Abstract
β-Arrestins 1 and 2 couple to seven trans-membrane receptors and regulate G protein-dependent signaling, receptor endocytosis and ubiquitylation. Recent studies have uncovered several unanticipated functions of β-arrestins, suggesting that the role of β-arrestins in cell signaling is much broader than originally thought.It is now recognized that β-arrestins can transduce receptor signaling independent of G proteins. The expression of β-arrestins is differentially regulated in immune cells and tissues in response to specific inflammatory stimuli, and β-arrestins are critical regulators of the inflammatory response. This review will focus on β-arrestins in immune cells and the impact of altered expression on the pathogenesis of specific inflammatory diseases. Understanding the role of β-arrestins in inflammation may lead to new strategies to treat inflammatory diseases, such as sepsis, rheumatoid arthritis, asthma, multiple sclerosis, inflammatory bowel disease and atherosclerosis.
Keywords: β-Arrestins, G protein-coupled receptors, immune cells, inflammation, inflammatory diseases
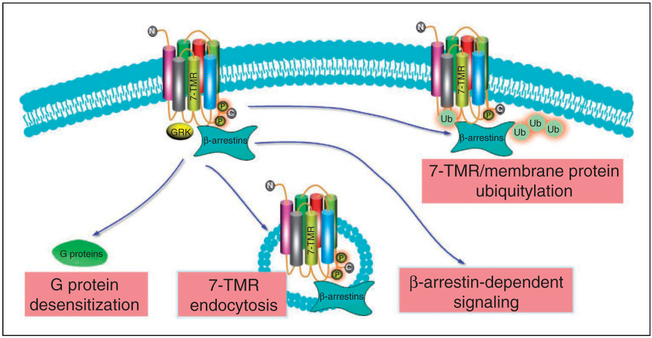
β-Arrestins mediate receptor endocytosis, ubiquitylation and G protein-dependent and G protein-independent signaling
The seven trans-membrane receptors (7-TMRs), also referred to as G protein-coupled receptors (GPCRs), are the largest class of cell surface receptors in the human genome. The 7-TMRs have immense therapeutic importance and regulate diverse physiological and pathophysiological processes.1 Ligand binding to 7-TMRs activate heterotrimeric G proteins that transiently drive second messengers (e.g. cAMP, inositol triphosphate and intracellular Ca2+) and initiates intracellular signals.2 β-Arrestins, which are nonvisual arrestins, are referred to as β-arrestin 1 and β-arrestin 2. β-Arrestins were initially characterized in the context of β-adrenergic receptor signaling as ‘arresting’ proteins, which desensitize agonist-induced 7-TMR signaling.3 Following agonist binding to 7-TMRs, the receptors are phosphorylated at the carboxyl terminus or intracellular loops by GPCR kinases.4 Phosphorylation promotes recruitment of β-arrestins to 7-TMRs, which, in turn, serve as a scaffolding protein. Clathrin and beta 2-adaptin are recruited to the plasma membrane β-arrestin complex and initiate inward endocytosis.5,6 The endocytotic vesicle with the 7-TMR can be targeted for lysosomal degradation or the receptor can be recycled back to the plasma membrane. β-Arrestin-dependent clathrin-mediated internalization of 7-TMRs appears to be a general mechanism of receptor desensitization for the majority of 7-TMRs. Depending upon the stability of interaction between β-arrestins and receptors, the receptors are distinguished as class A and class B receptors. Class A receptors, such as the β2-adrenergic receptor (β2-AR), transiently interact with β-arrestins and undergo rapid recycling. Class B receptors, such as the angiotensin receptor subtype 1 a (AT1aR), stably interact with β-arrestins and exhibit slow recycling.7
β-Arrestins also serve as adaptor proteins for E3 ubiquitin ligase, which mediates ubiquitylation and degradation of 7-TMRs.8 They interact with several E3 ubiquitin ligases, including Mdm2, neural precursor cell expressed developmentally down regulated 4 (NEDD4) and AIP4 to promote receptor ubiquitylation and degradation, such as β2-AR and chemokine receptor CXCR4.9–11 Recently, it has been determined that β-arrestins regulate ubiquitylation and internalization of certain non-7-TMR membrane proteins, such as TGF-β, insulin-like growth factor I receptor, calcium channels, the Na(+)/H(+) exchanger 1 and vascular endothelial cadherin.12–16
β-Arrestins function as signalosome adaptor/scaffolding proteins that regulate 7-TMR activation of MAPKs, including ERK1/2,17 JNK,18 p38 kinases,19 and Src family kinases.20,21 In addition to MAPK regulation, β-arrestins also scaffold AKT, PI3 kinase and phosphodiesterase 4 upon activation of specific receptors.22–25 In contrast to the established function of β-arrestin as a G protein signaling terminator, 7-TMR can signal through β-arrestins without coupling to G proteins, a phenomenon referred to as ‘G protein-independent signaling’. β-Arrestin-mediated G protein-independent signaling exhibits distinct spatial temporal differences in the signaling cascade compared with G protein-dependent signaling. For example, β-arrestin-dependent activation of ERK 1/2 is more localized in the cytoplasm and exhibits a prolonged activation. In contrast, G protein-dependent transient activation of ERK 1/2 is distributed in both the cytoplasm and nucleus, and activates the transcription factor ELK-1.26 β-Arrestin-dependent ERK activation activates cytoplasmic transcription factors signaling to the cell survival pathway.
The finding that β-arrestins can mediate G protein-independent signaling led to the discovery of ‘biased agonists’. Biased agonists can selectively activate G protein-dependent signaling or β-arrestin-dependent signaling. For example, stimulation of AT1aR by SII (Sar1, Ile4, Ile8-angiotensin II), a peptide analog of angiotensin II, led exclusively to β-arrestin-dependent signaling without activating G proteins.27 Biased agonists can also activate G protein-dependent signaling. An example of the latter biased agonist is an agonist of the nicotinic acid receptor GPR109A, a 7-TMR which couples to Gi/Go proteins. Nicotinic acid activation of the GPR109A receptor has been used to treat dyslipidemia and appears to lower triglycerides via G protein-dependent signaling. However, a side effect of nicotinic acid is cutaneous flushing, which depends on β-arrestin 1.28 Agonists of GPR109A, which selectively trigger G protein signaling without engaging β-arrestin 1, can maintain drug efficacy with fewer side effects. Taken together, the general functions of β-arrestins are summarized in Figure 1.
Figure 1.
β-Arrestin 1 and 2-mediated 7-TMR endocytosis, receptor and membrane protein ubiquitylation, and G protein-dependent and G protein-independent signaling. Ub: ubiquitylation.
Isoform specific regulation of immune cells by β-arrestins
Macrophages and monocyte
Myeloid cells, for example macrophages/monocytes, are the first line of defense against bacterial invasion and produce a plethora of cytokine, chemokine and lipid mediators. Isoform-specific expression of β-arrestins is altered by inflammatory stimuli, and isoformspecific regulation of β-arrestin macrophage/monocyte inflammatory responses have recently been investigated. An example of the former is the observation that β-arrestin 1, but not β-arrestin 2, expression was decreased in response to the TLR2 ligand Pam3CSK4 and TLR4 ligand LPS stimulation in peritoneal macrophages.29 The expression of β-arrestin 1 was decreased at both transcriptional and post-translational levels. Interestingly, LPS-mediated decreases of β-arrestin 1 levels were reversed by the JNK inhibitor (SP600125) suggesting an important role for LPS-induced JNK activation in suppressing β-arrestin 1 expression.29 The altered expression of β-arrestins in inflammation is summarized in Table 1.
Table 1.
β-Arrestin (β-arr) was altered in inflammation.
| Cell type /tissue | β-arr | Inflammation | Reference |
|---|---|---|---|
| Peritoneal macrophage | 1 | Pam3CSK4 and LPS decrease β-arr 1 | 29 |
| Raw 264.7 | 2 | LPS decreases β-arr 2 | 31 |
| Lung and liver | 2 | Mouse subjected to CLP | 55 |
| CD4+T cell | 2 | Mouse with allergic asthma | 58 |
| Joint tissue | 1/2 | CIA mice and TNFtg mice | 64 |
| FLS | 1/2 | CIA mice and TNFtg mice | 64 |
| FLS | 1/2 | TNF-α, HA and HMGB1-induced expression | 64 |
| Splenocytes | 1 | Adjuvant arthritis rat | 65 |
| Mesenteric lymph nodes | 1 | Adjuvant arthritis rat | 65 |
| FLS | 1/2 | CIA rat | 63 |
| Mesenteric lymph nodes | 1/2 | Adjuvant arthritis rat | 66 |
| PMBC | 1 | RA patients | 67 |
| FLS | 1/2 | RA patients | |
| CD4+T cell | 1 | MS patients | 46 |
| Splenocytes | 1 | EAE rat | 46 |
| Brain | 1 | MS patients | 71 |
| Spinal cord | 1 | EAE mice | 71 |
| Coronary arteries | 2 | Atherosclerotic patients | 79 |
TLR-induced inflammation is regulated by β-arrestins. Wang et al.30 reported that LPS-, polyI:C-, CpG DNA- and CD40L-induced TNF-α, IL-6 and IL-12p40 production were increased in bone marrow-derived macrophages isolated from β-arrestin 2 knockout (KO) mice. β-Arrestins directly interact and inhibit ubiquitination of TRAF6 following TLR or IL-1 receptor activation preventing TRAF6-mediated NF-κB activation. β-Arrestin 2 interacts with cytosolic IκBα and inhibits NF-κB activation and expression of its target gene, inducible NO synthase (iNOS), in response to LPS stimulation. These findings suggest that β-arrestin 2 stabilizes the NFκB/IκBα complex preventing NF-κB activation in macrophages.31 Parameswaran et al.32 demonstrated that in the Raw264.7 cells, knockdown of β-arrestin 1 leads to enhanced LPS-induced phosphorylation and degradation of NFκB1 p105, and enhanced MEK 1/2 and ERK 1/2 phosphorylation. Sender et al.33 observed that surfactant protein-A enhances alveolar macrophage β-arrestin 2 expression, and inhibits LPS-induced TLR4 expression and colocalization of TLR4 with early endosome antigen 1 through β-arrestin 2. In this study, LPS-induced TNF-α release in bronchoalveolar lavage fluid was increased in β-arrestin 2 KO mice compared with WT mice. In splenocytes, β-arrestin isoform-specific regulation of cytokines. LPS-induced TNF-α, IL-6 and others were decreased in splenocytes from β-arrestin 1 KO mice compared with WT mice, while LPS-induced TNF-α and IL-6 were increased in splenocytes from β-arrestin 2 KO mice.34 However, Porter et al.35 showed that LPS-induced IL-1 β, IL-12p70, IL-5 and INF-γ were decreased in CD11b + /CD11b− splenocytes from β-arrestin 2 KO mice compared with WT mice. In β-arrestin 1 KO mice, LPS-induced inflammatory gene expression was decreased in CD11b− splenocytes.35 The disparate findings among these studies may be, in part, a result of different cells, for example, mixed cellular phenotypes in splenocytes versus CD11b + /CD11b− cells. β-Arrestins may regulate inflammatory responses in a cell phenotype and β-arrestin isoform-dependent manner.
In addition to purified TLR ligands, β-arrestins 1 and 2 regulate adenovirus-induced inflammatory responses. In peritoneal macrophages isolated from wild type (WT) and β-arrestin 1 or 2 KO mice, β-arrestin 1 was demonstrated to be a positive regulator of adenovirus-induced inflammatory cytokine and chemokine production, and β-arrestin 2 appears to be a negative regulator.36 In another study, β-arrestin 1 interacted with CD74 receptor upon macrophage migration inhibitory factor (MIF) stimulation leading to CD74-MIF endocytosis and downstream ERK activation.37 Thus, β-arrestin 1 appears to positively regulate and β-arrestin 2 appears to negatively regulate specific macrophage inflammatory responses. The differential regulation of β-arrestin isoforms may reflect differing specificity in interacting with specific signaling proteins. β-Arrestin regulation of inflammatory responses is summarized in Table 2.
Table 2.
Altered expression of β-arrestins (β-arr) regulates inflammation.
| Cell type/ Animal | β-arr | Stimulation | Reference |
|---|---|---|---|
| BMDM | 2 KO | LPS, polyI:C, CpG DNA and CD40L-induced TNF-α, IL-6 and IL-12p40 | 30 |
| RAW 264.7 | 1 knockdown | LPS-induced ERK 1/2 activation, Degradation of NF-κB1 p105 | 32 |
| Splenocytes | 2 KO | LPS-induced TNF-α, IL-6 and IL-10 | 34 |
| CD11b+ splenocytes | 2 KO | LPS-induced IL-1 β, IL-12p40, IL-12p70, IFN-γ | 35 |
| CD11b- splenocytes | 1 KO 2 KO | LPS-induced IL-1 β, IL-12p70, IL5 and IFNγ | 35 |
| Peritoneal macrophage | 1 KO | Adenovirus-induced inflammatory response | 36 |
| Peritoneal macrophage | 2 KO | Adenovirus-induced inflammatory response | 36 |
| Raw 264.7 | 2 overexpress | LPS-induced iNOS, NO, NF-κB and degradation of IκBα | 31 |
| THP-1 | 2 knockdown | Inhibitory effect of fenoterol on LPS-induced TNF-α | 38 |
| PMN | 2 KO | LPS-induced IL-6 | 39 |
| PMN | 2 KO | ICAM-1 and L-selectin expression | 39 |
| PMN | 2 KO | PMN recruitment | 42 |
| PMN | 1 DN plasmid | Chemotaxis | 43 |
| PMN | 2 DN plasmid | Granule release | 45 |
| CD4+T cell | 1 KO | Bcl2 expression CD4+T cells survival Resistant to EAE | 46 |
| NK | 2 KO | Cytotoxicity of NK cells Resistant to virus infection | 48 |
| T and B lymphocytes | 2 KO | CXCR4-mediated chemotaxis | 49 |
| T lymphocytes | 2 KO | Recruitment to lung | 50 |
| MEF | 2 KO | LPS-induced IL-6 | 51 |
| HEK 293-TLR4/MD2 | 2 knockdown | LPS-induced ERK 1/2 and IL-8 | 51 |
| Hela Jurkat HEK 293 | 1 and 2 overexpress | IκBα degradation NF-κB activation | 52, 53 |
| Mouse | 2 KO | Endotoxin shock-induced TNF-α, IL-1 β and IL-6, and susceptible to endotoxin shock | 30 |
| Mouse | 1 and 2 KO | LPS-induced IFNγ, IL-5, IL-1 β, IL-12p40 and IL-6, and mortality | 35 |
| Mouse | 2 KO | CLP-induced mortality, plasma IL-6, lung and cecal MPO and bacterial load | 34 |
| Mouse | 2 KO | Polymicrobial infection-induced mortality, neutrophil sequestration in the lungs | 56 |
| Mouse | 2 KO | OVA-induced asthma | 50 |
| CD4+ T cells | 2 knockdown | Phosphorylated ERK 1/2 and IL-17 production | 59 |
| FLS | 1 overexpress | HA-induced TNF-α, IL-6 | 64 |
| FLS | 2 overexpress | HA-induced TNF-α, IL-6 | 64 |
| Mouse | 1 KO | Resistant to EAE | 46 |
| Mouse | 1 transgenic | Resistant to EAE | 46 |
| U937 | 1 overexpress | A1AR expression | 71 |
| Ldlr−/− mice | 2 KO | Reduce aortic atherosclerosis | 80 |
| Mouse | 1 KO | Neointimal hyperplasia | 80 |
| Mouse | 2 KO | Neointimal hyperplasia | 80 |
BMDM: bone marrow-derived macrophages; PMN: polymorphonuclear cells; OVA: ovalbumin.
Certain 7-TMRs can regulate TLR4 signaling through β-arrestins and vice versa. For example cross talk can occur between β2-AR and TLR signaling pathways in murine macrophage RAW 264 cells.31 The expression of β-arrestin 2, which is required for β2-AR signaling, is reduced in RAW 264 cells after stimulation with LPS.31 However, Fenoterol, a β2-AR agonist, inhibited LPS-induced TNF-α and IL-8, and decreased membrane TLR4/CD14 complex in THP-1 cells. The latter occurs through β-arrestin 2-mediated signaling as knockdown of β-arrestin 2 abrogated the suppression effect of fenoterol on LPS responses.38 In general, the macrophage/monocytes studies reviewed suggest that β-arrestins 1 and 2 have distinct, and sometime opposing functions, in regulating inflammatory responses.
Neutrophils
Neutrophils are recruited to the sites of infection upon bacterial invasion and participate in the first line of defense through phagocytosis and bacterial killing. LPS-stimulated IL-6 production was increased in neutrophils from β-arrestin 2 KO mice compared with WT cells. β-Arrestin 2 deficiency resulted in an augmented expression of adhesion receptor ICAM-1 and L-selectin.39 These data suggest that β-arrestin 2 may negatively regulate neutrophil cytokine production and adhesion receptor expression in response to inflammation.
β-Arrestins are essential regulators of neutrophil chemotaxis.40 Both β-arrestin 1 and 2 are required for IL-8-induced CXCR1 internalization, which, together with CXCR2, is responsible for IL-8-induced neutrophil chemotaxis.41 In vivo studies in WT and β-arrestin 2 KO mice revealed that CXCR2-mediated neutrophil recruitment to sites of inflammation was increased in β-Arrestin 2 KO mice compared with WT mice. Su et al.42 demonstrated that the deletion of β-arrestin 2 resulted in increased Ca2+ mobilization, superoxide anion production and GTPase activity in neutrophils, but decreased CXCR2 internalization relative to WT mice. CXCL1-induced recruitment of neutrophils was increased in β-arrestin 2 KO mice in two animal models: the dorsal air pouch model and the excisional wound healing model.42 Ge et al.43 demonstrated that neutrophil protease-activated receptor-2 (PAR-2) activation promotes ERK 1/2 and β-arrestin 1 dependent re-organization of the actin cytoskeletion, responsible for pseudopodia extension and chemotaxis. Prolonged ERK 1/2 activation in the pseudopodia is associated with a PAR-2/β-arrestin complex, which promotes chemotaxis.43 Therefore, β-arrestins may regulate chemotaxis in a cell phenotype and receptor-dependent manner. For a more in depth review of roles of β-arrestins in neutrophils, lymphocytes, and macrophage chemotaxis and migration, the reader is referred to comprehensive reviews by Luttrell and Gesty-Palmer.,26 DeFea40 and DeWire et al.44
β-Arrestins not only regulate chemotaxis to specific chemokines, but are also essential for chemokine-induced granule exocytosis, which is important for neutrophil function. Chemoattractant-stimulated granule release from neutrophils, basophils and eosinophils is critical for innate immune responses. In addition to chemotaxis, β-arrestins 1 and 2 regulate neutrophil degranulation.45 Barlic et al.45 demonstrated that IL-8 stimulates rapid formation of β-arrestin complexes with tyrosine kinase Hck or cFgr. Formation of β-arrestin-Hck complexes leads to Hck activation and trafficking of the complexes to granule-rich regions. Granulocytes expressing a dominant-negative β-arrestin mutant did not release granules or activate tyrosine kinases after IL-8 stimulation.45
Lymphocytes
CD4+ T cells are essential for adaptive immunity, and dysregulation of CD4+ T cell has been implicated in autoimmunity. Shi et al.46 demonstrated that β-arrestin promotes CD4+ T cell survival. Enhanced cell survival was thought to be a consequence of β-arrestin 1-dependent expression of proto-oncogene Bcl2.
NK cells are also crucial in the innate immune responses through surveillance of transformed and infected cells.47 NK cells contain abundant granules with cytolytic proteins, which induce the death of target cells.47 Yu et al.48 demonstrated that β-arrestin 2 associates with KIR2DL1, an inhibitory receptor of NK cells. The receptor recognizes MHC class I on target cells, which confer protection to healthy cells. Upon ligand activation, β-arrestin 2 mediates recruitment of the tyrosine phosphatases SHP-1 and SHP-2 to KIR2DL1, and facilitates inhibitory signaling. β-Arrestin 2 KO mice exhibit increased cytotoxicity of NK cells, and these mice are less susceptible to murine cytomegalovirus infection. The latter effects were abolished by depletion of NK cells compared with WT mice. These composite findings suggest that β-arrestin 2 inhibits activation of NK cells.48
β-Arrestin 2 positively regulates T lymphocyte trafficking: using β-arrestin 2 deficient mice, Fong et al.49 demonstrated that β-arrestins are required for the chemokine receptor CXCR4-mediated CD3+ T and B lymphocyte chemotaxis in vitro. Another in vivo study demonstrated that CD4+ T cell recruitment to the lung was impaired in β-arrestin 2 KO mice with allergic asthma.50
Other cells
Mouse embryonic fibroblasts (MEF) from WT, β-arrestins KO, β-arrestins 1 and 2 double KO, and MEFs with reconstituted WT β-arrestins in the double KO cells have been studied. β-Arrestin 2 positively regulates LPS-induced IL-6 production.51 The effect of small interfering RNA (siRNA)-specific knockdown of β-arrestins or overexpression of WT β-arrestins in HEK 293 cells co-transfected with TLR4 and MD2 was also investigated. These studies demonstrated that β-arrestin 2 positively regulates LPS-induced ERK 1/2 activation and IL-8 production. However, both β-arrestins 1 and 2 negatively regulated LPS-induced NF-κB activation.51 The multiple effects of β-arrestins on ERK 1/2 signaling pathway activation in cells exceeds the scope of the present review and has been comprehensively reviewed by Luttrell and Gesty-Palmer26 and DeWire et al.44 The inhibitory effect of β-arrestins 1 and 2 on NF-κB activation is consistent with other studies. Witherow et al.52 demonstrated that both β-arrestins 1 and 2 interact with IκBα and inhibit NF-κB activation in Hela cells and the T lymphocyte cell line Jurkat cells. Over-expression of β-arrestins 1 and 2 attenuated activation of NF-κB.52 Gao et al.53 also reported that β-arrestin 2 interacting with IκBα prevents the phosphorylation and degradation of IκBα and inhibits NF-κB activation in HEK 293 cells. Additionally, stimulation of β2-AR enhances β-arrestin 2 IκBα interaction and promotes stabilization of IκBα.53 The divergent effect of β-arrestins on ERK activation versus NF-κB signaling in the context of gene expression is uncertain. However, considering the scope of inflammatory genes regulated by NF-κB, β-arrestin inhibition of this signaling pathway may be predominately anti-inflammatory.
β-Arrestins regulate inflammatory disease
Endotoxemia and sepsis
Studies have implicated β-Arrestins in TLR signaling and gene activation. β-Arrestins 1 and 2 interact directly with TRAF6, a critical mediator in TLR signaling, preventing auto-ubiquitination of TRAF6 and activation of NF-κB and AP-1.30 Compared with WT mice, β-arrestin 2 KO mice subjected to endotoxin shock exhibit higher expression of pro-inflammatory cytokines, such as TNF-α, IL-1 β and IL-6. β-Arrestin 2 KO mice sensitized to LPS by D-galactosamine were more susceptible to endotoxin shock-induced lethality. Wang et al.30 concluded that β-arrestin 2 is a negative regulator of innate immune activation by TLRs. Porter et al.35 reported that LPS-induced inflammatory cytokines IFN-γ, IL-5, IL-1β, IL-12p40 and IL-6 levels in plasma were decreased in β-arrestin 1 and 2 KO mice compared with WT mice. β-Arrestin 1 and 2 KO mice exhibited improved survival to endotoxic shock.35 These different results may be a consequence of different endotoxemia models. Wang et al.30 employed D-galactosamine-endotoxin sensitized mice as opposed to endotoxin alone in the Porter et al.35 study.
In contrast to LPS shock, polymicrobial sepsis induced by cecal ligation and puncture (CLP) is generally accepted as a clinical relevant animal model.54 Fan et al.34 demonstrated that β-arrestin 2 KO mice were more susceptible to CLP-induced sepsis. When β-arrestin 2 KO and WT mice were subjected to CLP, survival rate was significantly decreased in β-arrestin 2 KO mice compared with WT mice. Subsequent studies demonstrated that CLP-induced plasma IL-6 and pulmonary and cecal myeloperoxidase (MPO) activity were significantly increased in the β-arrestin 2 KO mice. Also bacterial load in peripheral blood, peritoneal fluid and lung tissue were also significantly increased in the mice.34 Recent observations in another CLP sepsis study demonstrated that β-arrestin 2 levels were decreased in the lung and liver post-CLP. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX, preserved β-arrestin 2 expression and decreased NF-κB activation.55 Another recent article supports the notion that β-arrestin 2 negatively regulates systemic inflammation in response to polymicrobial infection. β-Arrestin 2 KO mice exhibit increased neutrophil sequestration and inflammation in the lungs following polymicrobial infection.56 Thus, β-arrestin 2 appears to be a negative regulator of inflammation in polymicrobial sepsis.
Asthma
Allergic asthma is a chronic inflammatory disorder of the airways that is characterized by migration of T helper cells and eosinophils into the lung. Migration of Th2 cells and eosinophils to the lung is important to their inflammatory function and is regulated, in large part, by chemokine receptors. Compared with WT mice, allergen-sensitized β-arrestin 2 KO mice do not accumulate CD3+ T lymphocytes in their airways, nor do they demonstrate other inflammatory features characteristic of asthma. In contrast, the airway inflammatory response to LPS, an event not coordinated by Th2 cells, was unaffected in β-arrestin 2 KO mice. In another asthma study β-arrestin 2 KO mice demonstrated defective chemokine-mediated CD4+ T cell migration to the lung.50 In subsequent studies the authors demonstrated that β-arrestin 2 also mediates the migration of hematopoietic-derived eosinophils to the lung.57 Additionally, it has been demonstrated that CD4+ T lymphocytes of mice with allergic asthma express higher levels of β-arrestin 2 mRNA and protein.58 Treatment of CD4+ T lymphocytes with siRNA targeting the β-arrestin 2 down-regulated phosphorylated ERK1/2 and IL-17 production essential to the pathogenesis of asthma.59 A recent study by Nichols et al.60 clarified the role of β-arrestin 2 in proteinase-activated receptor-2 (PAR2) signaling in the ovalbumin-induced murine model of allergic asthma. PAR2 is reported to have both protective and pro-inflammatory effects in the airway. The pro-inflammatory responses are mediated by β-arrestin 2, whereas the protective effects are not.60 As β-arrestin 2 appears to augment allergic inflammation, therapies focused on β-arrestin 2 may prove useful in the treatment of asthma.
Chronic use of β-agonists by asthmatics is associated with a loss of bronchodilator effects. β-Arrestin 2 KO mice exhibit augmented β-agonist-mediated airway smooth muscle relaxation and β-agonist-stimulated cAMP production.61 Moore et al.62 demonstrated that Salmeterol, a long-acting β2-AR agonist, did not induce significant β2-AR internalization or degradation, and was incapable of stimulating the translocation of β-arrestin 2 to the cell surface. Thus, the differing efficacy of β-agonists in asthma may be a consequence of differences in activation of β-arrestin-dependent signaling. These studies suggest a role of the β2-AR/ β-arrestin 2 axis in regulation of bronchial smooth muscle relaxation in experimental models of asthma.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune joint disease involving activation of innate immunity and dysfunction of adaptive immunity. A study by Wang et al.63 demonstrated that both β-arrestin 1 and 2 levels were increased in fibroblast-like synoviocytes (FLS) from collagen-induced arthritis (CIA) rats. Using a CIA mouse model and a human TNF-α transgenic (TNFtg) mouse model, Li et al.64 demonstrated that β-arrestin 1 and 2 expression was significantly increased in joint tissue of both CIA and TNFtg mice.64 The protein and mRNA levels of β-Arrestin 1 and 2 were also increased in FLS isolated from knee joints of CIA mice. TNF-α and low molecular mass hyaluronan (HA) induced an increase of β-arrestin 1 and 2 expression in FLS, while high mobility group box (HMGB)-1 selectively stimulated β-arrestin 1 expression. In FLS, HA-induced TNF-α and IL-6 production were increased by over-expression of β-arrestin 1, but decreased by overexpression of β-arrestin 2. Thus, β-arrestin expression is altered in FLS during inflammation and β-arrestins isoform specifically regulate FLS inflammatory responses.64 To examine the in vivo role of β-arrestin 2 in pathogenesis of arthritis, WT and β-arrestin 2 KO mice were subjected to collagen antibody-induced arthritis (CAIA). β-Arrestin 2 KO mice exhibited more severe arthritis in CAIA. Therefore, β-arrestin 2 may be anti-inflammatory in CAIA.64
Splenocytes from adjuvant arthritis rats on d 18 after induction of the disease exhibit increased β-arrestin 1 expression compared with controls. The level of β-arrestin 1 in splenocytes returned to normal at d 45. A similar time course for β-arrestin 1 expression was observed in mesenteric lymph nodes.65 In another study, the total glucosides of paeony (TGP), a herbal drug, decreased hind paw swelling and arthritis severity of CIA rats, and significantly reduced the expression of β-arrestins.63 In a subsequent study, it was demonstrated that β-arrestin 1 and 2 levels were increased in mesenteric lymph nodes from adjuvant arthritis rats.66 Although the data obtained with TGP are only correlative with reduction in both β-arrestin isoforms, the combined results with other studies with in vitro overexpression studies and β-arrestin 2 KO suggest that β-arrestin 1 may be pro-inflammatory versus β-arrestin 2, which maybe anti-inflammatory in experimental arthritis.
The relevance of all these experimental findings in animal models to human RA remains to be determined. However, β-arrestin 1 and 2 expression in tissues from RA patients has been examined. Recent studies demonstrate that both β-arrestin 1 and 2 expression is increased in FLS from RA patients compared with healthy controls (unpublished data). However, Lombardi et al.67 observed that β-arrestin 1 expression exhibited no difference in PBMC from RA patients compared with healthy donors. It is possible that in clinical RA localized changes in joint tissue β-arrestin expression may be more predictive of pathologic outcome.
Multiple sclerosis
Multiple sclerosis (MS) is characterized by the presence of plaques of demyelination throughout the central nervous system. Although the etiology of the disease has not been established, it is believed to involve autoimmune mechanisms. Ohguro et al.68 discovered that sera from MS patients formed an autoimmune complex with β-arrestin 1, while such serum auto-Abs were not detected from patients with other neurological diseases. The authors suggested that serum β-arrestin 1 Ab titers may be useful for the diagnosis and evaluation of the disease’s course.68,69 Forooghian et al.70 demonstrated that MS patients had a greater prevalence of positive T cell proliferative responses to β-arrestin 1 than healthy controls. Thus, β-arrestin has been identified as a novel non-myelin auto-antigen in MS. Such a notion is supported by an animal model of MS. In an experimental autoimmune encephalomyelitis (EAE) rat model, it has been demonstrated that β-arrestin 1 levels were significantly increased in the splenocytes from EAE compared with controls. β-Arrestin 1 positively regulates naïve and activated CD4+ T cell survival through regulation of acetylation of histone H4 at the Bcl2 promoter. β-Arrestin 1 KO mice exhibit significantly decreased numbers of CD4+ T cells in the spleen and lymph nodes. β-Arrestin 1 KO mice were much more resistant to EAE, whereas overexpression of β-arrestin 1 increased susceptibility to this autoimmunity.46 The authors also observed that CD4+ T cells from patients with MS had much higher expression of β-arrestin 1, whereas knockdown of β-arrestin 1 in those cells increased apoptosis. Therefore, the authors concluded that β-arrestin 1 is a critical for CD4+ T cell survival and is a factor in susceptibility to autoimmunity.46
In another clinical study, Tsutsui et al.71 further demonstrated that β-arrestin 1 expression was increased in brains of MS patients and varied inversely with the A1 adenosine receptor (A1AR). In vitro studies demonstrated that β-arrestin 1 overexpression down-regulated A1AR expression.71 In murine EAE, β-arrestin 1 and A1AR expression in the spinal cord displayed a similar inverse pattern compared with that observed in MS brain. EAE-induced neuroinflammation and neurobehavioral deficits were suppressed by glucocorticoid treatment, and correlated with concurrent reductions in β-arrestin 1, but enhanced A1AR expression. These studies suggest that β-arrestin 1 and A1AR expression and function are critical determinants in MS neuroinflammation.71
Studies have shown that β-arrestin 1 may down-regulate other receptors relevant to MS pathogenesis. β2-AR regulates astrocyte glucose metabolism to maintain brain activity in both normal and stress conditions.72 Clinical studies demonstrated that β2-AR is decreased in plaques and white matter of MS patients in postmortem brain sections compared with non-neurologic disease patients.73,74 As β-arrestin 1 regulates β2-AR internalization and degradation, increased β-arrestin 1 expression may result in decreases of β2-AR and reducing its neuroprotective effect.31,75
Inflammatory bowel disease
Inflammatory bowel disease includes both Crohn’s disease and ulcerative colitis, which are characterized by chronic relapsing inflammation of the gastrointestinal tract.76 Lee et al.77 demonstrated that β-arrestin 1 KO mice exhibit attenuated disease pathology in the experimental ulcerative colitis induced by trinitrobenzene sulfonic acid (TNBS) or dextran sulfate sodium.77 IL-6 expression in the plasma and colon tissue were also decreased in β-arrestin 1 KO mice compared with WT mice subjected to experimental colitis. These data suggest that β-arrestin 1 mediates IL-6 expression in the colon in experimental colitis model.77 In addition to β-arrestin 1, Fan et al.78 demonstrated that in the experimental ulcerative colitis in rats induced by TNBS, β-arrestin 2 expression was significantly decreased in the splenic lymphocytes. As β-arrestin 2 is frequently anti-inflammatory in a variety of diseases, these data suggest that β-arrestin 2 may also play a role in ulcerative colitis development.
Atherosclerosis and neointimal hyperplasia
Atherosclerosis and associated cardiovascular diseases are among the leading causes of mortality and morbidity worldwide. Cardiovascular risk factors (e.g. dyslipidemia, hypertension, diabetes and smoking) initiate chronic inflammatory responses that cause endothelial dysfunction, a key process in the initiation and progression of atherosclerosis. Transcriptional profiling data in severely atherosclerotic and non-atherosclerotic human coronary arteries showed that β-arrestin 2 mRNA levels are twofold higher in atherosclerotic patients than non-atherosclerotic controls.79 Atherosclerosis and arterial injury-induced neointimal hyperplasia involve smooth muscle cell (SMC) proliferation and migration into the arterial intima. ldlr−/− mice have an elevated serum cholesterol level and are employed as an animal model of atherosclerosis. Kim et al.80 demonstrated that deficiency of β-arrestin2 in ldlr−/− mice reduced aortic atherosclerosis and decreased the prevalence of atheroma SMCs.
Carotid endothelial denudation is employed to induce neointimal hyperplasia and has been studied in congenic WT, and β-arrestin 1 2 KO mice.80 Neointimal hyperplasia was enhanced in β-arrestin 1 KO mice, but diminished in β-arrestin 2 KO mice. After carotid injury, SMC ERK 1/2 activation and proliferation were also increased in β-arrestin 1 KO and decreased in β-arrestin 2 KO mice. Concordantly, SMC thymidine incorporation, ERK 1/2 activation and SMC migration evoked by 7-TMRs were greater in β-arrestin 1 KO than WT, but less in β-arrestin 2 KO. These studies support the hypothesis that SMC proliferation and migration in atherosclerosis are regulated reciprocally by β-arrestin 2 and β-arrestin 1.
Conclusions
The expression of β-arrestins 1 and 2 is regulated in immune cells in response to diverse inflammatory conditions. In turn, β-arrestin 1 and 2 regulate diverse inflammatory responses and contribute to multiple inflammatory diseases. Further evaluation of the effect of β-arrestins 1 and 2 in different cells at different stages of inflammatory diseases may generate a clearer picture how these unique proteins change in inflammation. The function of β-arrestins 1 and 2 in regulation of inflammation can be quite different. β-Arrestin isoforms regulate distinct spatial and temporal activation of signaling proteins that activate different target genes. β-Arrestin 1 or 2 KO mice have provided an important investigative approach and have been employed extensively. However, it is also possible that the β-arrestin isoforms can compensate for each other as knockout of single β-arrestin 1 or 2 in mice appears not to affect the mice, while knockout of both β-arrestin 1 and 2 is lethal. The availability of conditional β-arrestin 1 and 2 KO mice could help resolve this problem. Differentiation of signaling mediated by β-arrestins 1 and 2 in different relevant immune cells remains a huge challenge. Additionally, how β-arrestin levels are regulated at the transcriptional and/or translational level in inflammation remains poorly understood. Further investigations of the role of β-arrestins in inflammation are warranted and may lead to novel approaches to treat a variety of inflammatory diseases.
Acknowledgements
Thanks to Drs James Cook and Perry Halushka for their helpful suggestions.
Funding
This work was supported, in part, by GM27673 (JAC) and AI079248 (HF).
References
- 1.Bjarnadottir TK, Gloriam DE, Hellstrand SH, et al. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 2006; 88: 263–273. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaumer L, Yatani A, VanDongen AM, et al. G protein coupling of receptors to ionic channels and other effector systems. Soc Gen Physiol Ser 1990; 45: 169–183. [PubMed] [Google Scholar]
- 3.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-Arrestins in receptor signaling and desensitization. J Biol Chem 1998; 273: 18677–18680. [DOI] [PubMed] [Google Scholar]
- 4.Pitcher JA, Tesmer JJ, Freeman JL, et al. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem 1999; 274: 34531–34534. [DOI] [PubMed] [Google Scholar]
- 5.Goodman OB Jr, Krupnick JG, Santini F, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 1996; 383: 447–450. [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz RJ and Whalen EJ. β-Arrestins: traffic cops of cell signaling. Curr Opin Cell Biol 2004; 16: 162–168. [DOI] [PubMed] [Google Scholar]
- 7.Oakley RH, Laporte SA, Holt JA, et al. Association of betaarrestin with G protein-coupled receptors during clathrinmediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 1999; 274: 32248–32257. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy SK. Seven-transmembrane receptors and ubiquitination. Circ Res 2007; 100: 1142–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shenoy SK, McDonald PH, Kohout TA, et al. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 2001; 294: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 10.Shenoy SK, Xiao K, Venkataramanan V, et al. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem 2008; 283: 22166–22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchese A, Raiborg C, Santini F, et al. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell 2003; 5: 709–722. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Kirkbride KC, How T, et al. Beta-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science 2003; 301: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 13.Girnita L, Shenoy SK, Sehat B, et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem 2007; 282: 11329–11338. [DOI] [PubMed] [Google Scholar]
- 14.Puckerin A, Liu L, Permaul N, et al. Arrestin is required for agonist-induced trafficking of voltage-dependent calcium channels. J Biol Chem 2006; 281: 31131–31141. [DOI] [PubMed] [Google Scholar]
- 15.Simonin A and Fuster D. Nedd4-1 and beta-arrestin-1 are key regulators of Na + /H+ exchanger 1 ubiquitylation, endocytosis, and function. J Biol Chem 2010; 285: 38293–38303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavard J and Gutkind JS. VEGF controls endothelial-cell per-meability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 2006; 8: 1223–1234. [DOI] [PubMed] [Google Scholar]
- 17.Luttrell LM, Roudabush FL, Choy EW, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA 2001; 98: 2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald PH, Chow CW, Miller WE, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 2000; 290: 1574–1577. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Cheng Z, Ma L, et al. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem 2002; 277: 49212–4929 [DOI] [PubMed] [Google Scholar]
- 20.Luttrell LM, Daaka Y and Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 1999; 11: 177–183. [DOI] [PubMed] [Google Scholar]
- 21.Luttrell LM, Ferguson SS, Daaka Y, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999; 283: 655–661. [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu JM, Sotnikova TD, Marion S, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005; 122: 261–273. [DOI] [PubMed] [Google Scholar]
- 23.Povsic TJ, Kohout TA and Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem 2003; 278: 51334–51339. [DOI] [PubMed] [Google Scholar]
- 24.Bjorgo E, Solheim SA, Abrahamsen H, et al. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Mol Cell Biol 2010; 30: 1660–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Baillie GS and Houslay MD. Mdm2 directs the ubiquitination of beta-arrestin-sequestered cAMP phosphodiesterase-4D5. J Biol Chem 2009; 284: 16170–16182. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Luttrell LM and Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev 2010; 62: 305–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall RT, Strungs EG, Rachidi SM, et al. The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1,Ile4,Ile8]angiotensin II regulates a robust G protein-independent signaling network. J Biol Chem 2011; 286: 19880–19891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walters RW, Shukla AK, Kovacs JJ, et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 2009; 119: 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loniewski K, Shi Y, Pestka J, et al. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol Immunol 2008; 45: 2312–2322. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Tang Y, Teng L, et al. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 2006; 7: 139–147. [DOI] [PubMed] [Google Scholar]
- 31.Kizaki T, Izawa T, Sakurai T, et al. Beta2-adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin 2. Immunology 2008; 124: 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parameswaran N, Pao CS, Leonhard KS, et al. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem 2006; 281: 34159–34170. [DOI] [PubMed] [Google Scholar]
- 33.Sender V, Lang L and Stamme C. Surfactant protein-A modulates LPS-induced TLR4 localization and signaling via beta-arrestin 2. PLoS One 2013; 8: e59896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan H, Bitto A, Zingarelli B, et al. Beta-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology 2010; 130: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter KJ, Gonipeta B, Parvataneni S, et al. Regulation of lipo-polysaccharide-induced inflammatory response and endotoxemia by β-arrestins. J Cell Physiol 2010; 225: 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seregin SS, Appledorn DM, Patial S, et al. β-Arrestins modulate adenovirus-vector-induced innate immune responses: differential regulation by beta-arrestin-1 and beta-arrestin-2. Virus Res 2010; 147: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie L, Qiao X, Wu Y, et al. beta-Arrestin1 mediates the endocytosis and functions of macrophage migration inhibitory factor. PLoS One 2011; 6: e16428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Xu M, Zhang YY, et al. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol Sin 2009; 30: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basher F, Fan H, Zingarelli B, et al. beta-Arrestin 2: a negative regulator of inflammatory responses in polymorphonuclear leukocytes. Int J Clin Exp Med 2008; 1: 32–41. [PMC free article] [PubMed] [Google Scholar]
- 40.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol 2007; 69: 535–560. [DOI] [PubMed] [Google Scholar]
- 41.Barlic J, Khandaker MH, Mahon E, et al. β-Arrestins regulate interleukin-8-induced CXCR1 internalization. J Biol Chem 1999; 274: 16287–16294. [DOI] [PubMed] [Google Scholar]
- 42.Su Y, Raghuwanshi SK, Yu Y, et al. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol 2005; 175: 5396–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge L, Ly Y, Hollenberg M, et al. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudo-podia during protease-activated receptor-2-induced chemotaxis. J Biol Chem 2003; 278: 34418–34426. [DOI] [PubMed] [Google Scholar]
- 44.DeWire SM, Ahn S, Lefkowitz RJ, et al. β-Arrestins and cell signaling. Annu Rev Physiol 2007; 69: 483–510. [DOI] [PubMed] [Google Scholar]
- 45.Barlic J, Andrews JD, Kelvin AA, et al. Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol 2000; 1: 227–233. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, Feng Y, Kang J, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol 2007; 8: 817–824. [DOI] [PubMed] [Google Scholar]
- 47.Bryceson YT and Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol 2008; 20: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu MC, Su LL, Zou L, et al. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol 2008; 9: 898–907. [DOI] [PubMed] [Google Scholar]
- 49.Fong AM, Premont RT, Richardson RM, et al. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci U S A 2002; 99: 7478–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker JK, Fong AM, Lawson BL, et al. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 2003; 112: 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan H, Luttrell LM, Tempel GE, et al. β-Arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol 2007; 44: 3092–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witherow DS, Garrison TR, Miller WE, et al. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci USA 2004; 101: 8603–8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao H, Sun Y, Wu Y, et al. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 2004; 14: 303–317. [DOI] [PubMed] [Google Scholar]
- 54.Dejager L, Pinheiro I, Dejonckheere E, et al. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 2011; 19: 198–208. [DOI] [PubMed] [Google Scholar]
- 55.Bitto A, Minutoli L, David A, et al. Flavocoxid, a dual inhibitor of COX-2 and 5-LOX of natural origin, attenuates the inflammatory response and protects mice from sepsis. Crit Care 2012; 16: R32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Sharma D, Malik A, Lee E, et al. Gene dosage dependent negative regulatory role of beta-arrestin-2 in polymicrobial infection induced inflammation. Infect Immun 2013; 81: 3035–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollingsworth JW, Theriot BS, Li Z, et al. Both hematopoietic-derived and non-hematopoietic-derived {beta}-arrestin-2 regulates murine allergic airway disease. Am J Respir Cell Mol Biol 2010; 43: 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G, Liu Y, Yang M, et al. Effects of beta-arrestin 2 on cytokine production of CD4+ T lymphocytes of mice with allergic asthma. Indian J Exp Biol 2011; 49: 585–593. [PubMed] [Google Scholar]
- 59.Liu Y, Wang GY, Liu SK, et al. beta-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model. Iran J Allergy Asthma Immunol 2011; 10: 171–182. [PubMed] [Google Scholar]
- 60.Nichols HL, Saffeddine M, Theriot BS, et al. beta-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci USA 2012; 109: 16660–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deshpande DA, Theriot BS, Penn RB, et al. β-Arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J 2008; 22: 2134–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore RH, Millman EE, Godines V, et al. Salmeterol stimulation dissociates beta2-adrenergic receptor phosphorylation and internalization. Am J Respir Cell Mol Biol 2007; 36: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang QT, Zhang LL, Wu HX, et al. The expression change of β-Arrestins in fibroblast-like synoviocytes from rats with collageninduced arthritis and the effect of total glucosides of paeony. J Ethnopharmacol 2011; 133: 511–516. [DOI] [PubMed] [Google Scholar]
- 64.Li P, Cook JA, Gilkeson GS, et al. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol 2011; 49: 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lombardi MS, Kavelaars A, Cobelens PM, et al. Adjuvant arthritis induces down-regulation of G protein-coupled receptor kinases in the immune system. J Immunol 2001; 166: 1635–1640. [DOI] [PubMed] [Google Scholar]
- 66.Wu H, Wei W, Song L, et al. Paeoniflorin induced immune tolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergic receptor desensitization in rats with adjuvant arthritis. Int Immunopharmacol 2007; 7: 662–673. [DOI] [PubMed] [Google Scholar]
- 67.Lombardi MS, Kavelaars A, Schedlowski M, et al. Decreased expression and activity of G-protein-coupled receptor kinases in peripheral blood mononuclear cells of patients with rheumatoid arthritis. FASEB J 1999; 13: 715–725. [DOI] [PubMed] [Google Scholar]
- 68.Ohguro H, Chiba S, Igarashi Y, et al. Beta-arrestin and arrestin are recognized by autoantibodies in sera from multiple sclerosis patients. Proc Natl Acad Sci USA 1993; 90: 3241–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeda Y, Sudoh A, Chiba S, et al. Detection of serum antibody against arrestin from patients with acute disseminated encephalomyelitis. Tohoku J Exp Med 1999; 187: 65–70. [DOI] [PubMed] [Google Scholar]
- 70.Forooghian F, Cheung RK, Smith WC, et al. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol 2007; 27: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsutsui S, Vergote D, Shariat N, et al. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. FASEB J 2008; 22: 786–796. [DOI] [PubMed] [Google Scholar]
- 72.Dong JH, Chen X, Cui M, et al. Beta2-adrenergic receptor and astrocyte glucose metabolism. J Mol Neurosci 2012; 48: 456–463. [DOI] [PubMed] [Google Scholar]
- 73.De Keyser J, Wilczak N, Leta R, et al. Astrocytes in multiple sclerosis lack beta-2 adrenergic receptors. Neurology 1999; 53: 1628–1633. [DOI] [PubMed] [Google Scholar]
- 74.Zeinstra E, Wilczak N and De Keyser J. [3H]dihydroalprenolol binding to beta adrenergic receptors in multiple sclerosis brain. Neurosci Lett 2000; 289: 75–77. [DOI] [PubMed] [Google Scholar]
- 75.Kizaki T, Shirato K, Sakurai T, et al. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol Immunol 2009; 46: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 76.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–429. [DOI] [PubMed] [Google Scholar]
- 77.Lee T, Lee E, Irwin R, et al. beta-Arrestin-1 deficiency protects mice from experimental colitis. Am J Pathol 2013; 182: 1114–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan H, Liao Y, Tang Q, et al. Role of beta2-adrenoceptor-beta-arrestin2-nuclear factor-kappaB signal transduction pathway and intervention effects of oxymatrine in ulcerative colitis. Chin J Integr Med 2012; 18: 514–521. [DOI] [PubMed] [Google Scholar]
- 79.Archacki SR, Angheloiu G, Tian XL, et al. Identification of new genes differentially expressed in coronary artery disease by expression profiling. Physiol Genomics 2003; 15: 65–74. [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Zhang L, Peppel K, et al. β-Arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res 2008; 103: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]