Abstract
Objectives
Brown adipose tissue (BAT) and BAT-like adipose tissues, referred to as ‘beige’ adipose tissues uncouple respiration from ATP synthesis via uncoupling protein one (UCP-1). There is a sexual dimorphism with respect to beige and BAT tissues; pre-menopausal women have more BAT and are more sensitive to BAT activation than men or postmenopausal women. We hypothesized selective activation of adipose tissue estrogen receptor alpha (ERα) induces beiging of WAT through induction of lipolysis mediated by adipose tissue triglyceride lipase (ATGL).
Methods
3T3-L1 and primary adipocytes were treated with the selective ERα agonist pyrazole triol (PPT), and selection deletion of ERα (using siRNA) was used to determine if selective ERα activation, or inhibition, influences the adipose tissue expression of genes associated with beiging. In a second series of experiments, ERα was selectively added back to adipose tissue of mice lacking total body ERα (ERKO) to determine if add back of ERα changed the morphology of adipose tissue to resemble beige tissues. Additionally, WT and ERKO mice were exposed to cold and FDG labeled glucose uptake was measured to determine the ability of cold to induce UCP-1 in ERKO mice. To begin to mechanistically probe how activation of ERα facilitates beiging, we tested the influence of PPT to activate the lipolytic pathway through ATGL. Finally, since ERα exerts its effects both at the genomic and non-genomic level depending on its cellular location, we determined in vivo if beiging occurs in mice expressing ERα only at the plasma membrane (MOER mice) or only at nucleus (NOER mice).
Results
Selective ERα activation by PPT increased markers of beiging in vitro in 3T3-L1 and primary adipocytes, whereas, knockdown of ERα with siRNA reduced the ability of PPT to induce beiging in vitro. ERα add back to the adipose tissue of ERKO mice resulted in multilocular adipose tissue resembling a beige phenotype. Following cold exposure, FDG labeled glucose in BAT tissues of ERKO mice was reduced when compared to weight-matched controls. Glycerol release and ATGL expression were increased after PPT treatment, while pre-treatment with the ATGL inhibitor prevented PPT's ability to increase UCP-1 expression. Finally, MOER mice were more sensitive to beiging of adipose tissues when compared to NOER mice.
Conclusion
Our results demonstrate for the first time that selective-activation of ERα in adipocytes increases markers of beiging and this is likely through induction of AMPK and ATGL-mediated lipolysis providing FFAs as a fuel to activate UCP-1.
Keywords: Beige adipocytes, Lipolysis, Estrogen receptor alpha (ERα), Obesity, Type II diabetes mellitus (T2DM)
Highlights
-
•
Selective activation of estrogen receptor alpha (ERα) increases markers of beiging in white adipocytes.
-
•
Selective ERα activation increases glycerol release, lipolysis, markers of beiging of adipose tissues.
-
•
Mice lacking ERα are cold intolerant demonstrating the necessity of ERα to facilitate brown adipose tissue activation.
-
•
Mice with ERα only in the membrane (MOER mice) are more sensitive to a β3-adrenergic receptor induced beiging when compared to mice that express nuclear only ERα (NOER).
1. Introduction
Obesity is characterized by an excessive accumulation of white adipose tissue (WAT), which is a predisposition for the development of insulin resistance and type 2 diabetes mellitus (T2DM) and causes an increased risk of morbidity and mortality [1]. Caloric restriction and physical exercise can reduce obesity-related comorbidities; however, these interventions require rigorous, long-term adherence for success, making compliance challenging. One possibility to reduce obesity and T2DM is to convert WAT storage tissues into metabolically active tissues analogous to brown adipose tissue (BAT), and this process is known as beiging of adipose tissues [2]. BAT and beige tissues are functionally characterized by their unique ability to uncouple mitochondrial respiration from ATP synthesis via uncoupling protein 1 (UCP-1), allowing for enhanced free fatty acid (FFA) oxidation and heat production [3]. The recent discovery that BAT/beige tissues exist in adult humans has led to the concept that increasing their activity is a therapeutic strategy to reduce body weight and improve insulin sensitivity in humans [4], [5], [6]. Although several approaches have been proposed to induce beiging of WAT, most of them rely on activation of the sympathetic nervous system (SNS) [7], [8], [9]. Briefly, activated SNS releases norepinephrine, which binds to adrenergic receptors in BAT and beige tissues, promoting UCP-1 activation and heat production. However, adrenergic activation cannot be used therapeutically in humans because of its adverse effects on other tissues; therefore, there is a critical need to identify novel targets to safely and specifically activate BAT and beige tissues to reduce obesity and its comorbidities.
Lipolysis is a key mechanism underlying activation of UCP-1 and heat production in BAT and beige tissues. There are data in humans and rodents indicating BAT and beige tissues utilize triglycerides stored in lipid droplets, and, following adrenergic stimulation, there is a release of FFAs, which are then used as a primary fuel for oxidation [6], [10]. Perturbations of FFA mobilization are hallmarks of obesity that are associated with insulin resistance and T2DM. Specifically, adrenergic stimulation initiates the canonical adrenergic receptor-Gs-adenylyl cyclase-cAMP-PKA pathway [11], which in turn activates adipose tissue triglyceride lipase (ATGL) and other lipases such as hormone sensitive lipase (HSL) [12]. While whole body or adipose tissue specific ATGL knockout mice are unable to maintain body temperature upon cold exposure by activation of adrenergic signaling, HSL knockout mice are not cold insensitive, suggesting that ATGL is crucial for beiging of adipose tissues and cold tolerance [12]. Interestingly, the addition of FFAs to the culture medium of brown adipocytes stimulates thermogenesis in the absence of adrenergic stimulation [11], suggesting there might be non-adrenergic mechanisms which induce lipolysis and thereby, BAT/beige tissue stimulation.
There is a sexual dimorphism with respect to BAT and beige tissues, as demonstrated by the fact that premenopausal women have more BAT and are more sensitive to BAT/beige activation than men [13] or postmenopausal women. These data further suggest that sex hormones, and perhaps specifically estrogens and/or estrogen receptors, may be involved in mediating BAT and beige tissue activity. Estrogen receptor alpha (ERα) exists in numerous cell types to include the central nervous system (CNS) [14], as well as adipocytes [15], two tissues critical for the activation of BAT and beige tissues. We and others have demonstrated that ERα impacts whole body energy expenditure and glucose homeostasis in a tissue-specific manner [16], [17]. Furthermore, glucose homeostasis is impaired in humans with polymorphisms in the ERα gene [15], [18], while global ERα knockout mice (ERKO) are obese, insulin resistant, and dyslipidemic [19]. We and others have shown that ERα is involved in regulating adipose tissue function [14], [20], [21], [22], and knockdown of ERα from adipocytes results in increased adipocyte size (hypertrophy) and number (hyperplasia), as well as increased adipose tissue inflammation and fibrosis [14], [23]. Additionally, activation of ERα induces lipolysis in adipose tissues [24]. Due to the sexual dimorphism in BAT and beige tissues in humans and the critical role of ERα to modulate adipose tissue function, we hypothesize that activation of adipose tissue ERα induces beiging of WAT through induction of lipolysis which is mediated by the lipolytic factor ATGL.
2. Material and methods
2.1. Cell culture
2.1.1. 3T3-L1 cells
Mouse 3T3-L1 pre-adipocytes (Cat. number CL-173TM) were obtained from American Type Culture Collection (ATCC®, Manassas, VA, USA), and their growth and differentiation were performed according to ATCC®ś protocol [25]. Briefly, cells were grown in DMEM high glucose (ATCC®, Cat. number 30-2002) supplemented with 10% Bovine Calf Serum (BCS) (ATCC®, Cat. number 30-2031), and after 48 h they reached confluency. Cells were induced to differentiation in DMEM containing 10% Fetal Bovine Serum (FBS) (Gibco™, Thermo Fisher Scientific™, Waltham, MA, USA, Cat. number 26140-079) and supplemented with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) (Sigma®, St Louis, MO, USA, Cat. number 858455), 1 μM dexamethasone (DEXA) (Sigma®, Cat. number D1756), and 1 μg/ml insulin (Sigma®, I0516). 48 hrs after induction of differentiation, cells were maintained in DMEM supplemented with 10% FBS and 1 μg/ml insulin for 8–10 days until they reached full differentiation and were ready to be used in experiments. Cells were then exposed to DMEM phenol-free medium (Gibco™, Cat. number 21063-029) supplemented with 10% FBS charcoal:dextran stripped (Gemini-Bio Products, West Sacramento, CA, USA, Cat. number 100-119) and 1% sodium-pyruvate (Gibco™, Cat. number 11360-070) for 24 h prior to treatments. To begin to determine if there is a correlation between ERα and UCP-1 expression, we assayed ERα and UCP-1 mRNA at the same time point across a time course during the differentiation process (days 0, 4 and 10) of 3T3-L1 cells. To test if ERα activation in mature adipocytes promotes markers of beiging, differentiated 3T3-L1 adipocytes were treated with the ERα agonist propyl-pyrazole (PPT) (Tocris Bio-Techne Corporation, Minneapolis, MN, USA, Cat. number 1426) in the concentration of 10 nM, according to our previous publication [21] for the duration of 5 min to 5 h. Gene expression for makers of beiging to include: Ucp1, Ppargc1α, Pdk4, Tnfrsf9, and Tbx1 was determined by qPCR, and UCP-1 protein was measured by WB, as described below.
2.1.2. Primary adipocytes
Pre-adipocytes from female WT C57BL/6J mice were obtained and differentiated into adipocytes according to published protocols [26], [27]. Briefly, dissected inguinal WAT (iWAT) from mice was minced in PBS and then digested for 2 h in buffer containing 100 mM HEPES (pH 7.4), 120 mM NaCl, 50 mM KCl, 5 mM glucose, 1 M CaCl2, 1.5% BSA, and 1 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN, USA, Cat. number 11088866001). Digested tissues were then diluted in Stromal Vascular Fraction (SVF) media composed of DMEM/F12 (Gibco™, Cat. number 10565-018) and 10% FBS, filtered through a 100 μm cell strainer, and spun down for 5 min at 600 g to pellet the SVF and separate the adipocytes. The SVF pellet was resuspended in SVF medium, filtered through a 40 μm cell strainer and centrifuged again. At the end, the final pellet was resuspended in SVF media and plated. Cells were grown in SVF media until confluency, and 48 h later, they were differentiated in SVF media containing 0.5 mM IBMX, 1 μM DEXA, 5 μg/ml insulin, and 1 μM rosiglitazone (Sigma®, Cat. number R2408). Cells were then maintained in SVF media supplemented with 5 μg/ml insulin for 8–10 days. Differentiated adipocytes were exposed to DMEM/F12 phenol-red free medium (Gibco™, Cat. number 21041-025) and supplemented with 10% stripped FBS for 24 h. In order to test the role of ERα activation to induce markers of beiging in primary adipocytes, differentiated cells were treated with PPT in the concentration of 1 nM. For this series of experiments we used 1 nM of PPT because we saw no effect using 10 nM of PPT to induce markers of beiging in primary adipocytes. Primary cells were treated for 5 h and probed for the following beiging markers: Ucp1, Ppargc1α, Pdk4, Tnfrsf9, and Tbx1 for UCP-1 protein by WB.
2.1.3. Small interfering RNA (siRNA) knockdown
In order to determine if ERα is necessary to induce markers of beiging in adipocytes, we used a small interfering RNA (siRNA) to knockdown the ERα gene (Esr1) in mature 3T3-L1 cells according to our previously published protocol [21], [28]. Briefly, 3T3-L1 differentiated adipocytes were transferred to 12-well plates and transfected with siRNA targeting murine Esr1 (siGENOME mouse Esr1 siRNA, GE Dharmacon, Lafayette, LA, USA, Cat. number M-058688-01-0005), at the concentration of 100 nM, using Opti-MEM non-phenol medium (Gibco™, Cat. number 11058-021). As a negative control, cells were treated with a scrambled sequence siRNA at the same concentration (siGENOME non-targeting siRNA, GE Dharmacon, Cat. number D-001206-13-05). Lipofectamine™ was used as the transfection reagent (RNA iMAX, Invitrogen™, Thermo™, Cat. number 13778-030), at the concentration of 20 рM/ml, according to the manufacturer's instructions. 72 hrs after siRNA transfection, cells were collected and assayed to test the efficacy of the ERα knockdown by ERα mRNA. Using this protocol, we were able to achieve approximately 40% knockdown of ERα consistent with our previous publications [21], [28]. Transfected cells were then treated with PPT (10 nM), or the β3-adrenergic receptor agonist CL316,243 (Sigma®, Cat. number C5976) at a dose of 100 nM, according to previous reports [29] and cells were collected for UCP-1 mRNA measurement.
2.1.4. Glycerol release in the culture medium and lipolysis markers
To determine if ERα is necessary for mediating lipolysis, 3T3-L1 differentiated cells were exposed to media supplemented with 2% fatty-acid free BSA for 4 h according to previously published protocols [29], [30], [31]. Cells were then pre-treated for 1 h with the ATGL lipolysis blocker Atglinstatin (Sigma®, Cat. number SML 1075) at the concentration of 20 μM, or Isoproterenol (Tocris, Cat. number 10009951), which was used as a positive control at the dose of 10 μM [30]. Another set of cells were pre-treated with a blocker of HSL, CAY 10499 (Cayman, Ann Arbor, MI, USA, Cat. number 359714-55-9) at the dose of 10 μM, followed by which cells were treated with PPT (10 nM) for 5 h and glycerol release was measured in the media by the Adipolysis Assay kit (Cayman, Cat. number 10009381). ATGL protein and AMPK phosphorylation were assayed by WB as described below.
2.2. In vivo studies
2.2.1. Total body ERα knockout mice (ERKO)
To begin to determine the necessity of ERα to mediate BAT/beige thermogenesis in vivo, weight matched C57BL/6J WT and ERKO male mice were used. Mice were housed in normal vivarium conditions (22 °C), exposed to a 12 h light/dark cycle, fed a standard chow diet and provided ad libitum water. Animal care and procedures were approved by the University of Texas Southwestern Medical Center (UTSW, Dallas, TX), and were performed in accordance to approved IACUC protocols. For the first experiment, male mice were maintained at room temperature (RT, 22 °C) for 16 h, and fasted for 12 h following which they were sacrificed and the interscapular adipose tissue (iBAT) was collected for ERα and UCP-1 protein measurement by WB. In a second experiment, weight-matched WT and ERKO male mice were exposed to PET/CT scan performed using Siemens Inveon PET/CT Multi Modality System (Siemens Medical Solutions, Knoxville, TN). Briefly, mice were exposed to thermoneutrality (TN 28 °C) for 16 h; 3 days later, they were exposed to room temperature (RT 22 °C) for 16 h; and 3 days later, to cold temperature (4 °C) for 16 h. At the end of 16 h in each temperature, mice received 150 μCi of 18F fluorodeoxyglucose (FDG) in 150 μl of saline intravenously via tail vein injection. PET images were acquired 1 h post-injection for each temperature. PET and CT images were co-registered in Inveon Acquisition Workplace (Siemens Medical Solutions, Knoxville, TN). Regions of interest (ROIs) were drawn around the iBAT and standardized uptake values (SUV) were established within this region and was recorded. SUV was defined by the FDG uptake in BAT and normalized by total FDG uptake and body weight of the animal.
2.2.2. ERα added back to ERKO mice (Ad-ERα)
To determine the role of adipose tissue ERα to mediate beiging, ERα was added back to iWAT of female ERKO mice. Briefly, C57BL6 WT and ERKO female mice, housed in normal vivarium conditions (22 °C), were injected with an ERα overexpression vector (Ad-ERα) in one iWAT fat pad and a control vector (Ad-GFP) in the contra-lateral iWAT using the methods previously reported [26]. iWAT was collected 6 days after injections and samples were fixed in 10% neutral buffered formalin for 48 h, then embedded in paraffin wax for histology performed by the Cedars-Sinai Biobank and Translational Medicine Core (Los Angeles, CA). Slide images were acquired using Apeiro AT Turbo (Leica Biosystems Inc., U.S.). The average fluorescence intensity of 10 randomly selected iWAT regions of interest (ROI) were measured in each sample using StrataQuest software (TissueGnostics, Austria). Results are expressed as arbitrary units comparing the Ad-ERα iWAT to the contralateral Ad-GFP fat pad.
2.2.3. Adipocyte-specific knockdown of ERα (AdipoERα) in vivo
In order to determine the influence of ERα activation on markers of lipolysis in vivo, we used mice with selective knockdown of ERα in adipocytes as previously published [23]. Briefly, adiponectin promoter driven-Cre transgenic mice were crossed with mice carrying the lox-P-flanked ERα allele (ERαlox/lox), producing ERαlox/lox/Adiponectin-Cre (AdipoERα) littermates on a C57BL/6 background. Mice were housed in vivarium standard temperatures (around 22 °C), exposed to a 12 h light/dark cycle, and were fed standard chow with ad libitum water. Animal care and procedures were approved by the UTSW Medical Center in accordance to IACUC approved protocols. For markers of lipolysis analysis samples of iWAT and visceral adipose tissue of female AdipoERα mice and weight-matched WT controls were processed for perilipin, HSL and β3-AR mRNA. In another set of experiments, female and male AdipoERα and weight-matched WT controls were treated with CL316-243 for 5 days at the concentration of 1 mg/kg/d i.p. [32], [33], [34], and fasting glycerol was determined.
2.2.4. Membrane (MOER) or nuclear (NOER) only estrogen receptor alpha
In order to begin to differentiate the genomic vs nongenomic ability of ERα to mediate adrenergic-stimulated beiging of adipose tissues in vivo we used mice expressing ERα only in the membrane (MOER), or only in the nucleus (NOER), and compared their response to CL316,243 to that of age matched WT mice (a gift by Dr. Ellis Levin, generated as previously published) [35], [36]. Mice were housed in standard vivarium temperatures (22 °C), fed with a standard chow diet, and were then treated with saline (vehicle) or CL316,243 for 5 days in the concentration of 1 mg/kg/d i.p. [32], [33], [34]. The Animal Studies and the Research and Development Committees at the Department of Veterans Affairs Long Beach Healthcare Facility, CA approved the experimental procedures. On the final and 5th day of injections, mice were sacrificed following a 4 h fast and iWAT was collected and processed at Cedars-Sinai Medical Center for UCP-1 immunofluorescence and mRNA expression of UCP-1 and ATGL.
2.2.5. RT-qPCR
mRNA expression of markers of beiging and lipolysis following PPT or CL316,243 treatments was determined by RT-qPCR. Adipose tissue samples were lysed in TRIzol® reagent (Invitrogen™, Thermo™, Cat. number 15596026) and RNA was extracted and isolated using phase separation reagent (BCP) (MRC®, Cincinnati, OH, USA, Cat. number BP151) and RNeasy kit (Qiagen®, Hilden, Germany, Cat. number 74104), according to the manufacturer's instructions. The concentration and purity of RNA were determined by spectrophotometric analysis (NanoDrop ND-1000, Thermo™), and all samples had an A260/A280 ratio around 2. cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems™, Thermo™, Cat. number 4387-406). Real time qPCR was performed using TaqMan® Universal MasterMix II, with UNG (Applied Biosystems™, Cat. number 4440038), and TaqMan® specific primers (Applied Biosystems™) (Table 1), on the QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems™). Mouse β-actin gene (Actb) was used as the reference gene, and data was normalized and relative expression determined from the threshold cycle (Ct) following the 2−ΔΔCT method.
Table 1.
List of TaqMan® primers.
| Ucp1 | Mm01244861_m1 |
| Actb | Mm02619580_g1 |
| Ppargc1a | Mm01208835_m1 |
| Pdk4 | Mm01166879_m1 |
| Tnfrsf9 | Mm00441899_m1 |
| Tbx1 | Mm00448949_m1 |
| Esr1 | Mm00433149_m1 |
| Lipe | Mm00495359_m1 |
| Adgrb3 | Mm00657451_m1 |
| Pnpla2 | Mm00503040_m1 |
2.2.6. Western blotting (WB)
Protein levels of UCP-1, ATGL and phosphorylation of AMPK were determined by WB in cell and adipose tissue lysates. To do this, cells and adipose tissues were lysed in Pierce™ RIPA® buffer (Thermo™, Cat. number 89900) supplemented with anti-phosphatase cocktail (PhosSTOP EASY pack, Roche, Cat. number 04-906-837-001) and anti-protease cocktail (cOmplete™ mini, Roche, Cat. number 11836153001). After sample centrifugation, the supernatant was collected and protein concentration was determined using Pierce® BCA sample assay (Thermo™, Cat. number 23228). Proteins (30 μg) from lysates were separated by electrophoresis and electrotransferred to nitrocellulose membranes. Later, membranes were stained with Ponceau-S (Sigma®, Cat. number P7170) to be used as the loading control. Membranes were blocked in TBS-T containing 5% non-fat milk powder, followed by overnight incubation with primary antibodies: anti-UCP1 (Abcam, Cambridge, MA, USA, Cat. number ab10983, 1:500), anti-ERα (Sta Cruz Biotechnologies, Dallas, TX, Cat. number sc-542), anti-AMPK (Cell-Signaling, Danvers, MA, USA, Cat. number 2532S), anti-phospho-AMPK Thr172 (Cell, Cat. number 2535S), and anti-ATGL (Cell, Cat. number 2138S). Blot's intensity was quantified by densitometry (ImageQuant LAS 4000, GE Healthcare, Pittsburg, PA, USA). Results were expressed in arbitrary units in comparison to controls.
2.2.7. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical significance between groups was determined by one-way ANOVA followed by Tukey post-test. Two-tailed paired Student's test was used as appropriate. P values < 0.05 were considered statistically significant. Statistical analyses and graphs were generated using GraphPad Prism 6 for Windows software (GraphPad software, San Diego, CA, USA).
3. Results
3.1. Activation of ERα induces markers of beiging in mature adipocytes
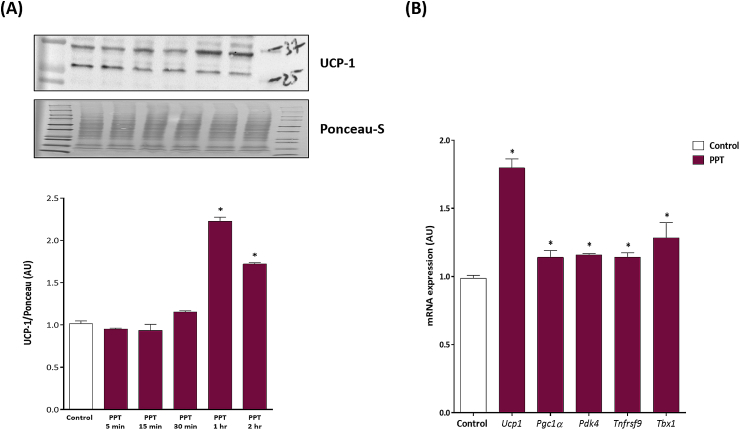
To begin to determine if there is correlation between ERα and UCP-1, we first characterized the expression level of ERα and UCP-1 in adipocytes throughout the differentiation process. Both ERα and UCP-1 mRNA expression were increased in parallel throughout differentiation, with the highest expression of both at day 10 (Suppl. Figure 1). Based on these results, only fully differentiated adipocytes were used for all future experiments. In 3T3-L1 cells treated with PPT there was a significant increase in UCP-1 protein following 1 h of PPT treatment which remained elevated for 2 h (Figure 1A). Following 5 h of PPT exposure, UCP-1 mRNA, as well as Ppargc1α, Pdk4, Tnfrsf9, and Pbx1, were elevated (Figure 1B). Importantly, where there is a trend for PPT to increase all markers of beige adipose tissue, the effect was most robust for UCP-1. Primary adipocytes extracted from iWAT of WT C57BL/6J female mice also had increased UCP-1 protein and mRNA levels following PPT treatment (Suppl. Figure 2).
Figure 1.
ERα activation increases markers of beiging in 3T3-L1 adipocytes. (A) 3T3-L1 differentiated cells were treated with the ERα agonist PPT at 5 min, 15 min, 30 min, 1 h, and 2 h for UCP-1 protein. (B) 3T3-L1 adipocytes were treated with PPT for 5 h to probe mRNA expression of beiging markers. Data on graphs represent mean ± SEM of 3 independent rounds of cells. *P < 0.05 vs control.
3.2. ERα expression in mature adipocytes facilitates PPT and CL316,243 induced UCP-1 upregulation
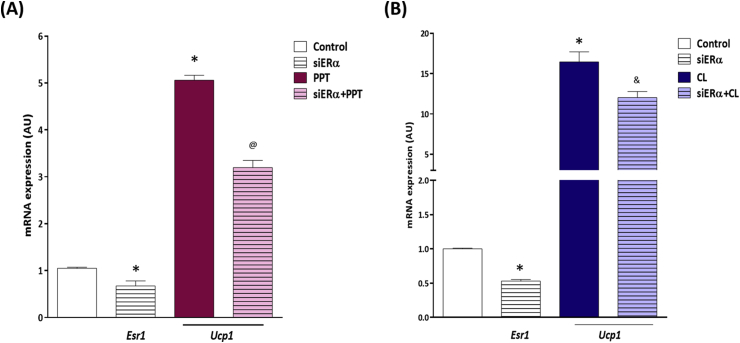
Suppressing the expression of ERα by 40% using siRNA in mature adipocytes reduced the ability of PPT to induce Ucp1 expression (Figure 2A). Additionally, adrenergic stimulation of β3-AR by CL316,243 increases UCP-1 expression in adipocytes [7], [8], [9], and in our model, treatment of mature 3T3-L1 adipocytes with CL316,243 increased UCP-1 expression (Figure 2B) and knockdown of ERα reduced CL316,243's ability to upregulate Ucp1 (Figure 2B) further suggesting that ERα in adipocytes is critical to mediate UCP-1 transcription, either following direct ERα stimulation or through activation of adrenergic receptors.
Figure 2.
ERα is necessary to induce Ucp1 expression in mature adipocytes. Esr1 and Ucp1 mRNA expression following siRNA knockdown (siERα) in mature 3T3-L1 adipocytes and treatment with (A) PPT or (B) CL316,243. *P < 0.05 vs control. @P < 0.05 vs PPT. &P < 0.05 vs CL.
Exposure to cold induces thermogenesis of BAT and beige tissues [32], [33], [34]; thus, we tested the role of ERα to mediate this process by exposing weight matched WT and ERKO male mice to cold. Mice were injected with 18F-fluorodeoxyglucose (FDG) after cold exposure which was followed by a PET/CT scan to measure glucose uptake in adipose tissues. ERKO mice had less FDG uptake in BAT upon cold exposure when compared to WT mice (Suppl. Figure 3A, B), and lower UCP-1 expression in BAT (Suppl. Figure 3C), further indicating ERα is necessary to mediate thermogenesis. To validate our model, we measured ERα levels in iBAT, and as expected, ERα was less expressed in iBAT of ERKO compared to WT mice (Suppl. Figure 3D).
To extend our knowledge on the role of ERα in adipocytes to mediate beiging, we overexpressed ERα (Ad-ERα) selectively in one iWAT fat pad while the contralateral iWAT pad served as the control (GFP control). This was done in WT and ERKO female mice. We observed that in the iWAT that received the control vector, the adipocyte morphology was different between ERKO and WT mice consistent with our previous reports [26]. Specifically, ERKO mice have significantly larger adipocytes than WT mice. ERα overexpression in the WT and ERKO iWAT reduced the size of the adipocytes. Notably, upon histological examination of the adipose tissue in the WT and ERKO Ad-ERα we observed multilocular cells resembling beige adipose tissues (Suppl. Figure 4A) [37], further suggesting ERα expression in adipose tissues is important to facilitate beiging of adipose tissue.
3.3. ERα activation induces beiging in adipocytes via ATGL-dependent lipolysis
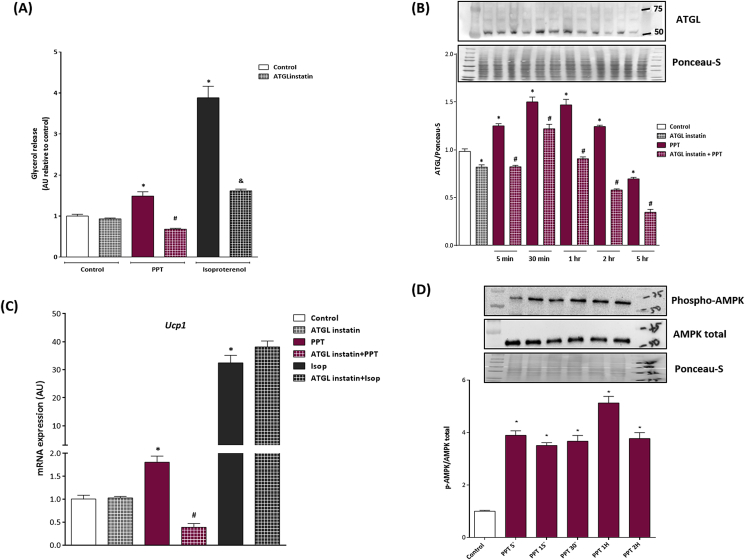
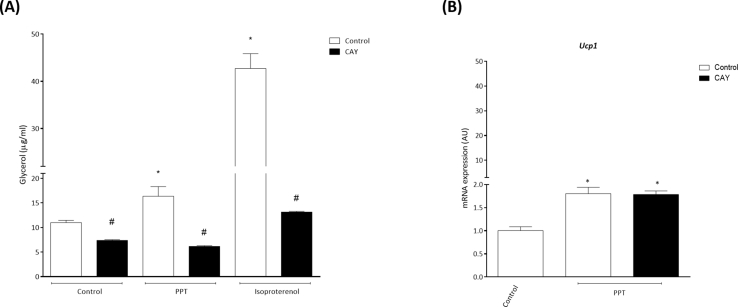
Adrenergic stimulation of mature adipocytes directly upregulates lipolysis [38], [39]. Liberation of FFAs from intracellular lipids has been shown to increase UCP-1 expression and activation. Activation of ERα has also been shown to increase lipolysis [24], although this is species and tissue specific. We hypothesized that selective ERα activation would enhance adipose tissue lipolysis and thereby promote UCP-1 expression. To test this, we determined glycerol release, an indicator of lipolysis, from adipocytes following PPT, isoproterenol (used as a positive control) or vehicle. Our data demonstrate increased glycerol release following PPT and isoproterenol in differentiated 3T3-L1 cells (Figure 3A). We next assayed ATGL protein levels in 3T3-L1 cells pre-treated with Atglinstatin, an ATGL inhibitor, prior to PPT treatment. We found that PPT increases ATGL protein rapidly (after 5 min of PPT treatment) and this was sustained over the course of 2 h, and the ability of PPT to increase ATGL protein was reduced by pre-treatment with Atglinstatin (Figure 3B). Furthermore, we tested if UCP-1 mRNA was upregulated by PPT following pretreatment of Atglinstatin, and found that Atglinstatin reduced the ability of PPT to increase UCP-1 expression (Figure 3C). Finally, we measured phosphorylation of AMPK, a key element in the lipolytic pathway that activates ATGL [12], and we found that PPT rapidly increased AMPK phosphorylation (after 5 min of PPT treatment) (Figure 3D), suggesting that rapid activation of ERα initiates ATGL-mediated lipolysis to induce markers of beiging in mature adipocytes.
Figure 3.
ERα activation increases ATGL-mediated lipolysis of adipocytes. 3T3-L1 adipocytes were pre-treated with the ATGL blocker, Atglistatin, for 1 h prior to PPT or isoproterenol treatments. (A) PPT treatment increased glycerol release in the medium while pre-treatment with Atglistatin prevented it. (B) PPT treatment increased ATGL protein while pre-treatment with Atglinstatin prevented its ability. (C) Atglinstatin prevented PPT's ability to increase Ucp1 mRNA expression. (D) PPT increased phosphorylation levels of AMPK, which activates ATGL, suggesting an involvement of membrane-initiated ERα signaling. Data on graphs represent mean ± SEM of 3 independent rounds of cells. *P < 0.05 vs control. #P < 0.05 vs PPT. &P < 0.05 vs isoproterenol.
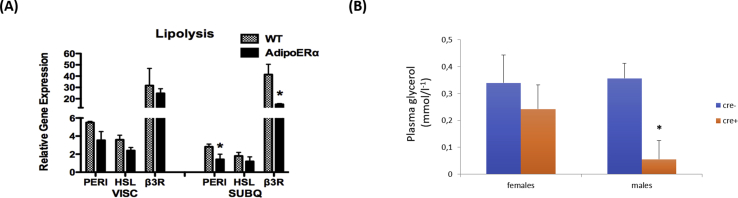
In order to better characterize if this is an adipocyte mediated phenomenum, we used iWAT from mice lacking ERα specifically from adipocytes (AdipoERα mice) and assayed perilipin, which regulates ATGL [40], and we found that perilipin mRNA was decreased in AdipoERα compared to controls (Suppl. Figure 5A). Additionally we found a reduction in β3-AR mRNA in AdipoERα iWAT further suggesting the importance of ERα in adipocytes in facilitating the programming of thermogenic adipose tissues. In contrast to iWAT, there were no differences in the beiging markers in visceral adipose tissue comparing WT and AdipoERα mice (Suppl. Figure 5A). Interestingly, AdipoERα male and female mice treated with CL316,243 presented lower plasma glycerol concentration compared to WT mice treated with CL, although it was only statistically significant in male mice (Suppl. Figure 5B).
3.4. Mice expressing ERα in the plasma membrane (MOER) have increased UCP-1 upregulation following CL316,243 when compared to mice expressing ERα in the nucleus (NOER)
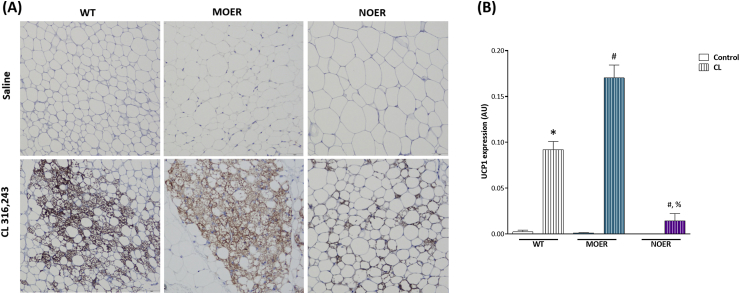
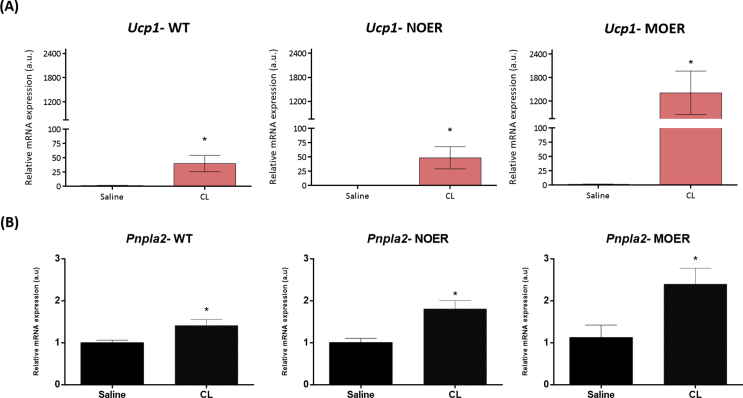
Based on our observation of a rapid increase in UCP-1 protein and phosphorylation of AMPK following 1 h of PPT treatment (Figure 2, Figure 3D), we sought to determine if these effects were mediated by membrane or nuclear ERα. We conducted these experiments in vivo using mice that express ERα predominantly in the cell membrane (MOER) or in the nucleus (NOER) [35], [36]. We treated these female MOER and NOER mice with CL316,243 for 5 days using the same protocol previously discussed. Upon analysis of the adipose tissue following the treatment protocol, we found large patches of UCP-1 positive cells in both WT and MOER mice but to a lesser degree in NOER mice (Figure 4A,B). These data suggest membrane ERα activation induces beiging of adipose tissues. This was supported by the fact that MOER mice had higher expression of UCP-1 (Suppl. Figure 6A) and ATGL mRNA (Suppl. Figure 6B) following CL316,243 treatment when compared to WT or NOER mice, further indicating the method by which ERα facilitates beiging of adipose tissues is through membrane-initiated lipolysis.
Figure 4.
UCP-1 expression is higher after CL treatment in ERα membrane only (MOER) compared to nucleus only ERα mice (NOER). (A) Representative images of UCP-1+ protein staining in female 1) WT mice, 2) mice with functional ERα only in the plasma membrane (MOER), or 3) only in the nucleus (NOER). (B) quantification of UCP-1 positive signal in WT, MOER, and NOER mice. Data on graphs represent mean ± SEM of 3–4 mice/group. *P < 0.05 vs WT control. #P < 0.05 vs WT CL. %P < 0.05 vs MOER CL.
4. Discussion
Our results demonstrate selective activation of ERα in mature 3T3-L1 adipocytes by the ERα agonist PPT, increased UCP-1 expression and markers of beiging. To our knowledge, this is the first-time selective activation of ERα in mature adipocytes has been used to induce beiging of adipose tissues. Our data further suggest that one of the mechanisms by which ERα mediates beiging of adipose tissues is through rapid activation of adipocyte AMPK, which promotes ATGL-dependent lipolysis, followed by increases in UCP-1 protein.
Early observational studies in humans demonstrated an inverse relationship between human brown adipose tissues (hBAT) mass and BMI [34] suggesting that hBAT may defend against weight gain. Moreover, cold exposure was shown to increase the amount and activity of detectable hBAT [34] as well as improve glucose uptake in hBAT [37]. Taken together, these data suggest that, in addition to its role as a heat generating organ, hBAT exerts a beneficial effect on glucose metabolism and could be harnessed as a novel obesity therapy. Pharmacological and genetic studies of classical BAT and inducible beige adipose tissues in rodents support this notion; however, a definitive hBAT-specific intervention for obesity or dysregulated glucose metabolism in humans has not yet been developed. Furthermore, studies have also demonstrated that hBAT generally resembles rodent beige adipose in terms of its molecular signature [9], [38]. As such, there is an intense effort to characterize the molecular pathways that guide beige adipose formation, activation, and function in human and rodent models, with the goal of their eventual translation to human obesity and metabolic therapies.
Interestingly, women have more hBAT mass and higher hBAT activity than similarly aged men [4], indicative of a critical link between sex and brown/beige adipose tissue development and function. Indeed, sex hormones, in particular estrogens, have already been shown to mediate a central mechanism of classical BAT activation in rodents, specifically, estradiol treatment was shown to increase BAT activity via hypothalamic ERα [14]. Though this study was conducted in rodents, the authors speculate that a similar mechanism may operate in humans and explain, at least partly, the reductions in energy expenditure and increases in adipose mass typical of the loss of ovarian estrogens during menopause. By contrast, our study reveals a direct, peripheral effect of ERα activation to promote UCP-1 expression in mature white adipocytes; therefore, it stands as a separate mechanism from centrally mediated estradiol brown/beige tissues activation.
Our findings suggesting that ERα activation increases UCP-1 protein in both mature 3T3-L1 and primary inguinal adipocytes from mice, and that knockdown of ERα reduces this response, indicates that activation of ERα can promote beiging of adipocytes. Admittedly, we were only able to achieve a 40% reduction in ERα using siRNA technology, this was still significant enough to reduce the efficacy of PPT and CL to induce beiging. The lack of complete knockdown of ERα may be due to the fact that mature adipocytes are difficult to efficiently transfect due to their large lipid droplets. PPT treatment throughout differentiation of adipocytes increased UCP-1 expression, further evidence that ERα activation has a clear ability to promote markers of beiging in adipocytes. We confirmed our cell culture data in vivo using mice lacking whole-body ERα (ERKO) and found that following cold exposure, ERKO mice had significantly lower glucose uptake when compared to WT mice. ERα added back to iWAT of ERKO mice enhanced the presentation of multilocular lipid droplets in iWAT suggestive of beiging [37].
Our findings also suggest the mechanistic pathway by which ERα promotes beiging of adipocytes is through rapid/membrane-initiated ERα signaling which induces lipolysis. While both extracellular FFAs taken up from the circulation and intracellular FFAs released by lipolytic enzymes fuel uncoupled respiration via UCP-1, it is known that intracellular FFAs both increase the expression and activation of UCP-1 [39], [41]. ATGL is a major intracellular lipase that functions in adipocytes to hydrolyze TGs to FFAs and diacylglycerides (DAGs). In vivo studies support a regulatory relationship between ATGL and adipose tissue thermogenesis. For example, adipocyte-specific overexpression of ATGL increased lipolysis and thermogenesis [42]. Adipocyte specific ATGL-KO mice have increased expression of WAT signature genes in BAT, and reduced UCP-1 expression [43]. Additionally, AMPK has been shown to activate ATGL, thereby increasing TG lipolysis [43], [44] and UCP-1 thermogenesis [45]. Our findings are consistent with these data in that, following selective ERα activation by PPT, AMPK phosphorylation increased, followed by increases in ATGL and UCP-1 proteins. Blockade of ATGL- using the ATGL-specific inhibitor Atglinstatin- blunted PPT-induced UCP-1 expression. Importantly, we found that blocking hormone sensitive lipase (HSL)- another critical modulator of lipolysis- did not prevent the ability of PPT to induce UCP-1 expression (Suppl. Figure 7), supporting the concept of an ERα-ATGL signaling pathway to promote beiging of adipocytes. We supported these findings in vivo using mice lacking ERα specifically in WAT (AdipoERα), by demonstrating that these mice had lower levels of fasting plasma glycerol and markers of lipolysis in iWAT when compared to WT mice.
Using the MOER and NOER mice, we were able to demonstrate that membrane ERα appears to be necessary to induce lipolysis and facilitate beiging. This is supported by our findings that treatment with CL316,243, a β-adrenergic receptor agonist known to induce beiging of WAT [46], increased UCP-1 expression in MOER mice to a level comparable to WT but not in NOER mice, suggesting that nuclear ERα signaling may be less important for the beiging response to adrenergic stimulation. Membrane ERα has been shown to participate in extra-nuclear signaling cascades [15] and has been implicated in various aspects of metabolism, and our data constitute evidence of a connection between membrane-initiated ERα signaling and UCP-1 induction. In a recent study of Ueda et al. (2018) [47], membrane-initiated ERα pathway was blocked in female mice and this lead to excessive weight gain, glucose intolerance and impaired adaptative thermogenesis which is in accordance to our study. Activation of central protein phosphatase (PP) 2A improves metabolic function suggesting an association between membrane-initiated ERα and PP-2A protein activation to promote metabolic homeostasis.
While our data are suggestive of a role for ERα to induce beiging, we admit there are limitations in our findings. First, we were unable to achieve a complete knockout of ERα using the siRNA approach; however, even with lack of a complete knockdown, we were still able to determine reductions in ERα reduce the ability of both PPT and CL to induce beiging. Additionally, all of our in vivo studies were conducted in vivarium's which do not house mice at thermoneutrality and therefore, introduce a ‘cold challenge’ to the mice even at baseline and within their normal housing conditions. Lastly, not all experiments were performed in all mouse models and therefore there are some gaps in knowledge gained which future studies will need to correct.
5. Conclusion
Our results suggest that selective-activation of adipocyte ERα increases markers of beiging and this appears to be through induction of AMPK and ATGL-mediated lipolysis providing free fatty acids as a fuel to activate UCP-1. Thus, selective activation of ERα represents a promising novel therapeutic target to promote beiging of adipose tissues to improve metabolic homeostasis.
Acknowledgments
We would like to than Dr. Ellis Levin for generously providing the MOER and NOER mice and his team for injecting the compound in the mice. We thank Margaret Elise Bullock, Ryan Surujdin and Saleh Ramezani for the in vitro and in vivo imaging studies in ERKO mice.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.09.002.
Conflict of interest
Authors declare there are no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig S1.
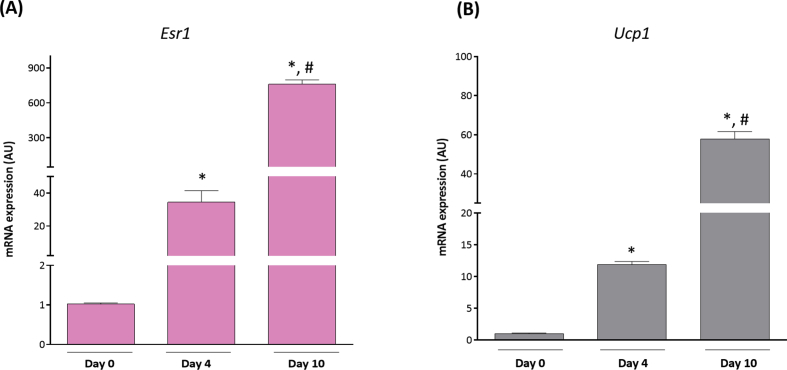
Suppl. Figure 1. Esr1 and Ucp1 expression increases during adipocyte differentiation. (A)Esr1 and (B)Ucp1 mRNA expression at Days 0, 4, and 10 of differentiation. Data on graphs represent mean ± SEM of 3 independent rounds of cells. *P < 0.05 vs day 0. #P < 0.05 vs control day 4.
Fig S2.
Suppl. Figure 2. ERα activation increases markers of beiging in primary adipocytes. (A) mRNA expression of beiging markers and (B) UCP-1 protein expression following 5 h of PPT treatment in primary, differentiated adipocytes obtained from iWAT of female C57/BL6J mice. Data on graphs represent mean ± SEM of 3 independent rounds of cells. *P < 0.05 vs control.
Fig S3.
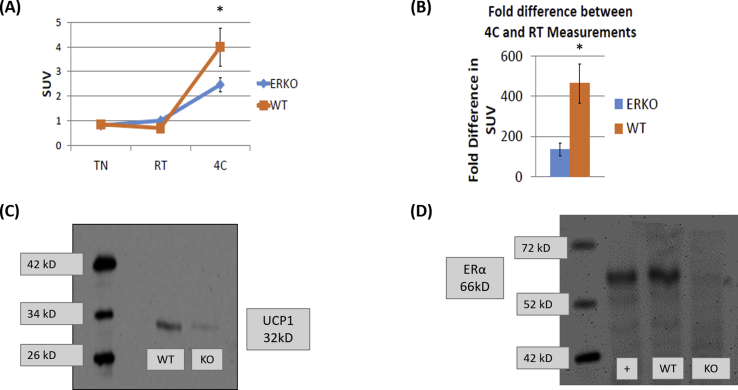
Suppl. Figure 3. FGD standardized uptake value (SUV) following cold exposure is impaired in ERKO mice. (A, B) WT and ERKO male mice were exposed to either thermoneunatrily (TN, 28 °C), room temperature (RT, 22 °C), or cold exposure (4 °C) for 16 h, followed by PET/CT scan to measure FGD uptake, demonstrated as SUV. In a second cohort of mice, WT and ERKO mice were exposed to room temperature (22 °C) for 16 h, and iBAT was collected for UCP-1 (C), and ERα protein determination (D). Uterus samples were used as positive control for ERα protein. Data on graphs represent mean ± SEM of 6 mice/group.
Fig S4.
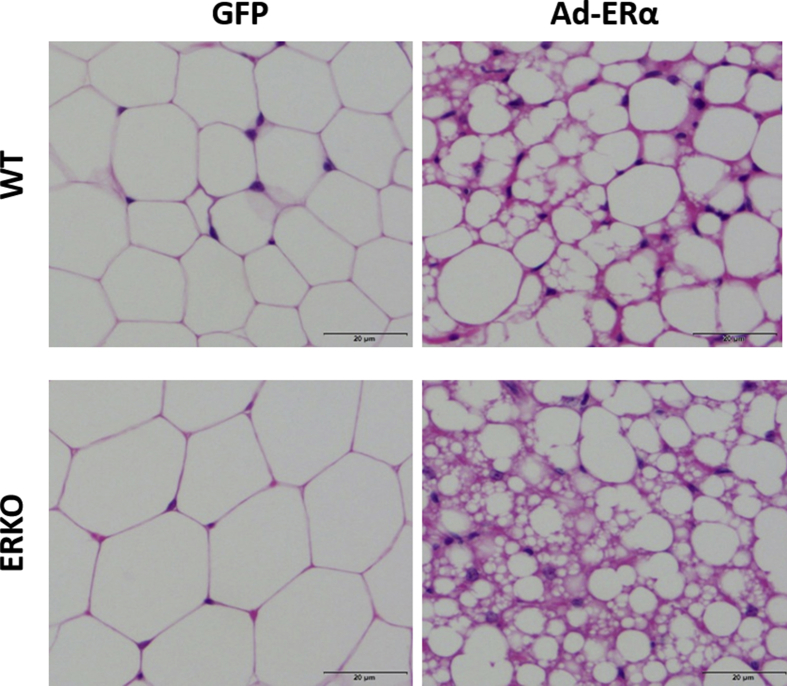
Suppl. Figure 4. ERα added back to adipose tissues of ERKO mice induced a beiging phenotype. WT and ERKO female mice were injected with a virus containing ERα in one iWAT fat pad and GFP in the contralateral iWAT pad in vivo, and tissues were collected for histology analysis. Data on graphs represent mean ± SEM of 3–4 mice/group.
Fig S5.
Suppl. Figure 5. AdipoERα present reduced expression of β3-AR and perilipin, a regulator of ATGL, and plasma glycerol release. (A) mRNA expression of lipolysis markers in female WT and AdipoERα mice. (B) Plasma glycerol release of WT and AdipoERα female and male mice. Data on graphs represent mean ± SEM of 3–4 mice/group.
Fig S6.
Suppl. Figure 6. UCP-1 and ATGL mRNA expression is upregulated to a higher extent in adipose tissue of female MOER than WT or NOER mice following CL treatment. Data on graphs represent mean ± SEM of 3–4 mice/group. *P < 0.05 vs saline control.
Fig S7.
Suppl. Figure 7. HSL-inhibitor pre-treatment does not prevent UCP-1 expression following PPT treatment. 3T3-L1 adipocytes were pre-treated with HSL blocker, CAY 10499, for 2 h prior to PPT or isoproterenol treatments. (A) PPT treatment increased glycerol release in the medium while pre-treatment with CAY prevented it. (B) CAY did not prevent PPT's ability to increase UCP-1 mRNA expression. Data on graphs represent mean ± SEM of 3 independent rounds of cells. *P < 0.05 vs control. #P < 0.05 vs PPT.
References
- 1.Centers for Disease Control and Prevention (CDC) 2018. Adult obesity facts.https://www.cdc.gov/obesity/data/adult.html Available at: [Google Scholar]
- 2.Kajimura S., Spiegelman B.M., Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metabolism. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 4.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. The New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheele C., Larsen T.J., Nielsen S. Novel nuances of human brown fat. Adipocyte. 2014;3:54–57. doi: 10.4161/adip.26520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouellet V., Labbe S.M., Blondin D.P., Phoenix S., Guerin B., Haman F. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. The Journal of Clinical Investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma S.W., Foster D.O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Canadian Journal of Physiology and Pharmacology. 1986;64:609–614. doi: 10.1139/y86-101. [DOI] [PubMed] [Google Scholar]
- 11.Braun K., Oeckl J., Westermeier J., Li Y., Klingenspor M. Non-adrenergic control of lipolysis and thermogenesis in adipose tissues. Journal of Experimental Biology. 2018;221 doi: 10.1242/jeb.165381. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber R., Diwoky C., Schoiswohl G., Feiler U., Wongsiriroj N., Abdellatif M. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metabolism. 2017;26:753–763. doi: 10.1016/j.cmet.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J., Cohen P., Spiegelman B.M. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & Development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez de Morentin P.B., Gonzalez-Garcia I., Martins L., Lage R., Fernandez-Mallo D., Martinez-Sanchez N. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morselli E., de Souza Santos R., Gao S., Avalos Y., Criollo A., Palmer B.F. Impact of estrogens and Estrogen Receptor Alpha (ESR1) in brain lipid metabolism. American Journal of Physiology-Endocrinology and Metabolism. 2018;315:E7–E14. doi: 10.1152/ajpendo.00473.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frank A.P., Palmer B.F., Clegg D.J. Do estrogens enhance activation of brown and beiging of adipose tissues? Physiology & Behavior. 2018;187:24–31. doi: 10.1016/j.physbeh.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Ropero A.B., Alonso-Magdalena P., Quesada I., Nadal A. The role of estrogen receptors in the control of energy and glucose homeostasis. Steroids. 2008;73:874–879. doi: 10.1016/j.steroids.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Motawi T.M., El-Rehany M.A., Rizk S.M., Ramzy M.M., El-Roby D.M. Genetic polymorphism of estrogen receptor alpha gene in Egyptian women with type II diabetes mellitus. Meta Gene. 2015;6:36–41. doi: 10.1016/j.mgene.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heine P.A., Taylor J.A., Iwamoto G.A., Lubahn D.B., Cooke P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hevener A.L., Clegg D.J., Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Molecular and Cellular Endocrinology. 2015;418:306–321. doi: 10.1016/j.mce.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatima L.A., Campello R.S., Santos R.S., Freitas H.S., Frank A.P., Machado U.F. Estrogen receptor 1 (ESR1) regulates VEGFA in adipose tissue. Scientific Reports. 2017;7:16716. doi: 10.1038/s41598-017-16686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza Santos R., Frank A.P., Clegg D.J. The impact of sex and sex hormones on cell function. Steroids. 2017;128:72–74. doi: 10.1016/j.steroids.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Davis K.E., Neinast M.D., Sun K., Skiles W.M., Bills J.D., Zehr J.A. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Molecular Metabolism. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedersen S.B., Kristensen K., Hermann P.A., Katzenellenbogen J.A., Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. The Journal of Clinical Endocrinology & Metabolism. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 25.ATCC . 2018. Chemically-induced differentiation of ATCC® CL-173™ (3T3-L1) using single-component commercially-available reagents.https://www.atcc.org/∼/media/PDFs/Technical%20Bulletins/tb09.ashx Available at: [Google Scholar]
- 26.Kim M., Neinast M.D., Frank A.P., Sun K., Park K., Zehr J.A. ERalpha upregulates Phd3 to ameliorate HIF-1 induced fibrosis and inflammation in adipose tissue. Molecular Metabolism. 2014;3:642–651. doi: 10.1016/j.molmet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao M., Vishvanath L., Busbuso N.C., Hepler C., Shan B., Sharma A.X. De novo adipocyte differentiation from Pdgfrbeta(+) preadipocytes protects against pathologic visceral adipose expansion in obesity. Nature Communications. 2018;9:890. doi: 10.1038/s41467-018-03196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos R.S., de Fatima L.A., Frank A.P., Carneiro E.M., Clegg D.J. The effects of 17 alpha-estradiol to inhibit inflammation in vitro. Biology of Sex Differences. 2017;8:30. doi: 10.1186/s13293-017-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klaus S., Seivert A., Boeuf S. Effect of the beta(3)-adrenergic agonist Cl316,243 on functional differentiation of white and brown adipocytes in primary cell culture. Biochimica et Biophysica Acta. 2001;1539:85–92. doi: 10.1016/s0167-4889(01)00093-3. [DOI] [PubMed] [Google Scholar]
- 30.Miller C.N., Yang J.Y., England E., Yin A., Baile C.A., Rayalam S. Isoproterenol increases uncoupling, glycolysis, and markers of beiging in mature 3T3-L1 adipocytes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy P.P., D'Souza K., Cuperlovic-Culf M., Kienesberger P.C., Touaibia M. New Atglistatin closely related analogues: synthesis and structure-activity relationship towards adipose triglyceride lipase inhibition. European Journal of Medicinal Chemistry. 2016;118:290–298. doi: 10.1016/j.ejmech.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Yao L., Cui X., Chen Q., Yang X., Fang F., Zhang J. Cold-inducible SIRT6 regulates thermogenesis of brown and beige fat. Cell Reports. 2017;20:641–654. doi: 10.1016/j.celrep.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 33.Bertholet A.M., Kazak L., Chouchani E.T., Bogaczynska M.G., Paranjpe I., Wainwright G.L. Mitochondrial patch clamp of beige adipocytes reveals UCP-1-positive and UCP-1-negative cells both exhibiting futile creatine cycling. Cell Metabolism. 2017;25:811–822. doi: 10.1016/j.cmet.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbatelli G., Murano I., Madson L., Hao Q., Jimenez M., Kristiansen K. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American Journal of Physiology. Endocrinology and Metabolism. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- 35.Pedram A., Razandi M., Kim J.K., O'Mahony F., Lee E.Y., Luderer U. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. The Journal of Biological Chemistry. 2009;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedram A., Razandi M., Lewis M., Hammes S., Levin E.R. Membrane-localized estrogen receptor alpha is required for normal organ development and function. Developmental Cell. 2014;29:482–490. doi: 10.1016/j.devcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of Clinical Investigation. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyers D.S., Skwish S., Dickinson K.E., Kienzle B., Arbeeny C.M. Beta 3-adrenergic receptor-mediated lipolysis and oxygen consumption in brown adipocytes from cynomolgus monkeys. The Journal of Clinical Endocrinology & Metabolism. 1997;82:395–401. doi: 10.1210/jcem.82.2.3738. [DOI] [PubMed] [Google Scholar]
- 39.Fedorenk A., Lishko P.V., Kirichok Y. Mechanism of fatty-acid-depenend UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Bell M., Screenivasan U., Hu H., Liu J., Dalen K. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. The Journal of Biological Chemistry. 2011;286 doi: 10.1074/jbc.M110.207779. 15707–15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholls D.G., Rial E. A novel regulatory mechanism for the brown-fat uncoupling protein? Nature Structural & Molecular Biology. 2016;23:364–365. doi: 10.1038/nsmb.3221. [DOI] [PubMed] [Google Scholar]
- 42.Ahmadian M., Duncan R.E., Varady K.A., Frasson D., Hellerstein M.K., Birkenfield A.L. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58:855–866. doi: 10.2337/db08-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmadian M., Abbott M.J., Tang T., Hudak C.S., Kim Y., Bruss M. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metabolism. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang D., Wang D., Zhuang X., Wang Z., Ni Y., Chen S. Barberine increases aipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids in Health and Disease. 2016;15:214. doi: 10.1186/s12944-016-0383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu L., Zhang L., Li B., Jiang H., Duan Y., Xie Z. AMP-Activated Protein Kinase (AMPK) regulates energy metabolism through modulating thermogensis in adipose tissue. Frontiers in Physiology. 2018;9:122. doi: 10.3389/fphys.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Z., Liu X., Zhao Q., Zhang L., Li C., Xue Y. Regulation of UCP1 in the browning of epididymal adipose tissue by β-3-adrenergic agonist: a role of microRNAs. International Journal of Endocrinology. 2014;2014:530636. doi: 10.1155/2014/530636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueda K., Takimoto E., Lu Q., Liu P., Fukuma N., Adachi Y. Membrane-initiated estrogen receptor signaling mediates metabolic homeostasis via central activation of protein phosphatase 2A. Diabetes. 2018;67:1524–1537. doi: 10.2337/db17-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]