Abstract
Purpose
In considering gene modification technologies, the priorities of patient communities must be a central consideration. The purpose of this study is to assess views of families with Down syndrome (DS) regarding potential genome-based interventions.
Methods
We constructed an anonymous online survey for family members of people with DS. Participants were asked to agree or disagree with scenarios describing hypothetical interventions to silence or significantly alter the physical and cognitive effects of a trisomy 21, and also with scenarios depicting currently available physical interventions.
Results
All 532 respondents were parents of people with DS. For each of the five scenarios, over half said they would approve the intervention or would advise their children with DS to do so. Responses to hypothetical prenatal and pediatric cognitive interventions were significantly affected by participants’ assessments of the impact of DS on their children’s and their families’ lives, while physical and adult cognitive scenarios were not.
Conclusion
Future interventions to address genetic conditions will impact patient communities and cannot succeed without their input and support. While many parents of people with DS indicated approval for hypothetical genetic therapies, these results indicate a need for continuing dialogue about benefits and drawbacks of gene modification technologies.
Keywords: CRISPR, gene editing, gene silencing, Down syndrome, intellectual disability
Introduction
Considerable progress has been made in recent years towards the successful use of gene therapy technologies to address genetic conditions, the most recent being the success of CRISPR/Cas9. These successes have generated expectations that “gene editing” may someday ameliorate or eliminate many genetic conditions.1–4 However, these rapid scientific advances have been accompanied by many ethical and social concerns about their implications.5–7 National and international professional and ethics bodies have issued statements calling for additional basic research before genetic modification technologies are translated into clinical applications in humans, and highlighting the need to hear from stakeholders in these technologies.8,9 In particular, the needs and priorities of communities with firsthand experience of potentially targeted conditions are a critical consideration in clinical translation.8,10
Down syndrome (DS), the most common genetic cause of intellectual disability,11,12 offers an interesting test case for stakeholder engagement in this area. In recent years, researchers have investigated mechanisms that may lessen the impact of trisomy 21 on physical and cognitive development.13,14 The DS community has also been vocal about the ways in which the condition is portrayed and the need for social integration and support.15–17 Parents, families, and self-advocates have publicly wrestled with the implications of widespread and expanding prenatal screening for DS, and made a strong case for preserving a safe and productive place in society for those with different physical and cognitive abilities and qualities.18,19
To explore the views of the DS community toward potential genetic interventions for DS, we designed a group of hypothetical scenarios that described interventions to silence or radically alter the physical and cognitive effects of a trisomy 21. Below we report quantitative results of an online mixed-methods survey in which parents of people with DS responded to these hypothetical scenarios.
Methods
Survey design
This online, anonymous survey was directed at parents or other caregivers for people with Down syndrome (DS). It was comprised of five hypothetical scenarios, based on interventions for symptoms of DS that either exist now or are being researched. Scenarios 2, 4, and 5 concerned hypothetical future interventions that would either prenatally “silence” the extra chromosome responsible for DS (Scenario 2) or significantly alter cognitive symptoms in pediatric (Scenario 4) or adult (Scenario 5) patients. For comparison, Scenarios 1 and 3 concerned currently available physical interventions for fetal bowel obstruction and newborn congenital heart defects, respectively. The survey did not specify which potential interventions were currently available; rather, participants were told, “Not all of the treatments we talk about here are real; researchers are studying some of them, and we don’t know whether those studies will ever become a real treatment.” Each scenario listed some likely benefits and risks in order to give participants a platform for weighing drawbacks and advantages; in the case of hypothetical interventions that would significantly alter the phenotype of DS, these were chosen to highlight previously documented positively- and negatively-viewed features of DS (e.g., intellectual delays, generally good physical health, typically friendly personality, typical inability to live independently as an adult). For each scenario, participants were asked whether they would choose to approve the intervention (or, in the case of an adult intervention, whether they would suggest their family member choose it), with a simple “Yes/No” binary option. (For text of scenarios, see Supplementary Materials and Methods.) Participants were also asked open-ended questions about why they made this choice; these data are being analyzed and published separately.
The survey also requested background information on participants and their family member with DS, including: the ages of the participant and the person with DS; distance to the family’s primary care doctor; frequency of medical visits for DS symptoms; whether they would describe their family member as “minimally,” “moderately,” or “very” affected by having DS; and whether their family member was diagnosed with DS prenatally or postnatally. Assessments of their family member’s and family’s quality of life and of the “burden” or “positive force” that DS represented in individuals’ and their family’s lives were captured using a five-point Likert scale.
Data collection
The anonymous survey was self-administered online using RedCap. A link to the survey was distributed to selected DS advocates (all employed by academic institutions) with sizable social media followings, and it was also publicly distributed via our own social media outlets (Twitter and Facebook). The survey remained available for 7 days in July, 2017. Due to its public distribution, it is impossible to estimate response rate. This analysis includes all completed surveys; however, response totals differ between individual questions.
Data analysis
The five hypothetical scenarios were summarized using frequencies and proportions of positive and negative responses. Parent/caregiver attitudes concerning perceived quality of life were likewise summarized with frequencies and proportions of responses across the cohort. Potential associations between response to scenario and quality of life attitudes were assessed using chi-square tests. Age of the caregiver and the person with DS were summarized using medians and interquartile ranges, and Wilcoxon ranksum tests were used to compare the two ages individually between those responding yes vs. those responding no to the five hypothesized scenarios. All analyses were performed with STATA, Version 14 (StataCorp, College Station, Texas). This study was declared exempt from further review by Mayo Clinic Institutional Review Board.
Results
Participants and their family member with Down syndrome
In total, 532 participants completed the online survey. Although response rate cannot be calculated, due to social media recruitment, 1093 individuals entered the survey (answered at least one question), for a completion rate of 48.7%. Only completed surveys are analyzed; these include seven surveys in which at least one question was left blank. Self-descriptions of participants are reported in Table 1. All respondents self-identified as a parent of a person with DS. Median (IQR) age of respondents was 41 (36, 47) years. Median (IQR) age of the children with DS was 5 (2, 11) years, with 8.6% reported to be 18 years or older. DS had been diagnosed prenatally for 39.3% of respondents’ families, and postnatally for 60.7%. Over half described their child as “moderately affected” by having DS, and over half said that this person had a clinical interaction at least once a month “for something associated with Down syndrome.” Most (89.3%) reported that they lived within half an hour of their family’s primary care doctor.
Table 1.
Self-reported demographics of respondents and their child with Down syndrome (DS).
| Age of respondent (n=528) | |
|
| |
| Median age (range) | 41 (17–74) |
|
| |
| Age of person with DS (n=532) | |
|
| |
| Median age (range) | 5 (<1–44) |
|
| |
| Time to primary care (n=531) | |
|
| |
| 1–15 minutes | 282 (53.11%) |
| 15–30 minutes | 193 (36.35%) |
| 30–60 minutes | 47 (8.85%) |
| Over 60 minutes | 9 (1.69%) |
|
| |
| How affected by DS (n=530) | |
|
| |
| Minimally | 149 (28.11%) |
| Moderately | 290 (54.72%) |
| Very | 91 (17.17%) |
|
| |
| Timing of diagnosis (n=532) | |
|
| |
| Pre-natal | 209 (39.28%) |
| Post-natal | 323 (60.72%) |
|
| |
| Frequency of clinical care (n=531) | |
|
| |
| Daily | 27 (5.09%) |
| Weekly | 214 (40.30%) |
| 1–3 times per week | 81 (15.25%) |
| Every few months | 162 (30.51%) |
| Less than once a year | 47 (8.85%) |
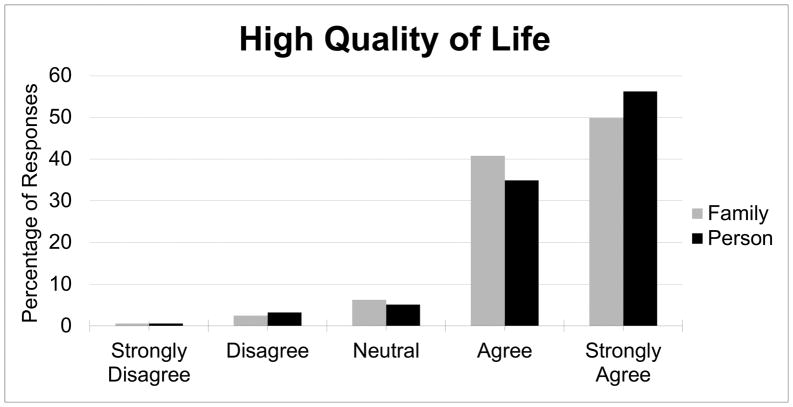
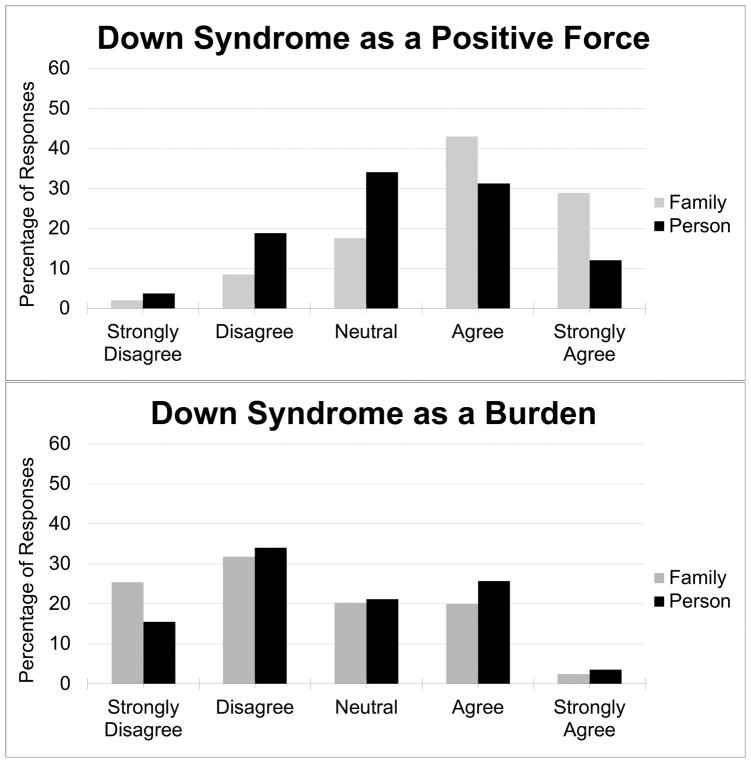
A strong majority of participants agreed or strongly agreed that their children and their families had a “high quality of life” (91.1% and 90.7%, respectively; see Figure 1). However, when participants were asked about the specific effects of DS on their children and their families, responses were more evenly distributed. While a majority of respondents agreed or strongly agreed (71.6%) that Down syndrome was “a positive force” in their families’ lives, fewer (43.2%) said the same for their children specifically (Figure 2). Nonetheless, most respondents were unwilling to characterize DS as a “burden” (Figure 2), with a minority agreeing or strongly agreeing that this was the case for their children with DS (29.1%) or their families (22.4%). Having received the DS diagnosis prenatally or postnatally had no statistical effect on participants’ assessments of the effects of DS on their children, their children’s and families’ quality of life, or the “burden” or “positive force” of DS on their children and families.
Figure 1.
Percentage of respondents reporting a high quality of life for their family and the person with DS.
Figure 2.
Percentage of respondents who view DS as a positive force or a burden on their family and on the person with DS.
Responses to hypothetical scenarios
For each of the five hypothetical scenarios, a majority of respondents said they would elect to undergo the intervention in question or would advise their children with DS to do so (see Table 2). The least agreed-with intervention was Scenario 2, a hypothetical prenatal intervention that would “silence” the extra chromosome causing DS with a series of injections (50.9% yes). Scenario 5, a hypothetical vaccine that could decrease a 35-year-old’s risk of DS-related Alzheimer’s disease (but could cause seizures), was acceptable to only 54.3% of the respondents. Scenario 1, depicting a prenatal surgery on fetal bladder outlet obstruction, received somewhat more positive responses (63.5% yes). Scenario 4, a hypothetical daily pill that could improve memory and attention in an 11-year-old with DS (but might also alter her personality to be more self-conscious and less outwardly affectionate) garnered agreement from over two-thirds of respondents (67.9% yes). Scenario 3, depicting surgery to repair a heart defect in a newborn with DS, was overwhelmingly supported (94.5% yes).
Table 2.
Responses to hypothetical scenarios.
| Yes | % | No | % | No answer | % | |
|---|---|---|---|---|---|---|
| Prenatal physical intervention | 338 | 63.5 | 187 | 35.2 | 7 | 1.3 |
| Prenatal cognitive intervention | 271 | 50.9 | 256 | 48.1 | 5 | 0.9 |
| Pediatric physical intervention | 503 | 94.5 | 28 | 5.3 | 1 | 0.2 |
| Pediatric cognitive intervention | 361 | 67.9 | 166 | 31.2 | 5 | 0.9 |
| Adult treatment to mitigate Alzheimer’s disease | 289 | 54.3 | 230 | 43.2 | 13 | 2.4 |
The influence of other factors on scenario responses
Responses to the prenatal and pediatric cognitive interventions described in Scenarios 2 and 4 were significantly affected by participants’ assessments of the effects of DS on their children’s and their families’ lives (see Table 3). Responses to the other three scenarios were not significantly affected by these factors. Other self-reported demographic factors (the ages of participants and their children with DS, the reported frequency of clinical interaction for the person with DS, and the families’ distance to their primary care doctor) did not have a statistically significant effect on responses to any of the five scenarios.
Table 3.
Influence of other factors on scenario responses (p-values).
| Prenatal physical | Prenatal cognitive | Pediatric physical | Pediatric cognitive | Adult | |
|---|---|---|---|---|---|
|
| |||||
| How affected by Down syndrome (minimally, moderately, very) | 0.98 | 0.001* | 0.02 | 0.01* | 0.35 |
| Down syndrome is a positive force in this person’s life | 0.7 | <0.0001* | 0.56 | <0.0001* | 0.33 |
| Down syndrome is a burden on this person | 0.89 | <0.0001* | 0.39 | 0.002* | 0.08 |
| Down syndrome is a positive force on our family | 0.44 | <0.0001* | 0.77 | 0.001* | 0.51 |
| Down syndrome is a burden on our family | 0.95 | <0.0001* | 0.38 | <0.0001* | 0.17 |
| This person has a high quality of life | 0.07 | 0.006* | 0.71 | 0.18 | 0.94 |
| Our family has a high quality of life | 0.11 | 0.007* | 0.93 | 0.04 | 0.88 |
p-value indicates statistical significance (≤0.01)
Participants who reported more intense effects of DS on their children were significantly more likely to agree with the cognitive interventions described in Scenarios 2 and 4. Those who viewed DS as a greater burden on their children were significantly more likely to agree with the Scenario 2 and 4 interventions, and while the same trend was seen for Scenario 5, it was not statistically significant. A similarly strong statistical correlation occurred between agreement with Scenario 2 and 4 interventions and self-reported burden of DS on the family. Participants were significantly less likely to agree with the Scenario 2 and 4 interventions if they saw DS as a positive force in their children’s lives, and the same relationship applied for those who saw DS as a positive force in their families’ lives. Only Scenario 2 showed a statistically significant relationship with self-reported quality of life; participants who rated their children’s and their families’ quality of life more highly were significantly less likely to agree with that hypothetical prenatal cognitive intervention (see Table 3).
Discussion
Parents responded in a range of ways to this set of hypothetical scenarios that depicted a future in which the phenotype of DS could be significantly altered. While the ability to lessen cognitive effects of DS appealed more to some than others, perhaps the most surprising finding of this survey was that over half of parents responded positively to every one of our hypothetical interventions. Nevertheless, these responses reflect an anecdotally-observed ambivalence among parents in the DS community about the meaning and value of potential treatments.
Varying perceptions of quality of life and the positive/negative effects of DS correlated strongly with responses to the hypothetical prenatal and pediatric cognitive interventions. Respondents with a generally more positive view of life with DS were significantly less willing to endorse interventions. This finding likely reflects a strong view among many families that DS is less a disease than a human variation with its own inherent value. However, the finding that over half of parents still agreed with these controversial hypothetical interventions suggests that this viewpoint is not uncontested, particularly among those who perceive more burdensome effects of DS on their child and family. As expected, the scenarios depicting purely physical interventions were generally less controversial and did not correlate statistically with views about DS or quality of life—particularly the newborn heart surgery scenario, which was likely familiar to many respondents since roughly half of babies born with DS have congenital heart defects. The ambivalent responses to the hypothetical adult cognitive intervention also did not correlate statistically with any particular perspective on DS or quality of life, perhaps reflecting either variable reactions to the hypothesized risks and benefits or the fact that very few respondents had yet seen their children with DS reach adulthood.
However, when parents considered potential interventions, these positive/negative value judgments about life with DS, along with subjective judgments of severity, carried more weight than the logistics of treatment or the timing of diagnosis. No statistical effects on scenario responses were observed for prenatally versus postnatally diagnosed DS. Similarly, scenario responses did not correlate with self-reported frequency or traveling distance for medical care. This finding perhaps suggests that the objective circumstances of life with a developmental genetic condition such as DS may matter less to parents than the positive or negative valence of their assessments of their children’s and families’ lives, at least when weighing the risks and benefits of cognitive interventions.
These results point to the need for more engagement with patient communities in the potential translation of interventions to alter the phenotype of DS, particularly for interventions designed to target cognitive elements of the phenotype. In particular, prenatal interventions to alter cognitive ability were considered the most controversial, perhaps reflecting the inability to predict many phenotypic aspects of DS before birth. Since populations who may face prenatal decision-making about gene modification are more likely to have only a prenatal diagnosis of a genetic condition without relevant life experience of that condition, future research should target populations who do not have a child with a genetic condition, or who have received prenatal genetic information but not yet completed their pregnancy.
Limitations
Hypothetical scenarios can never fully reflect the complex decision-making of a family facing real-life medical decisions. Rather, this survey was designed to elicit the factors that could affect decision-making if and when such decisions become reality. However, in the interest of brevity, the survey did not ask respondents for details about their many other demographic and socio-economic characteristics that could have shaped responses, such as income, education, race, gender, religion, political affiliation, or views of abortion or science. Open-ended responses in which participants discussed their reasoning can further enrich our understanding of how such decisions may be made; this qualitative analysis is still under way and will be published separately.
We listed hypothetical risks and benefits of each intervention based on necessarily limited knowledge of these potential futures; these fictional risks and benefits may have impacted responses in ways that we did not anticipate. While we recognized that offering specific risks and benefits of each scenario could limit participants’ imaginations in some ways, some framework for reflection was deemed necessary, for two reasons. First, risks and benefits offered participants ample reason both to consider an intervention attractive and to consider how worthwhile those benefits might be in relation to substantial drawbacks—since a lack of either risk or benefit could render serious reflection unnecessary, and what one family might consider a major risk or benefit might be less salient for another. Secondly, the recent history of science “hype,” particularly in the context of genomics, has tended to raise hopes for miraculous treatments or cures while glossing over potential downsides, both in research publications and in popular media.20–22 Though it would be impossible to invent objectively equivalent risks and benefits for each scenario to make them truly comparable, the possibility that participants might only imagine benefits without potential drawbacks outweighed our concerns about leading participants’ responses by articulating particular hypothetical risks. A useful avenue for future research might be to compare responses to scenarios with a variety of proposed risks and benefits, to assess ways that study populations weigh varying types and amounts of each.
Our questions about quality of life, the degree of “affected”-ness of DS, and the “burden” and/or “positive force” represented by DS were piloted with family members of people with DS to check that the language was self-explanatory and non-offensive; however, these measures were not validated and the terms used may have meant different things to different respondents. In addition, self-selection may have biased our sample toward younger parents with more access to technology, and possibly toward those who are more active in DS advocacy. The anonymity of an online interaction may also have influenced some people’s responses. Many participants volunteered their contact information for future research, and we will be conducting follow-up interviews with a distributed subset to explore these issues in more depth. Most importantly, we recognize that people with DS may have quite different views about potential interventions than their families do, and we are currently adapting this survey instrument in order to elicit the views of this population.
Conclusion
Although genetic interventions for DS and other developmental conditions are still hypothetical, the speed at which gene modification technologies are being developed necessitates a future-oriented assessment of the views of parents and other stakeholders. Disability communities have long voiced perspectives that differ sharply from those of clinicians and research scientists, speaking out about the positive aspects of their lives and arguing for the inherent value of many kinds of diversity. These findings indicate that, as technologies are developed that may radically alter the lives of those with genetic conditions, it will be essential to dialogue with a range of perspectives that illuminate both the benefits and the drawbacks of gene modification.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support from National Institutes of Health (NIH) grant R00HG006452, NIH grant K01HG009542, and the Mayo Center for Individualized Medicine Bioethics Program. The authors are also grateful for statistical analysis support from Dr. Nancy Hills. This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI grant number UL1TR001872. Its contents are solely the responsibility of the authors and do not represent the official views of the NIH.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213) doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 2.Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann M. CRISPR/Cas9 genome editing – new and old ethical issues arising from a revolutionary technology. NanoEthics. 2016;10(2):139–159. [Google Scholar]
- 6.Sankar PL, Cho MK. Engineering values Into genetic engineering: A proposed analytic framework for scientific social responsibility. Am J Bioethics. 2015;15(12):18–24. doi: 10.1080/15265161.2015.1104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosley KS, Botchan M, Bredenoord AL, et al. CRISPR germline engineering—the community speaks. Nat Biotech. 2015;33:478. doi: 10.1038/nbt.3227. [DOI] [PubMed] [Google Scholar]
- 8.National Academies of Sciences, Engineering, and Medicine. Human genome editing: Science, ethics, and governance. Washington, DC: The National Academies Press; 2017. http://nap.edu/24623. [PubMed] [Google Scholar]
- 9.National Academies of Sciences, Engineering, and Medicine. International Summit on Human Gene Editing: A Global Discussion. Washington, DC: National Academies Press; 2015. https://www.nap.edu/catalog/21913/ [PubMed] [Google Scholar]
- 10.Howard HC, van El CG, Forzano F, et al. One small edit for humans, one giant edit for humankind? Points and questions to consider for a responsible way forward for gene editing in humans. Eur J Hum Genet. 2018;26(1):1–11. doi: 10.1038/s41431-017-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Graaf G, Buckley F, Skotko BG. Estimation of the number of people with Down syndrome in the United States. Genet Med. 2017;19(4):439–447. doi: 10.1038/gim.2016.127. [DOI] [PubMed] [Google Scholar]
- 12.Patterson D, Costa ACS. Down syndrome and genetics—a case of linked histories. Nat Rev Genet. 2005;6(2):137–147. doi: 10.1038/nrg1525. [DOI] [PubMed] [Google Scholar]
- 13.Bartesaghi R, Haydar TF, Delabar JM, Dierssen M, Martínez-Cué C, Bianchi DW. New perspectives for the rescue of cognitive disability in Down syndrome. J Neurosci. 2015;35(41):13843–13852. doi: 10.1523/JNEUROSCI.2775-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guedj F, Bianchi DW. Noninvasive prenatal testing creates an opportunity for antenatal treatment of Down syndrome. Prenat Diagn. 2013;33(6):614–618. doi: 10.1002/pd.4134. [DOI] [PubMed] [Google Scholar]
- 15.Bérubé M. Life as we know it: A father, a family, and an exceptional child. New York: Pantheon Books; 1996. [Google Scholar]
- 16.Asch A, Wasserman DT. Where is the sin in synechdoche? Prenatal testing and the parent-child relationship. In: Wasserman D, Wachbroit R, Bickenbach J, editors. Quality of life and human difference: Genetic testing, health care, and disability. Cambridge: Cambridge University Press; 2005. pp. 172–216. [Google Scholar]
- 17.Phillips S. My son has Down’s syndrome - and he belongs in a mainstream school. [Accessed March 26, 2018];The Guardian. 2018 Mar 26; https://www.theguardian.com/commentisfree/2018/mar/26/downs-syndrome-inclusive-education-finland-schools-children-sally-phillips.
- 18.Friedersdorf C. ‘I Am a Man With Down Syndrome and My Life Is Worth Living’: Congressional testimony that illuminates what a developmental disability means—and doesn’t mean. [Accessed March 26, 2018];The Atlantic. 2017 Oct 30; https://www.theatlantic.com/politics/archive/2017/10/i-am-a-man-with-down-syndrome-and-my-life-is-worth-living/544325/
- 19.Kaposy C. A disability critique of the new prenatal test for Down syndrome. Kennedy Inst Ethics J. 2013;23(4):299–324. doi: 10.1353/ken.2013.0017. [DOI] [PubMed] [Google Scholar]
- 20.Brown N, Michael M. A sociology of expectations: Retrospecting prospects and prospecting retrospects. Technology Analysis and Strategic Management. 2010;15(1):3–18. [Google Scholar]
- 21.Caulfield T. Biotechnology and the popular press: Hype and the selling of science. Trends Biotechnol. 2014;22(7):337–339. doi: 10.1016/j.tibtech.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 22.O’Keefe M, Perrault S, Halpern J, Ikemoto L, Yarborough M. “Editing” genes: A case study about how language matters in bioethics. American Journal Of Bioethics. 2015;15(12):3–10. doi: 10.1080/15265161.2015.1103804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.