Abstract
Background:
Methylmercury (MeHg) is a pollutant of global concern. While there is a need to gauge early-life exposures, there remain outstanding ethical, financial, and practical challenges with using the preferred biomarker, whole blood, notably in pregnant women, infants, toddlers, and children. Dried bloodspots (DBS) may help overcome some of these challenges. Notably DBS are collected from newborns in many jurisdictions offering an institutionalized platform to efficiently characterize exposures.
Objective:
To develop, validate, and apply new method to measure MeHg levels in DBS with a specific aim to use this method to increase understanding of newborn exposures.
Methods:
Method development and validation was pursued by consulting U.S. EPA Method 1630 and other resources. The method was applied to measure MeHg levels in DBS from newborns (n=675) from the Michigan BioTrust for Health program.
Results:
The assay’s detection limit (0.3μg/L), accuracy (96–115% of expected), precision, linearity, and range met performance criteria guidelines. In the newborn DBS samples, the mean (SD) and geometric mean values of MeHg were 1.46 (0.90) and 1.25 μg/L respectively, and ranged from 0.09 to 9.97 μg/L. The values we report here are similar to cord blood mercury values reported elsewhere.
Conclusions:
This is the first characterization of MeHg exposure in newborns, and thus fills an important data gap as prior studies have focused on pregnant women, cord blood, or toddlers. This method helps overcome technical challenges associated with other proposed approaches, and moving ahead there is great promise for applying this DBS-based method for population-level surveillance, particularly in resource-limited settings and for children’s health.
Keywords: Mercury, biomonitoring, bloodspots, exposure science, methods development, surveillance, pregnancy, public health
1.0. INTRODUCTION
Mercury (Hg) is a pollutant of global concern now recognized under the multilateral UN Minamata Convention on Mercury (Evers et al., 2016; Gustin et al., 2016). Though Hg exists in multiple chemical forms the greatest concern is with the methylmercury (MeHg) form. Methylmercury is an established neurodevelopmental toxicant (Clarkson and Magos, 2006; Mergler et al., 2007), and a growing body of scientific evidence points to its disruptive effects on the cardiovascular, immune, and other physiological systems (Ha et al., 2016; Karagas et al., 2012).
Given the concerns over early-life exposures to MeHg there remains a need for suitable exposure science tools. While notable studies have characterized early-life exposures by sampling biomarkers from pregnant women or studying cord blood, thus allowing for assessment of exposure-outcome relationships and the establishment of reference doses and guideline values, the measurement and utility of MeHg biomarkers remain challenged owing to a range of technical, logistical, and biological factors (Basu et al., 2014a; Grandjean and Budtz-Jørgensen, 2011; Stern and Smith, 2003). In addition, beyond the prenatal period, there is little known about MeHg exposures during infancy (0–1 yr), which represents a tremendous knowledge gap. For example, MeHg is measured in the NHANES (NHANES, 2017) as well as the Canadian Health Measures Survey (CHMS) (Health Canada, 2013) the minimum age captured in these national surveys is 1 and 3 years, respectively.
Blood is the preferred biomarker to gauge MeHg exposures (Mergler et al., 2007) but it is not without its difficulties, particularly when trying to characterize early-life exposures. Blood sampling is invasive and ethically challenging for certain groups, including pregnant women, newborns, and infants. Sampling blood through venipuncture usually requires a clinical setting, as well as trained phlebotomists and specialized supplies (e.g., syringes, collection tubes). Storage and transport of blood necessitates cold-chain approaches and these add further logistical challenges and costs. Such challenges associated with blood-based measures, not only for MeHg but for other biomarkers, warrant the need to explore alternative methods in exposure science.
Dried blood spot (DBS) sampling emerged in the 1960s as a public health surveillance technique (Li and Lee, 2014). Over the past decade there has been an increasing interest in using DBS in exposure science (Olshan, 2007) particularly since DBS are a key component of newborn screening programs institutionalized in many jurisdictions and that residual DBS may be archived and available for research and surveillance purposes (Therrell et al., 2015). In addition, there are many ethical, practical, and economic advantages to using DBS to characterize exposures to toxic chemicals as well as biomarkers of health status (McDade et al., 2007). Sampling DBS can be more participant-friendly than venipuncture as less blood is collected in a less invasive manner. The approach is more conducive for field-based research, particularly in resource-limited settings (e.g., remote locations, developing countries) as there is no need for specialized equipment, cold-chain custody, and phlebotomists.
Researchers have started to develop methods to measure Hg in human DBS samples, and we are aware of four relevant studies (Supplemental Table 1). Chaudhuri et al. (2008) analyzed 18 newborn DBS samples from the U.S. Rocky Mountain region, Funk et al. (2013) analyzed 49 newborn DBS samples from North Carolina State Laboratory of Public Health, Funk et al. (2015) analyzed DBS samples from 82 young individuals from Chicago, and Nelson et al. (2016) analyzed DBS samples from 48 newborn-mother pairs from Minnesota. Despite signifying that Hg can be successfully measured in DBS, collectively these studies have key limitations that may prevent the widespread adoption of the reported methods. These include the fact that key aspects required for validating an analytical method (e.g., precision, linearity, accuracy) were not well covered in each of the studies. The reported detection limits in the studies overlapped with the mean blood total Hg values of U.S. citizens (NHANES, 2017). Also, inductively coupled plasma mass spectrometry (ICPMS) was used to measure Hg despite the fact that this instrumental platform can be problematic for the analyses of this particular element (e.g., high ionization potential, multiple isotopes, volatility, adsorption, polyatomic interferences). Finally, total Hg levels were measured in the DBS. While a majority of the total Hg in blood is found in the MeHg form, the proportion can vary tremendously (e.g., range from 0 to 100% in NHANES; Mahaffey et al., 2004). Without being able to speciate Hg into organic and inorganic fractions and/or carefully characterize potential exposure sources, the measurement of total Hg levels in a DBS likely suffers from random measurement error as well as potential bias associated with certain aspects of the analytical method and study design.
The objective of this study was to develop, validate, and apply a new method to measure MeHg levels in DBS with a specific aim to use this method to increase understanding of newborn exposures to MeHg. Specifically we used a commercially available gas chromatography–cold vapour atomic fluorescence spectroscopy (GC-CVAFS) instrumentation platform to separate Hg species and quantify MeHg levels at the low part per trillion concentrations. Method development was pursued by consulting U.S. EPA Method 1630, which details a GC-CVAFS method to measure MeHg in water samples. Method validation for these DBS samples was carefully monitored by reviewing performance criteria established in US EPA Method 1630 as well as test parameters for method validation outlined by ICH (Reports Q2A and Q2B) and ISO 17025 as summarized in a resource from Huber (Huber, 2007). Key test parameters we focused on included assay detection limit, linearity, range, precision, and accuracy. Assay specificity is not of concern as the instrumentation platform and method are designed solely for measurement of different Hg species, and here we focused on the MeHg spectral peaks. Following validation, we applied the method to the measurement of MeHg in DBS obtained from newborns (n=675) from the Michigan BioTrust for Health program which oversees newborn DBS collected in the State and their use in health research. This represents, to our knowledge, the first study to characterize MeHg exposures directly in newborns and thus helps improve our understanding of early-life exposures to MeHg by focusing on a lifestage that was previously unstudied. The study also provides a novel method that can be widely applied given the ubiquity of DBS sampling in newborn screening programs.
2.0. METHODS
2.1. General Overview
The study was conducted in two main phases. First we developed an analytical method to measure MeHg levels in DBS, and used a range of artificially created DBS for this study aspect (i.e., DBS created in the lab with blood from reference materials and other study populations). The development of the method was based on U.S. EPA Method 1630 (designed for water samples). During the methods development phase a range of experimental parameters were examined including punches and whole spots sampled from DBS (3 mm diameter punches that included 1, 2, 4, and 8 punches, and full blood spots of 35 μL and 50 μL), as well as DBS digestion times (2, 3, 4 and 6 hours), and digestion volumes and related dilutions (various conditions). The iterative and multi-factorial nature of methods development and resulting data are not conducive for simple representation here, and so we focus this paper on presenting the optimal method and reviewing its performance. Second, we applied this method to measure MeHg levels in DBS from newborns (n=675) from the Michigan BioTrust for Health program. These analyses took several months and spanned 20 batch runs. Each batch run contained a range of quality control samples (i.e., a minimum of at least 6 blood reference materials; 4 method blanks; usually 36 individual newborn DBS samples; and 2 DBS samples from which replicate punches were taken and run separately).
2.2. DBS Processing
All DBS cards in the methods development and application phases were Whatman 903 protein saver cards. For batches #1–6, two punches (3 mm diameter; 14.1 mm2 area) were taken from near the edge of a single spot of a DBS card. For batches #7–20, the Michigan BioTrust for Health program provided us with rectangular punches that were approximately 2mm x 6mm in size (mean area 14.2 mm2). We assumed that a single 3 mm punch contains 3.1 μL of blood based on Li and Lee (Li and Lee, 2014), and made this same assumption for the rectangular punches given the similar area. From 10% of all DBS cards a blank punch (i.e., did not contain blood) was taken from the edge of the card or supplied from the Michigan BioTrust for Health.
Punched samples from a single DBS card were placed into a single borosilicate glass vessel that was pre-cleaned with 10% HCl. The samples were digested using 8 ml of 25% potassium hydroxide (KOH) in methanol heated to a gentle boil (~140 °C) for 4 hours. Cooled digests were filled to 30 ml with methanol and stored at −20 °C until analysis. An aliquot of the sample digest (1.5 mL) was added to ultrapure water and adjusted to pH 4.0 – 4.5 using citrate buffer added in 200 μl increments. Thirty minutes prior to MeHg analyses, the sample was ethylated using 1% NaBEt4.
2.3. MeHg Analysis
The measurement of MeHg in the digests was carried out using a GC-CVAFS unit (Tekran 2700, Tekran Instruments Corporation, Toronto) as outlined by Siedlikowski et al. (Siedlikowski et al., 2016) as per U.S. EPA Method 1630. The volatile Hg species in the digest solution were purged, introduced to the machine via an autosampler (Model 2621-M, Tekran), and collected on a Tenax trap. The captured Hg compounds were thermally released from the trap (183 °C) and then separated in a GC column (90 °C). At the terminus of the GC column the gas-phase Hg compounds were reduced by pyrolysis at 800 °C, and the resulting elemental Hg vapour was detected by CVAFS at 253.7 nm. We detected distinct peaks for other mercury compounds (elemental or ionic forms) but ignored them here and focused on determining MeHg concentrations by comparing the peak fluorescence values against a standard reference curve that was comprised of a MeHgCl solution (0.02 – 2 ng/L; Alfa Aesar, certified >99.5%). We note that spectral peaks for other Hg compounds were distinct from that of MeHg, and that on-going efforts within our team aim to develop a method to speciate Hg from a DBS samples.
2.4. Assay Validation and Quality Control
Method validation was tested by comparing our measurements against a range of performance criteria listed in U.S. EPA Method 1630 as well as test parameters for method validation outlined by ICH (Reports Q2A and Q2B) and ISO 17025 as summarized in Huber (Huber, 2007). Noteworthy is our lab’s participation in the NCP/AMAP (Northern Contaminants Program and Arctic Monitoring and Assessment Program) inter-laboratory program that gauges performance of analytical labs. In Phase 10 of the NCP/AMAP program, which covers the period of this study, our analytical lab’s capabilities to measure MeHg and total Hg in fish and mussel tissues were ranked “excellent” with absolute z-scores less than <1.
Several quality control samples facilitated our validation work. Each batch run contained a range of quality control samples (i.e., a minimum of at least 6 blood reference materials; 4 method blanks; usually 36 individual newborn DBS samples; and 2 individual newborn DBS samples from which replicate punches were taken and run separately), and from these we characterized the assay’s detection limit, precision, linearity, range, and accuracy. Blank punches (i.e., not containing blood) were taken from 10% of the DBS cards to help establish a method detection limit. The method detection limit was defined as the minimum measured MeHg concentration that can be reported with 99% confidence from the method blank results. Assay precision evaluated closeness of agreement (or variability) of a series of measurements of the same homogeneous sample, and we addressed this using several approaches: a) initial and on-going precision and recovery (IPR and OPR respectively) schemes as outlined by U.S. EPA Method 1630; b) measurement of blank filter papers; c) measurement of the 5 blood reference materials; and d) running 10% of the DBS samples from the Michigan BioTrust for Health cohort in duplicate.
Standard Reference Materials consisted of whole blood from the Institut National de Santé Publique du Québec (INSPQ) Centre de Toxicologie du Québec (CTQ), and these were used to establish analytical accuracy and help gauge assay linearity and range. We used 5 blood reference materials (PC-B-MQ1101, PC-B-MQ1112, PC-B-MQ1210, PC-B-MQ1303, PC-B-MQ1510) with a range of assigned total Hg concentrations (1.7 – 37.9 μg/L) (Supplemental Table 2). These blood samples originated from unexposed volunteers and were spiked with varying concentrations of MeHg. The Hg levels assigned to the reference materials were based on results from independent analyses in at least 30 laboratories participating in the INSPQ-CTQ’s External Quality Assessment Scheme program. Note, however, the assigned Hg values refer to total Hg measurements and not MeHg as required here. As such, we measured MeHg in each of the whole blood reference materials in our lab and used the values as a reference point. We are not aware of any commercially available human blood reference materials with an assigned (let alone certified) MeHg value. Using these 5 INSPQ blood reference materials we created artificial DBS samples by pipetting 50 μl of reference material onto several Whatman 903 cards, and from these cards we punched spots as outlined earlier. Analytical accuracy was calculated as the difference between the test result (i.e., MeHg in the punched reference material-DBS cards) and the assigned reference value (i.e., MeHg measured in the whole blood reference materials). Assay linearity was assessed by determining if the instrument output (peak height) was directly proportional to the expected MeHg concentration in these reference materials as well as the MeHg standard solutions.
2.5. Michigan BioTrust for Health Samples
Institutional Review Board (IRB) approval for this work was obtained from McGill University, the University of Michigan, the Michigan Department of Health, and the Michigan BioTrust for Health. The samples investigated here are from a larger epidemiological study of risk factors for newborn hearing loss (manuscript in preparation). All the DBS hailed from the State of Michigan, and DBS collection year ranged from 2003 to 2015. For batches #1–10, the DBS were stored at ambient temperatures and the rest of the samples were stored at −20 °C prior to analyses.
2.6. Data Analyses
All data were initially reviewed through descriptive statistics and graphical plots. For the methods development and assay validation phases of this work, measures of central tendency (mean, median) and associated variances (standard deviation, inter-quartile ranges) were computed and compared against assay performance criteria (see Section 2.4) with additional specific details offered in Section 3. For the application phase of this work (i.e., analysis of DBS from Michigan BioTrust for Health), the MeHg levels were described using measures detailed above. In addition, t-tests and ANOVAs were run to test if MeHg levels varied according to batch number (n=20), punch types (circular vs. rectangular), and storage temperatures (ambient vs. −20 °C). Data are represented as mean ± standard deviation unless otherwise indicated.
3.0. RESULTS AND DISCUSSION
3.1. Detection Limits
The mean concentration of MeHg in the blank digest (i.e., KOH methanol) solution was 0.0092 (±0.0093) ng/L, thus resulting in a theoretical instrument detection limit (x̅ + 3*SD) of 0.02 ng/L. This value was derived from the 20 batch runs in which at least 3 blank samples were analyzed per run (n=63 blank samples over 20 batches). The detection limit calculated here is in line with the performance criteria listed in U.S. EPA Method 1630. The detection limit is at least one order of magnitude better than what can be achieved using other conventional total Hg measurement methods (e.g., atomic absorption spectroscopy (US EPA, 1998)).
A method detection limit was calculated by measuring the MeHg content in blank filter papers. Specifically, to match the workflow for the DBS samples we measured MeHg levels in two 3 mm circular punches of Whatman 903 filter paper from 10% of the DBS cards. These punches did not contain any blood but were taken from filter paper cards in which blood was collected. Similar to above, in each of the 20 batch runs we included 3 or 4 samples containing blank filter papers (n=78 samples over 20 batches). The MeHg content in a single blank filter paper punch (i.e., one 3mm punch) was calculated to be 0.5 (±0.15) pg with a corresponding theoretical detection limit (x̅ + 3*SD) of 0.97 pg. Assuming that a 3 mm punch may contain 3.1 μL of blood (Li and Lee, 2014), a concentration-based method detection limit of 0.165 (±0.049) μg/L was derived with a corresponding theoretical detection limit of 0.313 μg/L (x̅ + 3*SD). Ideally a method detection limit would be calculated using filter paper that was blotted with blood samples, but we are not aware of a blood source that is entirely devoid of MeHg.
The MeHg detection limits reported here using DBS are comparable to what has been previously reported in large national biomonitoring programs in which Hg was measured in whole blood samples. For example, the lower detection limit of total Hg and MeHg in whole blood from the 2011–2012 U.S. NHANES was 0.16 and 0.12 μg/L, respectively (Mortensen et al., 2014). The detection frequency of blood MeHg in NHANES 2011–2012 was 83.7% and this compares favourably to what we achieved here with the Michigan Biotrust DBS samples (i.e., 14 samples were below the theoretical detection limit resulting in a detection frequency of 98%). For samples that fell below the detection limit, the value obtained from the instrument was retained for analyses.
The method detection limit calculated in our study is more than two-fold better than reported in previous studies aiming to establish a DBS method to measure Hg. For example, 2 of the 4 studies cited in Supplemental Table 1 calculated detection limits of 0.65 μg/L (Chaudhuri et al., 2008) and 0.7 μg/L (Nelson et al., 2016). Application of these limits to their datasets resulted in detection frequencies of 38 to 72%.
3.2. Linearity and Range
The linearity of the method was assessed by measuring MeHg levels in DBS that had been artificially blotted with blood reference materials. The resulting linear regression from pooled data was Y = 14.3X +211, where Y=peak output and X was the MeHg content (ng), and the coefficient of determination was 0.999. Standard curves were also run in each batch with aqueous MeHg solutions with known concentrations (0.02 – 2 ng/L), and the resulting regressions of the pooled data also had coefficient of determinations of 0.999. These results indicate that the analytical procedure obtains results that are directly proportional to the concentration of MeHg in a sample with a known concentration.
The assay was also developed in consideration of relevant exposure levels. The standard curves used in this study spanned a relevant range once dilution factors had been considered. The results from the accuracy tests with reference materials (Section 3.4) and results from the application phase of the work (Section 3.5) show that the method covers a relevant range, though we articulate below a need to further improve the assay at the lower range.
3.3. Precision
As required by U.S. EPA Method 1630, initial and on-going precision and recovery (IPR and OPR respectively) is needed to show that the analytical system is in control and operating within specific limits. The IPR/OPR aqueous solution tested (0.5 ng/L) is the one recommended by U.S. EPA Method 1630. In our case, the mean concentration measured across the 20 batch runs was 0.49 (±0.03) ng/L, and this was well within the accepted performance criteria listed in U.S. EPA Method 1630. The coefficient of variation (i.e. relative standard deviation) for these measurements across days was 6.2%, which indicates good inter-day precision in reference to the acceptable performance criteria. Each batch run included 5 OPR measurements and the coefficient of variation for those measurements was 6.3% thus indicating good intra-day precision.
Precision was also determined from the results of the blank filter papers (intra-day and inter-day precision were 11% and 18%, respectively) and reference materials (13 to 20% across the 5 reference materials tested). All of these values were within the acceptable performance criteria listed in U.S. EPA Method 1630. Finally, assay precision was addressed via the DBS samples from the Biotrust samples. In each of the 20 batches we performed repeat measures on two samples, and thus duplicate analyses was performed on 40 samples. The mean precision (expressed as relative percent difference, RPD) was 20% and this is below the 35% performance criteria listed in the U.S. EPA 1630 document. We note, however, that there was variability in these measures with RPD values ranging from 0 to 76%, and there was no correlation between RPD and MeHg value. Five of the 40 repeated samples had RPD values above the 35% criteria value. Moving ahead it would be best to take multiple measures from a given DBS sample but we recognize limitations of doing so (e.g., newborn DBS are a limited resource; added financial costs). It is thus prudent for researchers to strive towards improving assay precision, but also carefully test and report upon pertinent quality control measures so that data end-users are best informed of the limitations.
3.4. Accuracy
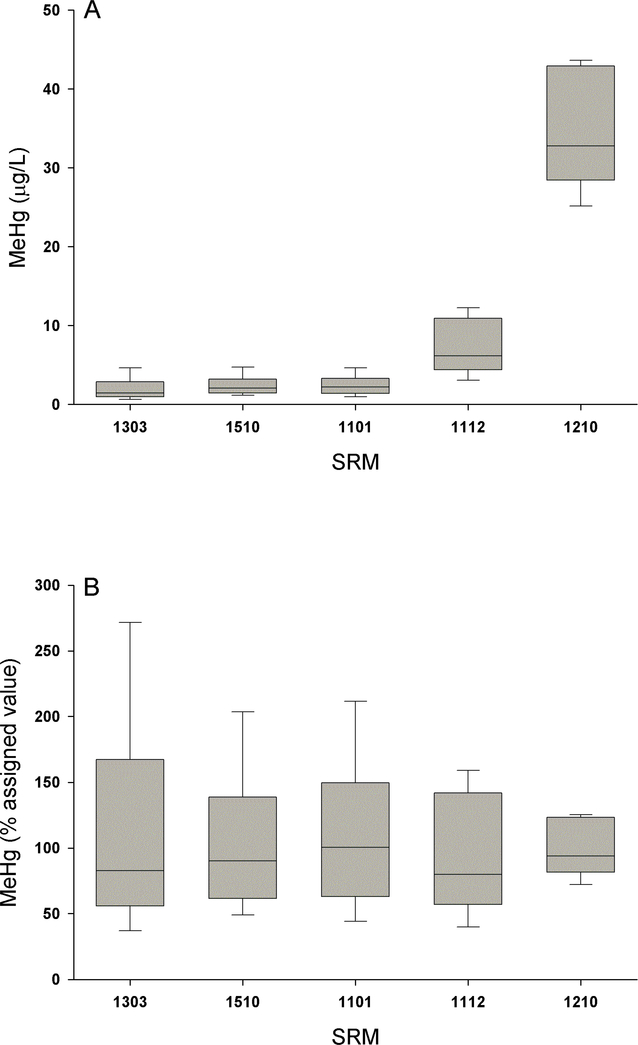
The accuracy of the method was assessed by measuring MeHg levels in DBS that had been artificially blotted with reference materials with a range of assigned total Hg levels (1.7 – 37.9 μg/L). In these reference materials we measured MeHg levels as a reference point as we are not aware of any commercially available human blood reference materials with an assigned or certified MeHg value (Supplemental Table 2). The mean measurements taken in these reference material DBS were within the expected range (i.e., ranged from 96 to 115% of expected; Figure 1) and fell between the acceptable performance range listed in U.S. EPA Method 1630 of 65 – 135%. Though, as illustrated in Figure 1B (and in reference to our discussion above in Section 3.3) the variance was relatively high thus warranting a need for further improvements to the assay particularly at the lower (albeit relevant) concentrations. As suggested by ICHQ2(R1) we tested at least 3 different reference material concentrations with more than 3 replicates per reference material.
Figure 1.
Analytical accuracy of methylmercury (MeHg) measurements taken in dried blood spots (DBS) with absolute (panel A) and relative (panel B) values shown as boxplots. Accuracy was determined by analyses of DBS that had been spotted with five different whole blood standard reference materials (SRM) from the Institut National de Santé Publique du Québec (INSPQ).
Method accuracy in the previous DBS papers concerning Hg also varied. The study from the U.S. Rocky Mountain region used a lyophilized human whole blood reference material purchased from Utak Laboratories (assigned value of 1.2 μg/L) as well one purchased from the INSPQ (4 μg/L), and their measured values fell within 13% of expected (Chaudhuri et al., 2008). The study from Minnesota used SRM 966 (not human blood but whole bovine blood with a certified total Hg value of 31.4 μg/L) obtained from the U.S. National Institutes of Standards and Technology (NIST) and spiked samples (5.4 and 15.9 μg/L) with recoveries within 24% of expected (Nelson et al., 2016). The study from North Carolina spiked samples and calculated recoveries were 48–230% (Funk et al., 2013) and in the study from Chicago we could not find information concerning analytical accuracy (Funk et al., 2015). While reference materials are important in helping establish a method and monitoring measurement quality, there are some outstanding concerns with this work. Most human blood reference materials for Hg focus on total Hg levels and not MeHg even though the ratio of MeHg to total Hg can vary widely (Mortensen et al., 2014). The only blood reference material we are aware of that provides a MeHg value is NIST SRM 966, though this particular reference material is from cattle and the MeHg level is very high (~30x higher than the U.S. population average). The community would benefit from additional human whole blood reference materials that cover relevant exposure levels (i.e., less than 1 μg/L). Most reference materials provide assigned instead of certified values, the latter that requires more rigor. Finally, none of the reference materials are provided in the most relevant matrix for the current study, namely as DBS cards.
3.5. Assay Application: Michigan BioTrust Cohort
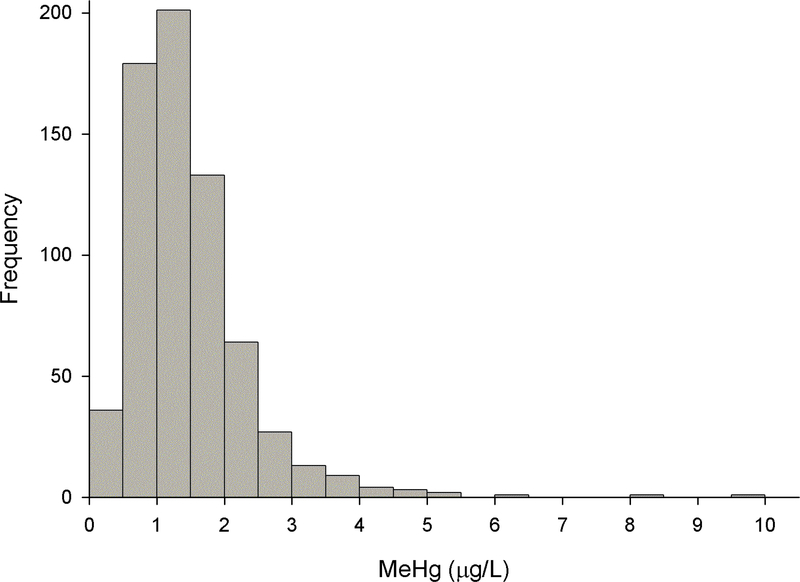
We measured MeHg levels in DBS samples obtained from 675 newborns through the Michigan BioTrust for Health program (Figure 2). The mean (SD) and geometric mean values of MeHg in the DBS were 1.46 (0.90) and 1.25 μg/L respectively, and they ranged from 0.09 to 9.97 μg/L. The 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentile values were 0.50, 0.64, 0.90, 1.29, 1.82, 2.39, and 3.00 μg/L respectively. There were no temporal differences in MeHg levels over the study period.
Figure 2.
Histogram of methylmercury (MeHg) measurements taken in dried blood spots (DBS) from 675 newborns through the Michigan BioTrust for Health program.
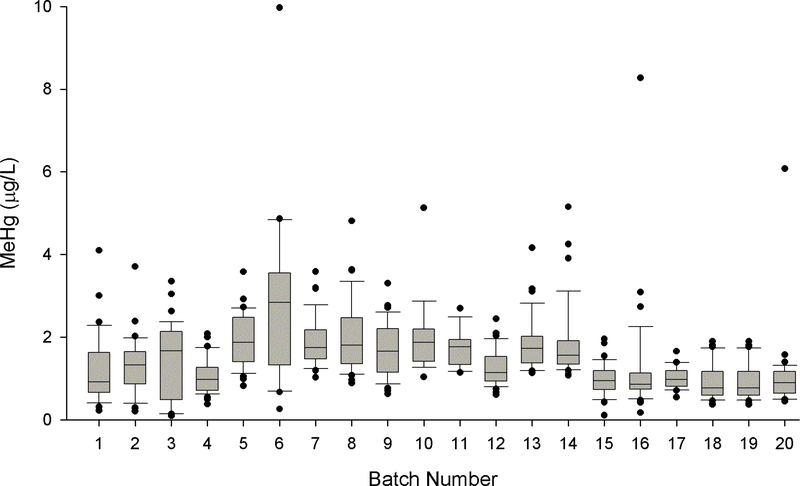
The analyses of these newborn DBS took several weeks and spanned 20 batch runs with each batch run consisting of upwards of 36 samples (Figure 3). While there was a statistically significant (p<0.001) batch effect we feel that there was no underlying bias as a range of quality control measures on those days (e.g., blanks, IPR/OPR, precision and accuracy tests with reference materials) performed consistently with other batches. We found no evidence of systematic drift in measurements (of newborn DBS samples and a range of quality control measures) across time as seen in a previous study on Hg analyses of DBS (Chaudhuri et al., 2008). The DBS cards that were kept stored at an ambient temperature (n=380) prior to analyses had significantly (p<0.001) higher MeHg levels that the n=295 cards that were kept frozen (1.64 ± 0.94 versus 1.25 ± 0.80 μg/L), and this difference warrants additional investigation. We have no reason to suspect that freezing would modify the MeHg concentration, though this find necessitates more work be done addressing sample stability. There was no difference (p=0.137) in MeHg levels in the circular (1.54 ± 1.10 μg/L; n=200) versus rectangular (1.43 ± 0.80 μg/L; n=475) punches.
Figure 3.
Boxplots of methylmercury (MeHg) measurements taken in dried blood spots (DBS) from 675 newborns through the Michigan BioTrust for Health program according to batch runs. Each batch contained upwards of 36 individual newborn DBS samples.
To our knowledge this is the first dataset of blood MeHg levels in newborns. Accordingly, there is no ideal comparison group and external validity of the work was pursued by comparing our results with a range of information streams. First, a panel of scientific experts concluded that blood Hg values in a background population falls between 1 and 5 μg/L which covers the range we report here (Mergler et al., 2007). Second, our review of the four earlier Hg DBS papers showed blood total Hg levels in DBS from newborns to be in the 0.3–0.5 μg/L range, with an upper bound of 6.5 μg/L (Supplemental Table 1). Third, the U.S. EPA’s MeHg reference dose is associated with a biomonitoring guideline value of 5.8 μg/L in cord blood (Mahaffey et al., 2004). In the Michigan BioTrust cohort, 3 of 674 (0.5%) samples were above this reference dose compared with U.S. NHANES 2011–2012 in which 0.05% of 1–5 year olds had blood MeHg values above the reference dose (Mortensen et al., 2014).
In addition to the above comparisons, we also relate our results with cord blood Hg measurements, as this may represent the most appropriate comparison group given the paucity of comparable data. DBS taken within 36 hrs of birth represents capillary blood from the newborn infant, and this seems most similar to cord blood that originates from the placenta and innervates the fetal circulatory system versus maternal blood sampled during pregnancy or blood taken from the toddler at age 1 and older. In doing so, the results from the current study overlap considerably with existing datasets. For example, median total Hg levels in cord blood taken from participants of the MIREC cohort (n=2,001 pregnant women from across 10 Canadian cities; Arbuckle et al., 2016) was 0.80 μg/L with a 95th percentile value of 3.61 μg/L. In more targeted studies across the U.S., average cord blood Hg measurements were 0.59 μg/L in San Francisco (Morello-Frosch et al., 2016), 0.94 μg/L in Baltimore (Wells et al., 2016), and 2.14 μg/L in New York (Geer et al., 2012). In other jurisdictions worldwide, the cord blood Hg levels are even higher with average values of 3.6 μg/L in Nigeria (Obi et al., 2015), 4.7 μg/L in Mexico (Basu et al., 2015b), and 8.2 μg/L in Spain (Ramon et al., 2011).
Taken together, there is good overlap between the results of our work and other studies though we acknowledge that there are pertinent challenges in making meaningful comparisons. It is unclear how Hg measurements in cord blood compare with newborn capillary blood, and moreover with venous blood which is most often sampled in Hg biomonitoring efforts. Most studies report upon total Hg values, and there is an increasing need to characterize MeHg levels as this is the toxicologically most relevant form. While a few studies have measured total Hg in newborn DBS (Supplemental Table 1), they are challenged and the work presented here on 675 newborns is greater than the 197 reported upon in the four previous studies combined.
3.6. Study Limitations
This study presents, validates, and applies a new method to analyze MeHg in DBS and increases understanding of newborn exposures, though there are notable study limitations that warrant attention. Several limitations have already been identified and discussed above in previous sections and will not be repeated here. Arguably the greatest challenge is not knowing the true blood volume in a DBS punch which is well articulated in key resources from the DBS community (Li and Lee, 2014)(Timmerman et al., 2011). This is certainly the case with DBS from newborns in which blood is taken shortly after birth by simply dropping it onto filter paper. Without sample volume information it is impossible to calculate an accurate concentration. In the current study we assumed that a single 3 mm punch contains 3.1 μL of blood (Li and Lee, 2014). Our own in-house estimates revealed a similar volume estimate (3.13 μL; see Supplemental Box 1 and Supplemental Table 3) though there can be tremendous variation across the punches, spots, and individuals. To minimize potential bias of variability in blood spreading across the spot, we always punched near the edge. Moving forward there will be a need to try and account for this variation, and this may include schemes to normalize the data using measures of additional blood constituents taken from the DBS.
Another limitation of this method is the possibility of MeHg contamination of the filter cards which may arise during the manufacture of the card as well as during blood collection and ensuing transport and storage of the cards. This possibility needs to be carefully monitored and accounted for, and ideally a measurement is taken from a blank punch from each filter card near the sampled DBS. Finally, improving analytical precision at the lower and relevant concentrations is necessary and may be achieved, for example, by running more replicates, improving the quality of the standard curve in the lower end, and continued training of personnel and improvement of the method.
3.7. Concluding Remarks
This study establishes a new method to measure MeHg levels in DBS, and applies this method to characterize for the first time MeHg levels in newborns. Specifically, this method was developed and validated very carefully considering performance criteria established in U.S. EPA Method 1630 as well as test parameters for method validation outlined by ICH (Reports Q2A and Q2B) and ISO 17025. We applied this method to characterize MeHg exposures in a relatively large study involving 675 newborn DBS samples from the State of Michigan. The results suggest that MeHg content in newborn capillary blood may be reflective of cord blood levels, though additional studies are needed to verify this assertion.
The method we developed helps overcome some challenges associated with other proposed approaches, and thus moving ahead there is great promise for adopting this DBS-based method in a range of settings. Similar to the current study, institutionalized newborn screening programs that are already collecting DBS in many jurisdictions can utilize archived samples for population biomonitoring or even research studies concerning exposure-outcome relationships that are hypothesized to have early-life aetiologies. Many communities challenged with Hg exposures (e.g., MeHg contamination of country foods in the Arctic or elemental Hg use in artisanal and small-scale gold mining communities in low- and middle-income countries) are often situated in resource-limited settings where it is difficult to sample blood. These examples highlight ethical, practical, and economic advantages to using DBS to characterize MeHg exposures.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge funding support from the Gerber Foundation, the Michigan Bloodspot Environmental Epidemiology Project (BLEEP), the Canada Research Chairs (CRC) program, infrastructure support from Canada Foundation for Innovation, and a Natural Sciences and Engineering Resaerch Council of Canada (NSERC) Discovery Grant. We thank the Michigan BioTrust for Health and Michigan Department of Health program staff for their support, with particular thanks to Carrie Langbo and Nancy Christ. We also thank Jessica Pawly, Stephane Bayen, Helene Lalande, and Jonathan Chevrier for discussing technical aspects of the work with our team.
Footnotes
CONFLICT OF INTEREST DECLARATION
The authors declare no conflicts of interest, including no competing financial interests.
REFERENCES
- Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, Legrand M, Davis K, Ettinger AS, Fraser WD, 2016. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 163, 270–282. doi: 10.1016/j.chemosphere.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Basu N, Goodrich JM, Head J, 2014a. Ecogenetics of mercury: From genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ. Toxicol. Chem 33, 1248–1258. doi: 10.1002/etc.2375 [DOI] [PubMed] [Google Scholar]
- Basu N, Tutino R, Zhang Z, Cantonwine DE, Goodrich JM, Somers EC, Rodriguez L, Schnaas L, Solano M, Mercado A, Peterson K, Sánchez BN, Hernández-Avila M, Hu H, Maria Téllez-Rojo M. 2014b. Mercury levels in pregnant women, children, and seafood from Mexico City. Environ Res. 135:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri SN, Butala SJM, Ball RW, Braniff CT, Rocky Mountain Biomonitoring Consortium, 2008. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J. Expo. Sci. Environ. Epidemiol 19, 298–316. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, 2006. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol 36, 609–62. doi: 10.1080/10408440600845619 [DOI] [PubMed] [Google Scholar]
- Evers DC, Keane SE, Basu N, Buck D, 2016. Evaluating the effectiveness of the Minamata Convention on Mercury: Principles and recommendations for next steps. Sci. Total Environ 569–570, 888–903. doi: 10.1016/j.scitotenv.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Funk WE, Pliel JD, Sauter DJ, McDade TW, Holl JL, 2015. Use of Dried Blood Spots for Estimating Children’s Exposures to Heavy Metals in Epidemiological Research. Environ. Anal. Toxicol doi: 10.4172/2161-0525.S6-001 [DOI] [Google Scholar]
- Funk WWE, McGee JJK, Olshan AFA, Ghio AAJ, 2013. Quantification of arsenic, lead, mercury and cadmium in newborn dried blood spots. Biomarkers 18, 174–177. doi: 10.3109/1354750X.2012.750379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer LA, Persad MD, Palmer CD, Steuerwald AJ, Dalloul M, Abulafia O, Parsons PJ. 2012. Assessment of prenatal mercury exposure in a predominately Caribbean immigrant community in Brooklyn, NY. J Environ Monit. 14(3):1035–43. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E, 2011. An ignored risk factor in toxicology: The total imprecision of exposure assessment. Pure Appl Chem 82, 383–391. doi: 10.1351/PAC-CON-09-05-04.An [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin MS, Evers DC, Bank MS, Hammerschmidt CR, Pierce A, Basu N, Blum J, Bustamante P, Chen C, Driscoll CT, Horvat M, Jaffe D, Pacyna J, Pirrone N, Selin N, 2016. Importance of Integration and Implementation of Emerging and Future Mercury Research into the Minamata Convention. Environ. Sci. Technol 50, 2767–2770. doi: 10.1021/acs.est.6b00573 [DOI] [PubMed] [Google Scholar]
- Ha E, Basu N, Bose-O’Reilly S, D??rea JG, McSorley E, Sakamoto M, Chan HM, 2016. Current progress on understanding the impact of mercury on human health. Environ. Res 152, 419–433. doi: 10.1016/j.envres.2016.06.042 [DOI] [PubMed] [Google Scholar]
- Canada Health, 2013. Second Report on Human Biomonitoring of Environmental Chemicals in Canada-Results of the Canadian Health Measures Survey Cycle 2ss (2009–2011).
- Huber L, 2007. Validation and Qualification in Analytical Laboratories. Informa Healthcarae, New York, USA. [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, 2012. Review Evidence on the Human Health Effects of Low-Level Methylmercury Exposure. Environ. Health Perspect 120, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lee MS, 2014. Dried Blood Spots Wiley Series on Pharmaceutical Science and Biotechnology : Practices, Dried Blood Spots - Applications and Techniques. John Wiley & Sons, Hoboken, New Jersey. [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC, 2004. Blood Organic Mercury and Dietary Mercury Intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ. Health Perspect 112, 562–570. doi: 10.1289/ehp.6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade T, Williams S, Snodgrass J, 2007. WHAT A DROP CAN DO: DRIED BLOOD SPOTS AS A minimally invasive method for integrating biomarkers into population-based research. Demography 44, 899–925. [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson H.a, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH, 2007. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio 36, 3–11. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch R, Cushing LJ, Jesdale BM, Schwartz JM, Guo W, Guo T, Wang M, Harwani S, Petropoulou SE, Duong W, Park JS, Petreas M, Gajek R, Alvaran J, She J, Dobraca D, Das R, Woodruff TJ. 2016. Environmental Chemicals in an Urban Population of Pregnant Women and Their Newborns from San Francisco. Environ Sci Technol. 50(22):12464–12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen ME, Caudill SP, Caldwell KL, Ward CD, Jones RL, 2014. Total and methyl mercury in whole blood measured for the first time in the U.S. population: NHANES 2011–2012. Environ. Res 134, 257–264. doi: 10.1016/j.envres.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Edhlund BL, Johnson J, Rosebush CE, Holmquist ZS, Swan SH, Nguyen RHN, 2016. Assessing a new method for measuring fetal exposure to mercury: Newborn bloodspots. Int. J. Environ. Res. Public Health 13 doi: 10.3390/ijerph13070692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHANES, 2017. Fourth national report on human exposure to environmental chemicals-Updated Tables, January 2017, Volume One Dep. Heal. Hum. Serv [Google Scholar]
- Obi E, Okafor C, Igwebe A, Ebenebe J, Afonne OJ, Ifediata F, Orisakwe OE, Nriagu JO, Basu N. 2015. Elevated prenatal methylmercury exposure in Nigeria: evidence from maternal and cord blood. Chemosphere. 119:485–9. [DOI] [PubMed] [Google Scholar]
- Olshan AF, 2007. Meeting report: the use of newborn blood spots in environmental research: opportunities and challenges. Environ. Health Perspect 115, 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon R, Murcia M, Aguinagalde X, Amurrio A, Llop S, Ibarluzea J, Lertxundi A, Alvarez-Pedrerol M, Casas M, Vioque J, Sunyer J, Tardon A, Martinez-Arguelles B, Ballester F 2011. Prenatal mercury exposure in a multicenter cohort study in Spain. Environ Int. 37(3):597–604. [DOI] [PubMed] [Google Scholar]
- Siedlikowski M, Bradley M, Kubow S, Goodrich JM, Franzblau A, Basu N, 2016. Bioaccessibility and bioavailability of methylmercury from seafood commonly consumed in North America: In vitro and epidemiological studies. Environ. Res 149, 266–273. doi: 10.1016/j.envres.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern AH, Smith AE, 2003. An assessment of the cord blood: Maternal blood methylmercury ratio: Implications for risk assessment. Environ. Health Perspect 111, 1465–1470. doi: 10.1289/ehp.6187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therrell BL, Padilla CD, Loeber JG, Kneisser I, Saadallah A, Borrajo GJC, Adams J, 2015. Current status of newborn screening worldwide: 2015. Semin. Perinatol 39, 171–187. doi: 10.1053/j.semperi.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Timmerman P, White S, Globig S, Lüdtke S, Brunet L, Smeraglia J, 2011. EBF recommendation on the validation of bioanalytical methods for dried blood spots. Bioanalysis 3, 1567–1575. doi: 10.4155/bio.11.132 [DOI] [PubMed] [Google Scholar]
- US EPA, 1998. Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry, Methods. [Google Scholar]
- Wells EM, Herbstman JB, Lin YH, Jarrett J, Verdon CP, Ward C, Caldwell KL, Hibbeln JR, Witter FR, Halden RU, Goldman LR. 2016. Cord Blood Methylmercury and Fetal Growth Outcomes in Baltimore Newborns: Potential Confounding and Effect Modification by Omega-3 Fatty Acids, Selenium, and Sex. Environ Health Perspect. 124(3):373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.