Abstract
Objective
Growing evidence supports that patients with Chronic Obstructive Pulmonary Disease (COPD) and coexisting Obstructive Sleep Apnea (OSA) have poor prognosis. This association is described as Overlap Syndrome. Positive Airway Pressure (PAP) therapy is now the preferred treatment for OSA. We hypothesized that use of PAP therapy in elderly patients with Overlap Syndrome would be associated with lower healthcare utilization.
Methods
In this retrospective cohort study, we analyzed data from 5% national sample of fee-for-service Medicare beneficiaries with a diagnosis of COPD who were newly started on PAP therapy in 2011. We examined the effect of PAP therapy on Emergency Room (ER) visits, hospitalizations for all cause and COPD-related conditions in the one year pre- and one year post-initiation of PAP therapy.
Results
In year 2011, we identified 319 patients with Overlap Syndrome who were new users of PAP therapy. In this cohort of patients, hospitalization rates for COPD-related conditions were significantly lower in the one year post-initiation of PAP therapy compared to the one year preinitiation period (19.4% vs 25.4%, p-value = 0.03). However, ER visits (for any cause or COPD-related conditions) and hospitalization rates for any cause did not differ significantly in the pre- and post-initiation periods. PAP therapy was more beneficial in older adults, those with higher COPD complexity and those with 3 or more comorbidities.
Conclusion
Initiation of PAP therapy in elderly patients with Overlap Syndrome is associated with a reduction in hospitalization for COPD-related conditions, but not for all cause hospitalizations and ER visits.
Keywords: COPD, OSA, Overlap Syndrome, PAP, hospitalization and healthcare utilization
Introduction
Chronic Obstructive Pulmonary Disease (COPD) and Obstructive Sleep Apnea (OSA) are both common breathing disorders in older adults.1,2 Moreover, the prevalence of these disorders increases with advancing age.1,2 COPD is a slowly progressive disease characterized by persistent airflow obstruction and OSA is defined as partial or total intermittent upper airway collapse resulting in hypoxemia and arousal during sleep.3
The coexistence OSA in patient with COPD has been noted4–7 and this association is described as the Overlap Syndrome.5,8 Recent studies have shown a higher prevalence of OSA in patients with moderate to severe COPD,4,9 in older patients with COPD,7 and in those requiring hospitalization for COPD.7,10 Also, the prevalence of coexisting COPD is higher in patients with OSA compared to the general population.7,9 These findings have raised the possibility of a common pathophysiological link between COPD and OSA.
Patient’s with Overlap Syndrome have worse nocturnal hypoxemia,9 increased oxidative stress and systemic inflammation,11 and a higher risk of Acute Exacerbation of COPD (AECOPD)10,12. There is increased risk of endothelial injury with development of cardiovascular comorbidities, including arrhythmias, hypoxia-induced pulmonary vasoconstriction resulting in pulmonary hypertension and right ventricular dysfunction.9 Patients with Overlap Syndrome have higher healthcare utilization10,12,13 and worse survival12–15 compared to patients with either COPD or OSA alone.
Positive Airway Pressure (PAP) therapy is the preferred treatment for OSA.3 PAP therapy, especially Bilevel Positive Airway Pressure therapy is also used effectively for acute respiratory failure following AECOPD;16 however, only a few controlled studies have investigated the role of PAP therapy in elderly patients with stable COPD with Overlap Syndrome, and none were randomized controlled trails.12,13,15 Marin et al., in a single center study of Overlap Syndrome patients followed over a 12-year period, showed that patients not treated with PAP therapy had higher risk of AECOPD and mortality.12 In another prospective study, COPD patients with hypoxemia and coexisting OSA showed a significantly lower risk of death with PAP therapy.13 We examined the role of PAP therapy in stable patients with Overlap Syndrome using a 5% national Medicare sample of patients from 2011. We hypothesized that health care utilization will be decreased after initiation of PAP therapy in elderly patients with Overlap Syndrome.
Materials and Methods
Data Source
In this retrospective population based cohort study, we used enrollment and claims data from a 5% national sample of Medicare beneficiaries from 2010–2012 provided by the Research Data Assistance Center.17 The Centers for Medicare and Medicaid Services (CMS) select a random sample of 5% Medicare beneficiaries based on the eighth and ninth digits (05, 20, 45, 70, 95) of their health insurance claim number. All records were de-identified prior to analysis.
Data used in this study were from multiple files: 1) Denominator file (Medicare enrollment information and demographic data); 2) Medicare Provider Analysis and Review file (claims for hospital inpatient and skilled nursing facility stays); 3) Outpatient Standard Analytic file (hospital outpatient services); 4) 100% Physician/Supplier file (physician and other medical services); 5) Durable Medical Equipment (DME) file; and 6) Prescription Drug Event (PDE) records. This study was approved by the University of Texas Medical Branch Institutional Review Board and informed consent was not needed due to the nature of the study.
Subjects were selected for this analysis if they were identified as COPD patients in the previous year (2010) and the current year (2011); were 66 years of age or older; received PAP therapy treatment in the current (2011) but not on PAP therapy in the previous year (2010); were enrolled in Medicare parts A and B but not in a Health Maintenance Organization (HMO); were not a resident of a nursing facility from one year prior to one year after the index PAP treatment or until death; and resided in one of nine United States geographic regions. The index PAP treatment was defined as the first PAP treatment in 2011.
A COPD patient was identified as having any of the following: 1) at least two outpatient visits (Evaluation and Management codes 99201 – 99205 or 99211 – 99215) with an encounter diagnosis of COPD [based on International Classification of Diseases, Ninth revision (ICD-9) codes 491.x, 492.x or 496.x] at least 30 days apart within a year; or 2) one acute care hospitalization with COPD diagnosis listed in the primary position as a discharge diagnosis; or 3) one acute care hospitalization with respiratory failure (518.81, 518.82, 518.84) listed in the primary position as a discharge diagnosis and COPD listed as the secondary diagnosis, similar to CMS methodology.17
Initiation of PAP therapy was identified based on the Healthcare Common Procedure Coding System PAP reimbursement codes E0470, E0471, E0561, E0562 and E0601 in DME files. We used monthly claims by DME for reimbursement of PAP equipment to examine the adherence to PAP therapy for the one year post-initiation of PAP. In this cohort of COPD patients who began index PAP therapy in 2011, we further looked for claims with a diagnosis of OSA based on ICD-9 codes 786.03, 780.51, 780.53, 780.57, 327.20 and 327.23.6,18
Variables
Medicare enrollment files were used to categorize subjects by age (66 – 74, 75 – 84, ≥85 years), gender, race, socioeconomic status, and nine CMS geographic regions. Low socioeconomic status was based on whether the patient was eligible for state buy-in coverage provided by the Medicaid program for at least one month during the calendar year. Comorbidity was examined as an index score generated based on the number of Elixhauser comorbidities (0, 1, 2, ≥ 3).19 COPD severity was categorized as low, moderate and high complexity as described by Mapel et al.20 Tobacco use was defined as ICD-9 diagnosis codes (305.1, 989.84 and V1582) in any position. In addition, we also examined for selected comorbidities including Hypertension, Diabetes Mellitus and Congestive Heart Failure in patients with Overlap Syndrome, as previous studies have reported high prevalence of these conditions in patients with OSA and COPD.21
Outcome
Our primary outcome of interest was to examine the impact of PAP on health care utilization [Emergency Room (ER) visits and hospitalization rate for all cause and COPD-related conditions] in the one year pre- and post-initiation of PAP therapy. The secondary study outcomes includes outpatient visits and use of inhaled steroids.
Statistical Analysis
Characteristics were expressed as mean ± standard deviation for continuous variables and as percentages for categorical variables. The percent of patients with ER visits and hospitalizations for all cause and for COPD-related conditions in the one year before and after PAP initiation were compared by McNemar test. For any significant outcomes found in McNemar test, we also conducted Time-to-Event analysis to examine the impact of PAP use on the timing of developing such an outcome. In these analysis, the index date was a year before PAP initiation in the pre-period and in the post-period it was the date of PAP initiation. Patients were censored at death or at 12 months follow up. The failure rate estimated from the Kaplan-Meier method was used to show the cumulative rate of the outcome over time. To account for the cluster effect of patients, we used the robust sandwich covariance estimation for the Cox Proportional Hazard model. We also examined the assumption of proportion hazard through the log baseline cumulative hazard plot. We performed subgroup analyses by age groups, gender, socioeconomic status, number of comorbidities and COPD complexity. All analyses were performed with statistical analysis system, version 9.4 (SAS Inc., Cary, NC). All reported P values were two-sided and P value <0.05 was considered statistically significant.
Results
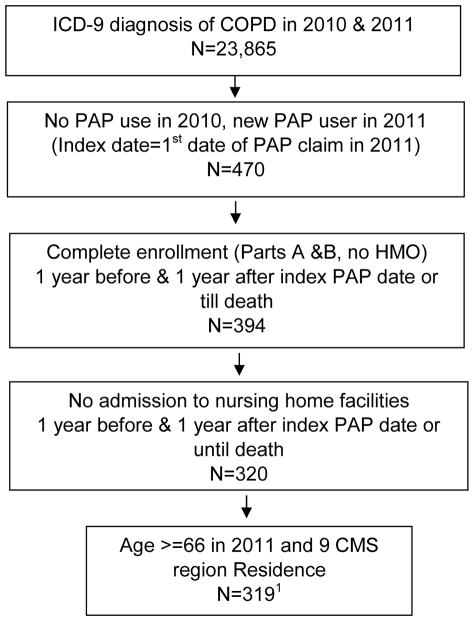
Between 2010 and 2011, we identified 319 fee-for-service Medicare patients with Overlap Syndrome who were new users of PAP therapy in 2011 (Figure 1). Of these, 292 (91.5%) patients had a visit for OSA during the same year. About 66 percent of patients who initiated PAP therapy had DME reimbursement claims for equipment for more than 9 months during the year post-initiation.
Figure 1. Flow chart showing the cohort selection.
1. Of these 319 COPD patients started on PAP therapy, 292 (91.5%) patients had a visit for OSA during the same year.
Abbreviations: ICD-9 = International Classification of Diseases, Ninth revision; COPD = Chronic Obstructive Pulmonary Disease; PAP = Positive Airway Pressure; HMO = Health Maintenance Organization; CMS = Centers for Medicare and Medicaid Services.
Table 1 shows the baseline characteristics of the cohort. The mean age was 74 ± 5 years, 60.2% were male, the majority were non-Hispanic white and 63% had three or more other comorbidities. The majority had moderate COPD complexity (57.7%) and 42.6% had used tobacco within 1 year prior to the initiation of PAP therapy. Hypertension (84%), Diabetes Mellitus (42%) and Congestive Heart Failure (32%) were the most common comorbidities in these patients with Overlap Syndrome.
Table 1.
Baseline Characteristics of patients with COPD and coexisting OSA (Overlap Syndrome) initiated on PAP in 2011
| Characteristic | Overall (%) | N =319 |
|---|---|---|
| Age in years | ||
| 66 – 74 | 173 (54.2) | |
| 75 – 84 | 135 (42.3) | |
| ≥85 | 11 (3.4) | |
| Gender | ||
| Female | 127 (39.8) | |
| Male | 192 (60.2) | |
| Race | ||
| White | 291 (91.2) | |
| Black | 20 (6.3) | |
| Other | 8 (2.5) | |
| Low Socioeconomic status1 | ||
| No | 263 (82.4) | |
| Yes | 56 (17.5) | |
| Region | ||
| New England | 9 (2.8) | |
| Middle Atlantic | 28 (8.8) | |
| East North Central | 60 (18.8) | |
| West North Central | 18 (5.6) | |
| South Atlantic | 85 (26.6) | |
| East South Central | 24 (7.5) | |
| West South Central | 39 (12.2) | |
| Mountain | 16 (5) | |
| Pacific | 40 (12.5) | |
| Number of Comorbidities2 | ||
| 0 | 13 (4) | |
| 1 | 43 (13.5) | |
| 2 | 62 (19.4) | |
| ≥ 3 | 201 (63) | |
| Comorbidity | ||
| Hypertension | 268 (84) | |
| Diabetes Mellitus | 134 (42) | |
| CHF | 102 (32) | |
| Anemia | 72 (22.6) | |
| Hypothyroidism | 61(19.1) | |
| Renal failure | 56 (17.5) | |
| Obesity | 56 (17.5) | |
| Depression | 31 (9.7) | |
| Psychiatric disorder | 16 (5.2) | |
| COPD complexity3 | ||
| Low | 83 (26.0) | |
| Moderate | 184 (57.7) | |
| High | 52 (16.3) | |
| Tobacco use 1 year prior to initiation PAP4 | 136 (42.6) | |
Definition of abbreviations: COPD = Chronic Obstructive Pulmonary Disease; OSA = Obstructive Sleep Apnea; PAP = Positive Airway Pressure; CHF = Congestive Heart Failure.
Socioeconomic status was based on whether the patient was eligible for state buy-in coverage provided by the Medicaid program for at least on year during the calendar year under study.
Comorbidities were identified using the Elixhauser Comorbidity Index (excluding COPD).
COPD complexity as defined by Mapel et al.20
Tobacco use was defined as ICD 9 diagnosis codes (305.1, 989.84 and V1582) on any position.
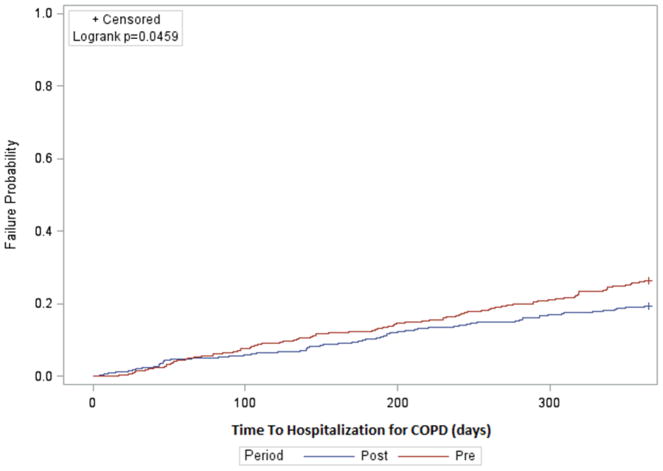
Table 2 presents the comparison of outcomes, ER visits and hospitalization for all cause and COPD-related reasons in the one year pre- and one year post-initiation of PAP therapy. ER visits for all cause (53.9% vs 54.9%, P value = 0.77) and COPD-related ER visits (16.6% vs 13.5%, P value = 0.18) were not significantly different in the one year pre- and one year post-initiation of PAP therapy. Also hospitalization for all causes was not significantly different during the pre- and post-initiation of PAP therapy (48% vs 45.8%, P value = 0.52). However, hospitalization for COPD-related conditions was significantly lower in the one year post-initiation of PAP therapy (25.4% vs 19.4%, P value = 0.03). The Kaplan-Meier analysis also shows that patients with Overlap Syndrome had a higher risk of COPD-related hospitalizations in the one year pre-initiation of PAP therapy compared to the one year post-initiation of PAP therapy (Figure 2). The non-proportional hazard of using of PAP therapy on the outcome of COPD hospitalization was detected through log baseline cumulative hazard plot. A Cox model with a time-dependent covariate of PAP therapy (up to 3 months and after 3 months) was conducted. During the one year period, the rate of COPD-related hospitalizations with use of PAP was not significantly different in the first 3 months [Hazard Ratio (HR) = 0.86, 95% Confidence Interval (CI): 0.48–1.54]. After 3 months of PAP initiation, patients had a significant lower COPD-related hospitalization rate (HR = 0.67, 95% CI: 0.48–0.94). From the McNemar test (Table 2), we found that PAP reduces COPD-related hospitalization, and from the Cox model, we identified that the benefit was seen mainly after 3 months of initiating PAP.
Table 2.
Outcomes in patients with Overlap Syndrome in one year pre- and one year post-initiation of PAP. (N=319)
| Outcomes | One year pre-initiation of PAP | One year post-initiation of PAP | P values |
|---|---|---|---|
| ER visit for all cause, (n) % | (172)53.9% | (175)54.9% | 0.77 |
| ER visit for COPD, (n) % | (53)16.6% | (43)13.5% | 0.18 |
| Hospitalization for all cause, (n)% | (153)48% | (146)45.8% | 0.52 |
| Mean ± SD | 0.97 ± 1.37 | 0.91 ± 1.34 | |
| Hospitalization for COPD, (n) % | (81)25.4% | (62)19.4% | 0.03 |
| Mean ± SD | 0.40 ± 0.83 | 0.31 ± 0.75 | |
| Outpatient visit, (n)% | (166)52% | (167)52.4% | 0.32 |
Definition of abbreviations: PAP = Positive Airway Pressure; ER = Emergency Room; COPD = Chronic Obstructive Pulmonary Disease.
Figure 2. COPD-related hospitalizations in patients with Overlap Syndrome in the one year pre- and one year post-initiation of PAP therapy*.
*Failure rates were estimated by Kaplan Meier method.
The number of outpatient visits at one year pre-initiation of PAP therapy compared to one year post-initiation of PAP therapy did not differ (52% vs. 52.4%, P value = 0.32). Of study subjects, 167 (52.4%) had complete Part D (drug benefit) enrollment during the study period. Of these 167, 71.3% were prescribed inhaled steroids (ICS, or LABA/ICS) at one year pre-initiation PAP and 68.3% at one year post-initiation of PAP; the difference was not significant. A total of 19 (6%) of 319 patients died within a year after initiation of PAP.
Table 3 presents the outcomes by subgroups in one year pre- and one year post-initiation of PAP. PAP therapy had a nonsignificant impact on COPD-related ER visits among those who were older (≥75 yrs), female, and had more comorbidities (≥3). As for COPD-related hospitalization, PAP therapy also had a nonsignificant impact for patients who were older (≥75 yrs), male, and had more comorbidities (≥3). The subgroups with moderate and high COPD complexity had statistically significant reductions in COPD related ER visits, hospitalizations and all cause hospitalization.
Table 3.
Pre- and Post-PAP use and outcomes by subgroups in patients with Overlap Syndrome.
| ER Visits | |||||||
|---|---|---|---|---|---|---|---|
| All Cause | COPD-related | ||||||
| One year pre-initiation of PAP | One year post-initiation of PAP | 1 P-Values | One year pre-initiation of PAP | One year post-initiation of PAP | 1 P-Values | ||
| Age group | 66–74 (N=173) | 92(53.18) | 88(50.87) | 0.59 | 28(16.18) | 26(15.03) | 0.71 |
| ≥75 (N=146) | 80(54.79) | 87(59.59) | 0.33 | 25(17.12) | 17(11.64) | 0.13 | |
| Low socioeconomic status | Yes (N=56) | 39(69.64) | 37(66.07) | 0.65 | 14(25.00) | 12(21.43) | 0.56 |
| No (N=263) | 133(50.57) | 138(52.47) | 0.59 | 39(14.83) | 31(11.79) | 0.23 | |
| Number of comorbidities | <3 (N=118) | 42(35.59) | 49(41.53) | 0.26 | 10(8.47) | 12(10.17) | 0.56 |
| ≥3 (N=201) | 130(64.68) | 126(62.69) | 0.63 | 43(21.39) | 31(15.42) | 0.07 | |
| Gender | Male (N=192) | 97(50.52) | 102(53.13) | 0.54 | 26(13.54) | 23(11.98) | 0.58 |
| Female (N=127) | 75(59.06) | 73(57.48) | 0.75 | 27(21.26) | 20(15.75) | 0.18 | |
| COPD complexity | Low (N=83) | 26(31.33) | 38(45.78) | 0.04 | 0 | 5(6.02) | - |
| Moderate and High (N=236) | 146(61.86) | 137(58.05) | 0.29 | 53(22.46) | 38(16.10) | 0.04 | |
| Hospitalization | |||||||
| All Cause | COPD-related | ||||||
| One year pre-initiation of PAP | One year post-initiation of PAP | 1 P-Values | One year pre-initiation of PAP | One year post-initiation of PAP | 1 P-Values | ||
| Age group | 66–74 | 84(48.55) | 79(45.66) | 0.51 | 47(27.17) | 39(22.54) | 0.19 |
| ≥75 | 69(47.26) | 67(45.89) | 0.80 | 34(23.29) | 23(15.75) | 0.07 | |
| Low socioeconomic status | Yes | 32(57.14) | 29(51.79) | 0.49 | 21(37.50) | 16(28.57) | 0.20 |
| No | 121(46.01) | 117(44.49) | 0.69 | 60(22.81) | 46(17.49) | 0.07 | |
| Number of comorbidities | <3 | 29(24.58) | 36(30.51) | 0.27 | 14(11.86) | 15(12.71) | 0.83 |
| ≥3 | 124(61.69) | 110(54.73) | 0.11 | 67(33.33) | 47(23.38) | 0.007 | |
| Gender | Male | 92(47.92) | 86(44.79) | 0.49 | 46(23.96) | 29(15.10) | 0.01 |
| Female | 61(48.03) | 60(47.24) | 0.88 | 35(27.56) | 33(25.98) | 0.72 | |
| COPD complexity | Low | 13(15.66) | 25(30.12) | 0.02 | 0 | 6(7.23) | - |
| Moderate and High | 140(59.32) | 121(51.27) | 0.04 | 81(34.32) | 56(23.73) | 0.003 | |
Definition of abbreviations: PAP = Positive Airway Pressure; ER = Emergency Room; COPD = Chronic Obstructive Pulmonary Disease.
P-values were generated from McNemar test.
Discussion
In this retrospective cohort study, we showed that the initiation of PAP therapy in patients with Overlap Syndrome was associated with a lower rate of COPD-related hospitalizations. However, hospitalizations for all cause and ER visits were not significantly different in the pre- and post-study periods. Patients with higher COPD complexity had more significant benefit with use of PAP therapy. The majority of Overlap Syndrome patients had a significant comorbidity burden, as noted in previous studies.6,12
One possible explanation for no significant reduction in all cause hospitalizations could be that the majority of patients with COPD are readmitted for causes other than COPD due to their high comorbidity burden. PAP therapy may reduce the risk for AECOPD but not for other causes.
Patient with Overlap Syndrome are at increased risk for AECOPD compared to patients with COPD alone. Complex bidirectional mechanisms have been proposed.5,22 Patients with COPD have hyperinflated lungs with reduced activity of respiratory muscles, which is worse in REM sleep due to hypotonia, and have blunted respiratory drive during sleep which results in worsening of nocturnal hypoxemia.5,9,22,23 In OSA, the anatomically collapsible pharyngeal airway, in combination with a sleep-induced reduced upper airway muscle tone, results in nocturnal hypoxemia. OSA may also augment vagal-mediated bronchoconstriction which leads to increased expiratory airflow resistance.22 Patients with Overlap Syndrome have both upper and lower airway obstruction and a reduction in respiratory drive during sleep, which results in more pronounced nocturnal hypoxemia and appears to have a synergistic effect on nocturnal hypoxemia.5,9,22,24
Similar to patients with COPD, those with OSA have increased expression of inflammatory mediators resulting in airway and systemic inflammation.25,26 In a recent study,11 inflammatory mediators (including neutrophils, TNF-alpha and IL-8) were significantly increased in the bronchoalveolar lavage (BAL) fluid from patients with Overlap Syndrome compared to patients with COPD alone. Investigators also found a significant correlation between nocturnal hypoxemia and airway inflammation. Also, the initiation of PAP therapy was associated with a significant reduction in markers of airway inflammation in BAL fluid. Another study has noted the beneficial effect of PAP therapy in reducing airway inflammation (documented by airway nitric oxide measurement) in OSA patients at three months.27
In the current literature, PAP therapy in patients with Overlap Syndrome has shown to improve hypoxemia28 and reduce systemic inflammation,11 which could be mechanisms by which the risk of AECOPD is reduced10,12 and survival improved.6,12,14 The reduction in hypoxemia and inflammation after initiation of PAP therapy in Overlap Syndrome may help explain the improvement in COPD-related hospitalization rates noted here and in previous studies.10,12 The benefits of PAP therapy in our study is noted after three months, similar to a prior study showing improvement in airway inflammation.27
Thirty-day hospital readmission after AECOPD is monitored by CMS as part of the hospital readmission reduction program which penalizes hospitals with excess readmissions. These penalties can have major financial implications for safety net hospitals as most operate at a small profit margin. 29 Based on results of our study and previous evidence in the literature, early diagnosis and treatment of OSA with PAP therapy in patients with COPD may help reduce the risk of AECOPD and subsequent readmission. Any intervention that can impact re-hospitalization in the bundle payment model era is worthy of further studies.
The results of this study may have been influenced by several limitations. First, our study cohort was limited to beneficiaries aged >66 years with Medicare Parts A and B coverage, and results may not be applicable to younger patients or patients enrolled in managed care.
Second, we used ICD-9 codes to identify patients with COPD and OSA; the use of information based on ICD-9 codes alone is problematic as a documented diagnosis does not confirm the accuracy of diagnosis. However, use of ICD-9 codes from claims data for COPD has been validated for data extraction.30 Also, recommendations by the American Academy of Sleep Medicine for hypopnea scoring rules have changed over time and ICD-9 codes for OSA have been modified over the last decade; these changes may interfere with the accuracy of the diagnosis of OSA. We used ICD-9 codes for OSA similar to previous studies in the literature.6,18
Third, we did not have access to information on severity of disease, air flow limitation by spirometry for COPD or severity of Apnea-Hypopnea Index (AHI) from previous polysomnograms for OSA. We therefore are unable to comment on the appropriateness of management and these factors may confound the risk of AECOPD and hospitalization. However, we evaluated the COPD complexity derived from administrative claims as an indirect measure of severity based on resource utilization.20
Fourth, the use of reimbursement data to study PAP therapy only accounts for the PAP machine being prescribed and provided to patient. We did not have access to individual PAP compliance data for these patients to ensure effectiveness of therapy by correcting AHI and nocturnal hypoxemia. We used monthly claims to DME as a surrogate for adherence with PAP therapy, though this methodology has not been validated in previous studies. Finally, given the observational design of the study, we cannot exclude unmeasured or residual confounding as a possible explanation for the observed association.
In conclusion, initiation of PAP therapy in elderly patients with Overlap Syndrome is associated with reduction in hospitalizations for COPD-related conditions but not for all cause hospitalizations or for ER visits.
Acknowledgments
Funding/Support: This work was supported by the Agency of Healthcare Research and Quality [Grant R01-HS020642] and the Patient-Centered Outcomes Research institute [Grant R24HS022134]. The sponsor had no role in the design or conduct of this research.
The authors thank Sarah Toombs Smith, PhD for her help with preparation of the manuscript.
Abbreviations
- AECOPD
Acute Exacerbation of Chronic Obstructive Pulmonary Disease
- AHI
Apnea-Hypopnea Index
- BAL
Bronchoalveolar Lavage
- CHF
Congestive Heart Failure
- CI
Confidence Interval
- CMS
Center of Medicare and Medicaid Services
- COPD
Chronic Obstructive Pulmonary Disease
- DME
Durable Medical Equipment
- ER
Emergency Room
- HMO
Health Maintenance Organization
- HR
Hazard Ratio
- ICD-9
International Classification of Diseases, Ninth revision
- OSA
Obstructive Sleep Apnea
- PAP
Positive Airway Pressure
Footnotes
Author contributions:
Dr. Gurinder Singh served as principal author, had full access to the data in the study and takes full responsibility for the content of the manuscript, including accuracy of data analysis.
Dr. Amitesh Agarwal, Mr. Wei Zhang, Dr. Yong-Fang Kuo, Dr. Rizwana Sultana and Dr. Gulshan Sharma contributed to the conception, study design, analysis, interpretation of results, drafting of manuscript and final approval of the manuscript.
Conflict of interest: Dr. Gulshan Sharma served on the advisory board of Sunovion and Mylan Pharmaceuticals. The remaining authors have no potential conflicts of interest related to the content of manuscript.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of University of Texas Medical Branch and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was not needed due to the nature of the study.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. American journal of epidemiology. 2013:kws342. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. The Lancet. 2011;378(9795):991–996. doi: 10.1016/S0140-6736(11)60990-2. [DOI] [PubMed] [Google Scholar]
- 3.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 4.Soler X, Gaio E, Powell FL, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Annals of the American Thoracic Society. 2015;12(8):1219–1225. doi: 10.1513/AnnalsATS.201407-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens RL, Macrea MM, Teodorescu M. The overlaps of asthma or COPD with OSA: A focused review. Respirology. 2017;22(6):1073–1083. doi: 10.1111/resp.13107. [DOI] [PubMed] [Google Scholar]
- 6.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9(8):767–772. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shawon MSR, Hamilton G, Perret J, Senaratna C, Lodge C, Dharmage SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: A systematic review. Sleep Medicine Reviews. 2016 doi: 10.1016/j.smrv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661. [PubMed] [Google Scholar]
- 9.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. American journal of respiratory and critical care medicine. 1995;151(1):82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 10.Konikkara J, Tavella R, Willes L, Kavuru M, Sharma S. Early recognition of obstructive sleep apnea in patients hospitalized with COPD exacerbation is associated with reduced readmission. Hospital Practice. 2016;44(1):41–47. doi: 10.1080/21548331.2016.1134268. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep medicine. 2015;16(9):1123–1130. doi: 10.1016/j.sleep.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. American journal of respiratory and critical care medicine. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 13.Machado ML, Vollmer W, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. European Respiratory Journal. 2010;35(1):132–137. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 14.Du W, Liu J, Zhou J, Ye D, OuYang Y, Deng Q. Obstructive sleep apnea, COPD, the overlap syndrome, and mortality: results from the 2005–2008 National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2018;13:665–674. doi: 10.2147/COPD.S148735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaoude P, Kufel T, El-Solh AA. Survival benefit of CPAP favors hypercapnic patients with the overlap syndrome. Lung. 2014;192(2):251–258. doi: 10.1007/s00408-014-9555-z. [DOI] [PubMed] [Google Scholar]
- 16.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. American journal of respiratory and critical care medicine. 2012;185(2):152–159. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers of Medicare and Medicaid Services. [Accessed May 2, 2016];Medicare data documentation. 2013 http://www.resdac.org/cms-data/variables/Hospital-Readmissions.
- 18.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. American journal of respiratory and critical care medicine. 2011;183(4):539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mapel DW, Dutro MP, Marton JP, Woodruff K, Make B. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC health services research. 2011;11(1):43. doi: 10.1186/1472-6963-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Paper presented at: Mayo Clinic Proceedings; 2004. [DOI] [PubMed] [Google Scholar]
- 22.McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. American journal of respiratory and critical care medicine. 2009;180(8):692–700. doi: 10.1164/rccm.200903-0347PP. [DOI] [PubMed] [Google Scholar]
- 23.Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest. 1996;109(2):387–394. doi: 10.1378/chest.109.2.387. [DOI] [PubMed] [Google Scholar]
- 24.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. American journal of respiratory and critical care medicine. 2003;167(1):7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 25.Ryan S, Taylor C, McNicholas W. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64(7):631–636. doi: 10.1136/thx.2008.105577. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. CHEST Journal. 2005;128(4):1995–2004. doi: 10.1378/chest.128.4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortuna AM, Miralda R, Calaf N, González M, Casan P, Mayos M. Airway and alveolar nitric oxide measurements in obstructive sleep apnea syndrome. Respir Med. 2011;105(4):630–636. doi: 10.1016/j.rmed.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Lacedonia D, Carpagnano G, Aliani M, et al. Daytime PaO 2 in OSAS, COPD and the combination of the two (overlap syndrome) Respiratory medicine. 2013;107(2):310–316. doi: 10.1016/j.rmed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Joynt KE, Jha AK. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. Jama. 2013;309(4):342–343. doi: 10.1001/jama.2012.94856. [DOI] [PubMed] [Google Scholar]
- 30.Stein BD, Bautista A, Schumock GT, et al. The Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Diagnosis Codes for Identifying Patients Hospitalized for COPD ExacerbationsValidity of Diagnosis Codes in COPD Exacerbations. CHEST Journal. 2012;141(1):87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]