Abstract
The tendency to engage in impulsive behavior in the context of negative affect, known as negative urgency, has emerged as a powerful transdiagnostic predictor of behavioral dysregulation. Although general vulnerability to negative affect (neuroticism) correlates with negative urgency, not all neurotic individuals engage in urgent behavior. Given prior experimental evidence that sympathetic nervous system (SNS) activation may promote emotion-related impulsivity, the present study examines tonic SNS activity as a moderator of the link between neuroticism and negative urgency. Participants (N = 194) completed measures of neuroticism and negative urgency, as well as a stress task. They also underwent assessment of tonic SNS activity (cardiac pre-ejection period). The link between neuroticism and negative urgency was strengthened for individuals with higher tonic SNS activity; however, this was not the case for behavioral performance on the task. A similar pattern was demonstrated for hostile reactivity to the stress task; increased hostile response partially explained the interaction between SNS activation and neuroticism on negative urgency. These findings suggest a potential facilitative role of the SNS in hostile reactivity and emotion-driven impulsivity among more neurotic individuals.
Keywords: Negative Urgency, Neuroticism, Impulsivity, Anger, Shame, Pre-Ejection Period
1. Introduction
The tendency to behave impulsively when distressed, known as negative urgency (Cyders and Smith, 2008; Whiteside and Lynam, 2001), has emerged as a strong transdiagnostic risk factor for a broad range of maladaptive behaviors often exhibited by psychiatric patients (Dir et al., 2013). Examples include reactive aggression and intimate partner violence (Derefinko et al., 2011), substance abuse (Fischer et al., 2012; Kaiser et al., 2012), risky sexual behavior (Deckman and Nathan DeWall, 2011; Derefinko et al., 2014), non-suicidal self-injury (Bresin et al., 2012; Peterson et al., 2014), suicidal behavior (Anestis and Joiner, 2011), and binge eating and purging (Fischer et al., 2013; Wenzel et al., 2014). While often conceptualized as a personality trait and used as a predictor in statistical models, negative urgency has increasingly been recognized as a potential modifiable mechanistic variable of interest for interventions targeting these disorders, with evidence that negative urgency decreases over the course of a range of interventions for individuals with substance use problems, disordered eating, and emotion regulation difficulties (e.g., Delgado-Rico et al., 2012; Littlefield et al., 2015; Zapolski et al., 2016). Clarifying the physiological and psychological attributes that characterize negative urgency may help identify both individuals at greater risk and potential targets for intervention development.
Among the broader population of individuals predisposed to experiencing high levels of negative affect (or neuroticism), only some also report high levels of emotion-driven impulsivity (Cyders and Coskunpinar, 2010). Although more frequent experience of negative affect is a risk factor for negative affect-related impulsivity, impulsive behavior is only one of a wide range of potential responses to difficult emotions (Gratz and Roemer, 2004). Little is known about the factors that may influence which neurotic individuals display this impulsive behavior in the context of distress and therefore are at increased risk for the wide range of negative-urgency related problems. Given experimental evidence that the sympathetic branch (SNS) of the autonomic nervous system plays a causal role in the connection between negative affect and emotion-related impulsive behavior (reviewed below), the present study examined whether elevated resting SNS activity strengthens the association between neuroticism and emotion-driven impulsivity, measured both by self-report and impulsive behavior during a stressful task. Given the behavioral and pharmacological interventions available that target elevated SNS activation (e.g., biofeedback, beta-blockers), understanding the role of SNS activation in negative urgency could have numerous clinical implications.
1.1. Pathophysiology of Negative Urgency: A Role for the Sympathetic Nervous System?
Experimental work points to the potential for heightened SNS activation to facilitate emotion-driven impulsive behavior. SNS input at beta-adrenergic receptors increases amygdala reactivity (Hurlemann et al., 2010; van Stegeren et al., 2005) and is associated with poorer executive cognitive functioning in response to stress (Ramos and Arnsten, 2007). Similarly, beta-adrenergic receptor blockade, which reduces SNS activity, attenuates amygdala reactivity to emotional stimuli (Hurlemann et al., 2010) and improves executive cognitive functioning (e.g., working memory, switching) in stressful circumstances (Alexander et al., 2007; Roozendaal et al., 2004). At the behavioral level, alpha adrenergic blockade reduces or eliminates stress-induced alcohol- and food-seeking in rats (Lê et al., 2011), and multiple randomized controlled trials indicate that beta adrenergic blockade reduces aggression in humans (Goedhard et al., 2006; Haspel, 2009). In sum, the experimental evidence suggests a potential facilitative role of heightened SNS activity in the pathophysiology of emotion-driven impulsivity.
Observational studies also suggest that SNS activity may be altered in clinical syndromes characterized by emotional impulsivity. Studies of individuals with borderline personality disorder, a psychiatric disorder characterized primarily by very high levels of neuroticism (Mullins-Sweatt et al., 2012) and impulsive behavior in response to negative emotion (Chapman et al., 2010; Peters et al., 2013), generally find greater tonic sympathetic activity (measured using cardiac pre-ejection period (PEP), a systolic time interval highly correlated with cardiac SNS innervation) in BPD patients compared to healthy controls (Ebner-Priemer et al., 2007; Kuo and Linehan, 2009; Weinberg et al., 2009). Taken together, findings suggest higher SNS arousal may contribute to emotion-driven impulsivity and associated syndromes.
In contrast, a number of studies have found seemingly opposite relations between SNS and generalized disinhibition, with suppressed baseline SNS characteristic of individuals with higher scores on unidimensional measures of impulsivity (Maniaci et al., 2017; Muñoz and Anastassiou-Hadjicharalambous, 2011), more errors on passive avoidance tasks (Muñoz and Anastassiou-Hadjicharalambous, 2011), and externalizing diagnoses in childhood (Beauchaine et al., 2001; Crowell et al., 2006), although findings are not consistent (Beauchaine et al., 2015; Karalunas et al., 2014; Musser et al., 2011). Several factors could explain these seemingly divergent findings. First, impulsivity is increasingly recognized as a multidimensional construct (Whiteside and Lynam, 2001), and suppressed tonic SNS findings may reflect forms of impulsivity not driven by emotion, such as a broader tendency toward disinhibited action. Indeed, the aforementioned studies use measures of impulsive behavior (both self-report and behavior) not linked to distress. Second, heightened SNS activation may only translate into greater emotion-driven impulsivity among individuals who tend to experience more intense and difficult to control negative affect, suggesting the need to consider trait-level moderators such as neuroticism.
1.2. The Current Study
Despite the evidence linking urgency-related phenotypes to SNS functioning, no study to date has directly examined associations of negative urgency with tonic SNS functioning. We sought to clarify the role of tonic SNS in facilitating the association between neuroticism and negative urgency, including examining the potential mechanism of hostile reactivity to stressors. Higher tonic SNS activation was hypothesized to moderate the association of neuroticism with negative urgency, as well as with impulsive behavior in the context of a distressing behavioral task. These interactive effects were also examined on affective reactivity to the behavioral task, given the potential for differences in affective reactivity to relate to differences in emotion-driven impulsivity.
2. Method
2.1. Participants
Participants were 203 introductory psychology students who completed the study in partial fulfillment of course requirements and $80 payment. Due to missing or unusable physiological data for several participants, the final sample N was 194. Participants’ ages ranged from 18–23 (M = 18.49 years, SD = 0.72), and 51% were female. The racial and ethnic characteristics were as follows: 78% White/Caucasian; 13% Black/African-American; 3% Asian; 3% Latinx; 2% Biracial; and 1% Other. “High risk” participants were over-recruited to ensure sufficient variability in impulsive behavior within this sample. Students completed a screening questionnaire during a mass testing early in the semester. This measure assessed conduct problem behaviors prior to age 18, such as stealing, lying, and fighting. Those whose scores fell within the top 25% for their gender were specifically invited to participate through email; in addition, the study was listed on a website accessible to the general research pool.
2.2. Procedure
All study procedures were approved by the local institutional review board. The current study was part of a larger longitudinal project designed to assess multiple predictors of impulsive behavior in young adults; the data for the present study are taken from the baseline visit. Other studies have been published using data from this project (Derefinko et al., 2016a; 2016b; Roberts et al., 2014); none include the PASAT-C task or examine the hypotheses in question. At the beginning of the session, participants were fitted with electrodes for the collection of impedance cardiography data, which were recorded using a custom bioamplifier from Mindware Technologies (Bionex Model 50–371100-00; Gahanna, OH). The signal was digitized with Mindware BioLab software at a sampling rate of 1 kHz. During the first session, participants completed a two-minute physiological baseline (a measure of tonic levels of SNS activity) while sitting quietly. Just prior to this baseline period, participants were given the following instructions: “Right now we are going to gather a ‘snapshot’ of your baseline state before the task begins. I need you to sit quietly for approximately two minutes while I record your readings.” Following these instructions, participants looked at a blank computer screen with a simple black-and-white fixation point for two minutes. Physiological measurements were also obtained during subsequent tasks (Derefinko et al., 2016b) not included in the present study. Next, participants completed personality measures, followed by a standardized laboratory stress task (see below).
2.3. Pre-ejection Period (PEP).
SNS influences on the heart were quantified using impedance cardiography. PEP was quantified as the period of time in milliseconds between the onset of ventricular depolarization (Q-wave of ECG) and the opening of the aortic valve (B point of dZ/dt waveform (Lozano et al., 2007). PEP reflects myocardial contractility and is commonly used as an index of sympathetic cardiac control (Berntson et al., 2004). Lower PEP values (i.e., shorter cardiac latencies) indicate higher cardiac sympathetic activity. Tonic PEP was computed by taking the average level of PEP during the two-minute period.
2.4. Psychological Measures
2.4.1. Neuroticism.
The Five Factor Model Rating Form (Mullins-Sweatt, 2006) asks participants to rate themselves on the 30 facets of the Five Factor Model of personality. Individual facets were combined to construct the domain of Neuroticism. To avoid criterion contamination, the impulsivity item was excluded from the measure in the present study. The Neuroticism subscale (without the impulsivity item) demonstrated good internal consistency in the current sample (α = 0.86).
2.4.2. Emotion-Driven Impulsivity.
Emotion-related impulsivity was measured using the negative urgency subscale from the UPPS Impulsivity scale (Whiteside and Lynam, 2001). This questionnaire provides a dimensional measure of the tendency to engage in regretted action during or following experiences of negative emotion. Example items include, “It was hard for me to resist acting on my feelings” and “I often make matters worse because I act without thinking when I am upset”. Internal consistency in the present sample was good (α = 0.89).
2.5. Stress Reactivity.
The Paced Auditory Serial Addition Task – Computerized Version (PASAT-C; Lejuez et al., 2003) is a computerized serial addition task that has been validated and widely used as a standardized laboratory stress induction. The task involves the rapid addition of numbers, involving the recall of previously presented numbers no longer on the screen. Explosion sounds occur when participants make mistakes or fail to respond quickly. The task involves three levels. The first level is 115 seconds in duration and the second level is 175 seconds in duration, with presentation of task stimuli increasing in speed from the first to the second level. The third level occurs at the same speed as the second and lasts as long as 240 seconds, but participants are told they may quit the task whenever they choose. The time spent in the third, most stressful level of the PASAT prior to quitting is used in the present study as a behavioral measure of impulsive behavior (premature quitting), likely as an attempt to avoid further distress. In addition, the PASAT-C includes a pre- and post-task version of the Positive and Negative Affect Scale (10-item PANAS; Watson et al., 1988) that asks participants to rate the extent to which they are currently experiencing 10 different distress-related emotions using a visual analog scale. In the present study, we utilized the full Negative Affect score from the PANAS as a manipulation check to verify that the PASAT-C was indeed distressing. We also used pre-to-post change scores in the items “distressed”, “afraid”, “hostile”, and “ashamed” as affective outcomes, to examine outcomes across a range of specific, potentially relevant affective states.
3. Results
3.1. Data Screening
Data were analyzed using SPSS 24. Descriptive statistics for primary study variables are presented in Table 1. Screening for skewness and kurtosis revealed that all variables fell within normal limits (Tabachnick and Fidell, 2000) with the exception of PASAT-C quit time, which was heavily skewed. A log transformation was applied to this variable, which was a sufficient correction to normalize residuals in later regression analyses.
Table 1.
Means, Standard Deviations, and Zero-Order Correlations among Primary Study Variables (N =194)
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Female Sex | ||||||||||
| 2. Age | −.07 | |||||||||
| 3. Neuroticism | −.06 | .004 | ||||||||
| 4. Negative Urgency | .01 | −.04 | .47*** | |||||||
| 5. PASAT Quit Time+ | .16* | .04 | .09 | .02 | ||||||
| 6. Baseline PEP | −.13 | .14 | .02 | −.13 | .02 | |||||
| 7. Δ PANAS Distress | .08 | .02 | −.11 | .10 | .15* | −.01 | ||||
| 8. Δ PANAS Afraid | .12 | −.11 | .16* | .05 | .07 | −.07 | .04 | |||
| 9. Δ PANAS Hostile | −.04 | −.03 | .16* | .26*** | .03 | −.09 | .17* | .08 | ||
| 10. Δ PANAS Ashamed | −03 | −.03 | .07 | .06 | −.004 | .06 | .14* | −.05 | .12 | |
| M (%) | 51% | 18.49 | 2.30 | 26.32 | 234.64 | 32.54 | 1.21 | 0.14 | 0.51 | 0.36 |
| SD | - | 0.72 | 0.65 | 6.33 | 175.79 | 9.93 | 1.09 | 0.53 | 0.89 | 0.69 |
Note:
p < .05
p < .01
p < .001
PASAT = Paced Auditory Serial Addition Task. +Variable represents the time spent in level 3 of the PASAT-C with values inverted so that higher scores reflect greater impulsive behavior. PEP = Cardiac Pre-ejection Period; Lower PEP scores indicate higher levels of sympathetic nervous system activation. Δ = Change During the PASAT. PANAS = Positive and Negative Affect Schedule.
3.2. Manipulation Checks: Impact of PASAT on Affect
Next, manipulation checks examined whether the PASAT-C had functioned as a stressor, increasing subjective distress. Consistent with previous reports, paired sample t-tests revealed that the PASAT-C was associated with increased global self-reported negative affect on the PANAS (Mean Difference Pre-to-Post = 1.21, t(193) = 15.71, p < .001). This demonstrates that the PASAT-C functioned, as expected, as a subjectively distressing task.
3.3. Zero-Order Correlations
Table 1 presents descriptive statistics for and zero-order correlations between primary study variables. Female sex was associated with longer persistence on the PASAT-C and no other variables; accordingly, female sex was only included as a co-variate in subsequent models involving the PASAT-C1. Age was not associated with any variables and therefore was not included in any subsequent analyses. Neuroticism was positively correlated with Negative Urgency; neither was correlated with PASAT-C quit time. Tonic PEP was not significantly correlated with any variables.
Changes in PANAS affective items were generally uncorrelated with one another, with the exception that changes in the Distress item were positively correlated with changes in both the Hostility and Shame items. Neuroticism was associated with greater increases in Fear and Hostility during the PASAT, whereas Negative Urgency was only associated with greater increases in Hostility. PASAT-C quit time (reverse-scored so that higher scores represented earlier quitting/more impulsive behavior) was also associated with a greater increase in distress during the PASAT-C. PEP was not correlated with changes on PANAS affect items.
3.4. Interactive Effects of Neuroticism and SNS Activity on UPPS Impulsivity and PASAT-C Quit-Time
To test the primary study hypotheses, OLS hierarchical multiple regression models predicted Negative Urgency and quit-time on the PASAT-C from 1.) PEP (measuring SNS activity, with greater PEP indicating lower levels of SNS activation) and Neuroticism, and 2.) interactions of Neuroticism with PEP (Table 2). Models were estimated and probed using the Process (Hayes, 2013) macro in SPSS. PEP and Neuroticism were z-scored prior to use in models. To illustrate the nature of interactions, significant interactions between the continuous variables were probed for simple slope estimates at −1 SD and +1 SD of PEP.
Table 2.
Hierarchical Multiple Regression Models Predicting Negative Urgency and PASAT-C Quit Time from Neuroticism, Pre-Ejection Period, and their Interactions (N=194)
|
Outcome |
||||
|---|---|---|---|---|
| Parameter | Negative Urgency | PASAT Quit Time+ | ||
| B | B | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Step 1 | ||||
| Female Sex++ | -- | -- | 0.21** | 0.20** |
| Neuroticism (N) | 0.49*** | 0.99*** | 0.12 | 0.49 |
| Baseline PEP | −0.13* | −0.14* | 0.03 | 0.02 |
| Step 2 | ||||
| N × Baseline PEP | −0.52* | −0.38 | ||
| R2 Δ | 0.02* | 0.011 | ||
| Model R2 | 0.26*** | 0.28*** | 0.054* | 0.065* |
Note:
p < .05
p < .01
p < .001
PEP = Cardiac Pre-ejection Period; lower PEP scores indicate higher levels of sympathetic nervous system activation. PASAT = Paced Auditory Serial Addition Task.
Variable was created by log transforming the time spent in level 3 of the PASAT and inverting values so that higher scores reflect greater impulsive behavior.
To reduce unnecessary error in estimates, female sex was only included in models where it demonstrated significant zero-order correlations with other included variables; however, patterns of significant findings remained the same female sex was included in other models.
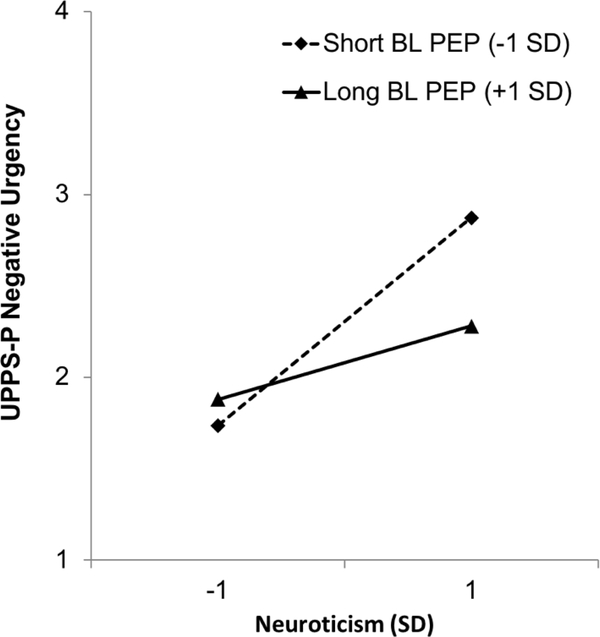
As hypothesized, PEP moderated the association between Neuroticism and Negative Urgency (Figure 1). Specifically, shorter tonic PEP (higher SNS activity) unmasked a strong positive association between Neuroticism and Negative Urgency (estimate for Neuroticism at −1 SD PEP = 0.66, t = 7.32, p < .001) that was less pronounced among those with longer tonic PEP (lower levels of SNS activity; estimate for Neuroticism at +1 SD PEP = 0.33, t = 3.66, p < .001). In sum, greater tonic sympathetic activity facilitated a relationship between Neuroticism and Negative Urgency, whereas Neuroticism was less robustly associated with Negative Urgency at lower levels of tonic sympathetic activity. The hypothesis that this pattern would extend to the behavioral outcome on the PASAT-C was not supported, with no significant interaction of Neuroticism with PEP predicting PASAT-C quit time (p = 0.13).
Figure 1.
Interaction of Neuroticism and Baseline Cardiac Pre-Ejection Period Predicting Negative Urgency (Interactions Between Continuous Variables Probed at −1/+1 SD).
Note: PEP = Pre-Ejection Period. Lower PEP scores indicate higher levels of sympathetic nervous system activation
3.5. Interactive Effects of Neuroticism and SNS Activity on Forms of Affective Reactivity
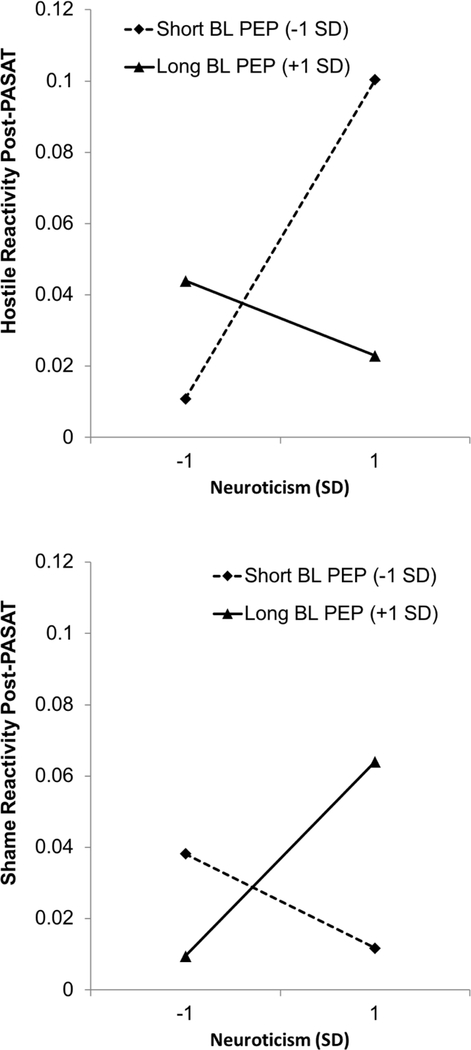
Models predicting each of the four forms of PASAT-C-related affective reactivity (Distress, Fear, Hostility, and Shame) can be found in Table 3. Models predicted post-task affect scores, controlling for pre-task affect in Step 1, to model task-based reactivity. Then similar to the previous tests, Step 2 added PEP and Neuroticism, and Step 3 the interaction of Neuroticism with PEP. Models revealed no significant interactive effects of Neuroticism and PEP on PASAT-C-related changes in distress or fear. However, PASAT-C-related changes in Hostility and Shame were predicted by the interaction of Neuroticism and PEP, in opposite directions (see Figure 2). Among individuals with shorter tonic PEP (higher SNS activity), Neuroticism was positively associated with Hostility reactivity during the PASAT-C (estimate for Neuroticism at −1 SD PEP = 0.31, t = 2.97, p = .003) but was not significantly associated with Shame reactivity (estimate for Neuroticism at −1 SD PEP = −0.05, t = −0.54, p = 0.59). However, among individuals with longer tonic PEP (lower levels of SNS activity), Neuroticism was positively associated with Shame reactivity during the PASAT-C (estimate for Neuroticism at +1 SD PEP = 0.25, t = 2.47, p = .02) and was not significantly associated with PASAT-C-related changes in Hostility (estimate for Neuroticism at +1 SD PEP = −0.01, t = −0.07, p = .95).
Table 3.
Hierarchical Multiple Regression Models Predicting Post PASAT-C Affect from Neuroticism, ANS Variables, and their Interactions, Controlling for Pre-Task Affect (N=194)
|
Outcome |
||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Post-Task Distress | Post-Task Fear | Post-Task Hostility | Post-Task Shame | ||||
| B | B | B | B | |||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Step 1 | ||||||||
| Baseline Affect | 0.35*** | 0.34*** | 0.36*** | 0.36*** | 0.28*** | 0.25** | 0.29*** | 0.28*** |
| Step 2 | ||||||||
| Neuroticism (N) | −0.30 | 0.29 | 0.16* | 0.53* | 0.14* | 0.65* | 0.10 | −0.39 |
| Baseline PEP | −0.03 | −0.03 | −0.08 | −0.08 | −0.08 | −0.09 | 0.05 | 0.06 |
| Step 3 | ||||||||
| N × Baseline PEP | −0.33 | −0.38 | −0.53* | −0.51* | ||||
| R2 Δ | 0.002 | 0.008 | 0.031* | 0.012 | 0.024 | 0.020* | 0.012 | 0.020* |
| Model R2 | 0.12*** | 0.13*** | 0.17*** | 0.18*** | 0.127*** | 0.148*** | 0.097*** | 0.12*** |
Note:
p = .05
p < .05
p < .01
p < .001.
PEP = Cardiac Pre-ejection Period; lower PEP scores indicate higher levels of sympathetic nervous system activation. PASAT = Paced Auditory Serial Addition Task. Model 1 = Steps 1 and 2, with change in R2 representing additional variance accounted for in Step 2; Model 2 = Steps 1, 2, and 3.
Figure 2.
Interaction of Neuroticism and Baseline Cardiac Pre-Ejection Period Predicting PASAT-Related Reactivity in Shame and Hostility (Interactions Between Continuous Variables Probed at −1/+1 SD).
Note: PEP = Pre-Ejection Period. Lower PEP scores indicate higher levels of sympathetic nervous system activation.
3.6. Post-hoc Mediated-Moderation of the Association Between Neuroticism and Negative Urgency by Affect and Sympathetic Activity
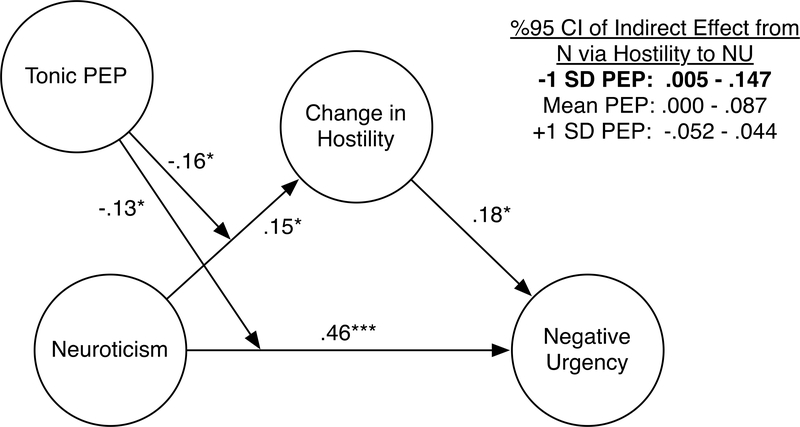
To examine the potential role of reactivity in Hostility and Shame as mechanism underlying the PEP-moderated association between Neuroticism and Negative Urgency, a post-hoc moderated-mediation model was estimated using Process (Hayes, 2013) producing 95% bias-corrected confidence intervals (CIs) with 5000 bootstrap samples. Indirect paths were modeled from Neuroticism to Negative Urgency via changes in Hostility, and PEP was entered as a moderator of 1.) the effect of Neuroticism on affect change and 2.) the direct effect of Neuroticism on Negative Urgency. Significant mediated-moderation was demonstrated from Neuroticism to Negative Urgency via increased Hostility, moderated by PEP (95% CI index of moderated mediation via Hostility = −0.088 to −0.001; see Figure 3). Moderation of the path from Neuroticism to Hostility by PEP was significant (B = −0.16, p = .019). Probing the indirect effect from Neuroticism to Negative Urgency via increased Hostility demonstrated a significant path at low levels of PEP (high levels of SNS activation; 95% CI for indirect effect at −1 SD PEP = 0.005 to 0.147), but not at mean (95% CI for indirect effect at mean PEP = −0.0003 to 0.087) or high levels of PEP (95% CI for indirect effect at +1 SD PEP = −0.052 to 0.044). PEP also moderated the direct path from Neuroticism to Negative Urgency (B = −0.13, p = .040), as well as a conditional effect of PEP on Negative Urgency (B = −0.13, p = .036). A similar model was examined substituting changes in Shame for Hostility, given the unexpected interaction found for Shame; however, no significant direct, indirect, or moderated effects were found. In sum, only for individuals with shorter PEP intervals at rest (higher tonic SNS activation), greater increases in Hostility accounted for part of the relationship between Neuroticism and Negative Urgency.
Figure 3.
Moderated Mediation Model Predicting Negative Urgency from Neuroticism via Changes in Hostility, Moderated by Pre-Ejection Period.
Note: Coefficient estimates are standardized. PEP = Pre-ejection Period. Lower PEP scores indicate higher levels of sympathetic nervous system activation.
4. Discussion
These findings add to a growing body of literature delineating the pathophysiology of emotion-driven impulsivity. As hypothesized, baseline PEP intervals moderated the association of neuroticism with self-reported negative urgency in the present sample. These findings suggest that for individuals broadly more prone to negative affect, those who also have higher levels of tonic SNS activation may be at greatest risk for impulsive behavior when upset or stressed. In a post-hoc test, these effects were accounted for in part by increased hostility in response to a stressful task, suggesting that greater susceptibility to angry affect in particular may play a role in how SNS activation facilitates vulnerability to affect-related impulsive behavior. This is consistent with previous research demonstrating that anger, unlike most forms of negative affect, is strongly linked to the behavioral approach system (Carver, 2004; Harmon-Jones, 2003) and approach-related neural activation (Harmon-Jones and Allen, 1998; Herrero et al., 2010). This potential emotion-driven increase in approach motivation could fuel the range of risky behaviors exemplified in negative urgency, such as reactive aggression (Derefinko et al., 2011), risky sexual behavior (Deckman and Nathan DeWall, 2011; Derefinko et al., 2014), and binge-eating (Fischer et al., 2013; Wenzel et al., 2014). While the present study was conducted in a non-clinical sample, this model is also consistent with clinical findings of heightened tonic or baseline SNS activation in BPD (Ebner-Priemer et al., 2007) and bulimia nervosa (Monteleone et al., 2011), disorders characterized by heightened neuroticism, anger reactivity (Engel et al., 2007; Gardner et al., 1991), and emotion-driven impulsivity (Claes et al., 2005; Peters et al., 2013). Given that this moderated-mediation model was conducted as a post-hoc test, further research specifically designed to examine this model further is warranted.
PEP moderated the association between neuroticism and increased elicitation of both hostility and shame during the PASAT-C, in opposite directions. For individuals with higher levels of neuroticism, lower baseline PEP (higher tonic SNS activity) was associated with greater reported increases in hostility following the task, whereas higher baseline PEP (lower tonic SNS) was related to greater increases in feeling ashamed. Anger-generating processes, such as anger rumination, have been shown to increase SNS activation (Ray et al., 2008); however, the present findings suggest that elevated tonic SNS activation may also facilitate hostile reactivity. Only increased hostile reactivity to the stressor was a significant factor in the larger moderated-mediation model predicting negative urgency; however, the higher neuroticism/lower SNS profile that may increase shame reactivity could in turn increase risk for other types of maladaptive behaviors and outcomes, perhaps including internalizing responses such as increases in avoidance and depressive symptoms.
Contrary to predictions, the interactive pattern of findings was not replicated for PASAT-C quit time. PASAT-C quit-time also did not demonstrate a significant association with negative urgency. It may capture a different aspect of impulsivity such as lack of perseverance, the tendency to give up on boring or difficult activities prematurely (Whiteside and Lynam, 2001), or may reflect low distress tolerance but not negative urgency per se. This may also be affected by the lack of contingencies used with the task, with no consequences applied to individuals who quit prematurely, which may not adequately model the rash or regrettable action component of negative urgency. Willingness to quit the task early might also reflect lower agreeableness (with agreeable people trying harder to persist with the requested task and disagreeable individuals demonstrating less concern for the research) for some individuals, rather than impulsivity or difficulty tolerating distress, especially given the over-recruitment for conduct problems for this sample. Future research should examine these interactive effects with other types of behavioral impulsivity tasks and across a broader range of samples. Findings to date across impulsivity research also generally demonstrate surprisingly low concordance between self-report and task-based measures (Cyders and Coskunpinar, 2012; 2011), so this null finding may reflect a common broader assessment issue. While the null findings for the PASAT-C warrants further investigation given that PASAT-C scores have demonstrated modest associations with psychopathology and real-world outcomes in a limited set of studies (Leyro et al., 2010), self-reported negative urgency is a more robust predictor of a wider range of real-world dysregulated behavior (Berg et al., 2015; Settles et al., 2012).
4.1. Does the Pathophysiology of Emotion-Related Impulsivity Differ from that of Non-Affective Impulsivity?
While preliminary and conducted in a non-clinical sample, the effects of tonic SNS activity on negative urgency in the present sample may help to clarify conflicting results in the literature regarding the role of the SNS in impulsivity. In contrast to the deleterious associations of higher tonic SNS activity in urgency-related phenotypes and facilitating the association between neuroticism and emotion-driven impulsivity in the present study, an extant body of literature suggests that lower tonic levels of SNS activity, as well as reduced SNS reactivity, characterizes clinical groups exhibiting non-affective impulsivity (i.e., lack of premeditation and sensation-seeking), such as attention deficit hyperactivity disorder, conduct disorder, and antisocial personality disorder (Beauchaine et al., 2013; Lorber, 2004). While on the surface, these forms of impulsive behavior can appear similar, they may involve different underlying biological alterations. Clearly differentiating between forms of impulsive behavior and symptoms in future research that utilizes clinical samples, as well as conducting studies clearly contrasting these forms of impulsive behavior, may clarify some of the divergent findings on physiology and impulsivity and related psychopathology.
4.2. Limitations
Several limitations of the present study should inform future work in this area. First, the cross-sectional nature of our sample precludes any conclusions about causal effects of the SNS on negative urgency. Experimental work examining the impact of beta adrenergic receptor blockade across various behavioral manifestations of negative urgency (e.g., aggression, self-injury, binge eating, substance abuse) would provide a clearer test of these hypotheses. Second, the present study utilized a student sample over-recruited for risky behavior; the results may not be fully generalizable to clinical samples. However, use of this sample allowed for examination of the constructs across a broader range than would be present in a purely clinical sample. Third, this sample was relatively small for the more complex models presented, with only 51% power to find some of these effects. Further work is needed to examine these models with more powerful designs. Also, only tonic SNS activation was assessed in the present study; further research should attempt to replicate these findings while adding assessment of SNS reactivity to various types of tasks and stimuli. Finally, the period of psychophysiological measurement was relatively short compared to other similar studies, lasting only two minutes; future research should examine physiological functioning over longer periods.
4.3. Conclusion
Among individuals with greater vulnerability to negative affect, those with generally elevated SNS arousal may be predisposed to hostile responses to stress and emotion-driven impulsive behavior. In contrast, those with generally lower SNS arousal may be more likely to display shame responses to stress. Further work is needed to examine how experimental manipulations of the SNS may influence affective reactivity and negative urgency and how elevated SNS activity may contribute to the expression of impulsive behavior and psychopathology in daily life.
Highlights.
Effects of tonic sympathetic (SNS) activation on negative urgency were examined
Higher SNS activity strengthened the association between neuroticism and urgency
SNS also strengthened the link between neuroticism and hostile reactivity to stress
Lower SNS strengthened link between neuroticism and shame reactivity to stress
Hostile reactivity partially mediated the moderating effect of SNS on urgency
Acknowledgements.
This work was supported by grants from the National Institute of Mental Health (T32MH019927; T32MH093315; K99MH109667; K23MH112889) and the National Institute of Drug Abuse (P50DA005312). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Inclusion of female sex in these models did not affect patterns of significance
References
- Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ, 2007. Beta-adrenergic modulation of cognitive flexibility during stress. Journal of Cognitive Neuroscience 19, 468–478. doi: 10.1162/jocn.2007.19.3.468 [DOI] [PubMed] [Google Scholar]
- Anestis MD, Joiner TE, 2011. Examining the role of emotion in suicidality: Negative urgency as an amplifier of the relationship between components of the interpersonal–psychological theory of suicidal behavior and lifetime number of suicide attempts. Journal of Affective Disorders 129, 261–269. doi: 10.1016/j.jad.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Neuhaus E, Chipman J, Reid MJ, Webster-Stratton C, 2013. Sympathetic- and parasympathetic-linked cardiac function and prediction of externalizing behavior, emotion regulation, and prosocial behavior among preschoolers treated for ADHD. Journal of Consulting and Clinical Psychology 81, 481–493. doi: 10.1037/a0032302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J, 2001. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology 110, 610–624. doi: 10.1037//0021-843X.110.4.610 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Neuhaus E, Gatzke-Kopp LM, Reid MJ, Chipman J, Brekke A, Olliges A, Shoemaker S, Webster-Stratton C, 2015. Electrodermal responding predicts responses to, and may be altered by, preschool intervention for ADHD. Journal of Consulting and Clinical Psychology 83, 293–303. doi: 10.1037/a0038405 [DOI] [PubMed] [Google Scholar]
- Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO, 2015. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assess 27, 1129–1146. doi: 10.1037/pas0000111 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, Cacioppo JT, 2004. Where to Q in PEP. Psychophysiol 41, 333–337. doi: 10.1111/j.1469-8986.2004.00156.x [DOI] [PubMed] [Google Scholar]
- Bresin K, Carter DL, Gordon KH, 2012. The relationship between trait impulsivity, negative affective states, and urge for nonsuicidal self-injury A daily diary study. Psychiatry Research 1–5. doi: 10.1016/j.psychres.2012.09.033 [DOI] [PubMed] [Google Scholar]
- Carver CS, 2004. Negative Affects Deriving From the Behavioral Approach System. Emotion 4, 3–22. doi: 10.1037/1528-3542.4.1.3 [DOI] [PubMed] [Google Scholar]
- Chapman AL, Dixon-Gordon KL, Layden BK, Walters KN, 2010. Borderline personality features moderate the effect of a fear induction on impulsivity. Personality Disorders: Theory, Research, and Treatment 1, 139–152. doi: 10.1037/a0019226 [DOI] [PubMed] [Google Scholar]
- Claes L, Vandereycken W, Vertommen H, 2005. Impulsivity-related traits in eating disorder patients. Personality and Individual Differences 39, 739–749. [Google Scholar]
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J, 2006. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology 115, 174–178. doi: 10.1037/0021-843X.115.1.174 [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A, 2012. The relationship between self-report and lab task conceptualizations of impulsivity. Journal of Research in Personality 46, 121–124. doi: 10.1016/j.jrp.2011.11.005 [DOI] [Google Scholar]
- Cyders MA, Coskunpinar A, 2011. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review 31, 965–982. doi: 10.1016/j.cpr.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A, 2010. Is urgency emotionality? Separating urgent behaviors from effects of emotional experiences. Personality and Individual Differences 48, 839–844. doi: 10.1016/j.paid.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, 2008. Emotion-based dispositions to rash action: positive and negative urgency. Psychological bulletin 134, 807–828. doi: 10.1037/a0013341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckman T, Nathan DeWall C, 2011. Negative urgency and risky sexual behaviors: A clarification of the relationship between impulsivity and risky sexual behavior. Personality and Individual Differences 51, 674–678. doi: 10.1016/j.paid.2011.06.004 [DOI] [Google Scholar]
- Delgado-Rico E, Río-Valle JS, Albein-Urios N, Caracuel A, González-Jiménez E, Piqueras MJ, Brandi P, Ruiz-López IM, García-Rodríguez I, Martín-Matillas M, Delgado-Fernández M, Campoy C, Verdejo-García A, 2012. Effects of a multicomponent behavioral intervention on impulsivity and cognitive deficits in adolescents with excess weight. Behav Pharmacol 23, 609–615. doi: 10.1097/FBP.0b013e328356c3ac [DOI] [PubMed] [Google Scholar]
- Derefinko KJ, Charnigo RJ, Peters JR, Adams ZW, Milich R, Lynam DR, 2016a. Substance Use Trajectories From Early Adolescence Through the Transition to College. J Stud Alcohol Drugs 77, 924–935. doi: 10.2307/1131226?ref=search-gateway:a887d211d04825d6400ba16cbb268358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefinko KJ, DeWall CN, Metze AV, Walsh EC, Lynam DR, 2011. Do different facets of impulsivity predict different types of aggression? Aggress Behav 37, 223–233. doi: 10.1002/ab.20387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefinko KJ, Eisenlohr-Moul TA, Peters JR, Roberts W, Walsh EC, Milich R, Lynam DR, 2016b. Physiological response to reward and extinction predicts alcohol, marijuana, and cigarette use two years later. Drug and Alcohol Dependence 163, S29–S36. doi: 10.1016/j.drugalcdep.2016.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derefinko KJ, Peters JR, Eisenlohr-Moul TA, Walsh EC, Adams ZW, Lynam DR, 2014. Relations between trait impulsivity, behavioral impulsivity, physiological arousal, and risky sexual behavior among young men. Arch Sex Behav 43, 1149–1158. doi: 10.1007/s10508-014-0327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dir AL, Karyadi K, Cyders MA, 2013. The uniqueness of negative urgency as a common risk factor for self-harm behaviors, alcohol consumption, and eating problems. Addict Behav 38, 2158–2162. doi: 10.1016/j.addbeh.2013.01.025 [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Welch SS, Grossman P, Reisch T, Linehan MM, Bohus M, 2007. Psychophysiological ambulatory assessment of affective dysregulation in borderline personality disorder. Psychiatry Research 150, 265–275. doi: 10.1016/j.psychres.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Engel SG, Boseck JJ, Crosby RD, Wonderlich SA, Mitchell JE, Smyth J, Miltenberger R, Steiger H, 2007. The relationship of momentary anger and impulsivity to bulimic behavior. Behav Res Ther 45, 437–447. doi: 10.1016/j.brat.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Fischer S, Peterson CM, McCarthy D, 2013. A prospective test of the influence of negative urgency and expectancies on binge eating and purging. Psychol Addict Behav 27, 294–300. doi: 10.1037/a0029323 [DOI] [PubMed] [Google Scholar]
- Fischer S, Settles R, Collins B, Gunn R, Smith GT, 2012. The role of negative urgency and expectancies in problem drinking and disordered eating: Testing a model of comorbidity in pathological and at-risk samples. Psychol Addict Behav 26, 112–123. doi: 10.1037/a0023460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DL, Leibenluft E, O’Leary KM, Cowdry RW, 1991. Self-ratings of anger and hostility in borderline personality disorder. J Nerv Ment Dis 179, 157–161. [DOI] [PubMed] [Google Scholar]
- Goedhard LE, Stolker JJ, Heerdink ER, Nijman HLI, Olivier B, Egberts TCG, 2006. Pharmacotherapy for the treatment of aggressive behavior in general adult psychiatry: A systematic review. J Clin Psychiatry 67, 1013–1024. [DOI] [PubMed] [Google Scholar]
- Gratz K, Roemer L, 2004. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the Difficulties in Emotion Regulation Scale. Journal of Psychopathology and Behavioral Assessment 26, 41–54. [Google Scholar]
- Harmon-Jones E, 2003. Anger and the behavioral approach system. Personality and Individual Differences 35, 995–1005. doi: 10.1016/S0191-8869(02)00313-6 [DOI] [Google Scholar]
- Harmon-Jones E, Allen JJB, 1998. Anger and frontal brain activity: EEG asymmetry consistent with approach motivation despite negative affective valence. Journal of Personality and Social Psychology 74, 1310. [DOI] [PubMed] [Google Scholar]
- Haspel T, 2009. Beta-blockers and the Treatment of Aggression. Harvard review of psychiatry 2, 274–281. [DOI] [PubMed] [Google Scholar]
- Hayes AF, 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis. Guilford Press. [Google Scholar]
- Herrero N, Gadea M, Rodríguez-Alarcón G, Espert R, Salvador A, 2010. What happens when we get angry? Hormonal, cardiovascular and asymmetrical brain responses. Hormones and Behavior 57, 276–283. doi: 10.1016/j.yhbeh.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, Schnell K, Musshoff F, Keysers C, Maier W, Kendrick KM, Onur OA, 2010. Human amygdala reactivity is diminished by the β-noradrenergic antagonist propranolol. Psychological Medicine 40, 1839–1848. doi: 10.1017/S0033291709992376 [DOI] [PubMed] [Google Scholar]
- Kaiser AJ, Milich R, Lynam DR, Charnigo RJ, 2012. Negative Urgency, Distress Tolerance, and substance abuse among college students. Addictive Behaviors 37, 1075–1083. doi: 10.1016/j.addbeh.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT, 2014. Subtyping Attention-Deficit/Hyperactivity Disorder Using Temperament Dimensions. JAMA Psychiatry 71, 1015–10. doi: 10.1001/jamapsychiatry.2014.763 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuo JR, Linehan MM, 2009. Disentangling emotion processes in borderline personality disorder: Physiological and self-reported assessment of biological vulnerability, baseline intensity, and reactivity to emotionally evocative stimuli. 118, 531–544. doi: 10.1037/a0016392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Kahler CW, Brown RA, 2003. A modified computer version of the Paced Auditory Serial Addition Task (PASAT) as a laboratory-based stressor. The Behavior Therapist. [Google Scholar]
- Leyro TM, Zvolensky MJ, Bernstein A, 2010. Distress tolerance and psychopathological symptoms and disorders: A review of the empirical literature among adults. Psychol Bull 136, 576–600. doi: 10.1037/a0019712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y, 2011. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 218, 89–99. doi: 10.1007/s00213-011-2178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Stevens AK, Cunningham S, Jones RE, King KM, Schumacher JA, Coffey SF, 2015. Stability and change in multi-method measures of impulsivity across residential addictions treatment. Addictive Behaviors 42, 126–129. doi: 10.1016/j.addbeh.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Lorber MF, 2004. Psychophysiology of Aggression, Psychopathy, and Conduct Problems: A Meta-Analysis. Psychol Bull 130, 531–552. doi: 10.1037/0033-2909.130.4.531 [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG, 2007. Where to B in dZ/dt. Psychophysiol 44, 113–119. doi: 10.1111/j.1469-8986.2006.00468.x [DOI] [PubMed] [Google Scholar]
- Maniaci G, Goudriaan AE, Cannizzaro C, Holst RJ, 2017. Impulsivity and Stress Response in Pathological Gamblers During the Trier Social Stress Test. Journal of Gambling Studies 1–14. doi: 10.1007/s10899-017-9685-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Scognamiglio P, Canestrelli B, Serino I, Monteleone AM, Maj M, 2011. Asymmetry of salivary cortisol and α-amylase responses to psychosocial stress in anorexia nervosa but not in bulimia nervosa. Psychological Medicine 41, 1963–1969. doi: 10.1017/S0033291711000092 [DOI] [PubMed] [Google Scholar]
- Mullins-Sweatt SN, 2006. Psychometric Properties of an Abbreviated Instrument of the Five-Factor Model. Assessment 13, 119–137. doi: 10.1177/1073191106286748 [DOI] [PubMed] [Google Scholar]
- Mullins-Sweatt SN, Edmundson M, Sauer-Zavala S, Lynam DR, Miller JD, Widiger TA, 2012. Five-Factor Measure of Borderline Personality Traits. J Pers Assess 94, 475–487. doi: 10.1080/00223891.2012.672504 [DOI] [PubMed] [Google Scholar]
- Muñoz LC, Anastassiou-Hadjicharalambous X, 2011. Disinhibited behaviors in young children: relations with impulsivity and autonomic psychophysiology. Biological Psychology 86, 349–359. doi: 10.1016/j.biopsycho.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT, 2011. Emotion Regulation via the Autonomic Nervous System in Children with Attention-Deficit/Hyperactivity Disorder (ADHD). J Abnorm Child Psychol 39, 841–852. doi: 10.1007/s10802-011-9499-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JR, Upton BT, Baer RA, 2013. Brief report: Relationships between facets of impulsivity and borderline personality features. Journal of Personality Disorders 27, 547–552. [DOI] [PubMed] [Google Scholar]
- Peterson CM, Davis-Becker K, Fischer S, 2014. Interactive role of depression, distress tolerance and negative urgency on non-suicidal self-injury. Personality and Mental Health 8, 151–160. doi: 10.1002/pmh.1256 [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AFT, 2007. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology & Therapeutics 113, 523–536. doi: 10.1016/j.pharmthera.2006.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Wilhelm FH, Gross JJ, 2008. All in the mind’s eye? Anger rumination and reappraisal. J Pers Soc Psychol 94, 133–145. doi: 10.1037/0022-3514.94.1.133 [DOI] [PubMed] [Google Scholar]
- Roberts W, Peters JR, Adams ZW, Lynam DR, Milich R, 2014. Identifying the facets of impulsivity that explain the relation between ADHD symptoms and substance use in a nonclinical sample. Addict Behav 39, 1272–1277. doi: 10.1016/j.addbeh.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, de Quervain DJF, Schelling G, McGaugh JL, 2004. A systemically administered β-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiology of Learning and Memory 81, 150–154. doi: 10.1016/j.nlm.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Settles RE, Fischer S, Cyders MA, Combs JL, Gunn RL, Smith GT, 2012. Negative urgency: A personality predictor of externalizing behavior characterized by neuroticism, low conscientiousness, and disagreeableness. 121, 160–172. doi: 10.1037/a0024948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, 2000. Using Multivariate Statistics, 4 ed. Allyn & Bacon. [Google Scholar]
- van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JPA, Rombouts SARB, 2005. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. NeuroImage 24, 898–909. doi: 10.1016/j.neuroimage.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klonsky ED, Hajcak G, 2009. Autonomic impairment in Borderline Personality Disorder: A laboratory investigation. Brain and Cognition 71, 279–286. doi: 10.1016/j.bandc.2009.07.014 [DOI] [PubMed] [Google Scholar]
- Wenzel KR, Weinstock J, Vander Wal JS, Weaver TL, 2014. Examining the role of negative urgency in a predictive model of bulimic symptoms. Eating Behaviors 15, 343–349. doi: 10.1016/j.eatbeh.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Whiteside S, Lynam D, 2001. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences 30, 669–689. [Google Scholar]
- Zapolski T, Nursing GSTJOS, 2017, 2016. Pilot study: implementing a brief DBT skills program in schools to reduce health risk behaviors among early adolescents. Journal of School Nursing 33, 198–204. doi: 10.1177/1059840516673188 [DOI] [PMC free article] [PubMed] [Google Scholar]