenesis. There is currently no study assessing the role of iron chelators in the development of PTE.
2.6. Inflammatory processes
2.6.1. Relevance to epilepsy and TBI
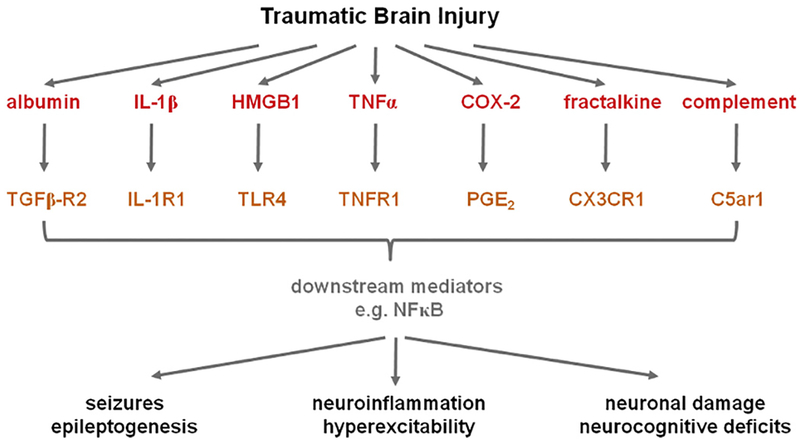
Neuroinflammation is a hallmark not only of neurotrauma but also of various epileptic disorders and their etiologies (Barker-Haliski et al., 2017; Galanopoulou et al., 2012b; Shandra et al., 2017; Terrone et al., 2017; Vezzani and Viviani, 2015; Webster et al., 2017). In response to epileptogenic insults, including TBI, there is a robust cellular response involving the activation of resident astrocytes and microglia, vascular changes including disruption of the BBB, and release of a plethora of soluble inflammatory mediators with neuromodulatory capacities. Inflammatory mediators including cytokines and chemokines are increasingly understood to both directly and indirectly influence neuronal excitability and survival, thus contributing to the epileptogenic process by promoting seizure initiation and propagation or epileptogenesis. Several key mediators implicated in epileptogenesis after TBI are summarized in Fig. 1.
Fig. 1.
A TBI triggers the upregulation of a wide range of pro-inflammatory mediators and their signaling pathways. Many of these, including those noted here, have been implicated in the development of epilepsy based on observations in patient samples as well as experimental manipulations in small animal models. See text for further details and source citations. Abbreviations: COX-2 = cyclooxygenase-2, CX3CR1 = CX3C chemokine receptor 1, C5ar1 = complement component 5a receptor 1, HMGB1 = high mobility group box protein-1, IL-1β = interleukin-1 beta, IL-1R1 = interleukin-1 receptor 1, NFκB = nuclear factor kappa-B; PGE2 = prostaglandin E2, MAPK = p38 mitogen activated protein kinase, TGFβ = transforming growth factor beta, TLR4 = toll-like receptor 4, TNFα = tumor necrosis factor alpha, TNFR1 = tumor necrosis factor receptor 1.
Leakage of the BBB and subsequent activation of neuroin-flammatory processes has been implicated in the establishment and progression of epilepsy (Bar-Klein et al., 2014; Friedman et al., 2009; Oby and Janigro, 2006; Seiffert et al., 2004; van Vliet et al., 2007). Seizures themselves or their etiologies can increase permeability of the BBB, therefore promoting extravasation of peripheral leukocytes into the brain parenchyma which then release more inflammatory cytokines (Alyu and Dikmen, 2017; Gorter et al., 2015). BBB leakage also exposes the brain to serum albumin, which binds to transforming growth factor beta (TGFβ) receptors to activate downstream signaling cascades (Bar Klein et al., 2014; Webster et al., 2017). Albumin uptake by astrocytes reduces their capacity to buffer extracellular potassium levels, increasing neuronal excitability and epileptiform activity. The prevention of experimental BBB dysfunction by blocking of albumin binding to TGFβ receptors provides strong evidence of a central role for this pathway in PTE (Bar-Klein et al., 2014; Friedman et al., 2009; Ivens et al., 2007).
Interleukin-1 (IL-1) is a prototypical pro-inflammatory cytokine, and one of the earliest responders to a traumatic insult (Rothwell, 2003). IL-1 beta (IL-1β) binds to its widely-expressed receptor IL-1 receptor 1 (IL-1R1), to initiate nuclear factor-kB (Nf-kB) dependent transcription of many other pro-inflammatory genes to amplify the subsequent neuroinflammatory response (Webster et al., 2017). Upregulation of IL-1β signaling has been observed in epilepsy patients and experimental models, with activation detected even prior to the diagnosis of epilepsy, thereby implicating it in epileptogenesis (Shandra et al., 2017; Vezzani et al., 2008). IL-1R1 activation may augment neuronal calcium influx by Src-mediated hyperphosphorylation of the N-methyl-D-aspartate (NMDA) receptor 2B (NR2B) subunit of NMDA receptor channels (Viviani et al., 2003) and reduces voltage sensitive K+ channels (Vezzani and Viviani, 2015). Cell type and receptor-specific effects of IL-1β have been reported on the gamma-aminobutyric acid A receptor (GABAAR) signaling. IL-1β enhances tonic GABAAergic inhibitory signaling, while it reduces synaptic GABAAR-mediated inhibition in the CA3 pyramidal neurons or in vitro but increases synaptic GABAAR inhibition in the CA1 pyramidal neurons (Roseti et al., 2015; Vezzani and Viviani, 2015). These pro-epileptogenic properties are largely shared by the activation of toll-like receptor 4 (TLR4), upon binding by the endogenous damage-associated molecule, high mobility group box protein 1 (HMGB1) (Roseti et al., 2015). HMGB1 is released both passively, during necrosis, as well as actively from immune cells following post-translational oxidation and acetylation in response to cytokine stimulation (Webster et al., 2017). Various rodent models have demonstrated a pro-ictogenic role for HMGB1 in experimental seizures (Balosso et al., 2014; Maroso et al., 2010; Ravizza et al., 2017).
TBI may also induce tumor necrosis factor alpha (TNFα), more commonly known for its role in the activation, proliferation and recruitment of immune cells during systemic inflammation (Croft et al., 2012). Interestingly, TNFα can have two distinct roles in the pathophysiology of seizures and epilepsy, dependent upon the receptor it binds; TNF receptor 1 (TNFR1, also known as p55) mediates pro-convulsive effects, while TNFR2 (a.k.a. p75) has predominantly anti-convulsive consequences (Balosso et al., 2013). A number of other cytokines or chemokines, which recruit and activate immune cells in the injured brain, have also been implicated in both seizures and epilepsy, as reviewed extensively elsewhere (Vezzani et al., 2008; Vezzani and Viviani, 2015). One chemokine in particular, fractalkine (CX3CL1), is robustly elevated after TBI in both patients and experimental models (Rancan et al., 2004), and has recently been shown to regulate synaptic transmission and neuronal excitability via CX3CR1-mediated activation of microglia (Ali et al., 2015; Roseti et al., 2013; Xu et al., 2012).
Cyclooxygenase (COX) is the rate-limiting enzyme required for the synthesis of prostanoids including prostaglandin E2 (PGE2). The COX-2 isoform is strongly upregulated in the human brain after seizures in experimental models of epilepsy (Desjardins et al., 2003; Serrano et al., 2011) COX-2 expression and activity is also enhanced for several days after TBI, increasing the levels of prostaglandins associated with neurodegeneration and neuroinflammation (Cernak et al., 2001; Dash et al., 2000; Figueiredo-Pereira et al., 2014). In a cortical stab injury adult rat model, COX-1+ microglia and macrophages increased by day 5–7 post-TBI and remained elevated till at least day 21, suggesting a potential role in secondary injury (Schwab et al., 2001). In experimental models of seizures and epilepsy, manipulation of COX-2 has been shown to suppress or enhance excitatory glutamatergic synaptic function, implicating a more direct role in neurotransmission (Takemiya et al., 2003; Yang and Chen, 2008).
2.6.2. Targeting neuroinflammatory pathways in models of TBI and PTE
The role of COX-1 or COX-2 has been tested in TBI models, using either inhibitors or knockout mice, with inconsistent results. In the adult rat CCI model, the COX-2 inhibitor celecoxib worsened the motor but not the cognitive outcomes, suggesting a COX-2 role in recovery (Dash et al., 2000). In the impact accelerator model of TBI, the COX-2 inhibitor nimesulide given after TBI (30 min and daily till 10 days post-TBI) improved motor and cognitive deficits (Cernak et al., 2001). The COX-2 inhibitor meloxicam, given 30 min after weight drop TBI, reduced BBB disruption and brain edema at 48 h (Hakan et al., 2010). However, neither meloxicam nor nimesulide improved functional outcomes or brain edema 6 and 24 h after percussion injury in 5–6 week old male Swiss mice, while indomethacin improved functional outcome only (Girgis et al., 2013). In contrast, carprofen, given for 7 days after CCI to adult male Sabra mice, reduced lesion, inflammation and edema, increased cellular proliferation, and improved neurological functional outcomes 3 months post-CCI (Thau-Zuchman et al., 2012). In PN17 rats subjected to CCI, the COX2 inhibitor SC58125 (15 min and 24 h post-CCI) prevented PGE2 increase without ameliorating lesion volume or performance in the Morris water maze (Hickey et al., 2007). In the rat CCI model, a less specific anti-inflammatory and anti-oxidant drug, alantolactone, given daily after CCI for 2 days, reduced neuroin-flammation markers (IL-1β, IL-6, PGE2), brain edema and improved neurological outcome at 48 h (Wang et al., 2018).
Cox-1 and/or Cox-2 null mice had no differences in brain injury or Morris water maze performance after CCI injury (Ahmad et al., 2008; Kelso et al., 2009). There are no antiepileptogenesis studies of COX inhibitors reported in TBI models. In another approach, pharmacological suppression of cytokine production by Minozac reduced susceptibility to electroconvulsive shock seizures 7 days post CCI (Chrzaszcz et al., 2010).
It is worth noting that the above-mentioned mechanisms are not mutually exclusive. In fact, there is considerable cross-talk between soluble mediators, their receptors, and downstream signaling cascades. For example, HMGB1 can enhance expression of IL-1β, while IL-1β can promote HMGB1 translocation from the nucleus to the cytoplasm in preparation for its release (Vitaliti et al., 2014). By activating NF-kB, IL-1β can initiate the transcription of many pro-inflammatory genes including TNFβ and COX-2. These interactions result in a very complex cellular environment post-TBI, with the net result being an increase in neuronal excitability and susceptibility to the development of chronic epilepsy.
A key disadvantage to broad-spectrum compounds with anti-inflammatory properties is that treatment will likely influence a broad range of pathways, both pathological and restorative, with a risk of off-target effects on normal brain function. As such, a more targeted approach focused on key inflammatory mediators or pathways implicated in the development of epilepsy, as described above, may hold greater promise for therapeutic potential. Specifically, in the context of PTE, the commercially-available IL-1R1 antagonist Kineret® (Anakinra) was recently shown to modulate the astrocytic response and influence long-term seizure susceptibility in an experimental model of pediatric TBI (Semple et al., 2017). Another approach, to target the signaling cascade downstream of albumin-TGFβ activation, was effective at preventing the development of epilepsy in a model of focal neocortical BBB disruption (Weissberg et al., 2015). Other targeted strategies, such as antagonism of HMGB1 with the BoxA pseudo-peptide, have demonstrated anti-ictogenic activity in experimental models and been proposed to have anti-epileptogenic potential in acquired epilepsy such as after a brain insult (Iori et al., 2016; Vezzani et al., 2011). Largely, however, targeted anti-inflammatory compounds have not yet been explored in preclinical models of PTE for antiepileptogenic potential, nor evaluated for anti-epileptogenic efficacy in patient populations. Further work is required to develop a better understanding of how neuroinflammation contributes to the onset and progression of acquired epilepsy, and to define the optimal therapeutic window for intervention with anti-inflammatory treatments.
2.7. Calcium channels
2.7.1. Relevance to epilepsy and TBI
Voltage-gated calcium (Ca2+) channels are classified as low-(LVA) or high-voltage-activated (HVA) channels. The monomeric LVA (T-type) Ca2+ channels are present in three different isoforms the CaV3.1 - CaV3.3 (Zamponi et al., 2010). HVA channels include CaV1 (L-type), CaV2.1 (P/Q-type) Cav2.2 (N-type) and Cav2.3 (R-type) channels which are composed of α2δ, β and γ subunits. HVA channels have higher depolarization threshold and are involved with neurotransmitter release in presynaptic terminals, whereas LVA channels are expressed mainly in neuron bodies and dendrites and have been linked to the pathogenesis of absence epilepsy and to a lesser extent to TLE (Zamponi et al., 2010). Mutations of the CACNA1A gene that codes for the HVA CaV2.1channel and in the CACNA1H gene that codes for CaV3.2 in humans and mice have been linked to absence epilepsy (Heron 2007, Chen 2003, Liang 2006–2007). Changes in intracellular Ca2+ levels and Ca2+ homeostatic mechanisms have been associated to seizures and acquired epileptogenesis (Raza et al., 2004). Evidence has shown that after an acute epileptogenic brain injury there are irreversible Ca2+ increases that lead to neuronal death, that is followed by sub-lethal, prolonged, but reversible, elevations in Ca2+ levels that trigger pathological changes which could ultimately lead to the development of epilepsy (Delorenzo et al., 2005; Nagarkatti et al., 2009). Low-threshold Ca2+−dependent burst firing in CA1 pyramidal cells seems to be important to epileptogenesis in TLE models (Casillas-Espinosa et al., 2015; Sanabria et al., 2001; Yaari et al., 2007). Moreover, T-type Ca2+ currents shifted from the normal steady-state inactivation to a more hyperpolarized membrane potential during the latent period, to a more depolarized membrane potential during the chronic period of epileptogenesis (Graef et al., 2009). CaV3.2 knockout mice are resistant to pilocarpine-induced SE and the development of chronic spontaneous occurring seizures. In addition, the development of hippocampal sclerosis and mossy fiber sprouting histopathological hallmarks of TLE were absent in these knockout mice (Becker et al., 2008). Moreover, T-type Ca2+ channels have a role in glutamate release (Casillas-Espinosa et al., 2012; Huang et al., 2011) and changes in T-type Ca2+ expression have been shown to affect regulation of expression of both glutamate and GABA receptors in limbic regions (Nagarkatti et al., 2009).
In TBI, intracellular Ca2+ accumulation contributes to neuronal death due to activation of apoptosis pathways (Gurkoff et al., 2017). Excessive absorption of Ca2+ by the mitochondrial membrane may trigger mitochondrial dysfunction (Lifshitz et al., 2004; Verweij et al., 1997) in both ipsilateral and contralateral to injury hemispheres (Verweij et al., 1997). Ca2+ dependent phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) may alter gene transcription, including of glutamate, GABA and cholinergic receptors that control neuronal excitability (Gurkoff et al., 2017). The abnormalities of Ca2+ homeostasis can be observed for up to 30 days post-TBI (Deshpande et al., 2008).
2.7.2. Targeting calcium channels in models of epilepsies and TBI
Blockers of T-type channels may modify epileptogenesis in other models of epilepsies. For example, early ethosuximide treatment has shown antiepileptogenic effects in absence epilepsy models (Blumenfeld et al., 2008; Dezsi et al., 2013; Russo et al., 2010). Z944, a selective pan-T-type channel blocker, delayed seizure progression in the amygdala kindling model (Casillas-Espinosa et al., 2015) and suppressed absence seizures via a mechanism distinct from ethosuximide (Tringham et al., 2012).
Investigation of these treatments in PTE models for their anti-epileptogenic potency is still lacking. However, studies with Ca2+ channel antagonists in TBI models help in understanding the role of Ca2+ channels in neurotrauma pathophysiology and their potential to alleviate the behavioral, structural, molecular sequelae of TBI (Casillas-Espinosa et al., 2015; Lee et al., 2004; Verweij et al., 2000). Combination of N-type calcium channel blocker (SNX-111, ziconotide) and anti-oxidant (U-101033E) improved TBI-induced mitochondrial dysfunction (Xiong et al., 1998). The optimal treatment window to restore mitochondrial function with ziconotide was found to be between 2–6 h post-CCI (Verweij et al., 2000). Ziconotide, given at 3, 5, and 24 h after TBI, improved motor and cognitive function in the acceleration-deceleration model of TBI in rats (Berman et al., 2000). Improved learning and neurological function was also reported with the N-type CaV2 in hibitor SNX-185 injected 5 min after LFPI injury in the CA2 pyramidal region (Lee et al., 2004). In an in vitro model of mechanical strain on mixed cortical and astroglial cultures, SNX-185 reduced calcium accumulation intracellularly and improved neuronal viability (Shahlaie et al., 2013).
2.8. Mechanistic target of rapamycin (mTOR)
2.8.1. Relevance to epilepsy and TBI
The mTOR signaling pathway controls cell growth and survival, proliferation and cellular metabolism (Laplante and Sabatini, 2012), cortical development, synaptic plasticity and learning (Tang et al., 2002), as well as neuronal morphology (Jaworski et al., 2005). Neuronal mTOR promotes axonal regeneration in response to CNS injury (Liu et al., 2010; Park et al., 2008) while it limits the ability of glial-mediated functional recovery in the injured brain and spinal cord (Codeluppi et al., 2009). Dysfunction of mTOR signaling is also implicated in a plethora of pathophysiological processes including cancer, diabetes, obesity, neurodegeneration but also seizures, epilepsy and a variety of etiologies causing them (Galanopoulou et al., 2012b; Zeng et al., 2009).
Overactivation of the mTOR pathway has been reported in a right parietal FPI rat model of TBI ipsilateral to the injury. This included phosphorylation of mTOR and downstream targets, like eukaryotic initiation factor 4E binding protein-1 (4E-BP1), p70 ribosomal S6 kinase (p70S6K), and ribosomal protein S6 (rpS6) within 30 min after the injury in the ipsilateral parietal cortex and hippocampus, lasting up to one day after the injury (Chen et al., 2007). This acute mTOR-mediated effect might represent a mechanistic strategy of affected neurons to respond and/or recover from injury. Activation of mTOR has been linked to reduced glutamate-induced excitoxicity after TBI due to the expression of the glutamate transporter in the glia (Wu et al., 2010).
2.8.2. Targeting mTOR in TBI models
The mTOR pathway may be critical for epileptogenesis in a variety of epilepsy models and may modify certain pathologies associated with epilepsy, such as mossy fiber sprouting in the dentate gyrus, neuronal morphology and connectivity. The effect however of mTOR inhibitors in suppressing epilepsy and associated pathologies can be time, species, dose or model dependent and may be reversed after withdrawal of the inhibitors, prompting the term “epileptostatic” rather than anti-epileptogenic [reviewed in (Galanopoulou et al., 2012b)].
In the closed head weight drop injury model, mTOR inhibition via rapamycin administration improved functional recovery and improvement of the ensuing behavioral impairment and inflammatory response (Erlich et al., 2007). Rapamycin administration increased neuronal survival in the injury site, reduced microglial activation and prevented p70S6K phosphorylation, serving as a candidate mechanism of neuro-protection following TBI. In a different study, inhibition of mTORC1 activity within hours after moderate CCI injury in mice, led to cognitive amelioration and significant reduction in neuronal apoptosis and gliosis, suggesting that acute intervention can enhance the recovery potential of the injured brain (Nikolaeva et al., 2016). In the CCI mouse model, overactivation of mTOR was found in the cortex and hippo-campus during the first post-CCI week (Guo et al., 2013). A four week course with rapamycin (6 mg/kg/day i.p.), starting 1 hour post-CCI, reduced neuronal degeneration in the hippocampus, transiently reduced mossy fiber sprouting during the treatment period, and reduced the percentage of mice that developed epilepsy at 4 months post-CCI (13% vs 50% in vehicle treated), suggesting antiepileptogenic effect, at least within the time frame of this study (Guo et al., 2013). In a different study using the CCI model, rapamycin (3 or 10 mg/kg i.p. starting the day of CCI and given till sacrifice) treated mice had reduced mossy fiber sprouting and neurogenesis but did not show significant reduction in neuronal loss or the observed behavioral seizures (i.e., tail stiffness and freezing for more than 30 s) compared to vehicle treated mice (Butler et al., 2015). However, vEEG monitoring was not performed. The difference in seizure outcomes between the two studies, could be due in model effects (i.e., severity of models), types of seizures recorded and methods of seizure monitoring.
Taken together, acute mTOR pathway activation after injury may be important for neuroprotection or regeneration but mTOR dysregulation resulting in overactivation may predispose to epilepsy. Most importantly, in a post-TBI brain, one needs to consider that mTOR modulators may have different effects upon the injured and the uninjured brain regions. Definition of the optimal treatment window and regimen that corrects mTOR dysregulation without impairing the mTOR signaling dependent processes that are necessary for normal brain function might be critical in shaping the recovery potential of the injured brain and possibly its vulnerability to epilepsy.
2.9. Central cholinergic pathways
2.9.1. Relevance to epilepsy and TBI
Most TBI patients are suffering from cognitive deficits reporting lasting problems in learning and memory (Hoofien et al., 2001). Acetylcholine is a neurotransmitter implicated in cognitive function and its release is found elevated in physiological processes such as fear and stress (Imperato et al., 1991) as well as during seizures (Gardner and Webster, 1977). Interestingly, TBI induces a profound cholinergic response, resulting in a massive release of acetylcholine immediately after the injury which seems to be transient reaching baseline levels within minutes or hours after the injury (Saija et al., 1988). Cholinergic stimulation results in seizure activity and significant neuronal damage in the hippocampus (Olney et al., 1983; Turski et al., 1983) suggesting that a TBI-induced cholinergic activation could have similar effects that might be relevant to post-traumatic epilepsy progression.
2.9.2. Targeting cholinergic system in TBI
Blockade of the muscarinic acetylcholine receptor (mAChR) acutely after moderate TBI showed neuroprotective effects enhancing the behavioral recovery after injury (Lyeth et al., 1993). Early suppression of muscarinic receptor activity and cessation of abnormally-elevated acetylcholine levels might prevent a TBI- and cholinergic-mediated acceleration of epileptogenic processes. Therefore, blockade of central cholinergic pathways might conceivably serve as a twofold therapeutic target for the prevention of post-traumatic cognitive deficits and the progression to post-traumatic epilepsy. However, there are no studies directly testing these effects in a PTE model.
2.10. Other pathways
Several therapeutic approaches have been tested in TBI models for their ability to modify the TBI-induced pathologies and functional recovery, although effects on PTE are lacking. Some of these studies are mentioned below. The cholesterol-lowering drug simvastatin (Wu et al., 2012) was administered in a 14 day treatment protocol following CCI injury, reduced axonal injury, decreased APP immunoreactivity, enhanced neurite outgrowth, and improved neurological functional recovery in adult Wistar rats. Melatonin activated mitophagy (elimination of damaged mitochondria) through the mTOR signaling and reduced inflammation in the CCI model (Lin et al., 2016). Subanesthetic doses of ketamine, an NMDA receptor inhibitor, reduced inflammation and autophagy and improved memory and behavior (Wang et al., 2017a). Another interesting target is the microRNAs (miRNAs). In a repetitive model of TBI, miR-124–3p increased following injury, which was proposed to be important in inhibiting inflammation and improving neurite outgrowth through mTOR inhibition (Huang et al., 2018). NaHS is a donor of hydrogen sulfide, which may preserve BBB integrity, exerts anti-inflammatory and anti-oxidant effects but also may inhibit autophagy by activating mTOR pathway. NaHS restored mitochondrial function and improved functional recovery in the CCI model (Xu et al., 2018). Most of these compounds interact with the pathways described above, making it therefore important to discern which effects could be a result of selected common pathways and which would require combination therapies to achieve maximal effect. Guanosine is an endogenous nucleoside with neuroprotection effects. A single injection of guanosine (7.5 mg/kg i.p.) administered 40 min after LFPI in adult Wistar rats improved the motor impairment 8hours after injury as well as reduced the ipsilateral cortical injury, glutamate uptake, Na+/K+-ATPase, glutamine synthetase activity, alterations in mitochondrial function, neuronal death, inflammation and brain edema (Gerbatin et al., 2017). This study did not assess the antiepileptogenic potential of guanosine.
Other treatments have also been tested for effects on post-TBI seizures (Table 1). Ceftriaxone is an antibiotic which is commonly used in the clinics but also in the perioperative prophylactic antibiotic treatment in survival surgeries in rodents. Ceftriaxone (200 mg/kg daily i.p. for 7 days, starting after TBI surgery) prevented the reduction in glutamate trasporter-1 (GLT-1) expression in the ipsilateral cortex after LFPI in rats, decreased GFAP expression in the lesioned cortex and reduced the cumulative seizure duration observed in EEG recording 12 weeks after TBI (Goodrich et al., 2013). Seizures in this study included spike runs that exceeded 10sec in duration although it is not clear what was their behavioral correlate. Of note, this dose and treatment duration is significantly higher and longer than the prophylactic treatment with ceftriaxone used prior to survival surgeries and there is no evidence that prophylactic antibiotic protocols may also alter PTE. Atipamezole selectively inhibits α2-adrenergic receptors and increases noradrenaline release in the brain. Chronic administration of atipamezole, starting at either 30 min or 7 days post-LFPI in adult rats and continuing for 9 weeks, improved functional motor outcome and beam-walking performance but not spatial learning and memory (Nissinen et al., 2017). Atipamezole did not affect the development of epilepsy but reduced seizure susceptibility in the PTZ testing at 14 weeks post-TBI. Ketogenic diet has also been tested in the LFPI model in its ability to modify flurothyl seizure threshold. The flurothyl seizure threshold was increased only during the time the animals were on the ketogenic diet but not after its discontinuation (Schwartzkroin et al., 2010). Hypothermia in LFPI rats may reduce subsequent PTZ seizure susceptibility (Atkins et al., 2010). Focal cortical cooling reduced the frequency of freeze-like arrest events associated with runs of spike-waves in the LFPI rat model compared to controls (Atkins et al., 2010; D’Ambrosio et al., 2013). In the undercut model, whereby partial isolation of sensorimotor cortex is done with trans-sections, gabapentin reduced the excitability, excitatory synapses, and GFAP expression; the authors proposed that these effects could be due to the reduction in thrombospondin, an astrocyte-released protein that participates in excitatory synapse formation (Li et al., 2012).
Table 1.
Preclinical treatment trials in rodent models of TBI to observe effects on seizures
| Treatment | Mechanism | Model | Species, strain, age, sex | Effect on spontaneous seizures | Effect on induced seizures | Other effects/comments | Reference |
|---|---|---|---|---|---|---|---|
| Atipamezole (1 mg/kg/i.p. 30min post-TBI followed by 100 μg/kg/hr SC minipump, for 9weeks; or 100 μg/kg/hr SC minipump for 7 days-9 weeks post-TBI) | α2-adrenergic antagonist | LFPI, severe | Rats, Sprague-Dawley, adult, male | No effect | Reduced PTZ seizure susceptibility | (Nissinen et al., 2017) | |
| Ceftriaxone (200 mg/kg/day i.p., 7 days post-TBI) | Increases expression of GLT-1 (glutamate transporter) | LFPI | Rats, Long Evans, Adult, male | Reduces cumulative seizure duration 12 weeks post-TBI | Prevents decrease of GLT-1. Seizures are spike runs > 10sec; behavioral correlate is not clear. | (Goodrich et al., 2013) | |
| Gabapentin (Starting 1 h post-TBI: −100 mg/kg, 3 times per day for 2 days or - 120 mg/kg/day for 13–15 days SC) | Inhibits α2δ subunit of L-type calcium channels; Inhibits thrombospondin-induced excitatory synapses formation | Undercut cortex model | Rats, Sprague-Dawley, 30 days old, male | N/A | Reduces evoked epileptiform discharges in cortical slices 1 day and 14 days post-gabapentin | Reduces, excitatory synapses, and GFAP expression | (Li et al., 2012) |
| Hypothermia | Hypothermia | LFPI, moderate | Rats, Sprague Dawley, Adult, male | N/A | Reduced number of PTZ seizures, 12 weeks post-TBI | Reduced mossy fiber sprouting | (Atkins et al., 2010) |
| Hypothermia (focal cooling by 2 °C for 5.5 weeks, starting 3 days post-TBI) | Focal cooling by 0.5–2 °C | Rostral parasagittal FPI | Rats, Sprague Dawley | Abolished ictal activity up to 10 weeks after cooling. Seizures are runs of spike waves with freezelike arrest. | N/A | (D’Ambrosio et al., 2013) | |
| Ketogenic diet (starting 3 weeks pre-TBI) | Ketosis, anti-inflammatory, multiple effects | LFPI | Rats, Sprague Dawley, 8 weeks, male | N/A | Increased threshold to flurothyl-induced seizures, 3 or 6weeks post-TBI while ketogenic diet is given but not after it is withdrawn. | (Schwartzkroin et al., 2010) | |
| Minozac (5 mg/kg i.p., 3 h and 6 h post-TBI) | Anti-inflammatory | CCI | CD1 mice, Adult, male | N/A | Reduced susceptibility to electroconvulsive shock seizures, 7 days post-TBI | Improved performance in Barnes maze testing (learning, memory) | (Chrzaszcz et al., 2010) |
| Rapamycin (6 mg/kg/day i.p, start 1 h post-TBI, for 4 weeks) | mTOR inhibition | CCI | CD1 mice, 8 weeks old, males | Reduced PTE risk at 4 months post TBI (13% vs 50% in vehicle treated) | N/A | VEEG monitoring for 16 weeks. Seizures show evolution on EEG, and result in arrest, clonus, rearing and/or falling. | (Guo et al., 2013) |
| Rapamycin (3 or 10 mg/kg i.p. starting the day of CCI and given till sacrifice) | mTOR inhibition | CCI | CD1 mice, 6–8 weeks old | No significant reduction in behavioral seizures | N/A | Seizures: behavioral tail stiffness and freezing > 10 sec | (Butler et al., 2015) |
| Rimonabant (SR141716A) (1 or 10 mg/kg i.p., immediately or 20 min after TBI) | CB1 receptor antagonist | LFPI, moderate | Rats, Wistar, 21 −22 days old | N/A | Reduced susceptibility to kainate induced seizures, 6 weeks post-TBI | (Echegoyen et al., 2009) | |
| Sodium selenate (1 mg/kg/day SC, minipumps, after TBI for 12 weeks) | Activates protein phosphatase 2A (PP2A) containing the PR55 regulatory subunit and decreases p-tau | LFPI, severe | Rats, Long Evans, adult, male | Reduced seizure frequency during treatment and 3–4 weeks postwashout | N/A | VEEG monitoring starting at 10 weeks post-TBI; monitoring done at 10–12 weeks (on treatment) and 15–16 weeks post-TBI (washout) | (Liu et al., 2016) |
CCI: controlled cortical impact; EEG: electroencephalography; FPI: fluid percussion injury; LFPI: lateral FPI; mTOR: mechanistic target of rapamycin; PP2A: protein phosphatase 2A; p-tau: hyperphosphorylated tau; PTE: post-traumatic epilepsy; PTZ: pentylenetetrazole; TBI: traumatic brain injury; vEEG: video EEG.
2.11. Considerations for future antiepileptogenic preclinical studies for PTE
Despite the large number of antiseizure medications used in patients or in animal models, medical treatments that prevent epilepsy is still lacking (Loscher and Schmidt, 2011). When designing an anti-epileptogenic drug trial it is important to plan on collecting adequate preclinical evidence that will guide future preclinical and clinical trials. From preclinical studies, we anticipate information that includes treatment indication and target population, dosing and route of administration, potential drug interactions, timing and duration of treatment and tolerability of the drug as well as tolerability (Galanopoulou et al., 2012a; Galanopoulou et al., 2013a; Schmidt, 2012). A number of proposals have been put forward to improve rigor (e.g., blinded, randomized design guided by power analysis) and transparency (e.g., detailed report of experimental conditions and variables that could affect results and their interpretation) in study design and reporting as well as to alleviate and remediate concerns that many promising preclinical therapies have not succeeded in transitioning to clinical practice (Brooks-Kayal et al., 2013; Galanopoulou et al., 2012a; Galanopoulou et al., 2013a; Lidster et al., 2016; O’Brien et al., 2013; Pitkanen et al., 2013). Recent efforts in harmonizing methodological approaches in preclinical research and generating preclinical common data elements attempt to provide helpful recommendations and improve comparisons among studies done in different laboratories (Galanopoulou et al., 2017a; Hernan et al., 2017; Kadam et al., 2017; Raimondo et al., 2017). We refer the reader to these reports which aim to improve the practices followed in biomedical animal research.
The development of antiepileptogenic and disease modifying treatments has been proposed as a priority for future epilepsy research (Galanopoulou et al., 2013b) (https://www.ninds.nih.gov/About-NINDS/Strategic-Plans-Evaluations/Strategic-Plans/2014-NINDS-Benchmarks-Epilepsy-Research). Overall, the existing TBI preclinical studies have mostly tested target modification against functional motor and behavioral recovery, pathological sequelae of TBI and to a lesser extent against the seizure susceptibility or epileptogenesis (listed in Table 1). Such seizure testing often examines the susceptibility to induced seizures, since it provides higher throughput testing compared to long-term monitoring for spontaneous seizures. However, clearer and more direct evidence for antiepileptogenic effect is obtained from studies utilizing long-term vEEG monitoring for spontaneous seizures. We will briefly list here some selected issues that may be considered in the design on future studies.
2.11.1. Target population
Most of the available studies are done in male rodents and usually at adult ages, with few exceptions on immature animals (Table 1). Age and sex differences exist not only in the neurobiological pathways that may affect drug effects but also on the consequences of seizures, their comorbidities and possibly pathways involved in epileptogenesis (Akman et al., 2014, 2015; Giorgi et al., 2014; Jones et al., 2014; Kight and McCarthy, 2014; Pitkanen et al., 2014). Studies that are powered to determine possible age and sex differences in treatment efficacy or tolerability in PTE models are needed. Although inclusion of such biological variables is currently strongly encouraged by funding agencies, meaningful conclusions on the potential age and sex differences may only be derived if studies are appropriately powered [see Table I in (Galanopoulou and Mowrey, 2016)], yet this is likely to severely affect the sample sizes and budget needed. The EpiBioS4Rx (Epilepsy bioinformatics study for antiepileptogenic therapy) Center Without Walls, which is designed to test new therapies for their potential to prevent PTE in the rat LFPI model, specifically adopted the multicenter pre-clinical trial design to address the issue of requiring large numbers of animals to rigorously test the effect of a candidate antiepileptogenic drug in PTE.
Another critical issue is the interpretation of data across various models of TBI and PTE. Ideally, findings confirmed across models are expected to confirm the validity of the results, yet in many situations conflicting results may be difficult to interpret due to the many species, model and experimental study design differences across studies (Galanopoulou et al., 2017b). Knowledge of the target mechanisms and interpretation of the treatment effects based on evidence for target relevance and modification in each model and study design may help distinguish between true and false negative studies. However, there have been exceptions when drugs that entered clinical practice in the absence of a clear target mechanism.
2.11.2. Study design
Most of the available trials take advantage of the identifiable time of the insult to initiate treatment soon after TBI (Table 1). More accurate definition of the therapeutic window, preferably guided by clinical surrogates or biomarkers, would greatly help guide treatment implementation in future clinical trials. Treatments may not have the same effect if given at different timepoints and it will ultimately be important to investigate whether subjects with established PTE will also respond to these treatments with cessation of seizures. Defining the minimal necessary duration of the candidate antiepileptogenic treatment based on its biological effects, will be important, both to allow testing for antiepileptogenic effects (i.e., persisting after treatment discontinuation) but also to minimize unnecessary exposure to drugs that can be potentially costly or harmful. Furthermore, one needs to eventually consider the cost and tolerability profiles of new therapies, which would be best served by delivering them only for the period that these are truly needed, based on the target relevance profile. Preclinical trials have the advantage that they allow collection of data from terminal procedures, that are needed to test target relevance and modification based on pathology findings, explore the BBB permeability of a drug, yet currently these cannot be easily applied in the clinics.
2.11.3. Biomarkers
While these are very useful to rationalize treatments, decide on best doses and protocols and interpret positive or negative results, exploration of the value of plasma, cerebrospinal fluid (CSF), imaging, and electrophysiological biomarkers or other clinical surrogates in de-fining treatment window and informing on target engagement or modification would greatly enhance efforts to translate current treatments to the clinics [(Engel Jr., 2018)(Pitkanen et al., 2018) and this supplement]. Plasma, urine and, in certain clinical situations, CSF are feasible to obtain and could potentially be used to either stratify patients by their risk for PTE or likelihood to respond to candidate treatments, once valid biomarkers become available. It is known that cytokines, such as TNF-α, IL-6, IL-8, IL-10, TGF-β, are elevated in CSF minutes after TBI (Sharma and Laskowitz, 2012). A study showed that IL-1β was elevated in the CSF, but not in the serum, in TBI patients compared to control (Diamond et al., 2014). Additionally, serum IL-1β levels were lower in patients that developed PTE compared to those who did not; no difference was found in CSF IL-1β levels (Diamond et al., 2014). In the same study, patients with high CSF/serum IL-1β ratios in the first week post-TBI had higher risk to develop PTE (Diamond et al., 2014). Apparently, IL-1β single-nucleotide polymorphism has an important role in PTE development, considering that individuals with TT genotype seem to be protected from PTE, however more studies are needed to better conclusions (Cotter et al., 2017). Various studies have also been assessing the value of miRNAs (small non coding RNAs), based on their implication in other models of epileptogenesis (Henshall et al., 2016).
Despite of the fact that there are no valid biomarkers available for post-TBI epileptogenesis, neuroimaging has been a widely applied technique to evaluate effectiveness of interventions against TBI-induced pathological changes including lesion volume or brain atrophy (Karve et al., 2016; Shultz et al., 2014; Sun et al., 2017; Yousuf et al., 2016), white matter integrity (Kim et al., 2015; Yousuf et al., 2016), metabolic changes (Brabazon et al., 2017; Kim et al., 2015; Vaquero et al., 2017), functional connectivity (McAllister et al., 2011; Roy et al., 2010) as well as neuroinflammation and BBB disruption (Corser-Jensen et al., 2014; Fukuda et al., 2013; Wang et al., 2017b) following brain injury. On the contrary, only few studies evaluating potential antiepileptogenic treatments have utilized neuroimaging brain outcomes and showed ablation or reduction in volumetric measurements on T2-weighted MRI (Semple et al., 2017; Shultz et al., 2015) as well as white matter integrity using diffusion weighted imaging (Liu et al., 2016; Shultz et al., 2015). It is evident that imaging modalities have not been evaluated to their full potential perhaps due to the logistic issues and related costs. Considering the potential advantages mainly of providing substantial insights into brain pathology and functioning in vivo at longitudinal time points and being of high translational value as they are already being used in the clinical management of TBI patients (Xu et al., 2017), applying these modalities will be of high value to the antiepileptogenic preclinical studies.
EEG is routinely used in the clinical practice and more invasive electrocorticography methods may be in use in certain neurointensive care unit centers that admit individuals with TBI, offering a promising tool for biomarker development. In the LFPI model, early predictors of PTE development are thought to be the emergence of pathological high-frequency oscillations (HFOs) (100–600 Hz) and repetitive HFOs with spikes (rHFOSs) (Bragin et al., 2016). TBI animals that present with early rHFOSs are more likely to manifest PTE at later time points suggesting that rHFOSs could be a candidate biomarker for epileptogenesis and therefore possible early selection and treatment of patients at high risk for PTE. For example, in the pilocarpine model of SE, levetiracetam suppressed both hippocampal HFOs and interictal spiking and led to a reduced seizure burden in the treated animals (Levesque et al., 2015). Furthermore, evidence from human patients suggests that HFOs become more pronounced after antiseizure medication reduction (Zijlmans et al., 2009). We refer the readers to the other reviews in this special issue that extensively cover the progress on such biomarkers (Engel Jr., 2018; Pitkanen et al., 2018).
2.11.4. Seizure outcome measures
Adoption of outcome measures that could be relevant to or in forming the clinical practice or trials would also be important (Galanopoulou and Mowrey, 2016). For antiepileptogenesis studies, seizure outcome assessment during and after treatment washout, guided by pharmacokinetic studies, is critical to determine true anti-epileptogenic from antiseizure effects. Common relevant efficacy outcomes include seizure freedom, measures of seizure severity and seizure duration, distinction of responders from nonresponders, and changes in seizure frequency.
Seizure scoring is an important element of the antiepileptogenesis studies. Seizures after TBI are usually categorized as early (≤7 days post-TBI, usually during the first 24 h) or late (> 1 week). PTE diagnosis lies in the occurrence of the first unprovoked (late) seizure which might become evident after several years following the initial brain insult. The existing preclinical studies have used either behavioral monitoring or vEEG monitoring to score seizures. Use of vEEG monitoring with appropriate cumulative durations of the monitoring sessions and at time points justified by the natural history of PTE in the selected model is paramount to enabling the study to detect meaningful differences in seizure outcomes. Use of vEEG recordings to identify seizures is more sensitive than behavioral monitoring, as demonstrated in (Cook et al., 2013; Goldenholz et al., 2017) and may allow the use of smaller sample sizes to detect differences. The behavioral correlate of the observed seizures is also important to consider and frame it in the context of the experimental group. It is well known that many studies have reported spike-wave bursts associated with behavioral arrest, sometimes associated with sudden movement at the end of the event in in rats or mice used as experimental controls, and these increase in frequency and duration with age [reviewed in (Kadam et al., 2017)]. Some investigators interpret these as absence-like seizures, responding to drugs usually treating absence seizures, and others as physiological oscillatory patterns associated with aging and not necessarily epileptic. Clear definitions of seizure types and criteria for seizure scoring are important since these various events may have different pharmacosensitivity profiles and prevalence in the various models, confounding the end effect of the treatments. Furthermore, their implications for assigning therapeutic indications of the tested drugs may differ. Preferably, distinction of treatment effects among these various event types may clarify the treatment indications. Importantly, it is critical that the duration and timing of the vEEG monitoring of controls (vehicle TBI or sham animals) is equivalent to the monitoring of experimental animals to determine whether the observed seizure effects are truly relevant to the TBI-induced seizures rather than to seizures or seizure-like events seen in shams or controls.
2.11.5. Measures of tolerability and functional outcome
Obtaining evidence of tolerability in an animal model of a disease is most helpful since drug tolerability may differ from control populations (Galanopoulou et al., 2013a). Tolerability or toxicity in an animal model may miss toxicities observed in humans, but it still offers valuable information if early toxicity is observed (Bracken, 2009; Galanopoulou et al., 2013a). Use of pharmacokinetic pharmacodynamics measures to correlate with efficacy and tolerability may enable better design of treatment protocols to be tested in humans. In TBI models, several measures of physical, motor, behavioral tests have been utilized to monitor tolerability, including but not limited to weight measurements and body conditioning scale, neuroscore, mortality (Galanopoulou et al., 2013a; Kharatishvili et al., 2006). For more specific functional outcomes, specialized behavioral tests can be utilized to evaluate effects on learning or memory, affective disorders, attention and these are reviewed in a different review in this special issue (Semple et al., 2018).
3. Conclusions
Animal models of TBI have provided useful information on target mechanisms and proof of concept studies for new candidate treatments of TBI sequelae and PTE. Several treatments have been tested against early pathology or functional outcomes. Very few have shown promise for their potential to reduce susceptibility to induced seizures and even fewer have shown evidence of antiepileptogenesis potential. In response to a call for development of antiepileptogenic or disease modifying treatments using rigorous methods, the EpiBioS4Rx (Epilepsy bioinformatics study for antiepileptogenic therapy) Center Without Walls has organized the first preclinical multicenter consortium to create a systematic platform for testing for new treatments to prevent PTE while collecting evidence for target and biomarker modification to guide future clinical trials. It is hoped that these efforts may deliver better future therapies to improve the life of those affected by PTE.
Acknowledgements:
PS and CL have no conflicts of interest in regards to this manuscript. IA has funding support from the Melbourne University Early Career Research grant. IA has no conflicts of interest in regards to the contents of this manuscript. PMCE acknowledges support from the Melbourne University Early Career Research grant. PMCE has no conflicts of interest in regards to the contents of this manuscript. BDS acknowledges support from the National Health and Medical Research Council of Australia (NHMRC) and Monash University. BDS has no conflicts of interest in regards to the contents of this manuscript. SLM is the Charles Frost Chair in Neurosurgery and Neurology and partially funded by grants from NIHU54 NS100064 and NS43209, US Department of Defense (W81XWH-13-1-0180), and the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. S.L.M. has no conflicts of interest in regards to this article. He is serving as Associate Editor of Neurobiology of Disease and is on the editorial board of Brain and Development, Pediatric Neurology and Physiological Research. He receives from Elsevier an annual compensation for his work as Associate Editor in Neurobiology of Disease and royalties from 2 books he co-edited. He received a consultant fee from UCB for participation in a DSMB. He has also received honorarium for participation in an advisory board meeting of Mallinckrodt, but there is no conflict of interest in regards to the contents of this manuscript. ASG acknowledges grant support by NINDSRO1 NS091170, the NINDS Center without WallsU54 NS100064 (EpiBioS4Rx), the United States Department of Defense (W81XWH-13-1-0180), research funding from the Heffer Family, the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. ASG has received royalties for publications from Elsevier and a one-time honorarium for participation at a scientific advisory board for Mallinckrodt and is a co-Editor-in-Chief at Epilepsia Open but has no conflicts in regards to this article.
Abbreviations:
- Aβ
amyloid beta (Aβ)
- APP
amyloid precursor protein
- BBB
blood brain barrier
- Ca2+
calcium
- C5ar1
complement component 5a receptor 1
- CCI
controlled impact
- COX
cyclooxygenase
- CSF
cerebrospinal fluid
- CX3CR1
CX3C chemokine receptor 1
- EEG
electroencephalography
- FPI
fluid percussion injury
- GABAAR
gamma-aminobutyric acid A receptor
- GLT-1
glutamate transporter 1
- HVA
high voltage activated
- HFOs
high-frequency oscillations
- HI-DEF
high dose deferoxamine in intracerebral hemorrhage
- HMGB1
high mobility group box protein-1
- HO-1
heme oxygenase-1
- IL
interleukin
- IL-1β
interleukin-1 beta
- IL-1R1
interleukin-1 receptor 1
- IRP
iron regulatory protein
- i.v.
intravenous
- LFPI
lateral FPI
- LVA
low-voltage-activated
- miRNA
micro RNA
- MRI
magnetic resonance imaging
- mTOR
mechanistic target of rapamycin
- mAchR
muscarinic ylcholine receptor
- NFκB
nuclear factor kappa-B
- NMDA
N-methyl-D-aspartate
- PGE2
prostaglandin E2
- PN
postnatal day
- PP2A
protein phosphatase 2A
- p-tau
hyperphosphorylated tau
- PTE
post-traumatic epilepsy
- PTZ
pentylenetetrazole
- rHFOSs
repetitive HFOs with spikes
- SC58125
COX2 inhibitor
- SE
status epilepticus
- TBI
traumatic brain injury
- TGFβ
transforming growth factor beta
- TLE
temporal lobe epilepsy
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor alpha
- TNFR
tumor necrosis factor receptor
- TRPC6
receptor potential canonical channel 6
- vEEG
video EEG
References
- Abou-Khalil BW, 2010. How important is Alzheimer’s disease as a risk factor for un-provoked seizures and epilepsy in the elderly? Epilepsy Curr 10, 36–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston D, 2017. Modeling the long-term consequences of repeated blast-induced mild traumatic brain injuries. Journal of Neurotrauma 34, S44–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Timothy J, Pandit L, Manju M, 2006. Post-traumatic epilepsy: an overview. Clinical Neurology and Neurosurgery 108, 433–439. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Rose ME, Vagni V, Griffith RP, Dixon CE, Kochanek PM, et al. , 2008. Genetic disruption of cyclooxygenase-2 does not improve histological or behavioral outcome after traumatic brain injury in mice. J Neurosci Res 86, 3605–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman O, Moshe SL, Galanopoulou AS, 2014. Sex-specific consequences of early life seizures. Neurobiol. Dis (72 Pt B), 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman O, Moshe SL, Galanopoulou AS, 2015. Early life status epilepticus and stress have distinct and sex-specific effects on learning, subsequent seizure outcomes, including anticonvulsant response to phenobarbital. CNS Neurosci Ther 21, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayram O, Kondo A, Mannix R, Smith C, Tsai CY, Li CY, et al. , 2017. Cis P-tau is induced in clinical and preclinical brain injury and contributes to post-injury sequelae. Nat. Commun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I, Chugh D, Ekdahl CT, 2015. Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain. Neurobiol. Dis 74, 194–203. [DOI] [PubMed] [Google Scholar]
- Ali I, Silva J, Liu S, Shultz S, Kwan P, Jones N, O’Brien T, 2018. Targeting neurodegeneration pathways to prevent post-traumatic epilepsy. Neurobiol. Dis in press (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyu F, Dikmen M, 2017. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29, 1–16. [DOI] [PubMed] [Google Scholar]
- Ates O, Ondul S, Onal C, Buyukkiraz M, Somay H, Cayli SR, et al. , 2006. Post-traumatic early epilepsy in pediatric age group with emphasis on influential factors. Childs Nerv Syst 22, 279–284. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, et al. , 2010. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur. J. Neurosci 32, 1912–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, et al. , 2014Ceruloplasmin and beta-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic. Biol. Med 69, 331–337. [DOI] [PubMed] [Google Scholar]
- Balosso S, Ravizza T, Aronica E, Vezzani A, 2013. The dual role of TNF-alpha and its receptors in seizures. Exp Neurol 247, 267–271. [DOI] [PubMed] [Google Scholar]
- Balosso S, Liu J, Bianchi ME, Vezzani A, 2014. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid Redox Signal 21, 1726–1740. [DOI] [PubMed] [Google Scholar]
- Barker-Haliski ML, Loscher W, White HS, Galanopoulou AS, 2017Neuroinflammation in epileptogenesis: Insights and translational perspectives from new models of epilepsy. Epilepsia 58 (Suppl. 3), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Klein G, Cacheaux LP, Kamintsky L, Prager O, Weissberg I, Schoknecht K, et al. , 2014. Losartan prevents acquired epilepsy via TGF-beta signaling suppression. Ann. Neurol 75, 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, et al. , 2008. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J. Neurosci 28, 13341–13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Verweij BH, Muizelaar JP, 2000. Neurobehavioral protection by the neuronal calcium channel blocker ziconotide in a model of traumatic diffuse brain injury in rats. J. Neurosurg 93, 821–828. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, et al. , 2008. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 49, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkvadze T, Pitkänen A, 2012. Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J. Neurotrauma 29, 789–812. [DOI] [PubMed] [Google Scholar]
- Brabazon F, Wilson CM, Jaiswal S, Reed J, Frey WHN, Byrnes KR, 2017. Intranasal insulin treatment of an experimental model of moderate traumatic brain injury. J. Cereb. Blood Flow Metab 37, 3203–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken MB, 2009. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med 102, 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RD, Casillas-Espinosa PM, Agoston DV, Bertram EH, Kamnaksh A, Semple BD, et al. , 2018. Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Li L, Almajano J, Alvarado-Rojas C, Reid AY, Staba RJ, et al. , 2016. Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia 57, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Bath KG, Berg AT, Galanopoulou AS, Holmes GL, Jensen FE, et al. , 2013. Issues related to symptomatic and disease-modifying treatments affecting cognitive and neuropsychiatric comorbidities of epilepsy. Epilepsia 54 (Suppl. 4), 44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CR, Boychuk JA, Smith BN, 2015. Effects of rapamycin treatment on neurogenesis and synaptic reorganization in the dentate gyrus after controlled cortical impact injury in mice. Front Syst. Neurosci 9, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas-Espinosa PM, Powell KL, O’Brien TJ, 2012. Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia 53 (Suppl. 9), 41–58. [DOI] [PubMed] [Google Scholar]
- Casillas-Espinosa PM, Hicks A, Jeffreys A, Snutch TP, O’Brien TJ, Powell KL, 2015. Z944, a Novel selective T-type calcium channel antagonist delays the progression of seizures in the amygdala kindling model. PLoS One 10, e0130012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castriotta RJ, Wilde MC, Lai JM, Atanasov S, Masel BE, Kuna ST, 2007. Prevalence and consequences of sleep disorders in traumatic brain injury. J. Clin. Sleep Med 3, 349–356. [PMC free article] [PubMed] [Google Scholar]
- Cernak I, O’Connor C, Vink R, 2001. Activation of cyclo-oxygenase-2 contributes to motor and cognitive dysfunction following diffuse traumatic brain injury in rats. Clin. Exp. Pharmacol. Physiol 28, 922–925. [DOI] [PubMed] [Google Scholar]
- Chen S, Atkins CM, Liu CL, Alonso OF, Dietrich WD, Hu BR, 2007. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J. Cereb Blood Flow Metab 27, 939–949. [DOI] [PubMed] [Google Scholar]
- Chrzaszcz M, Venkatesan C, Dragisic T, Watterson DM, Wainwright MS, 2010. Minozac treatment prevents increased seizure susceptibility in a mouse “two-hit” model of closed skull traumatic brain injury and electroconvulsive shock-induced seizures. J. Neurotrauma 27, 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, et al. , 2009. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J. Neurosci 29, 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook MJ, O’Brien TJ, Berkovic SF, Murphy M, Morokoff A, Fabinyi G, et al. , 2013. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol 12, 563–571. [DOI] [PubMed] [Google Scholar]
- Corcoran NM, Hovens CM, Michael M, Rosenthal MA, Costello AJ, 2010a. Open-label, phase I dose-escalation study of sodium selenate, a novel activator of PP2A, in patients with castration-resistant prostate cancer. Br. J. Cancer 103, 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran NM, Martin D, Hutter-Paier B, Windisch M, Nguyen T, Nheu L, et al. , 2010b. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J. Clin. Neurosci 17, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Corser-Jensen CE, Goodell DJ, Freund RK, Serbedzija P, Murphy RC, Farias SE, et al. , 2014. Blocking leukotriene synthesis attenuates the pathophysiology of traumatic brain injury and associated cognitive deficits. Exp. Neurol 256, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Kelso A, Neligan A, 2017. Genetic biomarkers of posttraumatic epilepsy: a systematic review. Seizure 46, 53–58. [DOI] [PubMed] [Google Scholar]
- Croft M, Duan W, Choi H, Eun SY, Madireddi S, Mehta A, 2012. TNF superfamily in inflammatory disease: translating basic insights. Trends Immunol 33, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Perucca E, 2004. Epilepsy after head injury. Curr. Opin. Neurol 17, 731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio R, Eastman CL, Darvas F, Fender JS, Verley DR, Farin FM, et al. , 2013. Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann. Neurol 73, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN, 2000. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J. Neurotrauma 17, 69–81. [DOI] [PubMed] [Google Scholar]
- Delorenzo RJ, Sun DA, Deshpande LS, 2005. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol. Ther 105, 229–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Sun DA, Sombati S, Baranova A, Wilson MS, Attkisson E, et al. , 2008. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci. Lett 441, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P, Sauvageau A, Bouthillier A, Navarro D, Hazell AS, Rose C, et al. , 2003. Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippo-campal sclerosis. Neurochem. Int 42, 299–303. [DOI] [PubMed] [Google Scholar]
- Dezsi G, Ozturk E, Stanic D, Powell KL, Blumenfeld H, O’Brien TJ, et al. , 2013. Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy. Epilepsia 54, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ML, Ritter AC, Failla MD, Boles JA, Conley YP, Kochanek PM, et al. , 2014. IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia 55, 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding K, Gupta PK, Diaz-Arrastia R, 2016. Epilepsy after traumatic brain injury In: Laskowitz D, Grant G (Eds.), Translational Research in Traumatic Brain Injury Frontiers in Neuroscience, Boca Raton (FL). [Google Scholar]
- Echegoyen J, Armstrong C, Morgan RJ, Soltesz I, 2009. Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcit-ability in a rat model. Epilepsy Res 85, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J Jr., 2018. Epileptogenesis, traumatic brain injury, and biomarkers. Neurobiol. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J, Bushnik T, Wright JM, Jamison L, Duong TT, 2009. Mortality in late post-traumatic seizures. J. Neurotrauma 26, 1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom ER, Hillered L, Flink R, Kihlstrom L, Lindquist C, Nie JX, et al. , 2001. Extracellular amino acid levels measured with intracerebral microdialysis in the model of posttraumatic epilepsy induced by intracortical iron injection. Epilepsy Res 43, 135–144. [DOI] [PubMed] [Google Scholar]
- Erkan Ustun M, Md AD, Oztin Ogun C, Sumer F, Gurbilek M, 2001. Effects of deferoxamine on tissue superoxide dismutase and glutathione peroxidase levels in experimental head trauma. J. Trauma 51, 22–25. [DOI] [PubMed] [Google Scholar]
- Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R, 2007. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol. Dis 26, 86–93. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R, 1989. The role of excitatory amino-acids and nmda receptors in traumatic brain injury. Science 244, 798–800. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Rockwell P, Schmidt-Glenewinkel T, Serrano P, 2014Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitinproteasome pathway and mitochondria to neurodegeneration. Front Mol. Neurosci 7, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Kaufer D, Heinemann U, 2009. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res 85, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Honig LS, Scarmeas N, 2012. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci. Ther 18, 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda AM, Adami A, Pop V, Bellone JA, Coats JS, Hartman RE, et al. , 2013. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J. Cereb. Blood Flow Metab 33, 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Mowrey WB, 2016. Not all that glitters is gold: a guide to critical appraisal of animal drug trials in epilepsy. Epilepsia Open 1, 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Buckmaster PS, Staley KJ, Moshe SL, Perucca E, Engel J Jr., et al. , 2012a. Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia 53, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Gorter JA, Cepeda C, 2012b. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia 53, 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Kokaia M, Loeb JA, Nehlig A, Pitkanen A, Rogawski MA, et al. , 2013a. Epilepsy therapy development: technical and methodologic issues in studies with animal models. Epilepsia 54 (Suppl. 4), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Simonato M, French JA, O’Brien TJ, 2013b. Joint AES/ILAE translational workshop to optimize preclinical epilepsy research. Epilepsia 54 (Suppl.4), 1–2. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, French JA, O’Brien T, Simonato M, 2017a. Harmonization in preclinical epilepsy research: A joint AES/ILAE translational initiative. Epilepsia 58 (Suppl. 4), 7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Mowrey WB, Liu W, Li Q, Shandra O, Moshe SL, 2017bPreclinical screening for treatments for infantile spasms in the multiple hit rat model of infantile spasms: an update. Neurochem. Res 42, 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CR, Webster RA, 1977. Convulsant-anticonvulsant interactions on seizure activity and cortical acetycholine release. Eur. J. Pharmacol 42, 247–256. [DOI] [PubMed] [Google Scholar]
- Garton T, Keep RF, Hua Y, Xi G, 2016. Brain iron overload following intracranial haemorrhage. Stroke Vasc. Neurol 1, 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbatin RDR, Cassol G, Dobrachinski F, Ferreira APO, Quines CB, Pace IDD, et al. , 2017. Guanosine protects against traumatic brain injury-induced functional impairments and neuronal loss by modulating excitotoxicity, mitochondrial dys-function, and inflammation. Mol. Neurobiol 54, 7585–7596. [DOI] [PubMed] [Google Scholar]
- Giorgi FS, Galanopoulou AS, Moshe SL, 2014. Sex dimorphism in seizure-controlling networks. Neurobiol. Dis (72 Pt B), 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis H, Palmier B, Croci N, Soustrat M, Plotkine M, Marchand-Leroux C, 2013. Effects of selective and non-selective cyclooxygenase inhibition against neurological deficit and brain oedema following closed head injury in mice. Brain Res 1491, 78–87. [DOI] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA, 2001. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci 21, 8523–8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenholz DM, Tharayil JJ, Kuzniecky R, Karoly P, Theodore WH, Cook MJ, 2017. Simulating clinical trials with and without intracranial EEG Data. Epilepsia Open 2, 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding EM, Robertson CS, Bryan RM, 1999. The consequences of traumatic brain injury on cerebral blood flow and autoregulation: a review. Clin. Exp. Hypertens 21, 299–332. [DOI] [PubMed] [Google Scholar]
- Goodrich GS, Kabakov AY, Hameed MQ, Dhamne SC, Rosenberg PA, Rotenberg A, 2013. Ceftriaxone treatment after traumatic brain injury restores expression of the glutamate transporter, GLT-1, reduces regional gliosis, and reduces post-traumatic seizures in the rat. J. Neurotrauma 30, 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, 2015. Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav 49, 13–16. [DOI] [PubMed] [Google Scholar]
- Graef JD, Nordskog BK, Wiggins WF, Godwin DW, 2009. An acquired channelopathy involving thalamic T-type Ca2+ channels after status epilepticus. J. Neurosci 29, 4430–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Zeng L, Brody D, Wong M, 2013. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 8, e64078–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkoff GG, Giza CC, Hovda DA, 2006. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res 1077, 24–36. [DOI] [PubMed] [Google Scholar]
- Gurkoff G, Shahlaie K, Lyeth B, Berman R, 2017. Voltage-Gated Calcium Channel Blockers for the Treatment of Traumatic Brain Injury New Therapeutics for Traumatic Brain Injury Elsevier, pp. 179–197. [Google Scholar]
- Hakan T, Toklu HZ, Biber N, Ozevren H, Solakoglu S, Demirturk P, et al. , 2010. Effect of COX-2 inhibitor meloxicam against traumatic brain injury-induced biochemical, histopathological changes and blood-brain barrier permeability. Neurol. Res 32, 629–635. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Pike BR, Temple MD, O’Dell DM, Lyeth BG, 1995. The effect of postinjury kindled seizures on cognitive performance of traumatically brain-injured rats. Exp. Neurol 136, 143–148. [DOI] [PubMed] [Google Scholar]
- Hawkins BE, Krishnamurthy S, Castillo-Carranza DL, Sengupta U, Prough DS, Jackson GR, et al. , 2013. Rapid accumulation of endogenous tau oligomers in a rat model of traumatic brain injury. J. Biol. Chem 288, 17042–17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshall DC, Hamer HM, Pasterkamp RJ, Goldstein DB, Kjems J, Prehn JHM, et al. , 2016. MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol 15, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Hernan AE, Schevon CA, Worrell GA, Galanopoulou AS, Kahane P, de Curtis M, et al. , 2017. Methodological standards and functional correlates of depth in vivo electrophysiological recordings in control rodents. A TASK1-WG3 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 58 (Suppl. 4), 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey RW, Adelson PD, Johnnides MJ, Davis DS, Yu Z, Rose ME, et al. , 2007. Cyclooxygenase-2 activity following traumatic brain injury in the developing rat. Pediatr. Res 62, 271–276. [DOI] [PubMed] [Google Scholar]
- Holth JK, Bomben VC, Reed JG, Inoue T, Younkin L, Younkin SG, et al. , 2013. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J. Neurosci 33, 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofien D, Gilboa A, Vakil E, Donovick PJ, 2001. Traumatic brain injury (TBI) 10–20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj 15, 189–209. [DOI] [PubMed] [Google Scholar]
- Huang Z, Lujan R, Kadurin I, Uebele VN, Renger JJ, Dolphin AC, et al. , 2011. Presynaptic HCN1 channels regulate Cav3.2 activity and neurotransmission at select cortical synapses. Nat. Neurosci 14, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, et al. , 2018. Increased miR-124–3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32, 512–528. [DOI] [PubMed] [Google Scholar]
- Hui Y, Wang D, Li W, Zhang L, Jin J, Ma N, et al. , 2011. Long-term overexpression of heme oxygenase 1 promotes tau aggregation in mouse brain by inducing tau phosphorylation. J. Alzheimers Dis 26, 299–313. [DOI] [PubMed] [Google Scholar]
- Hung C, Chen JW, 2012. Treatment of post-traumatic epilepsy. Curr. Treat Options Neurol 14, 293–306. [DOI] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN, 2010. Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J. Neurophysiol 103, 1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Haselhorst LA, Schoch KM, Bach EC, Rios-Pilier J, Scheff SW, et al. , 2012. Posttraumatic mossy fiber sprouting is related to the degree of cortical damage in three mouse strains. Epilepsy Res 99, 167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Angelucci L, 1991. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res 538, 111–117. [DOI] [PubMed] [Google Scholar]
- Iori V, Frigerio F, Vezzani A, 2016. Modulation of neuronal excitability by immune mediators in epilepsy. Curr. Opin. Pharmacol 26, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, et al. , 2007. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130, 535–547. [DOI] [PubMed] [Google Scholar]
- Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M, 2005. Control of dendritic arborization by the phosphoinositide-3’-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci 25, 11300–11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NC, Nguyen T, Corcoran NM, Velakoulis D, Chen T, Grundy R, et al. , 2012. Targeting hyperphosphorylated tau with sodium selenate suppresses seizures in rodent models. Neurobiol. Dis 45, 897–901. [DOI] [PubMed] [Google Scholar]
- Jones NC, O’Brien TJ, Carmant L, 2014. Interaction between sex and early-life stress: influence on epileptogenesis and epilepsy comorbidities. Neurobiol. Dis 72 (Pt B), 233–241. [DOI] [PubMed] [Google Scholar]
- Kadam SD, D’Ambrosio R, Duveau V, Roucard C, Garcia-Cairasco N, Ikeda A, et al. , 2017. Methodological standards and interpretation of video-electroencephalography in adult control rodents. A TASK1-WG1 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 58 (Suppl. 4), 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve IP, Zhang M, Habgood M, Frugier T, Brody KM, Sashindranath M, et al. , 2016. Ablation of type-1 IFN Signaling in hematopoietic cells confers protection following traumatic brain injury. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Scheff SW, Pauly JR, Loftin CD, 2009. Effects of genetic deficiency of cyclooxygenase-1 or cyclooxygenase-2 on functional and histological outcomes following traumatic brain injury in mice. BMC Neurosci 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendirli MT, Rose DT, Bertram EH, 2014. A model of posttraumatic epilepsy after penetrating brain injuries: effect of lesion size and metal fragments. Epilepsia 55, 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A, 2006. A model of post-traumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140, 685–697. [DOI] [PubMed] [Google Scholar]
- Kieslich M, Marquardt G, Galow G, Lorenz R, Jacobi G, 2001. Neurological and mental outcome after severe head injury in childhood: a long-term follow-up of 318 children. Disabil. Rehab 23, 665–669. [DOI] [PubMed] [Google Scholar]
- Kight KE, McCarthy MM, 2014. Using sex differences in the developing brain to identify nodes of influence for seizure susceptibility and epileptogenesis. Neurobiol. Dis 72 (Pt B), 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cam-Etoz B, Zhai G, Hubbard WJ, Zinn KR, Chaudry IH, 2015. Salutary effects of estrogen sulfate for traumatic brain injury. J. Neurotrauma 32, 1210–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE, 2005. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil 20, 229–238. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2012. mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LL, Galo E, Lyeth BG, Muizelaar JP, Berman RF, 2004. Neuroprotection in the rat lateral fluid percussion model of traumatic brain injury by SNX-185, an N-type voltage-gated calcium channel blocker. Exp. Neurol 190, 70–78. [DOI] [PubMed] [Google Scholar]
- Levesque M, Behr C, Avoli M, 2015. The anti-ictogenic effects of levetiracetam are mirrored by interictal spiking and high-frequency oscillation changes in a model of temporal lobe epilepsy. Seizure 25, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Graber KD, Jin S, McDonald W, Barres BA, Prince DA, 2012. Gabapentin decreases epileptiform discharges in a chronic model of neocortical trauma. Neurobiol. Dis 48, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hall AM, Kelinske M, Roberson ED, 2014. Seizure resistance without parkinsonism in aged mice after tau reduction. Neurobiol. Aging 35, 2617–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX, 2009. Dysregulation of tau phosphorylation in mouse brain during excitotoxic damage. J. Alzheimers Dis 17, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidster K, Jefferys JG, Blumcke I, Crunelli V, Flecknell P, Frenguelli BG, et al. , 2016. Opportunities for improving animal welfare in rodent models of epilepsy and seizures. J. Neurosci. Methods 260, 2–25. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK, 2004. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4, 705–713. [DOI] [PubMed] [Google Scholar]
- Lin C, Chao H, Li Z, Xu X, Liu Y, Hou L, et al. , 2016. Melatonin attenuates traumatic brain injury-induced inflammation: a possible role for mitophagy. J. Pineal Res 61, 177–186. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, et al. , 2010. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci 13, 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zheng P, Wright DK, Dezsi G, Braine E, Nguyen T, et al. , 2016. Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 139, 1919–1938. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Shen Y, Shultz SR, Nguyen A, Hovens C, Adlard PA, et al. , 2017aAccelerated kindling epileptogenesis in Tg4510 tau transgenic mice, but not in tau knockout mice. Epilepsia 58, E136–E141. [DOI] [PubMed] [Google Scholar]
- Liu X, Ou S, Yin M, Xu T, Wang T, Liu Y, et al. , 2017b. N-methyl-D-aspartate receptors mediate epilepsy-induced axonal impairment and tau phosphorylation via activating glycogen synthase kinase-3beta and cyclin-dependent kinase 5. Discov. Med 23, 221–234. [PubMed] [Google Scholar]
- Long DA, Ghosh K, Moore AN, Dixon CE, Dash PK, 1996. Deferoxamine improves spatial memory performance following experimental brain injury in rats. Brain Res 717, 109–117. [DOI] [PubMed] [Google Scholar]
- Loscher W, Schmidt D, 2011. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 52, 657–678. [DOI] [PubMed] [Google Scholar]
- Luo J, Nguyen A, Villeda S, Zhang H, Ding ZQ, Lindsey D, et al. , 2014. Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front. Neurol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyeth BG, Liu S, Hamm RJ, 1993. Combined scopolamine and morphine treatment of traumatic brain injury in the rat. Brain Res 617, 69–75. [DOI] [PubMed] [Google Scholar]
- Malpas CB, Vivash L, Genc S, Saling MM, Desmond P, Steward C, et al. , 2016. A phase IIa randomized control trial of VEL015 (sodium selenate) in mild-moderate Alzheimer’s disease. J. Alzheimers Dis 54, 223–232. [DOI] [PubMed] [Google Scholar]
- Marklund N, Hillered L, 2011. Animal modelling of traumatic brain injury in pre-clinical drug development: where do we go from here? Br. J. Pharmacol 164, 1207–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, et al. , 2010. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med 16, 413–419. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, McDonald BC, Ferrell RB, Tosteson TD, Yanofsky NN, et al. , 2011. Dopaminergic challenge with bromocriptine one month after mild traumatic brain injury: altered working memory and BOLD response. J. Neuropsychiatry Clin. Neurosci 23, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, Mucke L, 2011. The many faces of tau. Neuron 70, 410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccigrosso MM, Ford J, Benner B, Moussa D, Burnsides C, Fenn AM, et al. , 2016. Cognitive deficits develop 1 month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain Behav. Immun 54, 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti N, Deshpande LS, DeLorenzo RJ, 2009. Development of the calcium plateau following status epilepticus: role of calcium in epileptogenesis. Expert Rev. Neurother 9, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro N, Assal F, Seeck M, 2016. From here to epilepsy: the risk of seizure in patients with Alzheimer’s disease. Epileptic Disord 18, 1–12. [DOI] [PubMed] [Google Scholar]
- Nichols J, Perez R, Wu C, Adelson PD, Anderson T, 2015. Traumatic brain injury induces rapid enhancement of cortical excitability in juvenile rats. CNS Neurosci. Ther 21, 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaeva I, Crowell B, Valenziano J, Meaney D, D’Arcangelo G, 2016. Beneficial effects of early mTORC1 inhibition after traumatic brain injury. J. Neurotrauma 33, 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen J, Andrade P, Natunen T, Hiltunen M, Malm T, Kanninen K, et al. , 2017Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res 136, 18–34. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, Ben-Menachem E, Bertram EH 3rd, Collins SD, Kokaia M, Lerche H, et al. , 2013. Proposal for a “phase II” multicenter trial model for preclinical new antiepilepsy therapy development. Epilepsia 54 (Suppl. 4), 70–74. [DOI] [PubMed] [Google Scholar]
- Oby E, Janigro D, 2006. The blood-brain barrier and epilepsy. Epilepsia 47, 1761–1774. [DOI] [PubMed] [Google Scholar]
- Ogier M, Belmeguenai A, Lieutaud T, Georges B, Bouvard S, Carre E, et al. , 2017. Cognitive deficits and inflammatory response resulting from mild-to-moderate traumatic brain injury in rats are exacerbated by repeated pre-exposure to an innate stress stimulus. J. Neurotrauma 34, 1645–1657. [DOI] [PubMed] [Google Scholar]
- Okubo S, Xi G, Keep RF, Muraszko KM, Hua Y, 2013. Cerebral hemorrhage, brain edema, and heme oxygenase-1 expression after experimental traumatic brain injury. Acta Neurochir. Suppl 118, 83–87. [DOI] [PubMed] [Google Scholar]
- Olney JW, de Gubareff T, Labruyere J, 1983. Seizure-related brain damage induced by cholinergic agents. Nature 301, 520–522. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, et al. , 2008. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccenna L, Shears G, O’Brien TJ, 2017. Management of post-traumatic epilepsy: an evidence review over the last 5 years and future directions. Epilepsia Open 2, 123–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Nehlig A, Brooks-Kayal AR, Dudek FE, Friedman D, Galanopoulou AS, et al. , 2013. Issues related to development of antiepileptogenic therapies. Epilepsia 54 (Suppl. 4), 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Huusko N, Ndode-Ekane XE, Kyyriainen J, Lipponen A, Lipsanen A, et al. , 2014. Gender issues in antiepileptogenic treatments. Neurobiol Dis 72 (Pt B), 224–232. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Nnode-Ekane X, Lapinlampi N, Puhakka N, 2018. Epilepsy biomarkers-toward etiology and pathology specificity. Neurobiol. Dis (This special issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvenna V, Engeler M, Banjara M, Brennan C, Schreiber P, Dadas A, et al. , 2016. Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res 1630, 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Shi J, Yin X, Iqbal K, Grundke-Iqbal I, Gong CX, et al. , 2010. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J. Alzheimers Dis 19, 1221–1229. [DOI] [PubMed] [Google Scholar]
- Raimondo JV, Heinemann U, de Curtis M, Goodkin HP, Dulla CG, Janigro D, et al. , 2017. Methodological standards for in vitro models of epilepsy and epileptic seizures. A TASK1-WG4 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 58 (Suppl. 4), 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancan M, Bye N, Otto VI, Trentz O, Kossmann T, Frentzel S, et al. , 2004. The chemokine fractalkine in patients with severe traumatic brain injury and a mouse model of closed head injury. J. Cereb. Blood Flow Metab 24, 1110–1118. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Terrone G, Salamone A, Frigerio F, Balosso S, Antoine DJ, et al. , 2017. High Mobility Group Box 1 is a novel pathogenic factor and a mechanistic biomarker for epilepsy. Brain Behav. Immun 10.1016/j.bbi.2017.10.008. pii: S0889–1591(17)30464–6, (Epub ahead of print), Oct 13. [DOI] [PubMed] [Google Scholar]
- Raza M, Blair RE, Sombati S, Carter DS, Deshpande LS, DeLorenzo RJ, 2004. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl. Acad. Sci. U. S. A 101, 17522–17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan FR, Cheng IH, Wu T, et al. , 2007. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754. [DOI] [PubMed] [Google Scholar]
- Roseti C, Fucile S, Lauro C, Martinello K, Bertollini C, Esposito V, et al. , 2013. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia 54, 1834–1844. [DOI] [PubMed] [Google Scholar]
- Roseti C, van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, et al. , 2015. GABAA currents are decreased by IL-1beta in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol. Dis 82, 311–320. [DOI] [PubMed] [Google Scholar]
- Rostgaard N, Waldemar G, Nielsen JE, Simonsen AH, 2015. Cerebrospinal fluid biomarkers in familial forms of Alzheimer’s disease and frontotemporal dementia. Dement Geriatr. Cogn. Disord 40, 54–62. [DOI] [PubMed] [Google Scholar]
- Rothwell N, 2003. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav. Immun 17, 152–157. [DOI] [PubMed] [Google Scholar]
- Roy MJ, Francis J, Friedlander J, Banks-Williams L, Lande RG, Taylor P, et al. , 2010. Improvement in cerebral function with treatment of posttraumatic stress disorder. Ann. N. Y. Acad. Sci 1208, 142–149. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, et al. , 2010. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 51, 1560–1569. [DOI] [PubMed] [Google Scholar]
- Saija A, Hayes RL, Lyeth BG, Dixon CE, Yamamoto T, Robinson SE, 1988. The effect of concussive head injury on central cholinergic neurons. Brain Res 452, 303–311. [DOI] [PubMed] [Google Scholar]
- Sanabria ER, Su H, Yaari Y, 2001. Initiation of network bursts by Ca2+−dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J. Physiol 532, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Elexpuru G, Serratosa JM, Sanchez MP, 2017. Sodium selenate treatment improves symptoms and seizure susceptibility in a malin-deficient mouse model of Lafora disease. Epilepsia 58, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, 2012. Is antiepileptogenesis a realistic goal in clinical trials? Concerns and new horizons. Epileptic Disord 14, 105–113. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Seid K, Schluesener HJ, 2001. Traumatic brain injury induces prolonged accumulation of cyclooxygenase-1 expressing microglia/brain macrophages in rats. J. Neurotrauma 18, 881–890. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Wenzel HJ, Lyeth BG, Poon CC, Delance A, Van KC, et al. , 2010. Does ketogenic diet alter seizure sensitivity and cell loss following fluid percussion injury? Epilepsy Res 92, 74–84. [DOI] [PubMed] [Google Scholar]
- Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, et al. , 2018. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain 141, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, et al. , 2004. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci 24, 7829–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, O’Brien TJ, Gimlin K, Wright DK, Kim SE, Casillas-Espinosa PM, et al. , 2017. Interleukin-1 receptor in seizure susceptibility after traumatic Injury to the pediatric brain. J. Neurosci 37, 7864–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Zamani A, Raynor G, Shultz SR, Jones NC, 2018. Neurobehavioral, neurocognitive and psychosocial disorders associated with traumatic brain injury and post-traumatic epilepsy. Neurobiol. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Thom M, Martinian L, Harding B, Cross J, Nikolic M, et al. , 2007. Pathological tau tangles localize to focal cortical dysplasia in older patients. Epilepsia 48, 1447–1454. [DOI] [PubMed] [Google Scholar]
- Seo JS, Lee S, Shin JY, Hwang YJ, Cho H, Yoo SK, et al. , 2017. Transcriptome analyses of chronic traumatic encephalopathy show alterations in protein phosphatase expression associated with tauopathy. Exp. Mol. Med 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, et al. , 2011. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J. Neurosci 31, 14850–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahlaie K, Gurkoff GG, Lyeth BG, Muizelaar JP, Berman RF, 2013Neuroprotective effects of SNX-185 in an in vitro model of TBI with a second insult. Restor. Neurol. Neurosci 31, 141–153. [DOI] [PubMed] [Google Scholar]
- Shandra O, Moshe SL, Galanopoulou AS, 2017. Inflammation in Epileptic Encephalopathies. Adv. Protein Chem. Struct. Biol 108, 59–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Laskowitz DT, 2012. Biomarkers in traumatic brain injury. Curr. Neurol. Neurosci. Rep 12, 560–569. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott G, Leech R, 2014. Network dysfunction after traumatic brain injury. Nat. Rev. Neurol 10, 156–166. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Tan XL, Wright DK, Liu SJJ, Semple BD, Johnston L, et al. , 2014. Granulocyte-macrophage colony-stimulating factor is neuroprotective in experimental traumatic brain injury. J. Neurotrauma 31, 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz SR, Wright DK, Zheng P, Stuchbery R, Liu SJ, Sashindranath M, et al. , 2015. Sodium selenate reduces hyperphosphorylated tau and improves outcomes after traumatic brain injury. Brain 138, 1297–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Schultz SK, Seydel LL, Reist J, Kelly M, Weckmann M, et al. , 2013. Improving antipsychotic agent use in nursing homes: development of an algorithm for treating problem behaviors in dementia. J. Gerontol. Nurs 39, 24–35 quiz 6–7. [DOI] [PubMed] [Google Scholar]
- Statler KD, Swank S, Abildskov T, Bigler ED, White HS, 2008. Traumatic brain injury during development reduces minimal clonic seizure thresholds at maturity. Epilepsy Res 80, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler KD, Scheerlinck P, Pouliot W, Hamilton M, White HS, Dudek FE, 2009. A potential model of pediatric posttraumatic epilepsy. Epilepsy Res 86, 221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Brady RD, Wright DK, Kim HA, Zhang SR, Sobey CG, et al. , 2017. Treatment with an interleukin-1 receptor antagonist mitigates neuroinflammation and brain damage after polytrauma. Brain Behav. Immun 66, 359–371. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Suzuki K, Sugiura H, Yasuda S, Yamagata K, Kawakami Y, et al. , 2003. Inducible brain COX-2 facilitates the recurrence of hippocampal seizures in mouse rapid kindling. Prostaglandins Other Lipid Mediat 71, 205–216. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM, 2002. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U. S. A 99, 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrone G, Salamone A, Vezzani A, 2017. Inflammation and epilepsy: preclinical findings and potential clinical translation. Curr. Pharm. Des 23, 5569–5576. [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, Shohami E, Alexandrovich AG, Trembovler V, Leker RR, 2012. The anti-inflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J. Neurotrauma 29, 375–384. [DOI] [PubMed] [Google Scholar]
- Thom M, Liu JY, Thompson P, Phadke R, Narkiewicz M, Martinian L, et al. , 2011. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain 134, 2969–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, et al. , 2005. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75. [DOI] [PubMed] [Google Scholar]
- Tian FF, Zeng C, Ma YF, Guo TH, Chen JM, Chen Y, et al. , 2010. Potential roles of Cdk5/p35 and tau protein in hippocampal mossy fiber sprouting in the PTZ kindling model. Clin. Lab 56, 127–136. [PubMed] [Google Scholar]
- Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, et al. , 2012. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci. Transl. Med 4, 121ra19. [DOI] [PubMed] [Google Scholar]
- Turski WA, Czuczwar SJ, Kleinrok Z, Turski L, 1983. Cholinomimetics produce seizures and brain damage in rats. Experientia 39, 1408–1411. [DOI] [PubMed] [Google Scholar]
- van Eersel J, Ke YD, Liu X, Delerue F, Kril JJ, Gotz J, et al. , 2010. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc. Natl. Acad. Sci. U. S. A 107, 13888–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S., Redeker S, van Schaik R, Aronica E, Gorter JA, 2007. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130, 521–534. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Zurita M, Bonilla C, Fernandez C, Rubio JJ, Mucientes J, et al. , 2017. Progressive increase in brain glucose metabolism after intrathecal administration of autologous mesenchymal stromal cells in patients with diffuse axonal injury. Cytotherapy 19, 88–94. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP, 1997. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111). Neurol. Res 19, 334–339. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP, 2000. Improvement in mitochondrial dysfunction as a new surrogate efficiency measure for preclinical trials: dose-response and time-window profiles for administration of the calcium channel blocker Ziconotide in experimental brain injury. J. Neurosurg 93, 829–834. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B, 2015. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T, 2008. The role of cytokines in the pathophysiology of epilepsy. Brain Behav. Immun 22, 797–803. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T, 2011. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperactivity and seizures. Brain Behav. Immun 25, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Vitaliti G, Pavone P, Mahmood F, Nunnari G, Falsaperla R, 2014. Targeting in-flammation as a therapeutic strategy for drug-resistant epilepsies: an update of new immune-modulating approaches. Hum Vaccin. Immunother 10, 868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. , 2003. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci 23, 8692–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BC, Nieuwenhuijsen K, Sluiter JK, 2018. Consequences of traumatic brain injury in professional American football players: a systematic review of the literature. Clin. J. Sport Med 28, 91–99. [DOI] [PubMed] [Google Scholar]
- Wang L, Xi G, Keep RF, Hua Y, 2012. Iron enhances the neurotoxicity of amyloid beta. Transl. Stroke Res 3, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Ye Y, Chen F, Han WC, Sun JM, Lu X, et al. , 2017a. Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 343, 30–38. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Zhang H, Lee DH, Yu JT, Cheng T, Hong M, et al. , 2017b. Using functional and molecular MRI techniques to detect neuroinflammation and neuro-protection after traumatic brain injury. Brain Behav. Immun 64, 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lan YL, Xing JS, Lan XQ, Wang LT, Zhang B, 2018. Alantolactone plays neuroprotective roles in traumatic brain injury in rats via anti-inflammatory, anti-oxidative and anti-apoptosis pathways. Am. J. Transl. Res 10, 368–380. [PMC free article] [PubMed] [Google Scholar]
- Webster KM, Sun M, Crack P, O’Brien TJ, Shultz SR, Semple BD, 2017. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflamm 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, et al. , 2015. Albumin induces excitatory synaptogenesis through astrocytic TGF-beta/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dys-function. Neurobiol. Dis 78, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Hartings J, Lu XCM, Rolli M, Dave J, Tortella F, 2005. Characterization of a new rat model of penetrating ballistic brain injury. J. Neurotrauma 22, 313–331. [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Sypert GW, Munson JV, Hurd RW, 1978. Chronic focal epileptiform discharges induced by injection of iron into rat and cat cortex. Science 200, 1501–1503. [DOI] [PubMed] [Google Scholar]
- Wilson L, Stewart W, Dams-O’Connor K, Diaz-Arrastia R, Horton L, Menon DK, et al. , 2017. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 16, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H, 2010. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem. Biophys. Res. Commun 393, 514–518. [DOI] [PubMed] [Google Scholar]
- Wu H, Mahmood A, Qu C, Xiong Y, Chopp M, 2012. Simvastatin attenuates axonal injury after experimental traumatic brain injury and promotes neurite outgrowth of primary cortical neurons. Brain Res 1486, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Peterson PL, Verweij BH, Vinas FC, Muizelaar JP, Lee CP, 1998. Mitochondrial dysfunction after experimental traumatic brain injury: combined efficacy of SNX-111 and U-101033E. J. Neurotrauma 15, 531–544. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zeng K, Han Y, Wang L, Chen D, Xi Z, et al. , 2012. Altered expression of CX3CL1 in patients with epilepsy and in a rat model. Am. J. Pathol 180, 1950–1962. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Ou S, Liu X, Yuan J, Huang H, et al. , 2017. Risk factors for post-traumatic epilepsy: a systematic review and meta-analysis. Epilepsy Behav 67, 1–6. [DOI] [PubMed] [Google Scholar]
- Xu K, Wu F, Xu K, Li Z, Wei X, Lu Q, et al. , 2018. NaHS restores mitochondrial function and inhibits autophagy by activating the PI3K/Akt/mTOR signalling pathway to improve functional recovery after traumatic brain injury. Chem. Biol. Interact 286, 96–105. [DOI] [PubMed] [Google Scholar]
- Yaari Y, Yue C, Su H, 2007. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J. Physiol 580, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX, Cai Y, Shelton J, Deng SH, Luo XG, Oddo S, et al. , 2012. Chronic temporal lobe epilepsy is associated with enhanced Alzheimer-like neuropathology in 36 × Tg-AD mice. Plos One 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Chen C, 2008. Cyclooxygenase-2 in synaptic signaling. Curr. Pharm. Des 14, 1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts SD, Palesch YY, Moy CS, Selim M, 2013. High dose deferoxamine in intracerebral hemorrhage (HI-DEF) trial: rationale, design, and methods. Neurocrit. Care 19, 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf MA, Tan C, Torres-Altoro MI, Lu FM, Plautz E, Zhang S, et al. , 2016. Involvement of aberrant cyclin-dependent kinase 5/p25 activity in experimental traumatic brain injury. J. Neurochem 138, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yuan Q, Sun YR, Wu X, Du ZY, Li ZQ, et al. , 2017. Effects of deferoxamine mesylate on hematoma and perihematoma edema after traumatic intracerebral hemorrhage. J. Neurotrauma 34, 2753–2759. [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Lory P, Perez-Reyes E, 2010. Role of voltage-gated calcium channels in epilepsy. Pflugers Arch 460, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L-H, Rensing NR, Wong M, 2009. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J. Neurosci 29, 6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu R, Li M, Li F, Meng H, Zhu G, et al. , 2013. Deferoxamine attenuates iron-induced long-term neurotoxicity in rats with traumatic brain injury. Neurol. Sci 34, 639–645. [DOI] [PubMed] [Google Scholar]
- Zhao J, Chen Z, Xi G, Keep RF, Hua Y, 2014. Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl. Stroke Res 5, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Xi G, Wu G, Keep RF, Hua Y, 2016. Deferoxamine attenuated the upregulation of lipocalin-2 induced by traumatic brain injury in rats. Acta Neurochir. Suppl 121, 291–294. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J, 2009. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology 72, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]