Abstract
Introduction:
Lung cancer is the leading cause of cancer related deaths in the United States. Nearly 85% of all lung cancers are diagnosed at a late stage, with an associated five-year survival rate of 4%. Malignant central airway obstruction and malignant pleural effusions occur in upwards of 30% of these patients. Many of these patients are in need of palliative interventions for symptom control and to help improve their quality of life.
Areas covered:
This review covers the treatment modalities of malignant central airway obstruction and malignant pleural effusion. PubMed was used to search for the most up to date and clinically relevant articles that guide current treatment strategies. This review focuses on rigid bronchoscopy and the tools used for the relief of central airway obstruction, as well as intra-pleural catheter use and pleurodesis for the management of malignant pleural effusions.
Expert Commentary:
There are multiple treatment modalities that may be used to help alleviate the symptoms of malignant central airway obstruction and pleural effusion. The modality used depends on the urgency of the situation, and specific patient’s goals. An open dialogue to understand the patient’s end of life goals is an important factor when choosing the appropriate treatment strategy.
Keywords: Malignant Central Airway Obstruction, Malignant Pleural Effusion, Lung Cancer, Palliative Care, Interventional Pulmonology
1. Introduction
Lung cancer is currently the leading cause of cancer related deaths in the United States and will account for an estimated 154,050 deaths in 20181. The number of deaths from lung cancer exceeds the number of deaths from colon, breast, and prostate cancers combined. Over 234,000 new cases will be diagnosed in 20181, the majority of which will be non-small cell lung cancer. Nearly 85% of all lung cancers are diagnosed at an advanced stage portending a five-year survival rate of approximately 4.5%2,3(Figure 1). Recent advances in lung cancer screening, improved staging and novel therapies have resulted in patients living longer with advanced lung cancer. Patients with advanced cancers typically over-estimate their life expectancies4–6 and pursue costly, morbid and frequently ineffective treatments7,8 in attempt to treat their cancer and its associated complications.
Figure 1.
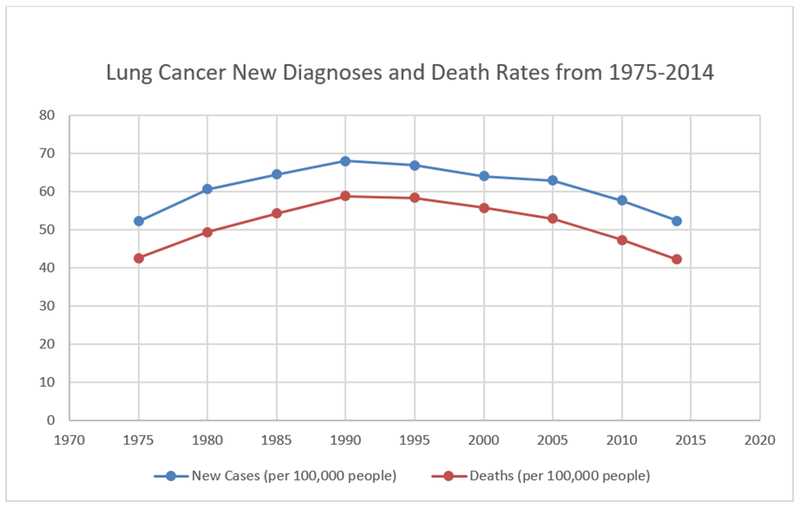
Survival graph of Lung cancer (Based on SEER Cancer Stat Facts: Lung and Bronchus Cancer).
Two of the most common and debilitating complications that occur in advanced lung cancer are central airway obstruction (CAO) and malignant pleural effusion (MPE). Both occur in about 25-30% of patients with advanced lung cancer9,10. Both CAO and MPE cause dyspnea, or breathlessness, which causes both physical and psychological distress11. Survival with one of these complications is typically less than 6 months12,13.
Central airway obstruction occurs when tumor obstructs the trachea and/or main stem bronchi. Patients with CAO commonly present with respiratory failure from near complete airway obstruction requiring emergent interventions to prevent death9,14. There are three types of CAO - intrinsic, extrinsic and mixed (Figure 2). Intrinsic occurs when the tumor grows from the bronchial wall and causes obstruction only within the confines of the airway. Extrinsic compression occurs due to mass growth or lymphadenopathy that leads to compression of the airway with no invasion into the airway wall. Lastly, mixed compression occurs when a mass invades the airway wall and causes both intrinsic blockage and extrinsic compression of the airway. Although lung cancer remains the most common cause of CAO, other etiologies should be considered during an initial presentation in patients with CAO. Table 1 contains a list of malignant causes of central airway obstruction. If death does not occur quickly, survivors suffer from refractory dyspnea, atelectasis and pneumonia15. There are numerous treatment options to combat CAO, which will be discussed in detail below.
Figure 2.
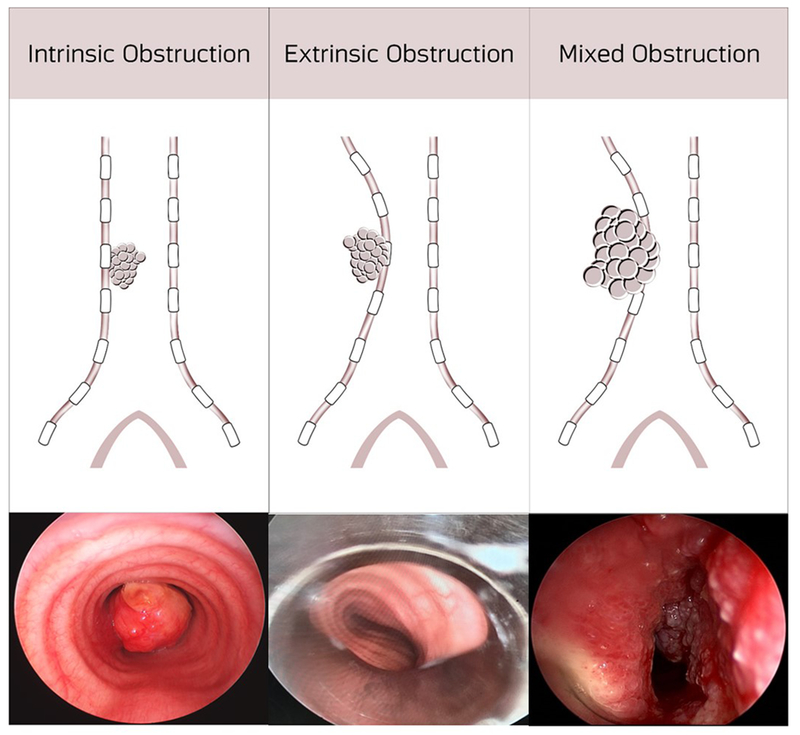
Intrinsic, Extrinsic and Mixed Central Airway Obstruction.
Table 1.
Causes of Malignant Central Airway Obstruction
| Malignant Causes of Central Airway Obstruction |
|---|
| Adenoid Cystic carcinoma |
| Breast cancer metastases |
| Bronchogenic carcinoma |
| Carcinoid |
| Colon Cancer metastases |
| Esophageal carcinomas |
| Laryngeal carcinoma metastases |
| Lymphadenopathy associated with any malignancy |
| Mediastinal Masses- Germ cell, Thymus, Thyroid, Lymphoma |
| Melanoma metastases |
| Mucoepidermoid carcinoma |
| Renal cell metastases |
| Sarcoma |
| Thyroid cancer metastases |
Malignant pleural effusions occur when cancer cells invade the pleural space, resulting in a marked increase in the collection of fluid around the lung. Lung cancer is the most common cause of malignant pleural effusion followed by breast cancer and lymphoma10. Patients found to have a MPE are diagnosed with metastatic cancer. MPEs cause debilitating dyspnea and chest pain, and if large enough can cause respiratory distress10,14. Current treatment options, which will be discussed below, include thoracentesis, indwelling pleural catheter (IPC) and pleurodesis.
Patients that suffer from CAO or MPE experience greatly impaired health related quality of life, as they require frequent hospitalizations and often intensive care level treatment. In the past, CAO and MPE would take many patients’ lives as the standard therapies of oxygen, narcotics, glucocorticoids and heliox16,17 provided no cure and often only very little relief of disabling dyspnea. Currently, with the growth of both palliative care as a specialty18 and the recognition that interventional therapies can be used for palliation14, there are more options for patients with both CAO and MPE. This article will review the current data related to the widely used palliative interventions for CAO and MPE.
2. Search Methods
We performed a comprehensive search of PubMed, EMBase, and Cochrane Library databases. Search terms included central airway obstruction, malignant airway obstruction, palliative care, radiotherapy, radiation therapy, external beam radiotherapy, and malignant pleural effusion. The searches were limited to human subjects and the English language. There was no start date limitation on the search and searches were completed in January 2018. Where applicable, bibliographies of relevant studies were searched by hand for other pertinent articles. Priority was given to randomized controlled trials, systematic reviews, and meta-analysis. RCT data were limited and as such we included well designed prospective and retrospective observational studies with a minimum sample size of 5 where needed. Exclusion criteria were non-English language, studies that included both benign and malignant diseases, and a sample size less than 50 for observational studies.
3. Central Airway Obstruction
The pathophysiology of dyspnea in central airway obstruction is thought to be due to the exertion it takes to overcome the flow limitation from the mass, rather than hypoxemia or hypercapnia9,19. Patients who develop air hunger with tracheal obstruction, typically don’t start to exhibit symptoms until the lumen of the trachea is less than 8mm9. The interventions for central airway obstruction can be divided into two main categories: bronchoscopic interventions and radiation therapy. Bronchoscopic interventions include laser, electrocautery, argon plasma coagulation, photodynamic therapy, cryoablation, balloon bronchoplasty and airway stenting. Radiation therapy includes both endobronchial brachytherapy (EBB) and external beam radiotherapy (EBRT).
3.1. Bronchoscopic Interventions
Bronchoscopy can be performed by either flexible or rigid bronchoscopy. Rigid bronchoscopy is a minimally invasive endoscopic procedure, which takes place in the operating room under general anesthesia. Patients are intubated with a rigid bronchoscope, which is a long metal tube with fenestrations in the distal end to allow ventilation (Figure 3). Rigid bronchoscopy is typically performed using jet ventilation, although spontaneous assisted ventilation (allowing general anesthesia) and controlled ventilation are also available strategies20. Visualization is accomplished using a rigid telescope camera inserted through the shaft of the rigid bronchoscope or a flexible bronchoscope placed through the inner lumen of the rigid bronchoscope. The larger inner diameter of a rigid bronchoscope allows for large instruments to be inserted to perform hemostasis or manage the central airway obstruction through recannulation via multiple different modalities, including airway stenting, electrocautery, argon plasma coagulation, laser, cryoablation or photodynamic therapy. Airway recanalization may occur via destruction of the tumor (no hemostasis), or vaporization, which leads to both destruction and hemostasis. All of these modalities will be described below, and although there is some data comparing techniques, it is important to remember that in most instances more than one of these therapies will be used in a single procedure.
Figure 3.
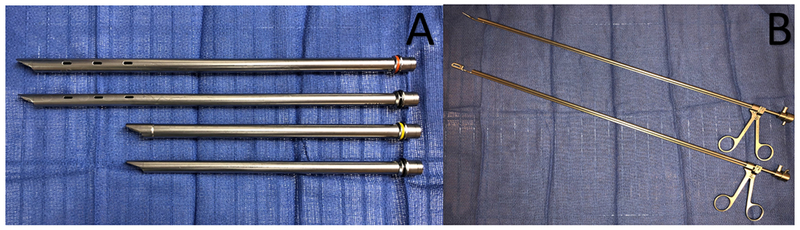
Panel A demonstrates multiple different sized rigid bronchoscopes. Panel B shows two different forceps used through the inner cannula of the rigid bronchoscope.
3.1.1. Laser
Laser therapy, or light amplification by stimulated emission of radiation, causes thermal ablation of tissue. The most commonly used laser is the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser, followed by the CO2 and argon lasers. The differences between the lasers are their operational wavelengths and bronchoscopic compatibility. The CO2 laser cannot be used via flexible bronchoscopy, only rigid bronchoscopy21,22. Argon is usable in a flexible bronchoscope, but its tissue penetration is shallow (1-2mm) 21,22. Nd:YAG can be used in a flexible bronchoscope, and has a tissue penetration up to 10mm21,22. Another advantage of Nd:YAG is that it can both resect (due to depth) and vaporize (due to the amount of energy used). The laser should be aimed parallel to the airway lesion, and quick pulses of laser therapy should be applied23,24. It is important that the FiO2 be kept less than 0.4, to avoid the risk of airway fire. Protective goggles should be worn by all staff and the patient’s eyes should be protected to avoid injury.
The advantage to using lasers for central airway obstruction are the ability to resect with hemostasis. Difficulty arises in the angles needed to perform laser resection because the laser needs to be parallel to the lesion. Because of this, it is used less frequently than other modalities. The main risks of laser therapy in addition to airway fire are hemorrhage and perforation of the airway. The degree of tissue destruction should be assessed frequently, in order to avoid airway perforation and evaluate bleeding.
Laser therapy has been shown to be an effective treatment for CAO. In a study of 86 patients treated with Nd:YAG for CAO, 76% had resolution of partial obstruction and 38% had resolution of complete obstruction25. Brutinel et al26 showed that 83% of 107 patients treated with Nd:YAG laser had an increase in their airway diameter after treatment. Additionally, the largest study (N=2008) showed that use of Nd:YAG laser relieved obstruction in greater than 90% of tracheal or main stem bronchi occlusion27.
3.1.2. Electrocautery
Electrocautery is the use of an electrical current kill cells through thermal ablation. The amount of heat generated depends on the current, type of tissue, and contact between the probe and tissue9,28. This is an immediate-acting technique, which primarily causes tissue destruction, but can cause vaporization as well due to high heat generation. Since this causes electrical current to flow, a grounding pad must be placed on the patient to avoid burning the airway. This procedure may be done through a rigid or flexible bronchoscope. One major advantage of electrocautery is that different electrodes, forceps, knife, wire snare or the blunt electrodes, can be used depending on the type of obstruction.
There are numerous advantages to using electrocautery, namely the ability to resect with hemostasis, it is compatible with the flexible bronchoscope, and it has numerous attachments to adapt to different types of obstructions. Common side effects of electrocautery are airway perforation, airway fires, and bleeding. It is important to keep FiO2 less than 0.4 peri-procedurally to avoid airway fires. In a retrospective review of 117 electrocautery procedures for both malignant CAO and non-malignant CAO, there was a 94% improvement in endoluminal patency and a 71% symptomatic improvement rate29.
3.1.3. Argon Plasma Coagulation
Argon Plasma Coagulation (APC) uses electrocoagulation (through argon gas) to produce tissue destruction. It has similar uses to electrocautery, with the difference being that APC uses a non-contact technique. In order to create argon plasma, the tip of the probe releases argon gas which is then ionized by an electrode at the tip of the catheter9. APC causes tissue coagulation without vaporization. Since this is a form of monopolar electrical surgery, grounding pads should be properly placed on the patient. The APC probe can be inserted into either a flexible or a rigid bronchoscope. The probe is held within 5mm of the target lesion, without coming into direct contact with the lesion or airway wall. It is important to remember that when applying APC, it will react with the closest piece of tissue to the tip of the probe. This modality is most commonly used for excision of smaller masses, since the lack of vaporization makes it a less ideal method for the debulking of larger masses.
The main advantage of APC is its compatibility with rigid and flexible bronchoscopes. Unlike laser therapy, the probe does not need to be parallel to the lesion, as it will react with the closest piece of tissue. The major limitation is that it is not ideal to debulk larger masses due to the lack of vaporization. The penetration depth of APC is approximately 2-3mm30, which decreases the risk of airway perforation, in comparison to laser therapy. Similar to laser therapy, FiO2 should be kept below 0.4 to reduce the risk of airway fire.
In a small retrospective study, APC with mechanical tumor resection was found to be more effective than cryotherapy recanalization in patients with CAO (97.3% vs 80.8%) 31. APC has great success in the treatment of hemoptysis, with one study of 60 patients showing complete resolution of bleeding after treatment32.
3.1.4. Photodynamic Therapy
Photodynamic Therapy (PDT) is a minimally invasive therapy that destroys cancer cells by using a non-thermal laser light administered bronchoscopically to activate a drug9. The most commonly used photosensitizer used is porfirmer sodium, which is given 48 hours prior to the procedure. During bronchoscopy, a specialized catheter and light source, in the working channel, is used to deliver specific wavelength of light. The procedure takes approximately 10 minutes to complete, and may be repeated multiple times, as the photosensitizing medication can last in the malignant cells for weeks. A routine airway inspection is recommended 24-48 hours after treatment to remove debris and secretions related to the procedure. Photosensitizing agents can be present in the skin for upwards of 6 weeks, so patients need to be counselled on the risks of excessive sun exposure33.
Photodynamic therapy less frequently used due to its delayed effects and need for repeated bronchoscopy. Due to this, it is also less ideal for large central airway obstructions. No studies have evaluated the effect of PDT on survival. One large prospective observational study of patients with lung cancer with CAO showed that PDT decreased obstruction by 67% and improved symptoms in all patients34. Despite these promising results, the majority (82%) of those had prior treatment with external beam radiotherapy/chemotherapy and 9% received YAG laser treatment.
3.1.5. Cryoablation
Cryoablation, or cryotherapy, is a technique in which tumors are destroyed by freezing, typically at temperatures less than −40°C9,35. The overall outcome of cryotherapy is dependent on the speed in which one freezes and thaws the tissue, the temperature used, the water content of the tissue and the number of cycles of freezing and thawing the tissue9,36. The probe is inserted through the bronchoscope with the tip of the cryoprobe in contact with the mass to allow for maximal surface area to be affected. The probe can be used for tissue destruction, debulking, biopsy or foreign body removal.
The safety profile of cryotherapy is the main advantage to using this technology. Cartilage and connective tissue are relatively cryoresistant due to the low water content, which makes airway perforation uncommon9,36,37. The primary disadvantage is that the maximal freezing effects are delayed, and benefits are not seen until after multiple treatments38. As with PDT, a follow-up bronchoscopy should be performed within a few days for airway inspection and removal of debris.
No studies have evaluated how cryoablation affects survival, but two larger studies have shown that cryoablation is a useful technique to relieve CAO and improve patient symptoms. In one of these studies, by Marasso et al37, 183 patients with malignant CAO were treated with cryotherapy; 57% had resolution of atelectasis, 93% had decreased hemoptysis, and 81% had improved dyspnea The most common complication was airway bleeding.
3.1.6. Balloon Bronchoplasty
Balloon bronchoplasty involves inserting a deflated balloon through a stenotic airway, and inflating the balloon to open the airway (Figure 4). Balloon dilation can be performed using rigid or flexible bronchoscopy. The balloon may be inserted through the working channel of the flexible bronchoscope, or adjacent to the scope, depending on the size of the working channel and size of the balloon. When the balloon catheter has traversed the stenosis, the balloon is then inflated and held, for anywhere from thirty seconds to one minute to dilate the airway. Although this method is effective for immediate relief of a severe stricture, it is rarely a definitive treatment. Thus, it is typically done in conjunction with other procedures, most commonly, stenting.
Figure 4.
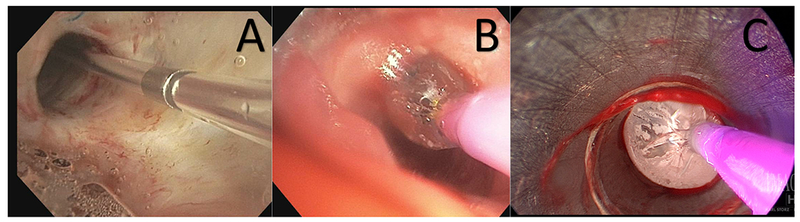
Balloon dilation. Panel A shows the catheter traversing the stenotic airway. Panel B demonstrates the initial inflation of the balloon to dilate the airway. Panel C displays maximal inflation of the balloon used to re-establish airway patency.
The major side effects of balloon bronchoplasty are bleeding, mediastinitis, and airway rupture leading to pneumothorax or pneumomediastinum39. In an analysis of 126 balloon dilation procedures, 79% of airway stenosis improved immediately following dilation40, although symptomatic improvement > 8 days was only present in 43% of cases.
3.1.7. Stenting
There are two main types of airway stents: silicone stents and self-expanding metallic stents (SEMS) commonly made of nitinol (either covered with a plastic membrane or uncovered) (Figure 5)41. Dr. J.F. Dumon, for whom a stent is named, was one of the first to show that silicone stents can be safely placed42. Airway stenting can be performed for all three causes of CAO. Typically, if the lesion is endoluminal (intrinsic disease), debridement and/or dilation is performed prior to stent placement. The goal of the stent is to keep an airway patent for a longer period of time, than an ablative procedure alone.
Figure 5.
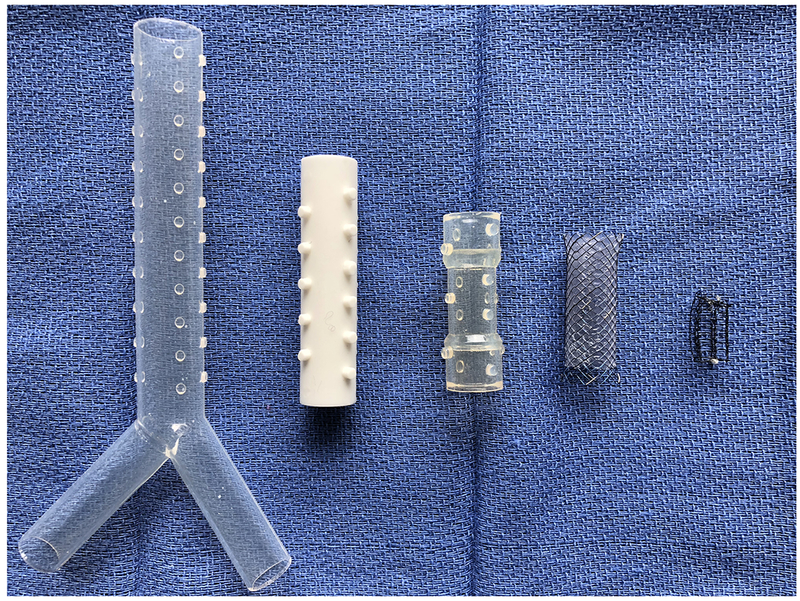
In order: Silicone Y stent, Silicone white stent, Silicone translucent stent, Covered self-expanding metallic stent, Uncovered self-expanding metallic stent.
Self-expanding metallic stents are made of stainless steel or nitinol, and as the name implies, expand after deployment into an airway. These stents, unlike silicone stents, can be deployed using a flexible bronchoscope. They should be sized appropriately to avoid airway necrosis and should fit tightly in the airway to prevent migration. Granulation tissue commonly forms around SEMS making them difficult to remove. There are two types of SEMS, covered and uncovered. A covered SEMS shares properties similar to a silicone stent (discussed below) - it is able to jail fistulas or bronchi (if needed), it has a higher rate of mucus plugging but less risk of granulation tissue growth. Uncovered SEMS, on the other hand, promote mucus clearance, but allow tumor regrowth through the stent. The risks of both types of SEMS include infection and stent fracture.
Unlike, SEMS, Silicone stents can only be placed with rigid bronchoscopy. These stents are more commonly used for extrinsic compression, to maintain patency after debridement of an intrinsic lesion, or to seal off fistulae (i.e. tracheoesophageal fistula). “Y” shaped silicone stents allow for treatment of the trachea and main stem bronchi with a single stent. Silicone stents can be easily readjusted once deployed in the airway. Benefits of these stents include a lower risk of infection, fracture and tumor regrowth/granulation growth into the stent, but they are more likely to migrate than SEMS. A large (> 1000 patients) multicenter observational trial by Dumon et al43, found that the average time a silicone stent was in place was 4 months, and the most common complications were stent migration followed by granuloma formation and stent obstruction. The estimated cost of a silicone stent is about $250 versus $1800 for a SEMS44.
Stenting has been shown to improve functional status, quality of life and dyspnea45–47. In a small study of 82 patients, 50 of whom had malignant CAO, 87% had improvement in their symptoms (dyspnea, cough, hemoptysis) immediately after stent placement48 In that study, which evaluated two different types of SEMS, the most frequent complications were infection, granuloma formation and stent migration. In another study of a 143 patients, 95% had effective palliation of symptoms post stenting49. This study also showed that airway stenting was frequently combined with other airway interventions. In a retrospective study of 50 patients, Razi et al50 demonstrated that airway stenting improved functional status and dyspnea scores. The authors also found that airway stenting improved survival in patients who had intermediate functional status versus patients who had poorer functional status, but this result may be due to how severely ill the patients were, not the stent itself.
3.2. Radiation
3.2.1. Endobronchial Brachytherapy vs. External Beam Radiotherapy
Endobronchial Brachytherapy (EBB) utilizes the placement of a radioactive substance within the airway, in order to treat a malignancy. In order for this to be effective, it must be placed in close proximity of the target lesion. A benefit of this procedure, is the ability to treat lesions external to the airway wall51,52. This procedure is performed using flexible bronchoscopy with a catheter to deliver the radioactive material next to the bronchoscope. Catheter position in close proximity to the target lesion is confirmed with fluoroscopy. Local radiation is then applied to the lesion, in an attempt to spare non-malignant tissue. The most common side effects include airway wall irritation/friability and stenosis at the treatment site.
This is in contrast to External Beam Radiation Therapy (EBRT), which involves external radiation application to a malignant lesion. Patients then receive multiple treatments to the malignancy over a short period of time (days to weeks). Since the radiation must pass through the chest wall, non-malignant tissues are affected leading to numerous potential side effects, including, pulmonary fibrosis, pneumonitis, esophagitis, chest wall pain, and rib fractures53,54.
According to the 2011 American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline, endobronchial brachytherapy is not recommended as either sole or adjunctive therapy for routine palliation of airway obstruction55. This recommendation is informed by four randomized trials evaluating endobronchial brachytherapy (EBB) and external beam radiation therapy (EBRT). In the study conducted by Mallick et al56, 45 patients were randomized to receive either EBRT and higher dose EBB, EBRT and lower dose EBB, or EBB alone. The authors assessed symptomatic improvement in dyspnea, cough, hemoptysis and obstructive pneumonia and found no difference between groups. In another RCT by Sur et al57, patients with stage III NSCLC with luminal disease received EBRT at varying dosing schedules and then were randomized to receive either EBRT or EBB. They showed that quality of life was equivalent in both groups but symptom free survival was longer in patients randomized to the EBRT (129 days vs. 77 days, p= 0.009). In a separate study of 95 patients randomized to receive either EBRT alone or EBRT and EBB58, it was shown that median survival was not statistically different (8.5 months for EBRT alone versus 7 months for EBRT and EBB, p = 0.21). There was, however, improved lung expansion in the group that received adjunctive EBB (35% for EBRT vs. 57% for EBRT and EBB, p=0.02). Finally in a study of 99 patients by Stout et al59 it was shown that patients who received EBRT had improved overall survival than patients who received EBB (1 year survival 37% vs. 22%, p =0.04) and a better improvement in their symptoms (83% vs. 59%, p= 0.03).
A Cochrane review evaluating different dosing regimens for non-small cell lung cancer showed that there is no survival advantage to dosing regimens, but higher dose regimens did have more toxicity, namely esophagitis60. One older retrospective observational study showed that in patients with CAO and resulting atelectasis who received EBRT only 12 out of 57 (21%) had improvement in atelectasis61. Those patients received greater than 50 Gy and four of them (33%) developed lung fibrosis, and two (17%) pneumonitis.
3.2.2. Bronchoscopic Interventions vs. Radiation
Due to ethical concerns, there have been few trials comparing bronchoscopic interventions to radiation therapy in patients with CAO. The largest study to be published is a retrospective study from Johns Hopkins Hospital where 237 patients were identified to have undergone bronchoscopic stenting, EBRT or both procedures. In this cohort, they found that patients who received both stenting and EBRT had a survival benefit over patients that received only one of the therapeutic options (Stenting only HR 2.12, 95% CI 1.02,4.39; EBRT only HR 1.62, 95% CI 0.93,2.83)62. One trial63 evaluating radiation versus bronchoscopy treatments for CAO enrolled 29 patients with non-small cell lung cancer in the central airways. They found that patients treated with Nd:YAG plus brachytherapy had more symptom free survival than those treated with only Nd-YAG (8.5 months versus 2.8 months, p< 0.05). This study is not widely generalizable given its small sample size as well as the fact that both Nd-YAG laser and brachytherapy seems to be used less frequently than other interventions.
4. Malignant Pleural Effusion (MPE)
A malignant pleural effusion occurs when pleural fluid production overwhelms pleural fluid absorption. The exact cause as to why certain cancers cause effusions is not clear, but the current pathogenesis theory revolves around both increased fluid production and impaired lymphatic drainage64. For symptomatic malignant pleural effusions, the three most common palliative therapeutic options are thoracentesis, pleurodesis, and placement of an indwelling pleural catheter12. Other therapies namely pleurectomy65 and pleuroperitoneal shunting66,67 that are rarely used will not be discussed.
4.1. Thoracentesis
Thoracentesis is the process of aspirating fluid from the pleural space for diagnostic or therapeutic purposes. This minimally invasive procedure is done under ultrasound guidance in both the inpatient and outpatient settings. Complications of this procedure include cellulitis at the site of needle insertion, bleeding and pneumothorax. Repeat thoracentesis may be done for patients with an expected survival less than one month. If patients are expected to live longer than one month, The British Thoracic Society pleural disease guidelines do not recommend repeat thoracentesis given that malignant pleural effusions recur quickly. If patients symptomatically improve with pleural drainage, longer term therapies discussed below should be considered13.
4.2. Pleurodesis
The mechanism by which the pleural space is eradicated is known as pleurodesis. Pleurodesis is performed via thoracoscopy, or through a small-bore chest tube. The two main types are chemical pleurodesis, which consists of placing a sclerosing agent into the pleural space, and mechanical pleurodesis, which consists of physically disrupting the pleura. Talc is the most effective and most well studied sclerosing agent68,69. The effect of talc on pleurodesis is more successful than bleomycin, tetracycline, mustine and doxycline68.
Talc or hydrated magnesium silicate, has been used since 1935, making it both cheap and readily available. Talc is available in two forms, small-particle and large particle. Small particle talc pleurodesis has been associated with the development of acute respiratory distress syndrome (ARDS) in 1-9% of patients70–72, presumed secondary to talc toxicity as smaller particles are more readily absorbed. In a large (N=558) prospective multicenter cohort study, 558 patients were treated with large particle talc pleurodesis and none of them (0%) developed ARDS73 although large particle Talc is not currently FDA approved in the United States.
Talc is instilled in one of two ways, either by slurry through a chest tube or by poudrage during thoracoscopy. Both the Cochrane review68,69 and the 2014 meta-analysis74 conclude that talc poudrage is more effective than talc slurry. If talc slurry is to be performed, small bore tubes (10-14 French) are preferred over large bore tubes (24-32 French)13 because they have equivalent efficacy and the smaller tubes cause less discomfort. 75,76,77.
Patients who undergo pleurodesis have an average length of stay of 3-5 days, as they need to remain in the hospital for chest tube drainage. Despite the evidence supporting talc pleurodesis, pleurodesis failure can occur13. Failure typically occurs when there is trapped lung, that is when the lung doesn’t re-expand78,79
4.3. Indwelling Pleural Catheter (IPC)
The use of indwelling pleural catheters (IPC), also known as tunneled pleural catheters, first gained popularity patients, who were not good candidates for pleurodesis80. Much like thoracentesis, IPC is performed as an outpatient procedure. Placement of these catheters is similar to placement of a pigtail catheter, using the seldinger technique, with the catheter tunneled anteriorly for easy patient access for drainage (Figure 6). Common side effects of indwelling pleural catheters are bleeding, skin infection, pleural infection, and retained catheter fragments after removal.
Figure 6.
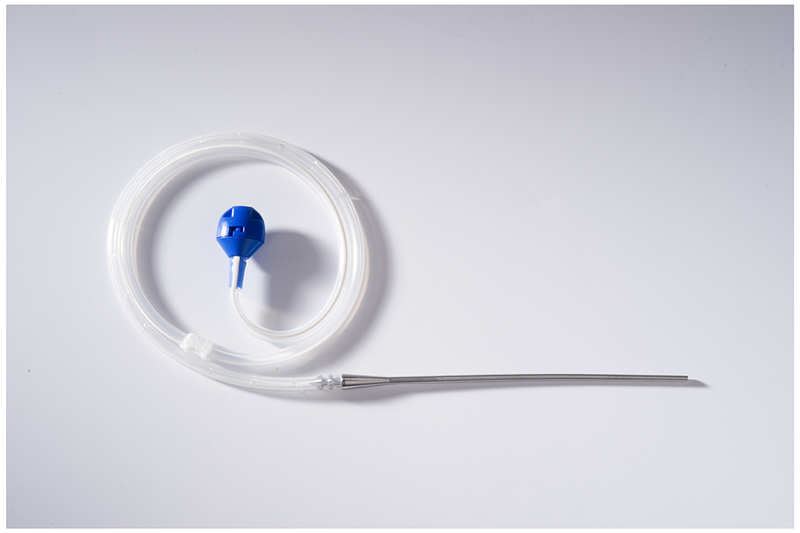
Rocket® Intra pleural catheter with attached metal tunneler and blue drainage port.
Indwelling catheters typically allow patients to spend fewer days in the hospital compared to pleurodesis, which is an important consideration for a palliative intervention. A randomized controlled trial81 in 2012 of 106 patients from 7 United Kingdom hospitals comparing IPC to talc pleurodesis via slurry showed that time in the hospital was significantly less for patients who received IPC (0 days versus 4 days, p< 0.001). There was also less dyspnea at 6 months in the IPC group (p=0.01). There were, however, more adverse events in the IPC group (21 vs. nine, p = 0.02) but no statistical difference in serious adverse events (9 in the IPC group vs. 5 in the talc group). The most concerning adverse event was pleural infection, which was serious in five patients in the IPC group, requiring intravenous antibiotics and in one patient leading to death. These results were echoed in a 2017 study showing that patients who receive IPC spend less time in the hospital (10 vs. 12 days, p=0.03), require less invasive pleural drainages (4.1% vs. 22.5%, p<0.05), but have more adverse events (30 vs. 18)82. In a large multi-center prospective non-randomized study of 65 patients, there was no difference in pleural infections (p=0.68) between the IPC (N=34) and pleurodesis groups (N=31)83. This study also demonstrated that patients who received IPC spent less time in the hospital than those who received pleurodesis (3 days vs. 10 days, p< 0.001). In an attempt to better define infections from IPCs, Fysh et al84 retrospectively evaluated 1021 with IPCs for MPEs patients from 11 centers in North America, Europe and Australia and found that only 50 patients (4.9%) developed an infection related to the IPC. In this study, the overall mortality risk from pleural infections in that population was only 0.29%. Photodynamic therapy is not commonly used
5. Conclusions
There are numerous different techniques that can be used to treat central airway obstruction. Although this variety exists, each modality has a niche for when it is most effective. Laser therapy is most effective if the mass is approachable parallel to the airway wall to avoid and can lead to both resection and vaporization of the mass. Electrocautery can also cause resection and vaporization with the benefit of having multiple electrodes that can be used in different circumstances. Argon plasma coagulation is ideal for smaller masses due to the lack of vaporization, and does not need to be parallel to the lesion as the argon plasma reacts with the closest tissue. Photodynamic therapy is more commonly used in adjunct to other therapies, given its lasting effects due to the photosensitizing medication that is used. Cryoablation allows for large, well-preserved biopsies or debulking, and due to cartilage and connective tissue resistance, has relatively fewer side effects than other treatment modalities. Balloon bronchoplasty allows for immediate relief of a stenotic or obstructed airway, although repeat procedures are frequently required. Stents are commonly used in conjunction with other techniques to lead to a longer lasting airway patency. External beam radiation therapy is the mainstay of radiation treatment and leads to improved survival over endobroncial brachytherapy. The combination therapy of EBRT with stenting seems to have the best outcomes on patient survival with the current available data.
For malignant pleural effusions, the data is still controversial. Recommendations for repeated thoracentesis are for patients with less than one-month survival, but to avoid repeated procedures, these patients may benefit from IPC placement or pleurodesis. When comparing IPC placement and pleurodesis, it appears that IPC has a shorter duration of hospital length of stay, although an increased risk in adverse events. Both of these procedures often lead to patient discomfort, leading the performing physician to manage patient’s acute pain. Overall, the use of IPC versus pleurodesis is still up for debate as to which is the superior therapy.
6. Expert Commentary
In the right setting, palliative procedures improve quality of life for patients with advanced lung cancer85. The challenge is choosing which procedure will be effective and thus it is extremely important that patients be referred to centers with multidisciplinary teams, including interventional pulmonologists, who can help identify the most appropriate procedures.
Bronchoscopic interventions are safe and effective for patients with central airway obstruction, but more randomized controlled trials are needed comparing interventions to improve our knowledge in this area. Although many modalities exist to treat CAO, it is important to remember that these are rarely used as a singular therapy and often times multiple techniques are used for the recanalization of an airway. Talc pleurodesis and indwelling pleural catheters are effective treatments for patients with malignant pleural effusions. Historically there is more experience with pleurodesis, but recent data on IPCs have been promising. It is important that patients be referred to centers with a high level of expertise in performing these procedures, to further decrease the risk of complications and increase the chance of procedural success. It is imperative to consider patient preference and quality of life when deciding on a treatment modality for MPE.
In emergency situations, we recommend immediate rigid bronchoscopy. This will allow the interventional pulmonologist to obtain an airway for ventilation, and use the larger tools discussed above to relieve the airway obstruction. Some of the modalities discussed have more delayed effects and will be less likely used in an emergent situation; these include PDT, cryotherapy, EBB and EBRT. Despite this, it is relatively common for these patients to start adjunctive EBRT within days after relief of a more acute airway obstruction. In the non-emergent situation, the therapy needs to be tailored to the individual patient, taking into account not just anatomic disease, but also likelihood of effectiveness, and most importantly overall patient goals (relief of dyspnea, less time in the hospital etc.).
7. Five-year view
The modality used to treat central airway obstruction will be relatively unchanged over the coming years. As technology advances and new techniques are developed then we may see shifts in treatment strategies. 3-D printed stents tailored towards an individual airway is currently being developed and may prove more beneficial than silicone or SEMS stents, but at this time it is unclear. The use of IPCs for malignant pleural effusions may become the mainstay of treatment, as new catheters are being developed to overcome the main serious adverse effect of pleural infection and numerous ongoing trials are focusing on both patient preference and quality of life.
Key issues.
Lung cancer is the leading cause of cancer related deaths in the United States, has a poor 5-year survival (4.5%), and these patients frequently experience central airway obstruction and/or malignant pleural effusions.
The treatment of malignant central airway obstruction can be divided into bronchoscopic or radiation therapy.
Bronchoscopic interventions are commonly done using rigid bronchoscopes and various techniques including laser, electrocautery, argon plasma coagulation, photodynamic therapy, cryoablation, balloon bronchoplasty and airway stenting.
The use of individual bronchoscopic interventions is dependent on the goal of the procedure.
External Beam Radiation Therapy is the preferred modality of radiation therapy for these patients, and has the strongest data to support its use.
Current data suggests that combination airway stenting and external beam radiation therapy is beneficial for patients that present with malignant airway obstruction.
The treatment of malignant pleural effusions can be treated via thoracentesis, intra-pleural catheter or pleurodesis.
Repeated thoracentesis is not recommended if the patient has an expected survival of greater than 1 month.
Talc is the preferred agent to be used in pleurodesis.
Indwelling pleural catheters lead to less time in the hospital, although a higher risk of adverse events, in comparison to pleurodesis alone.
Acknowledgements
The authors would like to thank Jon Baer for contributing to the medical image illustrations.
Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.American Cancer Society. Cancer Facts and Figures 2018. 2018. [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83(5):584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER Cancer Stat Facts: Lung and Bronchus Cancer. National Cancer Institute, Bethesda, MD: http://seer.cancer.gov/statfacts/html/lungb.html Accessed 2/15/2018. [Google Scholar]
- 4.Pronzato P, Bertelli G, Losardo P, Landucci M. What do advanced cancer patients know of their disease? A report from Italy. Support Care Cancer. 1994;2(4):242–244. [DOI] [PubMed] [Google Scholar]
- 5.Eidinger RN, Schapira DV. Cancer patients’ insight into their treatment, prognosis, and unconventional therapies. Cancer. 1984;53(12):2736–2740. [DOI] [PubMed] [Google Scholar]
- 6.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–1714. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol 2008;26(23):3860–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadakia KC, Moynihan TJ, Smith TJ, Loprinzi CL. Palliative communications: addressing chemotherapy in patients with advanced cancer. Ann Oncol 2012;23 Suppl 3:29–32. [DOI] [PubMed] [Google Scholar]
- 9.Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med 2004;169(12):1278–1297. [DOI] [PubMed] [Google Scholar]
- 10.American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162(5):1987–2001. [DOI] [PubMed] [Google Scholar]
- 11.Dunger C, Higginson IJ, Gysels M, Booth S, Simon ST, Bausewein C. Breathlessness and crises in the context of advanced illness: A comparison between COPD and lung cancer patients. Palliat Support Care. 2015;13(2):229–237. [DOI] [PubMed] [Google Scholar]
- 12.Yarmus L, Ernst A, Feller-Kopman D. Emerging technologies for the thorax: indications, management and complications. Respirology. 2010;15(2):208–219. [DOI] [PubMed] [Google Scholar]
- 13.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ, Group BTSPDG. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii32–40. [DOI] [PubMed] [Google Scholar]
- 14.Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e455S–497S. [DOI] [PubMed] [Google Scholar]
- 15.Beamis JF, Jr. Interventional pulmonology techniques for treating malignant large airway obstruction: an update. Curr Opin Pulm Med 2005;11(4):292–295. [DOI] [PubMed] [Google Scholar]
- 16.Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP. Dyspnea review for the palliative care professional: treatment goals and therapeutic options. J Palliat Med 2012;15(1):106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtis JL, Mahlmeister M, Fink JB, Lampe G, Matthay MA, Stulbarg MS. Helium-oxygen gas therapy. Use and availability for the emergency treatment of inoperable airway obstruction. Chest. 1986;90(3):455–457. [DOI] [PubMed] [Google Scholar]
- 18.Morrison RS, Maroney-Galin C, Kralovec PD, Meier DE. The growth of palliative care programs in United States hospitals. J Palliat Med 2005;8(6):1127–1134. [DOI] [PubMed] [Google Scholar]
- 19.Barach AL. The use of helium in the treatment of asthma and obstructive lesions in the larynx and trachea. Annals of Internal Medicine. 1935;9(6):739–765. [Google Scholar]
- 20.Pathak V, Welsby I, Mahmood K, Wahidi M, MacIntyre N, Shofer S. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc 2014;11(4):628–634. [DOI] [PubMed] [Google Scholar]
- 21.McDougall JC, Cortese DA. Neodymium-YAG laser therapy of malignant airway obstruction. A preliminary report. Mayo Clin Proc 1983;58(1):35–39. [PubMed] [Google Scholar]
- 22.Van Der Spek AF, Spargo PM, Norton ML. The physics of lasers and implications for their use during airway surgery. British journal of anaesthesia. 1988;60(6):709–729. [DOI] [PubMed] [Google Scholar]
- 23.Cavaliere S, Foccoli P, Farina PL. Nd:YAG laser bronchoscopy. A five-year experience with 1,396 applications in 1,000 patients. Chest. 1988;94(1):15–21. [DOI] [PubMed] [Google Scholar]
- 24.Dumon JF, Shapshay S, Bourcereau J, et al. Principles for safety in application of neodymium-YAG laser in bronchology. Chest. 1984;86(2):163–168. [DOI] [PubMed] [Google Scholar]
- 25.Hetzel MR, Nixon C, Edmondstone WM, et al. Laser therapy in 100 tracheobronchial tumours. Thorax. 1985;40(5):341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159–165. [DOI] [PubMed] [Google Scholar]
- 27.Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest. 1996;110(6):1536–1542. [DOI] [PubMed] [Google Scholar]
- 28.Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest. 2000;118(2):516–521. [DOI] [PubMed] [Google Scholar]
- 29.Wahidi MM, Unroe MA, Adlakha N, Beyea M, Shofer SL. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol 2011;6(9):1516–1520. [DOI] [PubMed] [Google Scholar]
- 30.Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endoscopic surgery and allied technologies. 1994;2(1):42–46. [PubMed] [Google Scholar]
- 31.Kizilgoz D, Aktas Z, Yilmaz A, Ozturk A, Segmen F. Comparison of two new techniques for the management of malignant central airway obstruction: argon plasma coagulation with mechanical tumor resection versus cryorecanalization. Surgical endoscopy. 2017. [DOI] [PubMed] [Google Scholar]
- 32.Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119(3):781–787. [DOI] [PubMed] [Google Scholar]
- 33.Moghissi K, Dixon K. Update on the current indications, practice and results of photodynamic therapy (PDT) in early central lung cancer (ECLC). Photodiagnosis Photodyn Ther 2008;5(1):10–18. [DOI] [PubMed] [Google Scholar]
- 34.Moghissi K, Dixon K, Stringer M, Freeman T, Thorpe A, Brown S. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15(1):1–6. [DOI] [PubMed] [Google Scholar]
- 35.Noppen M, Meysman M, Van Herreweghe R, Lamote J, D’Haese J, Vincken W. Bronchoscopic cryotherapy: preliminary experience. Acta Clin Belg 2001;56(2):73–77. [DOI] [PubMed] [Google Scholar]
- 36.Maiwand MO, Homasson JP. Cryotherapy for tracheobronchial disorders. Clinics in chest medicine. 1995;16(3):427–443. [PubMed] [Google Scholar]
- 37.Marasso A, Gallo E, Massaglia GM, Onoscuri M, Bernardi V. Cryosurgery in bronchoscopic treatment of tracheobronchial stenosis. Indications, limits, personal experience. Chest. 1993;103(2):472–474. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DA, Maiwand MO, Nath AR, Lockwood P, Lloyd MH, Saab M. Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax. 1990;45(7):509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller C, Frost A. Fiberoptic bronchoplasty. Description of a simple adjunct technique for the management of bronchial stenosis following lung transplantation. Chest. 1992;102(4):995–998. [DOI] [PubMed] [Google Scholar]
- 40.Hautmann H, Gamarra F, Pfeifer KJ, Huber RM. Fiberoptic bronchoscopic balloon dilatation in malignant tracheobronchial disease: indications and results. Chest. 2001;120(1):43–49. [DOI] [PubMed] [Google Scholar]
- 41.Casal RF. Update in airway stents. Curr Opin Pulm Med 2010;16(4):321–328. [DOI] [PubMed] [Google Scholar]
- 42.Dumon JF. A dedicated tracheobronchial stent. Chest. 1990;97(2):328–332. [DOI] [PubMed] [Google Scholar]
- 43.Dumon MC, Dumon JF, Perrin C, Blaive B. [Silicone tracheobronchial endoprosthesis]. Rev Mal Respir 1999;16(4 Pt 2):641–651. [PubMed] [Google Scholar]
- 44.Lund ME, Garland R, Ernst A. Airway stenting: Applications and practice management considerations. Chest. 2007;131(2):579–587. [DOI] [PubMed] [Google Scholar]
- 45.Bolliger CT, Probst R, Tschopp K, Soler M, Perruchoud AP. Silicone stents in the management of inoperable tracheobronchial stenoses. Indications and limitations. Chest. 1993;104(6):1653–1659. [DOI] [PubMed] [Google Scholar]
- 46.Bolliger CT, Heitz M, Hauser R, Probst R, Perruchoud AP. An Airway Wallstent for the treatment of tracheobronchial malignancies. Thorax. 1996;51(11):1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyazawa T, Yamakido M, Ikeda S, et al. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest. 2000;118(4):959–965. [DOI] [PubMed] [Google Scholar]
- 48.Saad CP, Murthy S, Krizmanich G, Mehta AC. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest. 2003;124(5):1993–1999. [DOI] [PubMed] [Google Scholar]
- 49.Wood DE, Liu YH, Vallieres E, Karmy-Jones R, Mulligan MS. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76(1):167–172; discussion 173-164. [DOI] [PubMed] [Google Scholar]
- 50.Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90(4):1088–1093. [DOI] [PubMed] [Google Scholar]
- 51.Klopp AH, Eapen GA, Komaki RR. Endobronchial Brachytherapy: An Effective Option for Palliation of Malignant Bronchial Obstruction. Clinical Lung Cancer. 2006;8(3):203–207. [DOI] [PubMed] [Google Scholar]
- 52.Seaman JC, Musani AI. Endobronchial Ablative Therapies. Clinics in Chest Medicine. 2013;34(3):417–425. [DOI] [PubMed] [Google Scholar]
- 53.Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys 2011;80(3):692–697. [DOI] [PubMed] [Google Scholar]
- 54.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24(30):4833–4839. [DOI] [PubMed] [Google Scholar]
- 55.Rodrigues G, Videtic GM, Sur R, et al. Palliative thoracic radiotherapy in lung cancer: An American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol 2011;1(2):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mallick I, Sharma SC, Behera D, Ghoshal S, Oinam AS. Optimization of dose and fractionation of endobronchial brachytherapy with or without external radiation in the palliative management of non-small cell lung cancer: a prospective randomized study. J Cancer Res Ther 2006;2(3):119–125. [DOI] [PubMed] [Google Scholar]
- 57.Sur R AS, Donde B et al. . Brachytherapy boost vs teletherapy boost in palliation of symptomatic, locally advanced non-small cell lung cancer: Preliminary analysis of a randomized, prospective study. 2001(17):309–315. [Google Scholar]
- 58.Langendijk H, de Jong J, Tjwa M, et al. External irradiation versus external irradiation plus endobronchial brachytherapy in inoperable non-small cell lung cancer: a prospective randomized study. Radiother Oncol 2001;58(3):257–268. [DOI] [PubMed] [Google Scholar]
- 59.Stout R, Barber P, Burt P, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol 2000;56(3):323–327. [DOI] [PubMed] [Google Scholar]
- 60.Lester JF, Macbeth FR, Toy E, Coles B. Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev 2006(4):CD002143. [DOI] [PubMed] [Google Scholar]
- 61.Chetty KG, Moran EM, Sassoon CS, Viravathana T, Light RW. Effect of radiation therapy on bronchial obstruction due to bronchogenic carcinoma. Chest. 1989;95(3):582–584. [DOI] [PubMed] [Google Scholar]
- 62.Mallow C, Thiboutot J, Semaan R, et al. Combination External Beam Radiation Therapy with Airway Stenting leads to Improved Survival in Patients with Malignant Central Airway Obstruction. Respirology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chella A, Ambrogi MC, Ribechini A, et al. Combined Nd-YAG laser/HDR brachytherapy versus Nd-YAG laser only in malignant central airway involvement: a prospective randomized study. Lung Cancer. 2000;27(3):169–175. [DOI] [PubMed] [Google Scholar]
- 64.Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med 2012;186(6):487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stather DR, Tremblay A. Use of tunneled pleural catheters for outpatient treatment of malignant pleural effusions. Curr Opin Pulm Med 2007;13(4):328–333. [DOI] [PubMed] [Google Scholar]
- 66.Schulze M, Boehle AS, Kurdow R, Dohrmann P, Henne-Bruns D. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg 2001;71(6):1809–1812. [DOI] [PubMed] [Google Scholar]
- 67.Genc O, Petrou M, Ladas G, Goldstraw P. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur J Cardiothorac Surg 2000;18(2):143–146. [DOI] [PubMed] [Google Scholar]
- 68.Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2016(5):Cd010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004(1):CD002916. [DOI] [PubMed] [Google Scholar]
- 70.Kennedy L, Rusch VW, Strange C, Ginsberg RJ, Sahn SA. Pleurodesis using talc slurry. Chest. 1994;106(2):342–346. [DOI] [PubMed] [Google Scholar]
- 71.Campos JR, Werebe EC, Vargas FS, Jatene FB, Light RW. Respiratory failure due to insufflated talc. Lancet. 1997;349(9047):251–252. [DOI] [PubMed] [Google Scholar]
- 72.de Campos JR, Vargas FS, de Campos Werebe E, et al. Thoracoscopy talc poudrage : a 15-year experience. Chest. 2001;119(3):801–806. [DOI] [PubMed] [Google Scholar]
- 73.Janssen JP, Collier G, Astoul P, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369(9572):1535–1539. [DOI] [PubMed] [Google Scholar]
- 74.Xia H, Wang XJ, Zhou Q, Shi HZ, Tong ZH. Efficacy and safety of talc pleurodesis for malignant pleural effusion: a meta-analysis. PLoS One. 2014;9(1):e87060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parulekar W, Di Primio G, Matzinger F, Dennie C, Bociek G. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest. 2001;120(1):19–25. [DOI] [PubMed] [Google Scholar]
- 76.Clementsen P, Evald T, Grode G, Hansen M, Krag Jacobsen G, Faurschou P. Treatment of malignant pleural effusion: pleurodesis using a small percutaneous catheter. A prospective randomized study. Respir Med 1998;92(3):593–596. [DOI] [PubMed] [Google Scholar]
- 77.Caglayan B, Torun E, Turan D, et al. Efficacy of iodopovidone pleurodesis and comparison of small-bore catheter versus large-bore chest tube. Ann Surg Oncol 2008;15(9):2594–2599. [DOI] [PubMed] [Google Scholar]
- 78.Antunes G, Neville E, Duffy J, Ali N, Pleural Diseases Group SoCCBTS. BTS guidelines for the management of malignant pleural effusions. Thorax. 2003;58 Suppl 2:ii29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Putnam JB, Jr. Malignant pleural effusions. Surg Clin North Am 2002;82(4):867–883. [DOI] [PubMed] [Google Scholar]
- 80.Sioris T, Sihvo E, Salo J, Rasanen J, Knuuttila A. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol 2009;35(5):546–551. [DOI] [PubMed] [Google Scholar]
- 81.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–2389. [DOI] [PubMed] [Google Scholar]
- 82.Thomas R, Fysh ETH, Smith NA, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. Jama. 2017;318(19):1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142(2):394–400. [DOI] [PubMed] [Google Scholar]
- 84.Fysh ET, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144(5):1597–1602. [DOI] [PubMed] [Google Scholar]
- 85.Amjadi K, Voduc N, Cruysberghs Y, et al. Impact of interventional bronchoscopy on quality of life in malignant airway obstruction. Respiration; international review of thoracic diseases. 2008;76(4):421–428. [DOI] [PubMed] [Google Scholar]


