Abstract
Myeloid derived suppressor cells (MDSC) are heterogeneous cell population consisting of myeloid progenitor cells and immature myeloid cells. These cells have essential immunoregulatory role in tumor bearing hosts and under different inflammatory conditions. No specific marker has been described to identify MDSC, which leaves their suppressor activity as their only hallmark function. In this review, we discuss the current in vivo and in vitro developed assays for elucidation of MDSC function and describe the discrepancies between murine and human MDSC in regard to their suppressor function. We also discuss antigen specificity of MDSC function and approaches to determine the effector function of these cells in vivo. Finally, we summarize different approaches currently being employed to target MDSC with the aim to enhance immune based therapies.
Keywords: Cancer, Tumor immunology, Tolerance
INTRODUCTION
Myeloid derived suppressor cells (MDSC) are composed of a variety of different cell types including precursors of granulocytes, macrophages and dendritic cells or early myeloid progenitor cells. Various studies have demonstrated the immunosuppressive function of MDSC in tumor bearing hosts (Bronte et al., 2001; Gabrilovich and Nagaraj, 2009; Kusmartsev and Gabrilovich, 2002; Marigo et al., 2008) as well as other pathological conditions such as inflammatory bowel disease, sepsis, trauma and transplantation (Cripps and Gorham, 2011; Delano et al., 2007; Haile et al., 2008; Makarenkova et al., 2006). In mice two distinct MDSC sub-populations have been identified: monocytic (CD11b+CD49d+Ly6Chigh) and granulocytic (CD11b+CD49d−Ly6Ghigh) MDSC (Haile et al., 2010; Movahedi et al., 2008; Youn et al., 2008).
MDSC have been shown to suppress immune responses through direct or indirect mechanisms (Bronte and Zanovello, 2005). The main proposed mechanisms for direct inhibition of immune response are production of NO through NOS2, release of ROS, depletion of arginine, secretion of immunosuppressive cytokines such as TGF-β and IL-10 as well as inducing apoptosis mediated by FAS-FASL pathway (Bronte et al., 2003; Corzo et al., 2009; Huang et al., 2006; Kusmartsev et al., 2004; Nagaraj et al., 2010; Sinha et al., 2011; Terabe et al., 2003). Hyperproduction of ROS and peroxynitrite by MDSC have been shown to directly disrupt antigen specific CD8+ T cell responses through nitration of tyrosines in a T-cell receptor TCR-CD8 complex, which affect the conformational flexibility of TCR-CD8 and its interaction with pMHC (Lu et al., 2011; Nagaraj et al., 2007). Recent reports suggest that MDSC down-regulate L-selectin on naive T cells, thereby interfering with their ability to home to their activation site (Hanson et al. 2009).
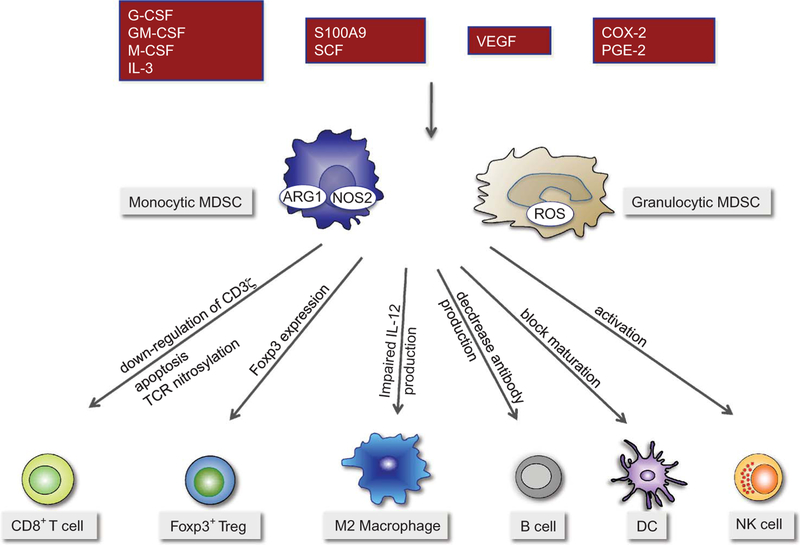
In addition, MDSC suppress indirectly by inducing other cell types with immune suppressor functions such as Tregs or M2 macrophages (Hoechst et al., 2011; Serafini et al., 2008; Sinha et al., 2007). Accumulation of MDSC is shown to be closely associated with inhibition of dendritic cell differentiation leading to disrupted antitumor immune responses (Cheng et al., 2008). Moreover, MDSC have been shown to suppress NK cell cytotoxicity through abrogation of perforin release in Stat5 dependent manner (Hoechst et al., 2009; Liu et al., 2007). Our group and others have demonstrated alteration of equilibrium between regulatory and effector T cells by MDSC through induction of Foxp3+ regulatory T cells both in vivo and in vitro (Hoechst et al., 2008; Serafini et al., 2008). A cross-talk between MDSC and macrophages have been proposed, whereby MDSC cause polarization of macrophages into M2 phenotype with reduced IL-12 production (Ostrand-Rosenberg, 2010) (Figure 1).
Figure 1:
MDSC inhibit multiple facets of the immune response. MDSCs are recruited by tumor derived soluble factors and inhibit the immune system through different pathways. Monocytic MDSC utilize NO and arginase 1, whereas granulocytic MDSC release ROS to exert their function. Monocytic MDSCs induce Foxp3 expression in CD4+ T cells, while it is not clear what MDSC subtype is responsible for the inhibition of other immune functions such as inhibition of macrophages, dendritic cells and NK cells.
Due to lack of specific makers for MDSC, they are mainly identified through their suppressor function but not by their phenotype. Therefore, in this review, we will summarize and discuss different assays, which have been used to demonstrate their suppressor function. We will discuss assays for human as well as for murine MDSC to demonstrate suppression of antigen-specific and non-specific T cell responses. Finally, we will summarize the current knowledge on evaluation of the suppressor function of MDSC in vivo, which is not only the most important assay, but also a very difficult task in the absence of specific markers for these cells.
Measurement of in vitro Function of Murine MDSC
The standard method in elucidating the function of MDSC is to perform in vitro co-culture experiments whereby purified CD11b+Gr-1+ cells (or MDSC sub-populations) are co-incubated together with T cells in the presence of a T cell stimulus. In this type of assay, the proliferation of T cells is assessed by thymidine incorporation or CFSE dilution (Dolcetti, Peranzoni, and Bronte, 2010; Watanabe et al., 2008). On the other hand, a pioneer work by Gabrilovich’s group has demonstrated that immature myeloid cells isolated from the fibrosarcoma bearing mice are able to suppress IFN-γ release by activated CD8+ T cells utilizing ELISPOT assay (Kusmartsev et al., 2004). Although some studies have shown that MDSC suppress the proliferation of antigen-specific T cells, other groups including ours did not observe an inhibition of IFN-γ release by antigen-specific T cells upon antigen stimulation.
This might indicate that MDSC may not impact the early events of effector T cell function such as cytokine production (IFN-γ secretion). It is possible that at the early time points, these cytokines might even induce the suppressive function of MDSC (Gallina et al., 2006). Recently, Watanabe et al demonstrated that despite a significant suppression of proliferation of T cells, MDSC could not impact the ability of IFN-γ secretion by T cells (Watanabe et al., 2008). In addition, MDSC cell lines were shown to prevent the generation of allo-specific CTL by blocking T cell proliferation, and induce apoptosis of Ag-stimulated CTL clones (Apolloni et al., 2000).
Measurement of the in vitro Function of Human MDSC
Although human MDSC are poorly characterized in terms of surface marker and function, progress has been made in identifying potential surface markers, which help to characterize this suppressive population in different cancer patients (Filipazzi, Huber, and Rivoltini, 2012; Greten, Manns, and Korangy, 2011; Zhao et al., 2012). In contrast to murine MDSC, studies using human MDSC have shown that they can interfere with both T cell proliferation as well as IFN-γ production (Table 1).
Table 1:
Comparison of human and murine MDSC.
| Murine MDSC | Human MDSC | |
|---|---|---|
| Proliferation | Inhibit proliferation but not IFN-γ release | Inhibit both proliferation and IFN-γ production |
| Antigen specific response | Mostly suppress antigen specific CD8+ T cell response | Inhibit both CD3/CD28 activated/antigen specific T cells |
| NK cells | Activate/inhibit NK cells | Inhibit NK cell cytotoxcity and IFN-γ release through NKp30 |
| Macrophages | Cross-talk exists: macrophages stimulate IL-10 production by MDSC which in turn interfere with IL-12 production by macrophages | not available |
| Regulatory T cells | Induce expression of FOXP3+ T reg through arginase 1 | Induce FOXP3+Treg via TGF-β/retinoic acid |
| Expansion of Subsets | monocytic < granulocytic | not available |
| Function of MDSC subsets | Monocytic > granulocytic1 | not available |
Youn and colleagues (Youn et al., 2008) found similar suppressor activity of monocytic and granulocytic MDSC, while a number of other studies demonstrated a more potent suppression of T cells by monocytic MDSC.
Earlier studies using CD11b+CD14− MDSC from renal cell carcinoma patients showed that, depletion of these cells from the culture enhances CD3/CD28 induced proliferation, cytokine production as well as CD3ζ chain expression (Zea et al., 2005). On the other hand, our group and others have shown that depletion of CD14+HLA-DR−/low MDSC enhanced antigen-specific T cell responses in vitro. Additionally, co-culturing of MDSC with CD3/CD28 stimulated T cells resulted in suppression of T cell proliferation and IFN-γ production. Human CD14+IL-4Rα+ MDSC from cancer patients are shown to have a potential to inhibit the MLR stimulated proliferation of PBMC (Mandruzzato et al., 2009).
Determination of Antigen Specificity of MDSC
A handful of experiments have shown that MDSC only suppress CD8+T cells in an antigen specific manner, which requires antigen presentation by MDSC to antigen specific CD8+ T cells. On the other hand, some studies provide evidence that murine MDSC suppress T cells activated through mitogens (Solito, Bronte, and Mandruzzato, 2011).
Initial reports by Gabrilovich et al. demonstrated that tumor-induced Gr-1 cells did not inhibit Con A stimulated or antigen specific CD4+ T cell responses, but inhibited antigen specific CD8+ T cell responses. This was attributed to the lack of MHC-II expression on MDSC and higher levels of MHC-I molecule. The same study showed that these Gr-1+ cells were able to inhibit only peptide but not mitogen stimulated CD8+ T cells (Gabrilovich et al., 2001) suggesting that these suppressor populations of cells presented the antigen through MHC-class I molecule. This indicates that antigen presentation by MDSC in the context of MHC-I molecule to CD8+ T cells is crucial in exerting their inhibitory function.
Recently, the same group has shown that the interaction between MDSC and CD4+ T cells in the context of MHC-II not only induces CD4+ T cell tolerance but also converts MDSC into non-specific suppressor cells (Nagaraj et al., 2012). Our unpublished observation and other reports have also shown that MDSC are unable to inhibit the proliferation of either Con-A or anti-CD3/ CD28 activated T cell responses (Kusmartsev, Li, and Chen, 2000). Furthermore, we have observed that an interaction between MDSC (particularly Gr-1high granulocytic MDSC) and T cells in the context of antigen presentation is required for efficient suppression by granulocytic MDSC (manuscript in preparation). A recent report also showed that both subsets of MDSC (Gr-1high and Gr-1low cells) were unable to inhibit T cell proliferation induced by CD3/CD28 antibody (Youn et al., 2008).
On the contrary, MDSC isolated from CSA1M fibrosarcoma tumor-bearing mice inhibited Con A induced T cell proliferation, in the presence of LPS and IFN-γ but not alone which was reversed by the addition of NOS2 inhibitor L-NMMA (Zhou et al., 2007). Apart from this, trauma-induced CD11b+/Gr-1+ cells were shown to significantly inhibit CD3/CD28-mediated T cell proliferation, TCRζ-chain expression as well as IL-2 production. The suppressive effects by the CD11b+/Gr-1+ cells were overcome with the arginase antagonist N-hydroxy-nor-L-arginine or extra supplementation of medium with L-arginine. Poor antigen-presenting capacity of control and trauma-induced CD11b+/Gr-1+ cells was detected in allogeneic murine leukocyte reaction (Makarenkova et al., 2006). Other reports have also suggested that sepsis induced MDSC inhibit both antigen specific as well as nonspecific (CD3/CD28) proliferative responses (Delano et al., 2007).
Similarly, in a colon carcinoma model, both spleen and bone marrow derived immature Gr-1+ myeloid cells were shown to strongly inhibit CD3/CD28-activated T cells in an IFN-γ-dependent manner. However, the same suppressive population failed to inhibit effector T cells that were pre-activated with anti-CD3 monoclonal antibody. These inhibitory effects of myeloid cells could be reversed by the addition of a combination of NOS inhibitor and SOD mimetic (Kusmartsev, Li, and Chen, 2000).
The ability of MDSC to inhibit in an antigen specific manner might be attributed to their capacity to present antigen to CD8+ T cells, which is further influenced by the setting from which the MDSC are isolated. However, further studies are required to prove this. Finally, a different study demonstrated that CD11b+ MDSC from LPS treated mice suppress contact dependent mixed lymphocyte responses through IL-10 and heme oxygenase-1 (De Wilde et al., 2009). In summary, more studies are needed to dissect the different aspects of inhibition of antigen-specific immune responses by MDSC.
Differential Effector Function of MDSC Subsets
In the last several years, based upon the expression of the two epitopes recognized by anti-Gr-1 antibody, two morphologically and functionally distinct subpopulations of murine MDSC have been identified. Our group has identified CD49d as a new marker, which can distinguish different MDSC subsets. CD11b+CD49d+ cells are similar in phenotype and function to CD11b+Ly6Chigh monocytic MDSC. On the other side CD11b+CD49d− and CD11b+Ly6Ghigh both represent the granulocytic MDSC subtype (Haile et al., 2010; Youn et al., 2008). CD49d can be used instead of Gr-1 as a marker for MDSC, which can circumvent potential interferences that occur when antibodies against Ly6C or Ly6G and Gr-1 are used in combination or to clearly distinguish between Gr-1high and Gr-1dull/int. cells. Based on these studies, monocytic MDSC are described as Ly6ChighCD49d+ cells, whereas granulocytic MDSC are Ly6GhighCd49d− cells (Haile et al., 2010).
Based upon these markers, in vitro functional experiments by our group and others have shown that monocytic MDSC posses a stronger suppressive ability dependent on IFN-γ and NO production. On the other hand, granulocytic MDSC displayed modest anti-proliferative effect on CD8+ T cells through the production of ROS (Dolcetti et al., 2010; Haile et al., 2010; Movahedi et al., 2008). However, one study has demonstrated that granulocytic MDSC have a more profound (although not statistically significant) suppressive activity per cell basis than monocytic or total population of MDSC (Youn et al., 2008). This controversy might result from the different cell lines used to induce the MDSC subsets in the study.
Approaches to Assess the in vivo Function of MDSC
Due to the lack of specific markers and transgenic murine models to selectively deplete or add MDSC, studies have used indirect approaches in order to prove their function in vivo. Such studies include adoptive transfer experiments, in which MDSC are harvested from tumor bearing mice and then co-transferred with antigen specific CD8+ T cells. Alternatively, multiple studies have tried to eliminate MDSC in vivo through pharmacological or antibody-mediated approaches.
Adoptive Transfer Approach
The first method used to quantify the in vivo function of MDSC is through co-injection of MDSC together with tumor cell lines. In a murine isograft model, combined co-injection of MC26 or 3LL cells with Gr-1+CD11b+ cells obtained either from naïve or tumor bearing mice subcutaneously, resulted in higher tumor volume and growth rate in mice co-injected with tumor derived MDSC (Yang et al., 2004). Similarly, in vivo determination of the cell mediated suppressive effect of MDSC was carried out to analyze the effect of nanoparticulated adjuvant induced MDSC used in vaccines.
Here, OT-I splenocytes were transferred to congenic mice on day 0. Mice were vaccinated with peptide pulsed DCs and MDSC on day 2 and MDSC were adoptively transferred again on day 4 before T cell function was tested on day 10. Interestingly, in this study, MDSC from tumor bearing mice were much more suppressive than CD11b+ cells from mice vaccinated with a nanoparticulatd adjuvant (Fernandez et al., 2011).
In line with this, using a model of intestinal inflammation, we have shown previously that adoptive co-transfer of antigen specific T cells with Gr-1+CD11b+ MDSC from mice with chronic inflammation can ameliorate severe gut- inflammation and body weight loss caused by CD8+ T cells. Although the in vivo mechanism behind such suppression is not well defined, these assays can be utilized to determine the in vivo function of immuno-regulatory MDSC (Haile et al., 2008).
To define the in vivo role of MDSC, a study has used allogenic transplantation models where by adoptive transfer of CFSE labeled spleen cells into irradiated allogenic recipient mice prevented the proliferation of T cell proliferation in vivo (Dugast et al., 2008). Reports suggest that MDSC inhibit proliferation but not IFN-γ secretion of T cells in vitro, however, there are discrepancies regarding the extrapolation of the in vitro results to in vivo obtained data. We have shown previously that MDSC can inhibit IFN-γ secretion but not proliferation in vivo (Haile et al., 2008), in line with a model of sepsis, where infusion of Gr-1+ cells obtained from the septic mice into mice which had previously received CD8+ T cells markedly reduced IFN-γ production by antigen specific CD8+ T in vivo (Delano et al., 2007). This might be attributed to differences between the inflammation-induced and tumor-induced MDSC.
Knock-out Mice/Transgenic Mice
The presence of IL-4Rα on CD11b+ cells appeared to be critical for their suppressive activity. Hence, the other approach used to verify the in vivo function of MDSC was indirect through the use of IL-4Rα−/− mice, where by transfer of CD8+ T lymphocytes purified from immunized γ-irradiated C26-GM cells marginally affected the tumor development in control mice but completely prevented the tumor growth in LysMCreIL-4R−/flox mice (Gallina et al., 2006). This is an indication that functional markers such as IL-4Rα on MDSC can be used to study the in vivo function of MDSC and hence useful for targeting MDSC in vivo.
Similarly, S100A9 was shown to be crucial for the in vivo increase in the number of MDSC in response to inoculation of mice with tumor cells. A study utilizing S100A9 knockout mice, has demonstrated that absence of S100A9 leads to aggressive tumor growth with high tumor volume and no infiltration of Gr-1+ cells (Cheng et al., 2008). Consistently, HIF-1α is involved in drastic alteration of the function of MDSC in the tumor microenvironment and influences their differentiation toward tumor-associated macrophages. MDSC from the HIF-1α knock-out mice displayed a more mature phenotype (express CD11C), had decreased viability, lower suppressive ability and lower expression of Arginase 1 and NOS2.
Comparison of the in vivo function of WT and HIF-1α deficient myeloid cells in vivo in harnessing the antitumor immune response revealed a substantially delayed tumor growth in the latter case (Corzo et al., 2010). Another mouse model used to rule out the in vivo function of MDSC is a model of tissue-restricted gene ablation where by Cebpb was deleted in all hematopoietic lineage cells. Cebpbflox/flox; Tie2cre(−/−) mice showed a decrease in CD11b+Gr-1+ cells after they were challenged with MCA203. In addition, CD11b+ splenocytes isolated from the spleen of these mice had completely lost their ability to inhibit antigen-specific CD8+ T cells in vitro (Marigo et al., 2010). Although these factors are not specific for MDSC, it is suggestive that C/EBPβ or HIF-1α can be used as one of the targets for potential intervention of MDSC function in vivo.
Pharmacological Manipulation
Chemotherapeutic agents such as gemcitabine have been shown to drastically and selectively reduce the number of splenic MDSC preserving CD4+, CD8+, NK and macrophages. These effects were accompanied by enhanced anti-tumor responses of CD8+ T cells and NK cells (Le et al. 2009; Suzuki et al. 2005). The molecular mechanism for this effect is not understood yet, however, the authors propose the selective killing of Gr-1+CD11b+ cells by gemcitabine with out affecting their influx and maturation as one explanation. In addition, in this study, only gemcitabine’s effect on splenic DC is shown; however, MDSC, which are located at other sites such as liver, might affect the conclusions drawn from such experiments.
Similarly, a single administration of 5-flurouracil, which results in MDSC depletion, elicited an increase in IFN-γ production by tumor-specific CD8+ T cells. This selective effect of 5-FU on MDSC is attributed to lower expression of thymidylate synthase (TS), a target enzyme for action of 5-FU (Vincent et al., 2010). In our own hands, a single injection of 5-FU (50 mg/kg) has led to a dramatic decrease in tumor size, so that it remains an open question whether the observed effects of 5-FU and possibly other cytotoxic reagents are direct effects on MDSC or an indirect effect due to targeting of tumor.
A promising approach could be differentiation of CD11b+Gr-1+ MDSC in to DC, macrophages or granulocytes with out affecting the growth of solid tumors is by using all-trans-retinoic acid (ATRA), a derivative of vitamin A (Gabrilovich et al., 2001; Ugel et al., 2009).
Pharmacological manipulation of MDSC function by targeting NOS and ARG1 using L-NMMA and nor-NOHA have also been tried. Although these drugs can effectively reverse inhibitory mechanisms of MDSC, their use in vivo is not favored due to side effects. However, administration of a clinically available PDE5 inhibitor, sildenafil, was shown to overcome MDSC mediated immunosuppression through down-regulation of IL-4α and ARG1 (Serafini, Borrello, and Bronte, 2006).
Depletion of MDSC
Gr-1 antibody depletion (clone RB6–8C5) has been widely used to deplete mice of MDSC. The Gr-1 epitope presented on MDSC is represented by the two molecules Ly-6G and Ly-6C, which potentially allow for co-staining of cells with anti-Gr-1 and anti-Ly-6G or anti Ly-6C. A study by Bronte et al. has used anti-Gr-1 antibody to deplete Mac+/Gr-1+CD11b+ cells and has shown that repeated i.p injection of antibody during the first days of infection with vaccina virus (VV)-encoding IL-2 and model antigen β-galactosidase (β-gal), mounted a potent immune response resulting in complete recovery of the deficient cytotoxic response, yet impaired immune response was observed in control antibody-treated group (Bronte et al., 1998).
However, the use of anti-Gr-1 antibody to deplete MDSC in vivo is highly uncertain, since, it recognizes both Ly6G and Ly6C, which leads to non-selective depletion of monocytes, T lymphocytes, NK cells and macrophages, and might impair host immunity and lead to opportunistic infection (Stewart and Smyth, 2011). In addition, results from our lab and others indicate that the antibody might by itself cause non-efficient depletion, aggregation and even activation of MDSC in different organs such as liver (Ribechini, Leenen, and Lutz, 2009) causing liver inflammation (manuscript submitted). Apart from this, incomplete and rebound accumulation of MDSC following depletion further complicates the analysis.
OUTLOOK
Immune suppression remains the hallmark of MDSC. Although different mechanisms have been identified as to how MDSC exert their suppressor function in different pathological settings, controversies regarding the best method to measure their immune suppressor function both in vitro and in vivo exist. The lack of specific markers as well as the existence of different MDSC subtypes make this task even more difficult and remain an important area of investigation.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal DM, Young HA, Zanovello P (2000). Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J. Immunol 165 (12):6723–6730. [DOI] [PubMed] [Google Scholar]
- Bronte V, Serafini P, Apolloni E, Zanovello P (2001). Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother 24 (6):431–446. [DOI] [PubMed] [Google Scholar]
- Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P (2003). IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol 170 (1):270–278. [DOI] [PubMed] [Google Scholar]
- Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NF (1998). Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol 161 (10):5313–5320. [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Zanovello P (2005). Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol 5 (8):641–654. [DOI] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI (2008). Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med 205(10):2235–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DJ (2010). HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med 207(11):2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI (2009). Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol 182(9):5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps JG, and Gorham JD (2011). MDSC in autoimmunity. Int. Immunopharmacol 11 (7):789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM, Cuturi MC, Goldman M, Le Moine A (2009). Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am. J. Transplant 9 (9):2034–2047. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL (2007). MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med 204 (6):1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti L, Peranzoni E, Bronte V (2010). Measurement of myeloid cell immune suppressive activity. Curr. Protoc. Immunol. Chapter 14: 14–17. [DOI] [PubMed] [Google Scholar]
- Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A., Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V (2010). Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol 40(1):22–35. [DOI] [PubMed] [Google Scholar]
- Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R., Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B (2008). Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol 180 (12): 7898–7906.18523253 [Google Scholar]
- Fernandez A, Mesa C, Marigo I, Dolcetti L, Clavell M, Oliver L, Fernandez LE, Bronte V (2011). Inhibition of tumor-induced myeloid-derived suppressor cell function by a nanoparticulated adjuvant. J. Immunol 186: (1):264–274. [DOI] [PubMed] [Google Scholar]
- Filipazzi P, Huber V, Rivoltini L 2012. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol. Immunother 61 (2):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol 9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM (2001). Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol 166 (9):5398–5406. [DOI] [PubMed] [Google Scholar]
- Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V (2006). Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest 116 (10):2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten TF, Manns MP, Korangy F (2011). Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol 11 (7):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile LA, Gamrekelashvili JM, Manns MP, Korangy F, Greten TF (2010). CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol 185 (1):203–210. [DOI] [PubMed] [Google Scholar]
- Haile LA, von Wasielewski R, Gamrekelashvili J, Kruger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F, Greten TF (2008). Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135 (3):871–881, 881 e1–5. [DOI] [PubMed] [Google Scholar]
- Hanson EM, Clements VK, Sinha P, Ilkovitch D, and Ostrand-Rosenberg S (2009). Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J. Immunol 183 (2):937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F (2011). Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood 117 (24):6532–6541. [DOI] [PubMed] [Google Scholar]
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F (2008). A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135(1):234–243. [DOI] [PubMed] [Google Scholar]
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F (2009). Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50(3):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH (2006). Gr-1 CD115 immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66 (2):1123–1131. [DOI] [PubMed] [Google Scholar]
- Kusmartsev SA, Li Y, Chen SH (2000). Gr-1 myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol 165 (2):779–785. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI (2002). Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol. Immunother 51(6):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI (2004). Antigen-specific inhibition of CD8 T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol 172 (2):989–999. [DOI] [PubMed] [Google Scholar]
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD (2009). Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol 9 (7–8):900–909. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG (2007). Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 109 (10):4336–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, and Gabrilovich D (2011). Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J. Clin. Invest [DOI] [PMC free article] [PubMed]
- Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB (2006). CD11b+/ Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol 176 (4):2085–2094. [DOI] [PubMed] [Google Scholar]
- Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P (2009). IL4Ralpha myeloid-derived suppressor cell expansion in cancer patients. J. Immunol 182 (10):6562–6528. [DOI] [PubMed] [Google Scholar]
- Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V (2010). Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32 (6):790–802. [DOI] [PubMed] [Google Scholar]
- Marigo I, Dolcetti L, Serafini P, Zanovello P, and Bronte V (2008). Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev 222:162–179. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA (2008). Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111(8):4233–4244. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI (2007). Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med 13(7):828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Nelson A, Youn JL, Cheng P, Quiceno D, Gabrilovich DI (2012). Antigen-specific CD4+ T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC Class II signaling. Cancer Res 72 (4):928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI (2010). Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol 184 (6):3106–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S (2010). Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother 59 (10):1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribechini E, Leenen PJ, Lutz MB (2009). Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur. J. Immunol 39(12):3538–3551. [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V (2006). Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol 16 (1):53–65. [DOI] [PubMed] [Google Scholar]
- Serafini P, Mgebroff S, Noonan K, Borrello I (2008). Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68 (13):5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S (2011). Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood 117(20):5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S (2007). Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol 179(2):977–983. [DOI] [PubMed] [Google Scholar]
- Solito S, Bronte V, Mandruzzato S (2011). Antigen specificity of immune suppression by myeloid-derived suppressor cells. J. Leukoc. Biol 90(1):31–36. [DOI] [PubMed] [Google Scholar]
- Stewart TJ, Smyth MJ (2011). Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev 30 (1):125–140. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM (2005). Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res 11 (18):6713–6721. [DOI] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA (2003). Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med 198 (11):1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, and Bronte V (2009). Therapeutic targeting of myeloid-derived suppressor cells. Curr. Opin. Pharmacol 9(4):470–481. [DOI] [PubMed] [Google Scholar]
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F (2010). 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70 (8):3052–3061. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LH, Cohen PA, Shu S (2008). Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J. Immunol 181(5):3291–3300. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC (2004). Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6 (4):409–421. [DOI] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol 181(8):5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AO (2005). Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65 (8):3044–3048. [DOI] [PubMed] [Google Scholar]
- Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, Greten TP, Korangy F (2012). S100A9 a new marker for monocytic human myeloid derived suppressor cells. Immunology [DOI] [PMC free article] [PubMed]
- Zhou R, He PL, Ren YX, Wang WH, Zhou RY, Wan H, Ono S, Fujiwara H, Zuo JP (2007). Myeloid suppressor cell-associated immune dysfunction in CSA1M fibrosarcoma tumor-bearing mice. Cancer Sci 98 (6):882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]