Abstract
Persistent adult anxiety disorders often begin in adolescence. As emphasis on early treatment grows, we need a better understanding of how adolescent anxiety develops. In the current study, we used a fear conditioning paradigm to identify disruptions in cue and context threat-learning in 19 high anxious (HA) and 24 low anxious (LA) adolescents (12–17 years). We presented three neutral female faces (conditioned stimulus, CS) in three contingent relations with an unconditioned stimulus (UCS, a shrieking female scream) in three virtual room contexts. The degree of contingency between the CSs and the UCSs varied across the rooms: in the predictable scream condition, the scream followed the face on 100% of trials; in the unpredictable scream condition, the scream and face appeared randomly and independently of each other; in the no-scream condition the CS was presented in the absence of any UCS. We found that the LA adolescents showed higher levels of fear-potentiated startle to the faces relative to the rooms. This difference was independent of the contingency condition. The HA adolescents showed non-differential startle between the CSs, but, in contrast to previous adult data, across both cue types displayed lowest startle to the unpredictable condition and highest startle to the no-scream condition. Our study is the first to examine context conditioning in adolescents, and our results suggest that high trait anxiety early in development may be associated with an inability to disambiguate the signalling roles of cues and contexts, and a mislabelling of safety or ambiguous signals.
Keywords: Adolescence, Anxiety, Context conditioning, Development, Individual differences
1. Introduction
Anxiety problems are common, persistent, and debilitating, exerting huge costs for individuals and society (Olesen, Gustavsson, Svensson, Wittchen, & Jonsson, 2012). Understanding the mechanisms by which anxiety problems arise or abate has therefore become a priority (Beddington et al., 2008). At least half of adult anxiety problems will have their onset by the age of 18 years (Gregory et al., 2007), and by focusing on early individual differences, we can develop a model that enables anxiety to be addressed early, with the aim of reducing long-term burden. In addition, on-going changes in brain function during adolescence exert long-term influences on patterns of behaviour in adulthood (Burnett, Sebastian, Cohen Kadosh, & Blakemore, 2011). This substantial neural development may shape how persistent fears emerge in a way that may contrast with how they emerge in adults.
In adult anxiety models, conditioning theory has highlighted the role of associative learning between explicit cues and aversive outcomes in producing fear responses. High-anxious adults show greater fear to a threat cue (conditioned stimulus (CS+); a stimulus which has been followed by an aversive unconditioned stimulus (UCS)) than their low-anxious counterparts (Lissek et al., 2005), and also manifest more fear to non-threat cues (CS–;a stimulus which is never followed by a UCS). Such fear responses to CS cues in high-anxious adults have been attributed to stimulus generalisation (Dunsmoor, Mitroff, & LaBar, 2009; Haddad, Pritchett, Lissek, & Lau, 2012; Lissek et al., 2008, 2009).
Although conditioning of discrete cues seems best suited for explaining transient fear states in both anxious and low-anxious individuals, context conditioning, or conditioning to diffuse non-specific ‘background’ cues, has been used to explain situations of more generalised and sustained fear responses, in other words, anxiety. Previous work with healthy adults suggests that contextual fear is greater under conditions when the CS/UCS association is less predictable, that is, when the UCS does not reliably follow the CS (Grillon, Baas, Cornwell, & Johnson, 2006). A small body of literature investigating contextual fear in anxious adults tentatively suggests that the enhanced response under unpredictable circumstances is even greater in high anxious individuals (Baas, 2012).
While developmental perspectives on anxiety emphasise the importance of extending this work to adolescents, the limited available data in this area focuses more on children than adolescents (Craske et al., 2008; Field & Storksen-Coulson, 2007; Waters, Henry, & Neumann, 2009) – and none, to our knowledge, pertains to contextual (as opposed to cued) fear responses. One reason for the paucity of fear conditioning studies in adolescents is balancing practical and ethical considerations. Electrical shocks, the most powerful UCS in adults, may not be appropriate for adolescents. Less noxious UCSs however, such as loud sounds and unpleasant photographs, whilst useful in working with children, provoke minimal fear in the adolescent age range (Lau et al., 2008). To address this problem, a paradigm which has recently been introduced uses a piercing female scream as the aversive UCS. The ‘screaming lady paradigm’ has been successfully used in both healthy and clinical non-adult populations both in the US and the UK (Haddad et al., 2012; Lau et al., 2008, 2011). Lau and colleagues used this procedure in a sample of anxious and low-anxious adolescents and found that while all adolescents could discriminate between threat and safety cues, anxious adolescents show enhanced fear to both (Lau et al., 2008). It remains to be determined whether high anxious adolescents generalise their fear to the context in which the fear conditioning occurs, and the extent to which anxiety-associated differences emerge under predictable and unpredictable conditions.
The current study combined the screaming lady paradigm (Lau et al., 2008) and a discriminative cued-conditioning paradigm, with measures of context learning (Grillon et al., 2006) to investigate differences in cue and context threat learning in high anxious and low anxious adolescents. Here, three neutral facial expressions appeared under one of three contingency conditions. In the predictable scream contingency condition (P), the CS+ was always followed by the scream, whereas in the unpredictable scream contingency condition (U), a different face and the scream appeared pseudo-randomly with a minimum gap of 2 s between them. In the no scream contingency condition (N), a third face served as the but no screams occurred. To index fear, we used fear-potentiated startle (FPS) that is electromyography of the eyeblink startle reflex to an airpuff. Fear-potentiated startle responses represent a low-invasive, easily controllable measure of fear. Moreover, the operational procedure is comparable in humans and animals, which is particularly attractive as the underlying neural networks are well-documented in the animal model (Grillon, 2002). Based on previous models of CS/Context conditioning interactions (e.g. Rescorla & Wagner, 1972), we expected to observe a differential FPS response to the discrete CS+ cue and the CS‒cues, and between the three contexts (independent of individual anxiety levels) due to the different CS–UCS contingencies. Specifically, we predicted greater contextual FPS to U relative to P and N, and expected to observe greater cued startle response to the CS+ relative to the CS–(independent of contingency condition). Importantly, based on prior data on cue conditioning and stimulus generalisation in anxious vs. low-anxious individuals, we predicted that (1) HA adolescents would show increased FPS responses to the discrete CS+ and both CS‒cues than LA adolescents independent of contingency condition and that this group difference may be particularly large for the CS+ cues and (2) HA adolescents would exhibit greater FPS responses to the room context cues, and based on adult data, tentatively, this anxiety-related difference would be greatest to the room in the unpredictable contingency condition.
2. Methods
2.1. Participants
For the current study, we created a large recruitment pool of 2000 children and adolescents from local schools, who were screened for state and trait anxiety levels (Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1970). Participant selection then focused on the highest and lowest scoring 5% of the sample (based on the trait anxiety score). The final sample reported here consisted of 43 participants, of whom 24 were low anxious (LA) (mean age: 14.0, SD: 1.2 years, 7 female) and 19 were high anxious (HA) (mean age: 14.6, SD: 1.6 years, 9 female). See also Table 1 for trait and state anxiety distribution in the two groups. An additional 6 LA and 11 HA participants were tested but not included in the current analyses due to technical difficulties leading to incomplete/invalid data (5 participants), or lack of reliable eye blink responses (i.e., blinks on less than 75% of trials, 12 participants). Participants self-reported no history of psychiatric illness or learning difficulty, which was corroborated by the school. Informed consent was obtained from the participant’s primary caregiver, and informed assent was obtained from all participants prior to testing. The study was approved by the local ethics committee at the University of Oxford. All participants received a £10 Amazon voucher for taking part in the study.
Table 1.
Age, gender and Spielberger State-Trait Anxiety Inventory scores for both anxiety groups (HA = High anxious; LA = Low anxious group).
| Group | Mean/SD | Between group comparison (t-test) | |
|---|---|---|---|
| Age | LA group HA group |
14.1/1.2 14.6/1.4 |
t(41) = 1.34, p = .186 |
| Gender | LA group HA group |
7 female/14 male 9 female/10 male |
X2(41) = 2.44, p = .118 |
| Trait | LA group HA group |
28/4.3 46/3.4 |
t(41) = 15.0, p < .001 |
| State | LA group HA group |
29/3.3 39/7.0 |
t(41) = 6.45, p < .001 |
2.2. Psychophysiological apparatus
We recorded the fear-potentiated eye-blink startle reflex in accordance with guidelines set out in Blumenthal et al. (2005). Stimulation and recording were controlled by Psychlab (Contact Precision Instruments, London, UK) and E-prime 2.0 software (Psychology Software Tools, Pittsburgh, PA). Electromyography (EMG) recordings of the eye-blink startle reflex were made with two 4 mm Ag–AgCl electrodes placed beneath the left eye over the orbicularis oculi muscle, approximately 25 mm apart. A third electrode was placed onto the tip of the nose and served as a baseline reference. EMG activity was sampled at 1000 Hz, with amplifier bandwidth set to 25–500 Hz. To elicit the fear-potentiated eye-blink response, we used a 40 ms air-puff startle probe of medical grade compressed air, delivered to the centre of the forehead through a polythene tube (2 m long, 32 mm inside diameter), affixed approximately 1 cm from the skin by way of a headpiece worn by the participant. The headpiece allowed the participants to move their head while maintaining constant placement of the air-puff. A visor was positioned between the polythene tube and the participant’s eyes to prevent the air-puff from reaching the cornea. A solenoid valve with an AC switch controlled delivery of the airpuffs. Prior to testing, air pressure was set at 0.7 bar initially (measured at the level of the regulator), but this was adjusted for each participant individually. Pressure was set at the minimal level required to elicit reliable blinking during a test block of six successive startle probe presentations.
2.3. Conditioning paradigm
We combined a classical, discriminative cued-conditioning paradigm (Lau et al., 2008) with a context variable. Photographs of three female faces with neutral expressions from the NimStim face stimulus set1 served as specific threat-cue (CS+) and safety-cues (CS−), each appearing in one of three contexts (three pictures of rooms in a house, see Fig. 1). The UCS was a 95 db. Female scream, lasting for 750 ms. Each face-room pairing appeared under one of three contingency conditions (Predictable (P), Unpredictable (U), No scream (N)), with different faces serving as CS+ and two CS‒cues. Specifically, in the P condition, the CS+ was always followed by the scream, whereas in the U condition, the face and the scream appeared pseudo-randomly with a minimum gap of 2 s between them. In the N condition, a different face served as the but no screams occurred. The room to cue allocation and allocation of face identities was counterbalanced across participants. Each trial began with the onset of the relevant room picture, which remained on the screen for 10 s. At a random time-point during each 10-s trial, the face picture appeared for 2 s, with the picture of the face superimposed on the picture of the room. In the predictable and unpredictable conditions, the unconditioned stimulus (the scream) was presented once for 750 ms either in conjunction with the face (CS+) (same offset time) or separate from the face . Within each 10 s trial, two air puffs were delivered, one when only the room was present, to provide a measure of startle to the room (i.e., room only baseline startle, to measure contextual fear) and a second one when the room and face were both present to provide a measure of the startle response towards the conditioned face stimulus (to measure cued fear). The air puffs were presented with a minimum time gap of 3 s between them to allow the EMG response to return to baseline. Further, the puffs never appeared within 2 s after stimulus onset (i.e. a room or face picture), so that the startle responses were not affected by the pre-pulse inhibition effect. To avoid predictability, we created 6 pseudo-randomised puff sequences (for baseline and face puffs), which were identical for the three contexts. Finally, the face puff was delivered for each face stimulus presentation, whereas the room-alone puff was delivered on 75% of the trials only, to avoid unnecessary habituation. More frequent airpuffs associated with the face cues than to the room only cues may have confounded the difference in startle between these stimuli. However it is not uncommon to find greater startle to discrete threat cues than to contextual cues (e.g. Brasser & Spear (2004)). Future studies could include pleasantness ratings of the air-puff probes to see whether there are differences across individuals in how aversively these were experienced. Each 10-s trial for each room/cue condition was shown 24 times, resulting in 72 trials. Presentation order of the three room/cue conditions was randomised across participants.
Fig. 1.
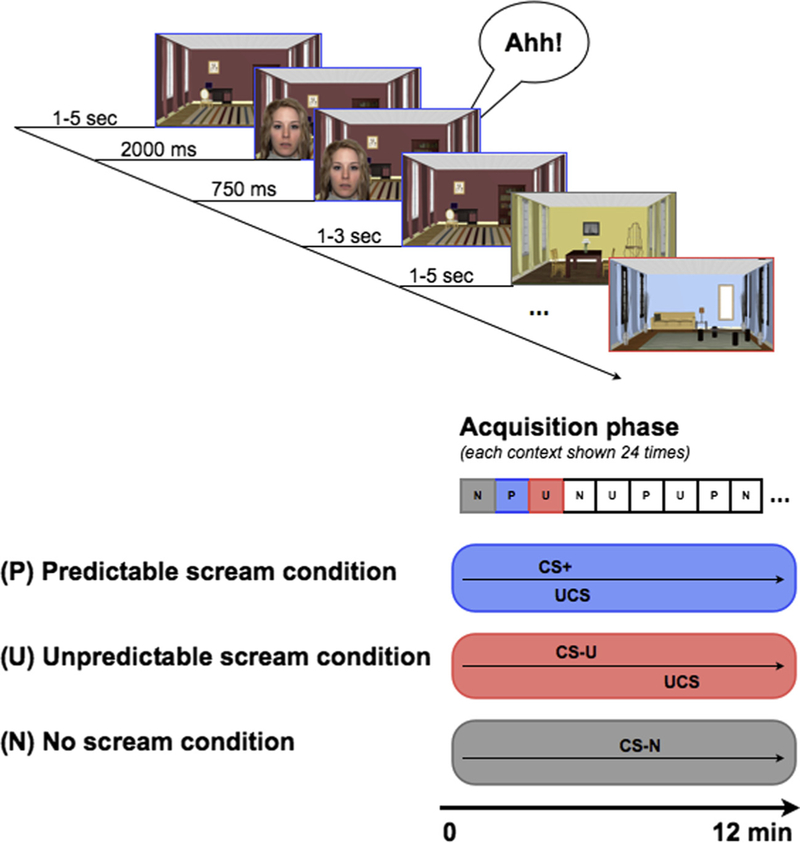
Experimental design used in the current study. Top panel: Time course of one sample trial showing the predictable scream condition. Bottom panel: Timing of CS+, UCS and CS− in the three contexts during the acquisition phase. Note that the three contingency conditions were shown in randomized order.
2.4. Procedure
All testing took place in a quiet room in the participant’s school. Prior to testing, the set-up and experimental procedure were explained in detail and informed assent was obtained. The EMG electrodes and the startle headpiece were then fitted, and the air-puff pressure was set at an appropriate level (see apparatus and materials). Next, three startle probes were delivered to habituate the startle reflex and to verify whether the air pressure was sufficiently strong to elicit a reliable blink response. The task consisted of a short practice phase, during which the participants experienced each of the three contexts once and heard the scream once, and an acquisition phase (72 trials).
2.5. Contingency awareness questions
Immediately following the acquisition phase, participants were shown a picture of the three rooms and asked the following questions: (1) Which room would you least want to go back into? (2) Which room had the least amount of screaming? Participants were then shown a picture of the three faces and asked the following questions: (1) Which person would you least like to see again? (2) Which person did the least amount of screaming? Participants’ room and face choices were recorded manually and entered into an Excel sheet for further analysis.
2.6. Data processing and analysis
For the EMG data pre-processing, the startle EMG was rectified and smoothed (20 ms moving window average). The onset latency window for the blink reflex was 20–100 ms following startle probe onset; blinks which began before or after this window were discarded (<10%), as these blinks were not deemed to represent genuine startle responses to the air puff probe. Excessively noisy trials were also discarded (<10%). Peak blink amplitude within 150 ms of startle probe onset was determined. The mean EMG level for the 50 ms preceding the onset latency window (i.e. baseline) was subtracted from the peak amplitude, generating the critical outcome measure for each trial. Raw EMG magnitudes were standardised using within-subject T-score conversions (see Figs. S1 and S2 for the mean startle response in all conditions, both t-scores (Fig. S1) and raw scores (Fig. S2)). Participants who had valid blinks for fewer than 75% of trials (n = 12) were excluded from the EMG analyses. For each participant, mean standardised blink amplitude was calculated for what we henceforth refer to as two ‘cue types’ in each contingency condition for the face startle (3 levels: ), and room only baseline startle (3 levels: Roomp, RoomU, RoomN).
3. Results
3.1. Psychophysiological responses
3.1.1. Fear responses differ for the three contingency conditions
In a first analysis, mean EMG startle amplitudes for each room and face in the three contingency conditions were subjected to a 3 2 Analysis of Variance (ANOVA), with contingency condition (3 levels: Predictable, Unpredictable, No-scream) and cue type (2 levels: face, room only (henceforth: room)) as the within-subject factors. All F-values are Huynh-Feldt corrected and all t-tests are two-tailed.
We found that both main effects for contingency condition and cue type were significant [Main effect of contingency condition: F(2, 84) = 9.14, p < .001, ᶯp2 = .171; Main effect of Cue type: (F(1, 42) = 13.32, p < .001, ᶯp2 = .024)]. The interaction between the two factors was also significant (F(2, 84) = 7.32, p < .001, ᶯp2 = .148) (Table 2) (Fig. 2). Further analysis established that this effect was driven by higher startle responses to the face cue in comparison to the room only in the P context and in the N context , whereas the face cue and the room only did not differ in the U context . We also found that fear responses to the face cue differed across the three contingency conditions (F(2, 84) = 14.05, p < .001, gp2 = .251), with and CS+P = CSN (t(42) = .562, p = .577, 95%CI [ 2.27, 4.15]) and and . Finally, fear responses to the three room-only cues did not differ (F(2, 84) = 2.60, p = .080, ᶯp2 = .058).
Table 2.
Acquisition phase: analysis of variance for the factors Contingency condition Cue type (collapsed across groups).
| Effect | Acquisition |
|---|---|
| Contingency condition | F(2,84) = 9.14, p < .001, ƞp2 = .171 |
| Cue | F(1,42) = 13.32, p < .001, ƞp2 = .024 |
| Contingency condition × cue | F(2,84) = 7.32, p < .001, ƞp2 = .148 |
Fig. 2.
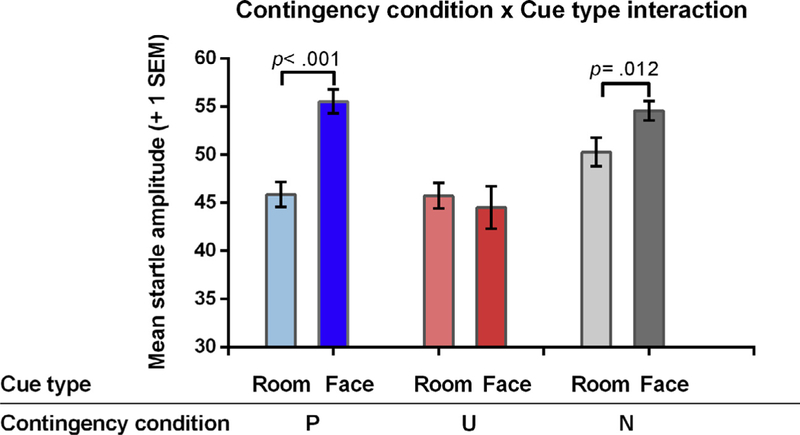
EMG startle responses show a significant contingency type × cue type interaction during the acquisition phase. Note that startle responses are collapsed across the two groups. Error bars depict 1 standard error of the mean.
3.1.2. Group differences in startle response in the three contingency conditions
Mean EMG startle amplitudes for each room and face in the three contingency conditions were subjected to a 3×2×2 Analysis of covariance (ANCOVA), with contingency condition (3 levels: Predictable, Unpredictable, No-scream) and cue type (2 levels: face, room) as the within-subject factors and anxiety group (2 levels: LA, HA) as the between-subject factor. Gender was included as a covariate in the analysis.
As with our first set of analysis, the 2-way interaction between contingency and cue-type emerged as significant. In addition, we found a main effect of anxiety group (see Table 3 for all effects and Fig. S1 for the mean startle response in all conditions for each group), that is, HA individuals showed greater startle responses overall than LA individuals. While the 3-way contingency condition cue type anxiety group interaction was not significant (F(2, 80) = 1.86, p = .162, ᶯp2 = .045), there was a significant cue type anxiety group interaction (F(2, 80) = 5.10, p = .029, ᶯp2 = .192) and a contingency condition anxiety group interaction (F(2, 80) = 5.22, p = .007, ᶯp2 = .178).
Table 3.
Acquisition phase: analysis of covariance (ANCOVA) for the factors Contingency condition Cue type Group + Gender Covariate.
| Effect | Acquisition |
|---|---|
| Contingency condition | F(2, 80) = 9.45, p < .001, ƞp2 = .191 |
| Group | F(1, 40) = 4.96, p = .032, ƞp2 = .061 |
| Cue | F(1, 40) = 0.85, p = .363, ƞp2 = .021 |
| Contingency condition × group | F(2,80) = 8.65, p < .001, ƞp2 =.178 |
| Cue × group | F(2,80) = 5.10, p = .029, ƞp2 =.192 |
| Contingency condition × cue | F(2,80) = 5.22, p = .007, ƞp2 =.113 |
| Contingency condition × cue × group | F(2,80) = 1.86, p = .162, ƞp2 = .045 |
To decompose the cue type anxiety group interaction, we compared the startle responses to the different cues (face, room) within each group (HA, LA) separately. This analysis showed that the LA group discriminated between the face (the CS) and the room only (t(23) = 4.40, p < .001, 95%CI [ 8.41, 3.83]) whereas the HA group did not (t(18) = 1.19, p = .250, 95%CI [ 6.66, 1.85]) (Fig. 3). Note that this analysis is collapsed across contingency conditions. In the LA group, participants exhibited stronger startle responses to the face cues (53 t-score) than to the room-only probes (47 t-score). Looking at anxiety-group differences to faces and rooms separately, we did not find any significant differences in startle response between the two groups to either face cues: (t(41) = 1.80, p = .079, 95%CI [ .358, 6.26]; or room cues: t(41) = .397, p = .693, 95%CI [ 2.19, 1.47]).
Fig. 3.
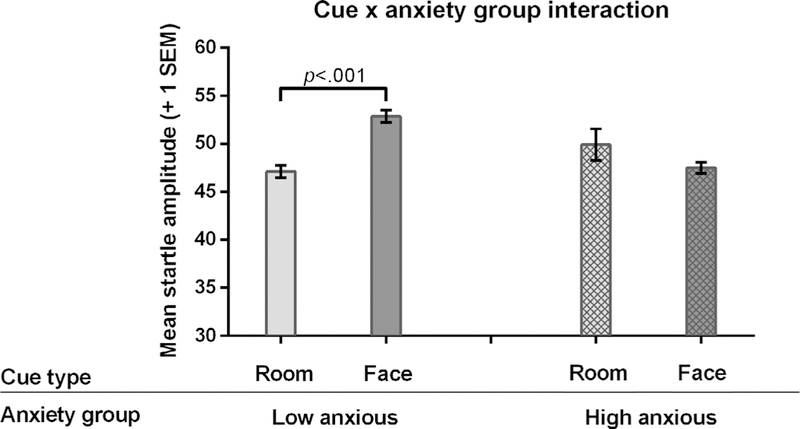
EMG startle responses show a significant cue type × anxiety group interaction during the acquisition phase. Note that startle responses are collapsed across the three contingency conditions. Error bars depict 1 standard error of the mean. Abbreviations: HA = high anxious group; LA = low anxious group.
To decompose the contingency condition anxiety group interaction, we compared the startle responses in the different contingency conditions (P, U, N) separately within each group (HA, LA) (see Fig. 4). Note that this analysis collapsed across cue type (i.e., faces vs. room only probes). The LA group exhibited similar startle responses in the three contingency conditions, i.e., the simple main effect of contingency condition was not significant in the LA group (F(2, 80) = 0.31, p = .735, 95%CI [ 5.98, 2.69]); how-ever the simple main effect of contingency condition was significant in the HA participants (F(2, 80) = 19.4, p < .001, 95%CI [ 15.4, 4.7]). Interestingly and in contrast to previous adult data (e.g., Haddad et al., 2012), this effect was primarily driven by a reduction in startle to the unpredictable condition (collapsed across face and room only), compared to the predictable condition (t(36) = 4.36, p < .001, 95%CI [5.70, 15.45]) and the no-scream condition (t(36) = 5.63, p < .001, 95%CI [ 20.34, 9.5]) (Fig. 4). Examining anxiety-group differences for each contingency condition separately, HA adolescents exhibited greater startle responses to the N condition (t(41) = 2.19, p = .034, 95%CI [ 7.79, .316]) and lower responses to the U condition relative to LA adolescents (t(41) = 4.06, p < .001, 95%CI [4.51, 13.43]).
Fig. 4.
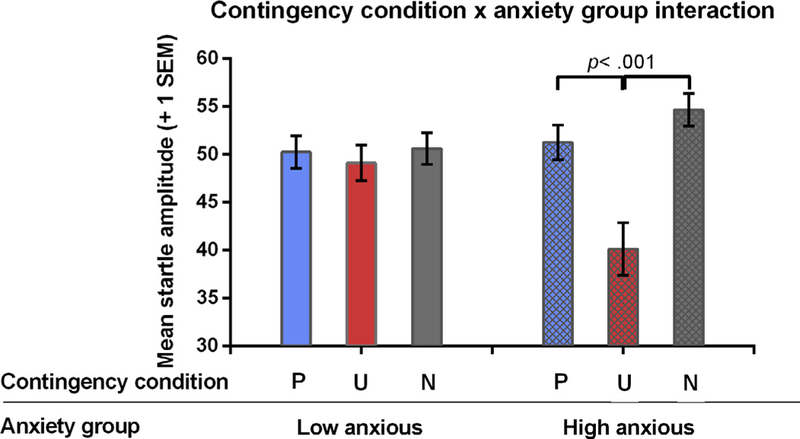
EMG startle responses show a significant contingency condition × anxiety group interaction during the acquisition phase. Note that startle responses are collapsed across cue type. Error bars depict 1 standard error of the mean. Abbreviations: HA = high anxious group; LA = low anxious group.
3.2. Contingency awareness questions
We used a Pearson Chi-square analysis to assess whether there were systematic differences in the response to the contingency awareness questions in both groups. Groups did not differ in how much they reported wanting to avoid rooms or faces across the three contingency conditions. However, compared to the HA group, the LA group was more aware that there were fewest screams in the no scream context (X2 (2, N = 42) = 5.79, p = .055) (see Table 4 for all results).
Table 4.
Pearson Chi-square analyses of the contingency awareness results. (HA = high anxious group; LA = low anxious group, N = no scream condition, P = predictable scream condition, U = unpredictable scream condition).
| Question | Group | P | U | N | Pearson c2 (2, N = 42) |
|---|---|---|---|---|---|
| Room cue | |||||
| Avoid | LA | 10 | 6 | 8 | =.307, p = .858 |
| HA | 6 | 5 | 7 | ||
| Least screams | LA | 2 | 5 | 17 | =5.79, p = .055 |
| HA | 7 | 2 | 9 | ||
| Face cue | |||||
| Avoid | LA | 9 | 6 | 9 | =.445, p = .801 |
| HA | 5 | 5 | 8 | ||
| Least screams | LA | 6 | 3 | 15 | =.700, p = .705 |
| HA | 4 | 4 | 10 | ||
4. Discussion
In the current study, we investigated fear conditioning to cues and contexts under predictable and unpredictable contingency conditions, using startle responses to CSs that were either paired or not paired with a UCS as indices of cued fear, and startle responses to rooms only as our measure of context fear. We predicted that combining a cued-conditioning paradigm with a con-textual contingency component would elicit differential fear responses in all participants. Associative learning theory predicts that conditioning to CSs should be stronger in contexts in which the CS is predictive of the UCS (e.g., Rescorla & Wagner, 1972). On the basis of this prediction and previous work with non-human animals and adult humans (for a review see Bouton, 2002), we predicted that across groups, participants would exhibit increased startle responses to the screaming face cue in the P contingency condition, intermediate startle responses in the U contingency condition, and lowest startle responses for the N contingency condition. These predictions were only partially sup-ported by our data. That is, while we did find increased fear-potentiated eye-blink startle responses for the screaming face in the P contingency condition and lower startle responses in the unpredictable contingency condition, face-cue dependent responses in the N contingency condition remained significantly increased (in comparison to the U contingency condition). These data suggest that while our sample of adolescents learned to fear a ‘predictable’ face-cue (i.e. the P contingency condition), they did not learn to fear a CS that was presented in a ‘safe’ context i.e. the N contingency condition.
However these findings were moderated by trait anxiety. In our main analysis, we addressed the question of how individual differences in trait anxiety modulated fear startle responses to the discrete face cues and the room context during development under different contingency conditions. Based on prior fear conditioning and stimulus generalisation data in high and low anxious adults, we predicted that HA adolescents would show increased fear responses across all the discrete cues (CS+ and both CS‒ faces) but particularly the CS‒ faces than LA adolescents. We also hypothesised that HA adolescents would exhibit higher contextual fear, and based on adult data, we tentatively predicted that HA adolescents would show increased contextual room fear in the unpredictable condition. Finally, we explored to what extent trait anxiety levels a priori determined contingency awareness during cue and context learning.
Our startle results showed a number of different patterns of fear responding in HA vs. LA adolescents. First there was a main group effect, which suggested HA showed greater fear responses than LA adolescents. This is perhaps not surprising seeing as anxiety is characterised by greater general fear. This result acts as supporting evidence for the validity of the paradigm to evoke relevant features of anxiety disorders in a controlled experimental setting. Second, while LA adolescents showed greater fear to the face cues than the room-only cues, HA adolescents displayed a non-discriminatory startle response to these cues. The non-significant 3-way interaction meant that this inability to differentiate occurred across all three contingency conditions. By contrast, our results showed that HA adolescents were instead more sensitive to the different contingency conditions – showing a greater fear to both cues in the predictable and no-scream contingency conditions relative to the unpredictable contingency conditions. Finally, we also found that HA adolescents were less aware that there were fewest screams in the N condition than their LA counterparts.
Our findings suggest that low-anxious participants were able to recognise the ‘‘belongingness’’ between the discrete faces and the UCS (but not the room contexts and the UCS), focusing fear responding on the stimuli that had higher belongingness with the scream. The concept of ‘belongingness’, which is sometimes also referred to as ‘preparedness’ (Hamm, Vaitl, & Lang, 1989; Seligman, 1971), refers to the finding that specific contingency associations between cues and aversive stimuli are more easily learned than others. In this case, LA participants may have reasonably expected that a face was more likely to start screaming than an empty room (because of previous experiences for example) thus giving rise to the increased fear of all the face cues compared to the rooms only. In contrast, high anxious adolescents generalised their fear across the two cue types (faces and rooms) by exhibiting an indiscriminate fear response between them. Such a pattern of results may also be consistent with stimulus generalisation (across discrete cues and context cues) seen in the HA group. Whereas previous research has shown that anxiety disorders are characterised by overgeneralisation of fear responses from threat to safety cues (Lissek et al., 2010), here we show that in high anxious adolescents, this fear to the threat cue may even generalise to diffuse non-specific room only baseline cues (i.e. the wider context in which the UCS appears).
Interestingly, this generalised and non-differential fear response to the face and room cues amongst HA adolescents did appear to vary across the three contingency conditions in unexpected ways. When the scream was predictable from the presence of the face cue, stronger startle responses emerged to both face and room, compared to when the scream was unpredictable. It may be that the HA adolescents had correctly learned that the face cue and scream were consistently paired in this predictable condition – and therefore generalised their fear to the face cue as well as the room cue. This finding suggests a fundamental disruption in the normal competitive process between learning about cues and contexts on the basis of predictiveness (e.g., Msetfi, Murphy, Simpson, & Kornbrot, 2005; Rescorla & Wagner, 1972).
The somewhat surprising finding was that HA adolescents showed significantly lower startle responses in the unpredictable context. This contrasts with the small number of adult studies which have reported potentiated startle in unpredictable contexts compared to predictable contexts (Grillon et al., 2006), and enhanced contextual fear under unpredictable circumstances in high anxious individuals in particular (Baas, 2012). Our results may be driven by anxious adolescents engaging in cognitive avoidance of the unpredictable condition, which superficially reduces the fear response in this context. Alternatively, enhanced vigilance in typical adults can sometimes also inhibit the startle reflex. Specifically, Löw and colleagues investigated changes in emotional arousal during the anticipation of potentially aversive events (Low, Lang, Smith, & Bradley, 2008) and found that immediately prior to aversive events, startle responses decreased, an effect that the authors suggested runs in line with the defense cascade model (Lang, Bradley, & Cuthbert, 1997). With regards to the substantial neural and cognitive development that occurs during adolescence, such a response pattern in high anxious individuals may well help shape the use of behavioural or cognitive strategies in a way that differs tos that low anxious adolescents (Blakemore, 2008; Burnett et al., 2011; Cohen Kadosh, Heathcote, & Lau, 2014; Cohen Kadosh, Linden, & Lau, 2013). If this result reflects an important developmental stage, tracking the emergence of anxiety responses using discrete cues and contexts may provide a unique window on the development of learning mechanisms.
Our results also indicate that startle responses in HA adolescents were significantly increased in the N contingency condition in comparison to the U contingency condition, despite the fact that no screams occurred in this context. It may be that neutral stimuli are viewed as more threatening in anxious samples – this would be consistent with other findings in the literature showing that neutral faces are experienced as more fearful in anxious than non-anxious individuals (Yoon & Zinbarg, 2008). Moreover, the contingency learning data found that fewer of our HA participants knew that the N condition was associated with fewer screams.
While our findings contrast somewhat with those of adults, in which anxious individuals show greater, generalised contextual fear under conditions of unpredictability, they nonetheless add to an important area of research. That is, our results seem to suggest that the generality of the conditioning account needs to be further differentiated to accommodate differences in the selectivity of associative learning for the two groups. That is, our LA group showed relatively flat discrimination across contingency conditions but could discriminate between face and room cues. Our HA group, however, were unable to differentiate between the face and room cues but were sensitive to contingency differences. It remains to be determined whether the observed differential learning pattern across trait anxiety is specific for adolescence as a developmental period. Alternatively the more surprising aspects of findings, namely the observed group differences may reflect specific design intricacies. We also note that the slightly skewed gender distribution in our sample (which is not representative of high anxiety samples in general) might have affected the results further and this should be addressed in future studies.
Few studies to date have investigated anxiety-group differences in fear conditioning during development (Craske et al., 2008; Lau et al., 2008, 2011; Liberman, Lipp, Spence, & March, 2006; Waters, Neumann, Henry, Craske, & Ornitz, 2008), and all of these studies have used discrete cued conditioning, a paradigm that is best suited for explaining transient fear states in both anxious and low-anxious individuals. The present study extends these previous findings by using a context conditioning paradigm with adolescents. This novel combination not only contributes to a better understanding of the developmental trajectory of anxiety in gen-eral, but also specifically to our understanding of more generalised and sustained fear responses.
5. Conclusions
The current study investigated anxiety-associated differences in threat-learning in different context conditions to gain a better understanding of how sustained anxiety develops in adolescents, a key developmental stage for the onset of anxiety disorders. We were particularly interested in investigating how individual differences shape fear-learning in a sample of adolescents. We were able to show several differences between the HA and LA adolescents: HA adolescents failed to discriminate between faces and room cues (generalising fear from one to the other) – but were sensitive to differences across contingency conditions albeit in an unexpected way. This paradigm has not yet been conducted in high and low anxious adults. Future research would need to verify whether these anxiety group differences also occur in adults to confirm whether the reported group differences here are genuinely developmental differences in the nature of how anxiety is expressed in fear learning – or in fact are differences associated with the particular paradigm used. Nonetheless, gaining a better understanding of how anxiety levels influence learning during development is important, because this will have a knock-on effect on behaviour downstream, particularly in the acquisition of stable behavioural response patterns.
Supplementary Material
Acknowledgements
This work was supported by a Economic and Social Research Council grant to Jennifer Y F Lau (ES/I032959/1). The authors would also like to thank Stasya Ng, Tom Sanders, and Sophie Williams for help with participant recruitment and testing.
Footnotes
Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development.
References
- Baas JM (2012). Individual differences in predicting aversive events and modulating contextual anxiety in a context and cue conditioning paradigm. Biological Psychology 10.1016/j.biopsycho.2012.02.001. [DOI] [PubMed]
- Beddington J, Cooper CL, Field J, Goswami U, Huppert FA, Jenkins R, et al. (2008). The mental wealth of nations. Nature, 455, 1057–1060. [DOI] [PubMed] [Google Scholar]
- Blakemore S-J (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9, 267–277. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, & van Boxtel A (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42(1), 1–15. doi:PSYP271 [pii] 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry, 52, 976–986. [DOI] [PubMed] [Google Scholar]
- Brasser SM, & Spear NE (2004). Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiology of Learning and Memory, 81, 46–59. [DOI] [PubMed] [Google Scholar]
- Burnett S, Sebastian C, Cohen Kadosh K., & Blakemore S-J (2011). The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies Neuroscience and Biobehavioral Reviews, 35(8). 1654–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Heathcote LC, & Lau JY (2014). Age-related changes in attentional control across adolescence: How does this impact emotion regulation capacities? Frontiers in Psychology [DOI] [PMC free article] [PubMed]
- Cohen Kadosh K., Linden DEJ, & Lau JY (2013). Plasticity during childhood and adolescence: Innovative approaches to investigating neurocognitive development Developmental Science, 16(4), 574–583. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Lindsey Bergman R., Naliboff B, Lipp OV, Negoro H, et al. (2008). Is aversive learning a marker of risk for anxiety disorders in children? Behaviour Research and Therapy, 46(8), 954–967. 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, & LaBar KS (2009). Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory, 16(7), 460–469. 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AP, & Storksen-Coulson H (2007). The interaction of pathways to fear in childhood anxiety: A preliminary study. Behaviour Research and Therapy, 45(12), 3051–3059. 10.1016/j.brat.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Moffitt TE, Koenen K, Eley TC, & Poulton R (2007). Juvenile mental health histories of adults with anxiety disorders. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. American Journal of Psychiatry, 164(2), 301–308. 10.1176/appi.ajp.164.2.301. [DOI] [PubMed] [Google Scholar]
- Grillon C (2002). Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry, 52, 958–975. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, & Johnson L (2006). Context conditioning and behavioral avoidance in a virtual reality environment: Effect of predictability Biological Psychiatry, 60(7), 752–759. 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Haddad ADM, Pritchett D, Lissek S, & Lau JYF (2012). Trait anxiety and fear responses to safety cues: Stimulus generalization or sensitization? Journal of Psychopathology and Behavioural Assessment, 34, 323–331. [Google Scholar]
- Hamm AO, Vaitl D, & Lang PJ (1989). Fear conditioning, meaning, and belongingness: A selective association analysis. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Journal of Abnormal Psychology, 98(4), 395–406. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert B (1997). Motivated attention: Affect, activation and action. In Lang PJ, Simons RF, & Balaban MT (Eds.), Attention and orienting: Sensory and motivational processes (pp. 97–135). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, et al. (2011).Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences, 108(11), 4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, et al. (2008). Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry, 47(1), 94–102. 10.1097/chi.0b01e31815a5f01S0890-8567(09)62089-X[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LC, Lipp OV, Spence SH, & March S (2006). Evidence for retarded extinction of aversive learning in anxious children. [Research Support, Non-U.S. Gov’t]. Behaviour Research and Therapy, 44(10), 1491–1502. 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, et al. (2008). Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy, 46(5), 678–687. 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. (2005). Classical fear conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy, 43(11), 1391–1424. 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, et al. (2010). Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry, 167(1), 47–55. 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, et al. (2009). Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy, 47(2), 111–118. 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A, Lang PJ, Smith JC, & Bradley MM (2008). Both predator and prey: Emotional arousal in threat and reward. [Research Support, N.I.H., Extramural]. Psychological Science, 19(9), 865–873. 10.1111/j.1467s-9280.2008.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msetfi RM, Murphy RA, Simpson J, & Kornbrot DE (2005). Depressive realism and outcome density bias in contingency judgments: The effect of the context and intertrial interval. Journal of Experimental Psychology: General, 134(1), 10–22. 10.1037/0096-3445.134.1.10. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, & Jonsson B (2012). The economic cost of brain disorders in Europe. [Research Support, Non-U.S. Gov’t]. European Journal of Neurology, 19(1), 155–162. 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Black AH & Prokasy WF (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York: Appleton-Century-Crofts. [Google Scholar]
- Seligman MEP (1971). Phobias and preparedness. Behavior Therapy, 2(3), 307–320. [Google Scholar]
- <B/>Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1970). State-Train Anxiety Inventory: Mind Garden Inc. 855 Oak Grove Ave., Suite 215, Menlo Park, CA: 94025. [Google Scholar]
- Waters AM, Henry J, & Neumann DL (2009). Aversive Pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. [Research Support, Non-U.S. Gov’t]. Journal of Abnormal Psychology, 118(2), 311–321. 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- Waters AM, Neumann DL, Henry J, Craske MG, & Ornitz EM (2008). Baseline and affective startle modulation by angry and neutral faces in 4–8-year-old anxious and non-anxious children. [Comparative Study]. Biological Psychology, 78(1), 10–19. 10.1016/j.biopsycho.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Yoon KL, & Zinbarg RE (2008). Interpreting neutral faces as threatening is a default mode for socially anxious individuals. Journal of Abnormal Psychology, 117(3), 680–685. 10.1037/0021-843X.117.3.680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


