Abstract
Background
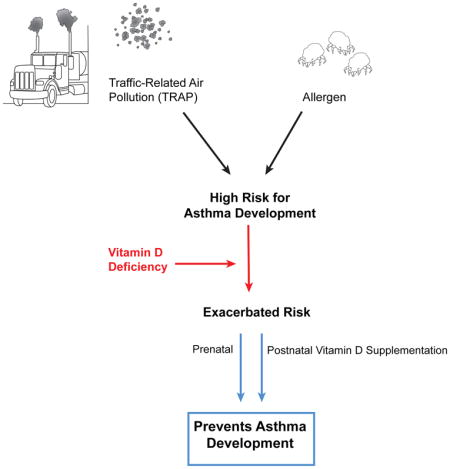
Recent literature suggests that children who are vitamin D deficient are uniquely susceptible to the effects of traffic-related air pollution (TRAP) exposure. This is highly significant because large segments of the population reside in zones of high TRAP exposure.
Objective
To determine whether vitamin D supplementation mitigates the impact of TRAP exposure on asthma development, asthma exacerbation, and/or airway inflammation, and to determine the timing of vitamin D supplementation that confers maximal health benefit.
Methods
Using established mouse models of asthma, we examined the impact of pre-and post-natal vitamin D supplementation on asthma development as well as the utility of vitamin D as a treatment for established asthma in the context of diesel-exhaust particle (DEP) exposure.
Results
DEP and allergen co-exposure resulted in increased airway hyperresponsiveness (AHR) and accumulation of pathogenic Th2/Th17 cells in the lungs of vitamin D deficient mice compared to control mice. Prenatal and postnatal vitamin D supplementation significantly attenuated the development of AHR, and decreased pulmonary accumulation of Th2/Th17 cells following co-exposure to TRAP and allergen, but not allergen alone. Restoration of normal vitamin D status had no impact on AHR once asthma was already established.
Conclusions
Our data establish that vitamin D confers protection against asthma development specifically in the context of TRAP exposure. While vitamin D replacement did not reverse established asthma, restoration of normal vitamin D status in early life significantly attenuated the development of AHR in DEP-exacerbated allergic asthma and reduced lung Th2/Th17 cells, which portend the development of severe asthma.
Keywords: Vitamin D, asthma, diesel exhaust particle, house dust mite, traffic pollution, allergen, prevention
Graphical Abstract
Introduction
Vitamin D insufficiency (<30ng/mL) affects at least 50% of children and 39% of adults within the United States(1, 2). Widespread vitamin D deficiency has been postulated to be a contributing factor to the rise in asthma seen in the past 2 decades(3). As such numerous trials have been conducted to examine the efficacy of vitamin D supplementation on preventing or attenuating asthma symptoms and the results have been mixed. A recent review concluded that “limited information is available addressing primary prevention of allergic diseases after vitamin D supplementation, and its potential impact remains uncertain(4).” Part of the reason for this, is the lack of understanding of who would benefit most from vitamin D supplementation and when supplementation would be most effective.
Traffic-related air pollution (TRAP) is a significant contributor to particulate matter (PM) exposure levels in children(5). A recent comprehensive and systematic review of worldwide traffic emissions and health science by a Special Panel convened by the Health Effects Institute found sufficient evidence that exposure to TRAP causes asthma exacerbation in children and likely promotes asthma development in early life(6). Diesel exhaust particles (DEP), the predominant particulate matter component of TRAP, have the unique ability to reach small airways including the alveolar gas exchange region further exacerbating asthma symptoms(7, 8). DEPs are composed of elemental carbon cores with large surface areas capable of binding organic polycyclic aromatic hydrocarbons, transition metals, and airborne allergens, which have the potential to induce reactive oxygen species and inflammation(9, 10). A recent study revealed that children who were both vitamin D deficient and living near a major roadway were 5 times more likely to experience asthma exacerbations in comparison to children who were vitamin D sufficient and living in the same high TRAP exposure regions(11). These data suggest that vitamin D deficiency may render children uniquely susceptible to the adverse effects of TRAP. This is highly significant because 45% of the US population, including children, resides in zones of high exposure(12), and TRAP exposure leads to more severe allergic asthma characterized by Th2/Th17 cells which are crucial in mediating DEP-exacerbated allergic asthma exacerbations(13–15). These double producing T effector cells have been associated with severe asthma by numerous studies and have been shown to be refractory to steroid treatment(16, 17). Th17 cells isolated from steroid refractory asthmatics and treated with vitamin D have been shown to produce significantly decreased IL-17A(18). This study and others have shown that vitamin D has immunosuppressive effects on Th17 cells(18–20).
Based on these data, we sought to establish whether vitamin D supplementation mitigates the impact of TRAP exposure on asthma development, asthma exacerbation, and/or airway inflammation, and to determine the timing of vitamin D supplementation that confers the most beneficial health impact. We also examined the impact of vitamin D supplementation on the accumulation of T effector cells in the lungs.
Methods
Murine Asthma Model
Two different models of vitamin D supplementation were used- postnatal and prenatal supplementation. For postnatal supplementation, wild-type 3-week-old BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and mice were placed on a normal chow diet. Mice were given either control water or water supplemented with 20,000 IU cholecalciferol (Sigma) ad libitum for 4 weeks prior to intratracheal challenges. After 4 weeks, mice underwent intratracheal challenges of saline, HDM (10μg comprising 3.3 μg of protein, 1.1 μg of Der p1), DEP (100 μg), or both and were challenged 3 times a week for 3 weeks as previously described(13). HDM extract (Dermatophagoides pteronyssinus) was purchased from Greer Laboratories (Lenoir, NC). DEPs were generated from a 4-cylinder Deutz diesel engine at the Environmental Protection Agency (Research Triangle Park, NC); detailed characterization of this compressor DEP has been described previously and compared with other sources of DEPs(21). The mice were sacrificed 24 hours after the last challenge. For the prenatal supplementation model, parents were placed on a normal chow diet and a subset of mice were given water supplemented with 20,000 IU cholecalciferol (Sigma) ad libitum. Pups were continued on supplemented or control water consistent with their parents’ diet and underwent the murine asthma model described above between 7–8 weeks of age. All mice were housed in a specific pathogen-free environment in the animal facility at Cincinnati Children’s Hospital Medical Center. All procedures were performed in accordance with the ethical guidelines in the Guide for the Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee approved by the Veterinary Services Department of the Cincinnati Children’s Hospital Medical Center.
Vitamin D Measurement
Plasma was obtained from mice retro-orbitally and 25(OH)D levels were measured by ELISA (DEIA 4458, Creative Diagnostics, Shirley, NY).
Airway responsiveness
To measure airway responsiveness, invasive measurements were made with the FlexiVent apparatus (SCIREQ, Montreal, Quebec, Canada). Mice were anesthetized with ketamine, xylazine, and acepromazine (100, 20, and 10 mg/mL, respectively, mixed at a ratio of 4:1:1). Mouse tracheas were cannulated with a 19-gauge blunt needle, and the mice were ventilated at 150 breaths/min and 3.0 cm H2O positive end-expiratory pressure. Two total lung capacity perturbations were then performed for airway recruitment before baseline measurement, and subsequent methacholine challenges were performed. Dynamic resistance was assessed after exposure to increasing concentrations of aerosolized methacholine (0, 3, 6.25, and 12.5 mg/mL). The average of the 3 highest dynamic resistance values with a coefficient of determination of 0.9 or greater (as determined by using FlexiVent software) was used to determine the dose-response curve.
Bronchoalveolar lavage fluid (BALF) collection and analysis
Bronchoalveolar lavage was performed by means of tracheal cannulation. Lungs were lavaged with 1 mL of MACs buffer (1X PBS, 0.5% BSA, 2mM EDTA). The collected BALF was centrifuged, and total cell numbers counted with a hemocytometer. Cells were spun onto slides and stained with the HEMA3 stain set (Fisher Scientific, Kalamazoo, MI). After the slides were cover slipped and de-identified, 200 cells were counted, and the total number of each cell type was calculated.
Flow Cytometry
Lungs were removed, and the upper right lobe was minced and incubated at 37°C for 30 minutes in 1 mL of RPMI 1640 containing Liberase TL (0.5 mg/mL; Roche Diagnostics, Indianapolis, IN) and DNAse I (0.5 mg/mL; Sigma, St Louis, MO). Lung cells were passed through a 70-μm cell strainer with a syringe rubber, and the strainer was washed with 5 mL of RPMI plus DNAse I media. Cells were centrifuged and re-suspended in 1 mL of PBS plus 0.5% BSA plus 2 mmol/L EDTA before being counted on a hemocytometer. To observe Th2/Th17 cytokine production, lung cells were ex vivo stimulated with phorbol 12-myristate 13-acetate (PMA) +ionomycin (.05 μg/mL and .5 μg/mL, respectively) for an hour prior to the addition of Brefeldin-A (Biolegend, San Diego, CA) for an additional two and a half hours. Approximately 500,000 lung cells were transferred to a 96-well plate with V-shaped wells on ice, centrifuged, and re-suspended in PBS containing Fc Block (2.4G2 mAb). T cells were stained with combinations of CD4–FITC, γδT-cell receptor–PE/Cy7, CD25-AF647, CD3-AF700, and CD44–Pacific Blue (BioLegend, San Diego, CA). Cells were labeled with the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit, according to the manufacturer’s instructions (Invitrogen by Life Technologies, Carlsbad, CA). Intracellular staining for IL-13–PE, IL-17A–AF647, IFN-γ–PerCP5.5, and forkhead box protein 3(FOXP3)–PerCP5.5 were conducted, according to the manufacturer’s instructions (eBioscience, San Diego, CA). Acquisition was done on a FACSCanto III (Becton Dickinson, Mountain View, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Statistical Analysis
All statistical analyses were done using PRISM software (GraphPad Software Inc., La Jolla, CA). Statistical significance was assessed using one-way ANOVA followed by a Bonferroni post-test, except when mentioned otherwise in the figure legend.
Results
Vitamin D supplementation significantly attenuates development of AHR in mice co-exposed to HDM+DEP
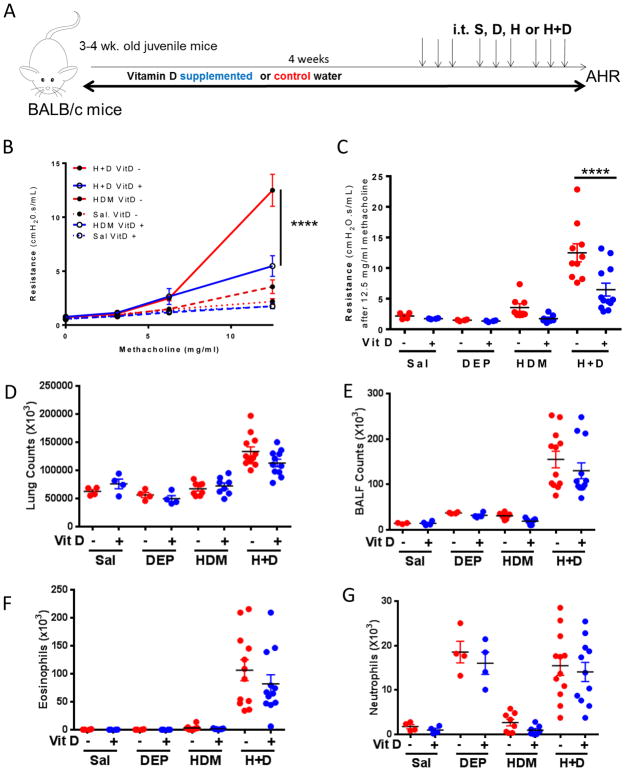
Three-week old BALB/c mice (juvenile age) were placed on a normal chow diet with vitamin D supplemented water or control water for 4 weeks to correct vitamin D levels to sufficient (>30ng/mL) or deficient (<20 ng/mL) levels(22). After 5 weeks, the mice underwent 9 intratracheal challenges to saline, diesel exhaust particles (DEP), house dust mite (HDM) or both HDM+DEP. The mice were continued on their respective diets throughout challenges to maintain vitamin D sufficiency or deficiency. Twenty-four hours after the last challenge, airway hyperresponsiveness was measured. Consistent with previously published data by our group and others, mice co-exposed to HDM+DEP develop significantly increased airway hyperresponsiveness in comparison to mice exposed to only HDM(13). Supplementation with vitamin D resulted in attenuated airway hyperresponsiveness in mice co-exposed to HDM and DEP, (Figure 1A, B), in comparison to co-exposed vitamin D deficient mice. Vitamin D supplementation had no/minimal impact on mice exposed to HDM alone suggesting that vitamin D supplementation offers protection specifically in the context of DEP exposure. Vitamin D supplementation had no impact on the total lung inflammation. There were no differences in the total lung cells, BALF cell counts, eosinophils or neutrophils present in the lungs between the groups (Figure 1D–G). There was a significant increase in lung neutrophilia and eosinophilia in HDM+DEP co-exposed mice compared to mice exposed to HDM alone as we have shown previously(13) (Figure 1F, G).
Figure 1. Postnatal vitamin D supplementation decreases AHR in mice co-exposed to HDM+DEP.
(A) Protocol: BALB/c mice were placed on a vitamin D supplementation or control water for 4 weeks, and then exposed intratracheally to saline, DEP (100μg) and/or house dust mite (HDM; 10μg) three times a week over 3 weeks. (B) Airway Resistance was measured 24 hours after last challenge. (n=4–12 mice per group; 2-way ANOVA, ****p< 0.0001, ***p <.001, **p <.01, *p < 0.05). (C) Resistance after exposure to 12.5 mg/mL methacholine. (D) Total lung cell counts. (E) BALF cell counts. (F) BALF eosinophils. (G) BALF neutrophils.
Vitamin D supplementation significantly decreases Th2 and Th2/Th17 double producing populations in the lungs
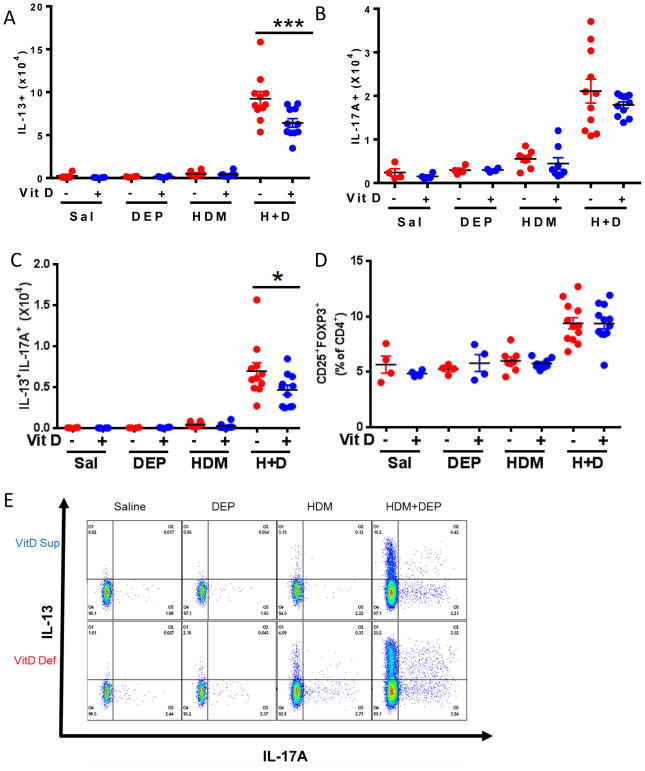
We have previously shown that Th2, Th17, and Th2/Th17 double-producing populations are increased in mice co-exposed to HDM+DEP(13). Lung cells were characterized by flow cytometry to elucidate the impact of vitamin D supplementation on the presence of effector T-cells in the lungs. Vitamin D supplementation resulted in significantly decreased numbers of Th2 and Th2/Th17 double-producing populations in the lungs of mice co-exposed to DEP and allergen (Figure 2A, C). The frequency of Treg cells in the lungs was not affected by vitamin D supplementation (Figure 2D). Also, vitamin D treatment did not significantly alter the MFI of the positive Th2 or Th17 cells within the challenge groups (data not shown).
Figure 2. Vitamin D deficiency increases the presence of IL-13+ and IL-13+IL-17A+ pulmonary CD4+ effector T-cells.
Intracellular staining for CD4+CD44+T-cells producing (A) IL-13, (B) IL-17A, or (C) IL-13/IL-17A; or (D) staining for CD25+Foxp3+ Treg cells. (E) Representative dot plots (n=4–12 mice per group). ****p< 0.0001, ***p <.001, **p <.01, *p < 0.05 using 1-way ANOVA with Bonferroni’s multiple comparison test. (n=4–12 mice per group)
Prenatal vitamin D supplementation also significantly decreases development of AHR in mice exposed to HDM and co-exposed to HDM+DEP
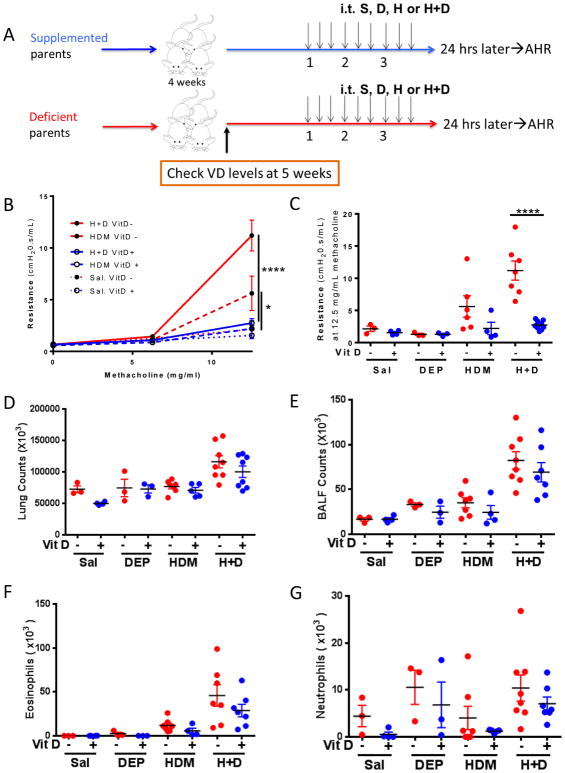
In the experiments above, vitamin D supplementation was initiated in early life, at 3 weeks of age. We next examined whether in utero prenatal supplementation was equivalent to postnatal early life supplementation or whether it may confer increased protection. BALB/c mice bred from parents on a vitamin D replete or control water underwent 9 intratracheal challenges around 6–7 weeks of age (Figure 3A). The mice continued on their respective water source and their serum vitamin D levels were >30 ng/mL and <20ng/mL, respectively, at the start of the challenges. Vitamin D supplemented mice co-exposed to HDM+DEP develop attenuated airway hyperresponsiveness compared to vitamin D deficient co-exposed mice (Figure 3B, C). A similar effect of vitamin D supplementation was observed in mice exposed to HDM alone, but the impact of vitamin D supplementation was most evident in the co-exposed group (Figure 3B, C). Mice co-exposed to HDM+DEP had increased BALF neutrophilia and eosinophilia compared to mice exposed to HDM alone, but vitamin D supplementation had no impact on total lung cell counts, BALF total cell counts, BALF eosinophils, or BALF neutrophils (Figure 3D–G).
Figure 3. Prenatal vitamin D supplementation attenuates the development of AHR in mice co-exposed to HDM+DEP.
(A) Protocol: BALB/c pups bred from vitamin D supplemented or deficient parents were subjected to 9 IT challenges of saline, DEP and or HDM over 3 weeks while continued on vitamin D supplemented or control water. (B) Airway Resistance. (n=4–8 mice per group; 2-way ANOVA, ****p< 0.0001, ***p <.001, **p <.01, *p < 0.05). (C) Resistance after exposure to 12.5 mg/mL methacholine. (D) Total lung cell counts. (E) Total BALF counts. (F) BALF eosinophils. (G) BALF neutrophils.
Prenatal vitamin D supplementation significantly decreases Th2 and Th2/Th17 double producing populations in the lungs
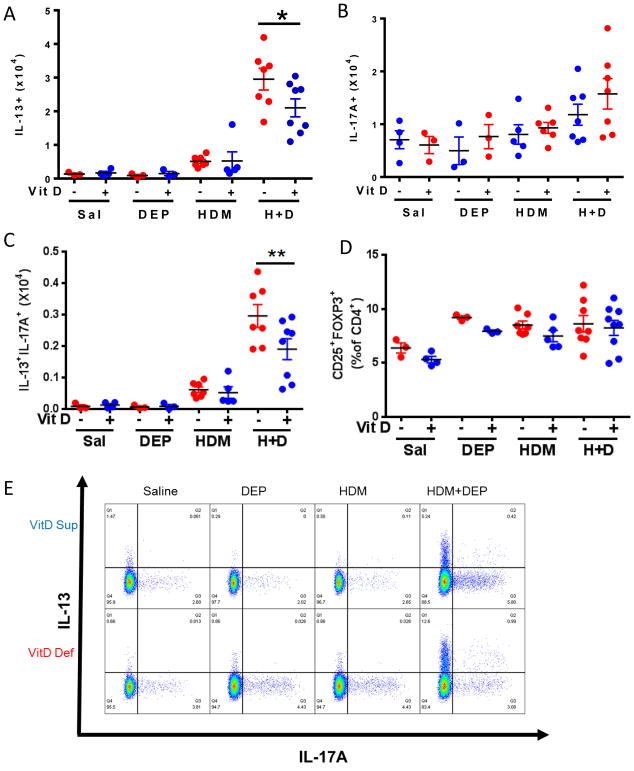
We next examined whether prenatal vitamin D supplementation impacted the pattern of lung effector T-cells similar to what we observed with postnatal vitamin D supplementation. Mice co-exposed to HDM+DEP had significantly increased Th2, Th17, and Th2/Th17 double producing cells as we previously published(13). Allergen and DEP co-exposed mice prenatally and continuously supplemented with vitamin D water had significantly decreased Th2 and Th2/Th17 double producing populations present in the lung (Figure 4A, C) compared to deficient mice. The frequency of Treg cells in the lungs was not different between the subgroups (Figure 4D).
Figure 4. Prenatal vitamin D supplementation decreases the presence of IL-13+ and IL-13+IL-17A+ lung CD4+ effector T-cells.
Intracellular staining for CD4+CD44+T-cells producing (A) IL-13, (B) IL-17A, or (C) IL-13/L-17A; or (D) staining for CD25+Foxp3+ Treg cells. (E) Representative dot plots. ****p< 0.0001, ***p <.001, **p <.01, *p < 0.05 using 1-way ANOVA with Bonferroni’s multiple comparison test. (n=4–8 mice per group).
Prenatal vitamin D supplementation is superior at attenuating the development of AHR in mice co-exposed to HDM+DEP
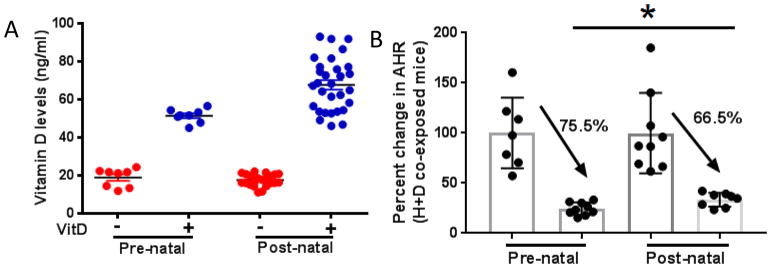
While both prenatal and postnatal supplementation were effective in preventing the development of AHR, we next directly compared the 2 treatments to determine if one conferred increased health benefit. The vitamin D levels were similar at the start of both the prenatal and postnatal experimental protocols (Figure 5A). Prenatal supplementation resulted in a 75.5% decrease in observed AHR between supplemented and deficient mice while postnatal supplementation resulted in a decrease of 66.5% (Figure 5B). Thus, while both prenatal and postnatal supplementation were effective, prenatal care conferred increased health benefit.
Figure 5. Prenatal vitamin D supplementation is superior at attenuating AHR in HDM+DEP co-exposed mice.
(A) Vitamin D levels of mice from both experimental models. (n=8–15) (B) The difference in AHR between vitamin D supplemented and deficient co-exposed mice was measured. The average AHR response of deficient co-exposed mice was normalized to 100 and compared to supplemented mice. ****p< 0.0001, ***p <.001, **p <.01, *p < 0.05; t-test.
Vitamin D does not confer protection against DEP-induced asthma exacerbation once asthma is established
To determine whether vitamin D supplementation would attenuate AHR once asthma was already established, BALB/c mice were exposed to allergen or allergen+DEP to induce asthma as previously published(13). Once asthma was established, the mice were started on vitamin D supplementation to achieve sufficient levels, >30 ng/mL (Supplemental Figure 1A). There were no significant differences observed in AHR or airway inflammation between vitamin D supplemented and deficient mice in either HDM or HDM+DEP co-exposed mice (Supplemental Figure 1B). Thus, while vitamin D supplementation prevented DEP-induced allergic asthma development, it had no benefit once asthma was established.
Discussion
Recent epidemiologic studies have suggested that vitamin D deficiency specifically makes children susceptible to the effects of TRAP exposure. Our results offer the first evidence that this is the case. Specifically, our data demonstrate that co-exposure to TRAP and allergen in context of the vitamin D deficiency results in increased asthma development and asthma severity. Further, prenatal or postnatal supplementation with vitamin D confers protection against this negative health effect of TRAP exposure. These findings have tremendous public health impact because in large cities in North America, up to 45% of the population resides in zones that are most impacted by TRAP and over 30% of schools are located in high TRAP exposure areas(12). Further, although truck engines are being designed to decrease particle emissions, air quality and engine exhaust control policies implemented in 2005–2010 have not produced significant changes in traffic air pollution levels(23).
Given the suggestive evidence of protective effects of vitamin D from observational studies, numerous clinical trials have been conducted to examine the impact of vitamin D supplementation on asthma outcomes. Tachimoto et al. found improved asthma control in asthmatic children supplemented with 800 IU vitamin D3 for 2 months(24). Other studies, however, show a lack of, or even adverse, effects of vitamin D in individuals with established asthma(25–28). This is not surprising given our findings that vitamin D confers no health benefit once asthma is established. Indeed, the recently published Vitamin D and Asthma (VIDA) trial in adults found no significant differences in treatment failure and time to first exacerbation(29). Recent studies have also examined the potential for prenatal vitamin D supplementation as a preventive strategy. The Vitamin D Antenatal Asthma Reduction Trial (VDAART) was designed to determine whether prenatal vitamin D (cholecalciferol) supplementation could prevent asthma or recurrent wheeze in early childhood(30). The incidence of asthma and recurrent wheezing in their children at age 3 years was lowered by 6.1% in the group supplemented with 4400 IU/d of vitamin D, however, this did not meet statistical significance. Unfortunately, the groups were not stratified by TRAP exposure and as the authors note, the study may have been underpowered. A recent meta-analysis was published to examine the current literature and observe the associations between 25(OH)D levels in cord or maternal blood and risk of childhood asthma, wheeze, and respiratory infections(31). Feng et al found that increased in utero exposure to 25(OH)D decreases the risk of persistent wheeze and asthma upon analysis of 16 birth cohorts(31). Similarly, another meta-analysis revealed that prenatal intake of vitamin D may protect against the development of recurrent wheeze in children(32). While these epidemiologic studies are consistent with our findings, our data suggest that the observed health benefit of vitamin D supplementation is likely to be most evident among children and mothers exposed to high levels of TRAP. Indeed, several recent reviews and editorials have concluded that additional studies are needed to determine whether vitamin D would benefit patients with specific exposures or asthma disease endotypes. Ethical issues also arise when participants remain vitamin deficient to serve as controls for these studies(33). Our data strongly support that children and pregnant mothers exposed to high levels of TRAP would benefit most from vitamin D intervention. This lack of attention to TRAP exposure may in part explain the disparate findings.
We found that both prenatal and early life continuous vitamin D supplementation attenuated the development of AHR in mice. Prenatal supplementation offered the most protection, but additional studies are necessary to determine whether the observed differences between prenatal and postnatal supplementation are clinically relevant. Future studies will also need to address how long the protection lasts following normalization of Vitamin D status and whether supplementation must be continued to protect against future persistent DEP challenges. Our data suggest that the optimal window for vitamin D’s protective effects is in early life, perhaps prior to allergen sensitization. To test this, we challenged vitamin D sufficient and deficient mice to saline, HDM, or HDM+DEP once a week for three weeks to determine whether vitamin D has an impact on sensitization (Supplemental Figure 2A). There were no significant differences in HDM-specific antibodies IgG1 and IgE (Supplemental Figure 2B, C). Lung, spleen, and mediastinal lymph nodes were then ex vivo stimulated with HDM for 6 days and culture supernatants were analyzed for IL-5 and IL-13 production. No significant differences were noted in spleen or lymph node cultures (data not shown). Thus, vitamin D does not have a major effect on allergen sensitization in this model. It remains possible that vitamin D has an impact on sensitization at lower doses. Interestingly, lung cells from mice challenged with H+D secreted significantly more IL-13 upon ex vivo stimulation and this was significantly decreased in mice that were supplemented with vitamin D (Supplemental Figure 2D). This further suggests that the protective effects of vitamin D are mediated, at least in part, by decreased numbers of effector Th2 and Th2/Th17 cells. While vitamin D was able to prevent asthma development, once asthma was established, there was no significant benefit of vitamin D supplementation on future asthma exacerbations in either HDM or HDM+DEP challenged mice (Supplemental figure 1B). These results may explain why vitamin D treatment has not been successful in treatment studies(25–28).
In children, TRAP exposure is associated with increased serum IL17A levels and increased asthma severity(13). Consistent with this, in mice, DEP exposure results in more severe allergic asthma, and the accumulation and persistence of allergen specific Th2/Th17 cells in the lungs(34). These cells rapidly produce Th2 and Th17 cytokines upon re-exposure to antigen. The persistence of these resident memory effector cells in the lungs following TRAP exposure may be responsible, in part, for asthma-promoting effects of TRAP exposure. Given recent reports demonstrating that vitamin D downregulates IL-17 production, it is possible that vitamin D may have a direct impact on Th17 cells. One study found that vitamin D deficiency is associated with enhanced production of IL-17A in patients with asthma and that IL-17A production is inhibited by vitamin D treatment independent of steroids(18). Another study found that vitamin D reduces the differentiation and expansion of Th17 cells in asthmatic children(19). Herein, we observed that Th2 and Th2/Th17 cells were uniquely affected by vitamin D supplementation. In contrast, eosinophils, neutrophils, and Treg numbers were unaffected. Th2/Th17 double producing cells have been shown to be more pathogenic in their ability to secrete cytokines and exacerbate allergic asthma(35). They have also been associated with severe steroid resistant asthma(16). In summary, our data reveal that in the context of vitamin D deficiency, these pathogenic effector cells, which promote the development and persistence of asthma, accumulate in the lungs. Early supplementation with vitamin D prevents the accumulation of these cells and prevents asthma development. These data have obvious clinical implications and support the need for a trial to examine the efficacy of vitamin D supplementation in preventing asthma development in children exposed to high levels of TRAP.
Supplementary Material
Clinical Implications.
Early vitamin D supplementation may be an effective preventive strategy for the development of TRAP-induced asthma.
Acknowledgments
Funding Source:
Supported by NIH AI1070235-11 (GKKH)
The DEP was kindly provided by Ian Gilmour (EPA, Research Triangle Park, NC 27711). We thank Ashley Ulm for graphical assistance and Angela Sadler for editorial assistance.
Abbreviations
- AHR
airway hyperresponsiveness
- BALF
bronchoalveolar lavage fluid
- DEP
diesel exhaust particle
- HDM
house dust mite extract
- PM
particulate matter
- TRAP
traffic-related air pollution
Footnotes
Conflict of interest: The authors have declared that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yetley EA. Assessing the vitamin D status of the US population. The American journal of clinical nutrition. 2008;88(2):558S–64S. doi: 10.1093/ajcn/88.2.558S. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocr Pract. 2012;18(6):914–23. doi: 10.4158/EP12072.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Yepes-Nunez JJ, Brozek JL, Fiocchi A, Pawankar R, Cuello-Garcia C, Zhang Y, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2017 doi: 10.1111/all.13241. [DOI] [PubMed] [Google Scholar]
- 5.Spira-Cohen A, Chen LC, Kendall M, Sheesley R, Thurston GD. Personal exposures to traffic-related particle pollution among children with asthma in the South Bronx, NY. J Expo Sci Environ Epidemiol. 2010;20(5):446–56. doi: 10.1038/jes.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. 45. Health Effects Institute; 2010. Pollution. HPotHEoT-RA; pp. 610–24. [Google Scholar]
- 7.Alessandrini F, Schulz H, Takenaka S, Lentner B, Karg E, Behrendt H, et al. Effects of ultrafine carbon particle inhalation on allergic inflammation of the lung. J Allergy Clin Immunol. 2006;117(4):824–30. doi: 10.1016/j.jaci.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Riedl M, Diaz-Sanchez D. Biology of diesel exhaust effects on respiratory function. The Journal of allergy and clinical immunology. 2005;115(2):221–8. doi: 10.1016/j.jaci.2004.11.047. quiz 9. [DOI] [PubMed] [Google Scholar]
- 9.Robinson RK, Birrell MA, Adcock JJ, Wortley MA, Dubuis ED, Chen S, et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosser F, Brehm JM, Forno E, Acosta-Perez E, Kurland K, Canino G, et al. Proximity to a major road, vitamin d insufficiency, and severe asthma exacerbations in puerto rican children. American journal of respiratory and critical care medicine. 2014;190(10):1190–3. doi: 10.1164/rccm.201408-1568LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appatova AS, Ryan PH, LeMasters GK, Grinshpun SA. Proximal exposure of public schools and students to major roadways: a nationwide US survey. J Environ Plann Man. 2008;51(5):631–46. [Google Scholar]
- 13.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. The Journal of allergy and clinical immunology. 2013;132(5):1194–204. e2. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Grove KC, Provoost S, Hendriks RW, McKenzie ANJ, Seys LJM, Kumar S, et al. Dysregulation of type 2 innate lymphoid cells and T(H)2 cells impairs pollutant-induced allergic airway responses. J Allergy Clin Immun. 2017;139(1):246. doi: 10.1016/j.jaci.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2014;134(1):63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. The Journal of allergy and clinical immunology. 2014;134(5):1175–86. e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, et al. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-gamma(high) immunophenotypes: Potential benefits of calcitriol. J Allergy Clin Immunol. 2015;136(3):628–37. e4. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1alpha,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132(2):297–304. e3. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Hamzaoui A, Berraies A, Hamdi B, Kaabachi W, Ammar J, Hamzaoui K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology. 2014;219(11):873–9. doi: 10.1016/j.imbio.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016;166–167:59–71. doi: 10.1016/j.clim.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Stevens T, Cho SH, Linak WP, Gilmour MI. Differential potentiation of allergic lung disease in mice exposed to chemically distinct diesel samples. Toxicol Sci. 2009;107(2):522–34. doi: 10.1093/toxsci/kfn248. [DOI] [PubMed] [Google Scholar]
- 22.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 23.Grinshpun SA, Yermakov M, Reponen T, Simmons M, LeMasters GK, Ryan PH. Traffic Particles in Ambient Air of a Major US Urban Area: Has Anything Changed over a Decade? Aerosol Air Qual Res. 2014;14(5):1344–51. [Google Scholar]
- 24.Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71(7):1001–9. doi: 10.1111/all.12856. [DOI] [PubMed] [Google Scholar]
- 25.Kerley CP, Hutchinson K, Cormican L, Faul J, Greally P, Coghlan D, et al. Vitamin D3 for uncontrolled childhood asthma: A pilot study. Pediatr Allergy Immunol. 2016;27(4):404–12. doi: 10.1111/pai.12547. [DOI] [PubMed] [Google Scholar]
- 26.Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs) Thorax. 2015;70(5):451–7. doi: 10.1136/thoraxjnl-2014-206449. [DOI] [PubMed] [Google Scholar]
- 27.Chawes BL, Bonnelykke K, Stokholm J, Vissing NH, Bjarnadottir E, Schoos AM, et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA. 2016;315(4):353–61. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 28.Bar Yoseph R, Livnat G, Schnapp Z, Hakim F, Dabbah H, Goldbart A, et al. The effect of vitamin D on airway reactivity and inflammation in asthmatic children: A double-blind placebo-controlled trial. Pediatr Pulmonol. 2015;50(8):747–53. doi: 10.1002/ppul.23076. [DOI] [PubMed] [Google Scholar]
- 29.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311(20):2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38(1):37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng H, Xun P, Pike K, Wills AK, Chawes BL, Bisgaard H, et al. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2017;139(5):1508–17. doi: 10.1016/j.jaci.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 32.Vahdaninia M, Mackenzie H, Helps S, Dean T. Prenatal Intake of Vitamins and Allergic Outcomes in the Offspring: A Systematic Review and Meta-Analysis. J Allergy Clin Immunol Pract. 2017;5(3):771–8. e5. doi: 10.1016/j.jaip.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Cabana MD, Kunselman SJ, Nyenhuis SM, Wechsler ME. Researching asthma across the ages: insights from the National Heart, Lung, and Blood Institute’s Asthma Network. J Allergy Clin Immunol. 2014;133(1):27–33. doi: 10.1016/j.jaci.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. The Journal of allergy and clinical immunology. 2015;136(2):295–303. e7. doi: 10.1016/j.jaci.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. The Journal of experimental medicine. 2010;207(11):2479–91. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.