Abstract
BACKGROUND:
Prophylactic anticoagulation is routinely used in the inpatient setting; however, the risk of venous thromboembolism (VTE) remains elevated after discharge. Extensive evidence and clinical guidelines suggest post-discharge VTE prophylaxis is critical in at-risk populations, but it remains severely underused in practice.
STUDY DESIGN:
We performed a single-institution retrospective, nonrandomized, pre- and post-intervention analysis of a systematic post-discharge pharmacologic prophylaxis program against the primary end point, which is post-discharge symptomatic VTE. An institutional American College of Surgeons NSQIP dataset was used to identify patients and outcomes. Patients undergoing major abdominal surgery for malignancy or inflammatory bowel disease were eligible for the post-discharge VTE prevention program.
RESULTS:
Among 1,043 patients who underwent abdominal surgery for malignancy or inflammatory bowel disease, 800 (77%) were in the pre-intervention cohort and 243 (23%) patients were in the post-intervention cohort. Rates of inpatient VTE did not significantly differ between cohorts (0.7%, n = 6 pre-intervention vs 1.7%, n = 4 post-intervention; p However, compared the pre-intervention cohort, patients in = 0.25). the post-intervention cohort demonstrated a significantly lower post-discharge VTE rate (2.5%, n = 20 pre-intervention vs 0.0%, n = 0 post-intervention; p < 0.01).
CONCLUSIONS:
A systematic post-discharge VTE prophylaxis program including provider education, local guideline adaptation, bedside medication delivery, and education for at-risk patients, was associated with significantly fewer post-discharge VTE events.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a well-described, frequently preventable complication of major abdominal and pelvic surgery.1 Venous thromboembolism is the most common preventable cause of death within 30 days of an operation for intra-abdominal malignancy, and rates of postoperative VTE are even higher in inflammatory bowel disease (IBD).2,3 Pharmacologic prophylaxis against VTE has repeatedly been demonstrated to be safe and effective and, as such, has been widely adopted in the inpatient setting.4,5 Critically, however, at least 30% to 50% of VTEs occur after discharge for patients undergoing abdominal surgery for malignancy or IBD.2,3,6,7
Randomized, double-blinded trials have demonstrated the efficacy of VTE prophylaxis in the post-discharge setting with a large body of evidence supporting the practice as safe and cost-effective.8–12 As a result, for the past decade multiple professional societies, including the American College of Chest Physicians, the National Cancer Care Network, and the American Society of Clinical Oncology, have recommended routine post-discharge prophylaxis after major abdominopelvic cancer surgery.4,13,14 Despite this, post-discharge prophylaxis remains underused.15,16 Barriers to improved post-discharge prophylaxis rates are not well described, but are posited to include costs, lack of awareness, lack of local adaptation of national guidelines, and logistical challenges associated with ensuring patient compliance.17,18
We sought to address these challenges through the implementation of a systematic post-discharge VTE prophylaxis program for high-risk patients after abdominal surgery for malignancy or IBD at an academic medical center. The purpose of this study was to examine the impact of post-discharge VTE prophylaxis implementation on eligible patients by comparing rates of post-discharge VTE before and after implementation of this initiative.
METHODS
Study population
We performed a retrospective analysis of a nonrandomized, pre- and post-intervention quality improvement protocol at a quaternary care center. The study was approved by the IRB of the Brigham and Women’s Hospital. An institutional American College of Surgeons (ACS) NSQIP dataset was used to identify patients who underwent a general surgery procedure for intra-abdominal malignancy or IBD between January 1, 2012 and June 30, 2015. The ACS NSQIP is a sample of all inpatient and outpatient operations requiring general, spinal, or epidural anesthesia, excluding trauma surgery and transplantation surgery.19 In the ACS NSQIP, preoperative risk factors, intraoperative factors, and postoperative outcomes are collected by trained research nurses. Demographic and clinical factors were compared between pre-intervention and post-intervention cohorts, including preoperative comorbidities, the American Society of Anesthesiologists Physical Status Classification and preoperative functional health status. Preoperative functional status is an ACS NSQIP variable defined as “independent,” “partially dependent,” “dependent,” or “unknown,” based on the best functional status in the 30 days before surgery.19
Inclusion criteria were diagnoses of malignancy or IBD (based on ICD-9 codes) among patients who underwent abdominal general surgery primary procedures (based on CPT codes). Among patients who underwent more than one admission for separate procedures, only the first admission was considered to be in the risk set. Exclusion criteria were used to improve external validity (ie general-izability) and included history of sepsis or systemic inflammatory response syndrome, dyspnea at rest, nonin-dependent functional status, nonelective case, American Society of Anesthesiologists class 5, and ventilator dependence. The ACS NSQIP database measures outcomes within a 30-day follow-up period, which potentially creates bias from censoring. Venous thromboembolism might be more likely to develop before discharge in participants with long postoperative lengths of hospital stay (left censoring). Additionally, the time at risk for a post-discharge event developing is decreased among participants with long postoperative length of stay. For example, a 29-day postoperative length of stay would leave only 1 day at risk for postoperative VTE (right censoring). To avoid differential lengths of stay and time at risk for events, participants with postoperative lengths of stay more than 14 days were excluded.
Quality improvement initiative
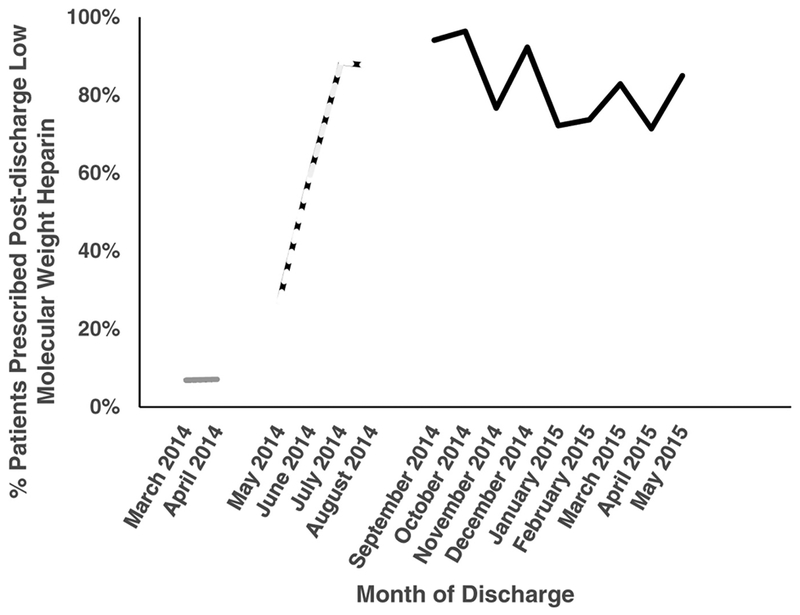
An institution-wide quality improvement initiative was undertaken to reduce post-discharge VTE. The initiative involved prescribing and delivering a 4-week outpatient supply of low molecular weight heparin (LMWH; including syringes) to the bedside of eligible patients before discharge. Patients were given predischarge instruction by trained nursing staff. The baseline, pre-intervention period for identification of VTE extended from January 1, 2012 to April 30, 2014. Patients who underwent surgery during a peri-implementation “wash-in” period, when compliance was <90% for prescribing post-discharge LMWH (May 1, 2014 to August 31, 2014), were excluded from analysis. The post-intervention period was defined as September 1, 2014 to June 30, 2015. Sensitivity analysis including the wash-in period in the analysis was performed. During the inpatient stay, patients received unfractionated heparin or LMWH pre- and postoperatively, although inpatient prophylaxis was not systematically controlled.
A hospital-wide multidisciplinary VTE prevention task force led collaboratively by the Department of Surgery and Department of Quality and Safety was created and charged with ensuring adherence to evidence-based best practices in VTE prophylaxis. The group contained broad expert representation from surgery, hematology, vascular medicine, internal medicine, anesthesia, clinical pharmacy services, and nursing. This task force developed a hospital-specific post-discharge prophylaxis algorithm for at-risk surgical patients. The algorithm explicitly addressed inclusion and exclusion criteria and included a detailed risk-assessment tool based on a well-validated instrumentdthe Caprini score.20 Patients were included if they underwent a major abdominal or pelvic surgical procedure for cancer or IBD and had a Caprini score ≥5 on discharge; patients with fluctuating renal function or concern for postoperative bleeding were excluded. Specific guidance on LMWH selection and dosing was also provided (enoxaparin 40 mg subcutaneously daily for 28 days postoperatively).
Dissemination of the algorithm took place in Department of Surgery clinical and quality committees, as well as morbidity and mortality conferences. To monitor adherence to the newly implemented algorithm, ACS NSQIP nurse reviewers collected real-time data on procedure, discharge risk score, and discharge medications in a separately maintained electronic database. The rate of post-discharge prophylaxis prescription in eligible patients was recorded in this database and regularly communicated to surgical teams during follow-up morbidity and mortality conferences.
During implementation of the new protocol, the high volume of LMWH prescriptions on discharge revealed the challenges in highly variable insurance coverage and frequent unavailability in commercial outpatient pharmacies. Accordingly, the Departments of Surgery and Quality and Safety, in collaboration with the hospital’s outpatient pharmacy, developed a bedside medication delivery program for post-discharge enoxaparin during the wash-in phase. This allowed surgical teams to ensure that patients had the medication in-hand at the time of discharge and that patients were well trained on prophylaxis self-administration. Additionally, the outpatient pharmacy was usually able to bill patients’ prescription benefit plans for the retail cost of the enoxaparin. This revenue stream, although variable depending on insurance coverage, resulted in substantial additional margin enabling both additional pharmacy staffing to cope with higher prescription volume, as well as financial assistance to patients with no insurance or high copays.
End points
The primary end point was symptomatic initial post-discharge VTE. Venous thromboembolism was defined as the presence of PE (defined by ACS NSQIP as a new diagnosis of a new thrombus in a pulmonary artery with evidence of PE on a definitive imaging study) and/or the presence of DVT (defined by ACS NSQIP as a new diagnosis of a new superficial or DVT that warrants treatment, confirmed by a definitive imaging modality). By NSQIP definition, all VTEs were clinically significant, requiring therapeutic doses of anticoagulation or IVC filter unless declined by the patient or clinically contraindicated. Secondary end points included initial inpatient VTE, unplanned readmission, mortality, and post-discharge bleeding events. An event occurring on the day of discharge was considered an inpatient event, given that the LMWH intervention did not begin until the day after discharge. In both pre- and post-intervention cohorts, follow-up time extended to 30 days postoperatively per ACS NSQIP protocol.
Statistical analysis
We stratified patients into 2 groups, defined in relation to the implementation of the post-discharge VTE prevention initiative: the pre-intervention cohort (January 1, 2012 to April 30, 2014) and the post-intervention cohort (September 1, 2014 to June 30, 2015). As mentioned, patients in the peri-implementation period were excluded from the main analysis, but included in the sensitivity analysis. Of note, given that this was a real-world implementation analysis, not all patients in the post-intervention cohort received enoxaparin (nor were all patients eligible for it based on our risk-assessment algorithm) and, conversely, patients in the pre-intervention cohort might have been prescribed post-discharge enoxaparin. In contradistinction, the study involves an intent-to-treat analysis of the implementation of a post-discharge enoxaparin intervention. Univariable analyses were performed to compare pre- and post-intervention cohorts with respect to the primary and secondary end points. Chi-square or Fisher’s exact tests were used to compare categorical variables between groups. Mann-Whitney U tests were used to compare continuous variables between groups.
We conducted sensitivity analyses among patients ineligible for the protocol. Specifically, we compared the rates of VTE during pre-intervention and post-intervention phases among patients who underwent nonabdominal surgery or who underwent abdominal surgery for indications other than malignancy or IBD and, therefore, did not systematically receive post-discharge LMWH as part of this quality improvement initiative. Additionally, we conducted a separate analysis including the wash-in period. Finally, we conducted a separate analysis including only 2014 to 2015 to better control for possible changes in VTE rate over time.
RESULTS
A total of 1,043 patients underwent abdominal surgery for malignancy (92% [n = 963]) or IBD (8%[n = 80]) during the study period. Of these, 800 (77%) were in the pre-intervention cohort and did not systematically receive post-discharge LMWH. The remaining 243 (23%) patients were in the post-intervention cohort and were exposed to the quality improvement initiative. Rates of compliance with enoxaparin prescription in the pre-, peri-, and post-intervention phases are displayed in Figure 1. Underlying diagnosis and rates of colectomy, hepatectomy, and pancreatectomy were similar between cohorts. At baseline, the post-intervention cohort was significantly more likely than the pre-intervention cohort to have a disseminated malignancy and to have higher (more severe) American Society of Anesthesiologists Physical Status Classification. Details of baseline characteristics in each cohort are displayed in Table 1.
Figure 1.
Monthly percentage of low molecular weight heparin prescriptions on discharge among eligible patients. Solid gray line, pre-intervention phase; dashed line, “wash-in” phase; solid black line, post-intervention phase.
Table 1.
Baseline Characteristics of Patients Undergoing Abdominal Surgery for Malignancy or Inflammatory Bowel Disease (n = 1,043)
| Variable | Pre-intervention | Post-intervention | p Value |
|---|---|---|---|
| n (%) | 800 (77) | 243 (23) | |
| Age, y, median (range) | 59.9 (50.3–69.2) | 58.6 (49.1–68.4) | 0.40 |
| Female, n (%) | 403 (50) | 119 (49) | 0.70 |
| White, n (%) | 728 (94) | 222 (95) | 0.59 |
| Procedure, n (%) | |||
| Colectomy | 435 (54) | 127 (52) | 0.56 |
| Hepatectomy | 119 (15) | 37 (15) | 0.89 |
| Pancreatectomy | 87 (11) | 30 (12) | 0.52 |
| Indication, n (%) | |||
| Malignancy | 739 (92) | 224 (92) | 0.92 |
| Inflammatory bowel disease | 61 (8) | 19 (8) | 0.92 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 91 (11) | 30 (12) | 0.68 |
| Smoking | 84 (11) | 36 (15) | 0.06 |
| Dyspnea | 62 (8) | 14 (6) | 0.30 |
| COPD | 15 (2) | 4 (2) | 0.99 |
| Hypertension | 324 (41) | 104 (43) | 0.52 |
| Disseminated malignancy | 162 (20) | 70 (29) | <0.01 |
| Chronic steroids | 53 (7) | 23 (9) | 0.13 |
| Weight loss | 52 (7) | 19 (8) | 0.47 |
| Bleeding disorder | 26 (3) | 13 (5) | 0.13 |
| BMI, kg/m2, median (range) | 26.9 (23.5–30.9) | 27.5 (23.8–32.4) | 0.24 |
| ASA classification, n (%) | <0.01 | ||
| I | 6 (1) | 2 (1) | |
| II | 367 (46) | 69 (28) | |
| III | 424 (53) | 172 (71) | |
| IV | 3 (0.4) | 0 (0) | |
| Operative duration, min, median (range) | 175 (120–258) | 173 (125–248) | 0.88 |
| Thrombocytopenia, n (%) | 54 (7) | 17 (7) | 0.87 |
| Thrombocytosis, n (%) | 29 (4) | 11 (5) | 0.51 |
| Albumin <3.0 g/dL, n (%) | 11 (2) | 1 (1) | 0.32 |
| International normalized ratio >1.5 U, n (%) | 1 (1) | 1 (1) | 0.40 |
ASA, American Society of Anesthesiologists Physical Status Classification.
The overall rate of VTE was 2.9% (n = 30), including 10 inpatient and 20 post-discharge events. There was no significant difference in inpatient VTE occurrence between the pre-intervention (0.7% [n = 6]) and post-intervention cohorts (1.7% [n = 4]) (p = 0.25). The pri mary end point of post-discharge VTE occurred in 20 of 800 (2.5%) eligible patients in the pre-intervention cohort, and no VTE events were reported among the 243 (0.0%) patients in the post-intervention group (p < 0.01). Of the 20 post-discharge VTEs, 25% (n = 5) occurred during post-discharge week 1, 30% (n = 6) occurred during post-discharge week 2, 30% (n = 6) occurred during post-discharge week 3, and 15% (n = 3) occurred during post-discharge week 4. Table 2 reports the details of VTE in each cohort.
Table 2.
Proportion of Venous Thromboembolism among Patients Eligible and Those Not Eligible for Post-Discharge Low Molecular Weight Heparin (n = 1,043)
| Variable | Pre-intervention | Post-intervention | p Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Eligible | |||||
| Post-discharge | |||||
| VTE | 20 | 2.5 | 0 | 0.0 | <0.01 |
| DVT | 15 | 1.9 | 0 | 0.0 | 0.03 |
| PE | 5 | 0.6 | 0 | 0.0 | 0.60 |
| Inpatient | |||||
| VTE | 6 | 0.7 | 4 | 1.7 | 0.25 |
| DVT | 6 | 0.7 | 2 | 0.8 | >0.99 |
| PE | 0 | 0.0 | 2 | 0.8 | 0.05 |
| Not eligible* | |||||
| Post-discharge | 12 | 0.5 | 3 | 0.6 | >0.99 |
| VTE | |||||
| DVT | 10 | 0.4 | 3 | 0.6 | 0.72 |
| PE | 5 | 0.2 | 0 | 0.0 | 0.59 |
| Inpatient | |||||
| VTE | 41 | 0.2 | 1 | 0.2 | >0.99 |
| DVT | 3 | 0.1 | 1 | 0.2 | 0.57 |
| PE | 1 | 0.04 | 0 | 0.0 | >0.99 |
Patients who underwent any surgery other than abdominal surgery for malignancy or inflammatory bowel disease; n 2,834. DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
We conducted 3 sensitivity analyses. First, we evaluated VTE rates among patients ineligible for post-discharge prophylaxis. The most common procedures in the group of patients who were ineligible for the post-discharge LMWH initiative were bariatric operations (34% [n = 1,074]), operations on small or large bowel for indications other than malignancy or IBD (20% [n = 636]), soft tissue procedures (13% [n = 415]), and hernia repair operations (11% [n = 351]). Among such patients who carried diagnoses or underwent procedures that rendered them ineligible for the post-discharge LMWH initiative, there were no signifi-cant differences in rates of outpatient VTE during the pre-intervention and post-intervention phases (Table 2). A second sensitivity analysis was conducted to control for possible temporal trends. A pre-intervention decrease in outpatient VTE rate from4.2% in the year 2012 to 0.9% in the year 2013 was noted. We performed a subset analysis including data closely surrounding the time of the intervention. In this analysis, limited to years 2014 and 2015, the pre-intervention VTE rate was 2.6% (n = 3), compared with post-intervention VTE rate of 0% (n = 0; p = 0.03). Finally, we performed a sensitivity analysis including the wash-in period. Including these patients, the pre-intervention post-discharge VTE rate was 2.5% (n = 20), compared with a post-intervention post-discharge VTE rate of 0.8% (n = 3; p 0.04).
There were no post-discharge bleeding events detected among patients in either the pre-intervention or post-intervention cohorts. Unplanned readmission was considerably higher among patients who sustained post-discharge VTE (85% [17 of 20]) compared with those who did not sustain post-discharge VTE (12% [125 of 1,023]; p < 0.01). Within the first 30 postoperative days, 4 deaths occurred in the pre-intervention period and none in the post-intervention period.
DISCUSSION
Prophylaxis against VTE after major abdominal surgery is a common, well-accepted practice in the inpatient setting.16,21 The risk of VTE, however, extends beyond discharge: at least 30% to 50% of postoperative VTE events occur in the outpatient setting.2,3,6,7 Patients with underlying malignancy or IBD are at particularly elevated risk.3,6 Despite more than a decade of strong evidence to support its use, post-discharge prophylaxis remains under-used in practice.15,16 The barriers to implementation have not been well described, but likely include cost, failure to recognize patient VTE risk, lack of local adaptation of evidence-based clinical practice guidelines and logistical challenges associated with maximizing patient compliance.17,18 Accordingly, we implemented and assessed a comprehensive post-discharge prophylaxis program for patients undergoing major abdominal surgery for cancer or IBD. We found that implementation of this program was associated with substantially reduced post-discharge VTE in eligible patients with no concomitant increase in bleeding events.
Venous thromboembolism event rates and the impact of post-discharge prophylaxis
Venous thromboembolism rates reported both pre- and post-intervention in this single-institution study are largely consistent with existing literature. We found an overall 30-day VTE rate of 3.1% pre-intervention, with 76% of events occurring post-discharge. Agnelli and colleagues,2 in a prospective study including >2,000 participants, reported a VTE rate of 2.8% after general surgery procedures for cancer with >70% of events occurring after postoperative day 5.2 In a larger, retrospective study, Hammond and colleagues22 reported an overall 30-day VTE rate of 3.5% after major surgery for cancer, with higher rates after colectomy, gastrectomy, and pancreatectomy. For IBD patients, Gross and colleagues3 recently documented a 30-day postoperative VTE rate of2.7% after abdominal surgery. The post-discharge event rate reported in the current analysis is congruent with the higher end of the published literature due to the comparatively large percentage of our patients with known risk factors, such as disseminated malignancies that might have necessitated complex resections, longer operative times, and steroid use.23–25 Recent trends in the use of laparoscopy and recovery pathways resulting in shorter length of stay might have also contributed to our high proportion of post-discharge events pre-intervention.26
The introduction of post-discharge prophylaxis was associated with a substantial decrease in post-discharge VTE events—from 2.5% pre-intervention to 0.0% post-intervention in our study. Although we observed no events in the post-intervention cohort, this does not indicate a probability of zero (given that the event is not physically impossible). Rather than emphasizing the absolute number found in this study, we focus on the significantly decreased VTE rate with prophylaxis that was achieved in a nonrandomized, real-world clinical setting. This reduction is consistent with clinical trial data showing decreases of VTE rates up to an order of magnitude in the post-discharge prophylaxis arms.9,10,23 Our findings are also consistent with a meta-analysis by Rasmussen and colleagues,8 which showed that symptomatic post-discharge VTE might be reduced to nearly zero by appropriate post-discharge prophylaxis.
Implications for practice
Despite compelling evidence surrounding the effectiveness of post-discharge VTE prophylaxis, the small proportion of eligible patients receiving this treatment nationally is both troublesome and illustrative of the need for surgeons to be actively involved in hospital-level quality improvement.15,16 We believe that many of the barriers to national implementation of evidence-based best practices for VTE prophylaxis lie in the details of local, organizational structure, and practice. Cabana and colleagues27 identified lack of awareness, familiarity, agreement, self-efficacy, and outcomes expectancy along with the inertia of previous practice and external barriers as the most common obstacles to successful guideline adoption. In our implementation, we sought to overcome many of these obstacles simultaneously.
National guidelines are often broadly written so as to be applicable across a wide range of patient populations and practice settings.18 For post-discharge VTE prophylaxis, the published guidelines often do not specify key details, such as patient risk assessment, drug selection and dosing, or exclusion criteria.4,13,14 As a result, in our institution there might have previously been low awareness of, and uncertainty as to how to implement, these guidelines in specific patient populations.
Additionally, many academic medical centers, including ours, are organized in traditional clinical departments. Development and implementation of hospital-specific post-discharge VTE prophylaxis protocols benefit from interdisciplinary expertise in surgery, hematology, vascular medicine, pharmacy, and nursing. Clinical and logistical factors including inpatient and outpatient pharmacy formularies (choice of medication), patient payer mix (which medications are covered; how the medications are covered), and surgical case mix (the volume of patients benefitting from VTE prophylaxis) are all important variables to consider in post-discharge VTE prophylaxis algorithm design. Traditional clinical “silos” can often present challenges to achieving the level of interdisciplinary coordination required to implement cross-disciplinary interventions. The creation of an interdisciplinary working group was instrumental in developing and disseminating VTE prophylaxis protocols and logistical solutions throughout our institution, ensuring surgeons were not left to address the details of post-discharge VTE prophylaxis in a silo.
A strategy to measure adherence was also likely critical to driving adoption once the new protocol was introduced. Several VTE-related metrics were collected and monitored at our institution by virtue of the Surgical Care Improvement Project’s process measures, ACS NSQIP, and AHRQ Patient Safety Indicators, among others. Post-discharge prophylaxis, however, was not routinely monitored before our intervention. We suspect rapid practice change was enhanced by the provision of nonpunitive feedback on post-discharge prophylaxis rates at the department and individual surgeon level.
Finally, external barriers to post-discharge prophylaxis in the form of low patient compliance due to commercial pharmacy stock outs and variable copays were rapidly identified once prophylaxis prescription at discharge began. The involvement of the hospital’s outpatient pharmacy was critical to addressing these challenges. By implementing a bedside medication delivery program, we were able to provide patient education before discharge, ensure receipt of medication, and capture additional revenue with which we provided financial assistance to uninsured or underinsured patients who might have been unable to pay for prescribed post-discharge prophylaxis.
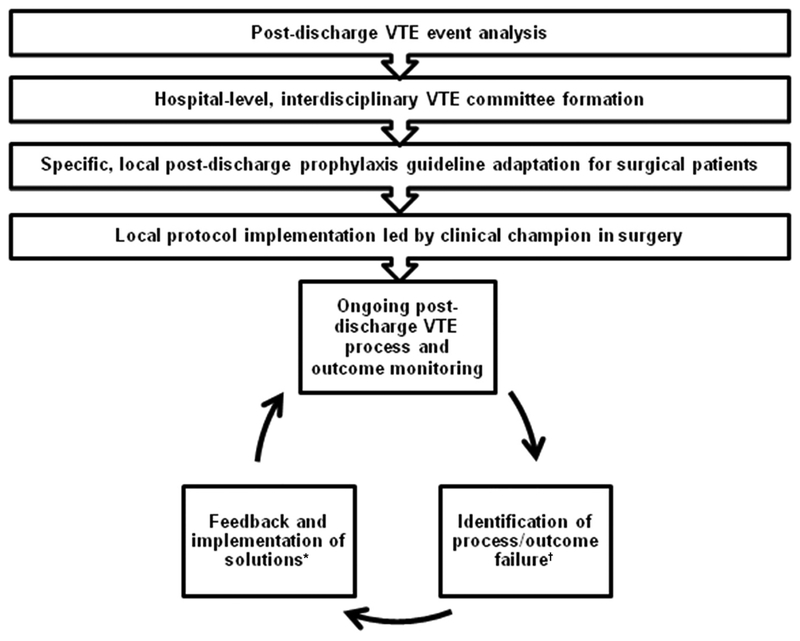
Figure 2 summarizes the overall quality improvement process. This process began with an institutional analysis of VTE events and ultimately led to the formation of our multidisciplinary VTE taskforce, as well as the development and implementation of local guidelines. Implementation feedback consisted of anecdotal front-line experiences and patient comments as well as process/outcomes metrics, including post-discharge LMWH prescription rates in eligible patients, bleeding events, and VTE events.
Figure 2.
Flow diagram of venous thromboembolism (VTE) quality improvement process. *For example, targeted surgeon outreach, bedside medication delivery program. †For example, low surgeon adherence, patient feedback.
Limitations
This was a retrospective analysis using the ACS NSQIP database and is subject to all limitations inherent to similar studies. Data collection was limited to ACS NSQIP variables. Accordingly, we were not able to detect changes in inpatient prophylaxis or other unexamined temporal trends that might have impacted post-discharge VTE rates. Although we were unable to randomize patients to prophylaxis and nonprophylaxis cohorts, we noted pre-implementation enoxaparin prescription rates of <5%. To further mitigate these uncertainties, we performed several sensitivity analyses, including an analysis limiting data to years closest to that in which the intervention began, and an analysis of post-discharge VTE rates among patients ineligible to receive post-discharge prophylaxis during the study period (ie patients undergoing nonabdominal surgery or abdominal surgery for indications other than malignancy or IBD). Surveillance for VTE was left to the clinician’s discretion. Although we note the stability of the inpatient VTE rate in the pre- and post-intervention groups during the study period, there is the possibility of surveillance bias (ie the phenomenon of an increased event rate attributable to increased testing) or unanticipated changes in inpatient prophylaxis patterns during our study period.
A second important limitation is that we lacked information about patient compliance (ie enoxaparin use) with prescribed prophylaxis. We used prescription rate as a proxy indicator for exposure to enoxaparin. It is unlikely that the robust reduction in post-discharge events observed subsequent to implementation of the intervention occurred in the absence of a high level of drug administration, which we believe was enhanced by bedside medication delivery and patient education before discharge. Additionally, monitoring patient adherence would be a logistical barrier to real-world implementation of post-discharge prophylaxis programs.
Finally, the patient population included in this single-institution academic medical center study can limit generalizability. It would be worthwhile to study similar initiatives in hospital systems with significantly different patient populations, both demographically and clinically.
CONCLUSIONS
We designed and implemented a multidisciplinary post-discharge VTE prophylaxis program for patients undergoing abdominal surgery for cancer or IBD and at high risk for VTE. The program consisted of local guideline adaptation and dissemination, measurement and feedback of provider adherence rates, and bedside medication delivery and patient education at discharge. Implementation was associated with a dramatic reduction in post-discharge VTE events in eligible patients. All healthcare systems performing major abdominal surgery for cancer or IBD should consider implementation of interventions to enhance post-discharge VTE prophylaxis for high-risk patients. Additional research is required to investigate local and systemic barriers to implementing comprehensive post-discharge VTE prophylaxis strategies in appropriate abdominal surgery patients, as well as to identify additional high-risk patient populations that also might benefit from predischarge bedside delivery of post-discharge VTE prophylactic therapies.
Acknowledgments
Disclosures outside the scope of this work: Dr Piazza received grants and research support from Bristol-Myers Squibb, Daiichi-Sankyo, British Technology Group, and Janssen; and he is on the advisory board of Merck and eXIthera.
Abbreviations and Acronyms
- ACS
American College of Surgeons
- DVT
deep vein thrombosis
- IBD
inflammatory bowel disease
- LMWH
low molecular weight heparin
- PE
pulmonary embolism
- VTE
venous thromboembolism
Footnotes
Disclosure Information: Nothing to disclose.
REFERENCES
- 1.Mukherjee D, Lidor AO, Chu KM, et al. Postoperative venous thromboembolism rates vary significantly after different types of major abdominal operations. J Gastrointest Surg 2008;12: 2015–2022. [DOI] [PubMed] [Google Scholar]
- 2.Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006;243: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross ME, Vogler SA, Mone MC, et al. The importance of extended postoperative venous thromboembolism prophylaxis in IBD: a National Surgical Quality Improvement Program analysis. Dis Colon Rectum 2014;57: 482–489. [DOI] [PubMed] [Google Scholar]
- 4.Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE Survey): findings in surgical patients. Ann Surg 2010;251:330–338. [DOI] [PubMed] [Google Scholar]
- 6.Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg 2011; 254:131–137. [DOI] [PubMed] [Google Scholar]
- 7.Alsubaie H, Leggett C, Lambert P, et al. Diagnosis of VTE postdischarge for major abdominal and pelvic oncologic surgery: implications for a change in practice. Can J Surg J Can Chir 2015;58:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen MS, Jørgensen LN, Wille-Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev 2009;[1]:CD004318. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen MS, Jorgensen LN, Wille-Jørgensen P, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost 2006;4:2384–2390. [DOI] [PubMed] [Google Scholar]
- 10.Kakkar VV, Balibrea JL, Martínez-González J, et al. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost 2010;8:1223–1229. [DOI] [PubMed] [Google Scholar]
- 11.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346: 975–980. [DOI] [PubMed] [Google Scholar]
- 12.Vedovati MC, Becattini C, Rondelli F, et al. A randomized study on 1-week versus 4-week prophylaxis for venous thromboembolism after laparoscopic surgery for colorectal cancer. Ann Surg 2014;259:665–669. [DOI] [PubMed] [Google Scholar]
- 13.Streiff M, Bockenstedt P, Cataland S. NCCN Clinical Practice Guidelines in Oncology: Venous Thromboembolic Disease. Version 2. Fort Washington, PA: NCCN; 2011. [Google Scholar]
- 14.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Am Soc Clin Oncol 2007;25: 5490–5505. [DOI] [PubMed] [Google Scholar]
- 15.Merkow RP, Bilimoria KY, Sohn MW, et al. Adherence with postdischarge venous thromboembolism chemoprophylaxis recommendations after colorectal cancer surgery among elderly Medicare beneficiaries. Ann Surg 2014;260: 103–108. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DW, Simianu VV, Bastawrous AL, et al. Thromboembolic complications and prophylaxis patterns in colorectal surgery. JAMA Surg 2015;150:712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockbain AJ, Singh-Sekhon N, Ilsley DW. Extended venous thromboembolism prophylaxis after colorectal cancer resection: a UK perspective. Ann Surg 2016;263:e26. [DOI] [PubMed] [Google Scholar]
- 18.Merkow RP, Bilimoria KY, Bentrem DJ. Reply to letter: “Extended Venous Thromboembolism Prophylaxis After Colorectal Cancer Resection: A UK Perspective”. Ann Surg 2016;263:e27. [DOI] [PubMed] [Google Scholar]
- 19.American College of Surgeons. ACS NSQIP. User Guide for the 2014 ACS NSQIP Participant Use Data File (PUF). Chicago, IL: American College of Surgeons; 2015. [Google Scholar]
- 20.Bahl V, Hu HM, Henke PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg 2010;251:344–350. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev 2013;7: CD008201. [DOI] [PubMed] [Google Scholar]
- 22.Hammond J, Kozma C, Hart JC, et al. Rates of venous thromboembolism among patients with major surgery for cancer. Ann Surg Oncol 2011;18:3240–3247. [DOI] [PubMed] [Google Scholar]
- 23.Bergqvist D. Venous thromboembolism: a review of risk and prevention in colorectal surgery patients. Dis Colon Rectum 2006;49:1620–1628. [DOI] [PubMed] [Google Scholar]
- 24.Bouras G, Burns EM, Howell AM, et al. Risk of post-discharge venous thromboembolism and associated mortality in general surgery: a population-based cohort study using linked hospital and primary care data in England. PLoS One 2015;10:e0145759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallaert JB, De Martino RR, Marsicovetere PS, et al. Venous thromboembolism after surgery for inflammatory bowel disease: are there modifiable risk factors? Data from ACS NSQIP. Dis Colon Rectum 2012;55:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehlet H, Harling H. Length of stay after laparoscopic colonic surgery—an 11-year nationwide Danish survey. Colorectal Dis 2012;14:1118–1120. [DOI] [PubMed] [Google Scholar]
- 27.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–1465. [DOI] [PubMed] [Google Scholar]