Abstract
Resting heart rate (RHR) is independently associated with cardiovascular disease (CVD) risk. We determined whether RHR, measured in mid-life, is also associated with cognitive decline. We studied 13,720 middle-aged white and black ARIC participants without a prior history of stroke or atrial fibrillation. RHR was obtained from a 12-lead resting electrocardiogram at the baseline visit (1990-1992) and categorized into groups as <60 (reference), 60-69, 70-79 and ≥80 bpm. Cognitive scores were obtained at baseline and at up to two additional visits (1996-1998 and 2011-2013). The primary outcome was a global composite cognitive score (Z-score) derived from 3 tests: delayed word recall, digit symbol substitution, and word fluency. The associations of RHR with cognitive decline and incident dementia were examined using linear mixed-effects and Cox hazard models, respectively, adjusting for socio-demographics, CVD risk factors and AV-nodal blockade use. Multiple imputation methods were used to account for attrition over follow-up. Participants had mean±SD age of 58±6 years; 56% were women, 24% black. Average RHR was 66±10 bpm. Over a mean follow-up of 20-years, those with RHR ≥80 bpm had greater global cognitive decline [average adjusted Z-score difference −0.12 (95% Cl −0.21, −0.03)] and increased risk for incident dementia [Hazard Ratio 1.28 (1.04, 1.57)], compared to those with RHR <60 bpm. In conclusion, elevated RHR is independently associated with greater cognitive decline and incident dementia over 20-years. Further studies are needed to determine whether the associations are causal or secondary to another underlying process, and whether modification of RHR can affect cognitive decline.
Keywords: Heart Rate, Cognitive Decline, Risk Factors
Introduction
In an aging population, preservation of cognitive function is a key focus for preventive measures.1 Cardiovascular diseases (CVD) and cardiovascular risk factors are associated with cognitive decline and with dementia.2 Resting heart rate (RHR) is an easily measured and independent predictor of mortality and CVD risk.3,4 It is also potentially modifiable through improved fitness and pharmacological agents. However, despite the established association of RHR with future CVD risk, there are very few studies examining RHR and cognitive decline. One study showed that lower RHR was associated with less cognitive decline in patients with prior ischemic strokes, but little is known about that relationship in the general population.5 Thus, we used data from the Atherosclerosis Risk in Communities (ARIC) study to examine the cross-sectional and longitudinal associations of RHR with cognitive decline in a community-based cohort. We hypothesized that elevated RHR will be independently associated with lower crosssectional cognitive function and with greater cognitive decline over 20 years.
Methods
The ARIC study is an ongoing prospective epidemiological study that recruited 15,792 men and women aged 44 to 66 years from four U.S. communities (suburbs of Minneapolis, MN; Washington County, MD; Forsyth County, NC; Jackson, MS) from 1987 to 1989 (visit 1). The ARIC design has been previously described.6 The initial cognitive testing in ARIC was performed at visit 2 from 1990 to 1992, and thus visit 2 represents the baseline for our analysis. Cognitive testing was then repeated at ARIC visits 4 and 5, which took place from 1996 to 1998 and from 2011 to 2013, respectively.
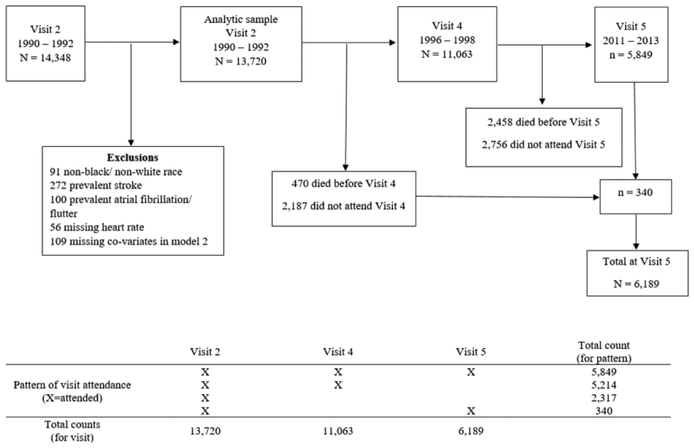
Visit 2 initially included 14,348 participants; however, we limited our analysis to 13,720 participants. We excluded 91 individuals who were neither white nor black and blacks from the MN and MD sites due to small numbers, 272 who had prior history of stroke, 100 with atrial fibrillation/flutter, 56 participants who had missing RHR, and 109 with missing information on key covariates. Participant inclusions/exclusions and participant flow throughout the study (visits 2 through 5) are shown in Figure 1. The ARIC Study was approved by the Institutional Review Boards at all ARIC centers, and all participants provided written informed consent at each study visit.
Figure 1.
Participants flow chart including exclusions
RHR was obtained from a standard 12-lead resting electrocardiogram (ECG) at visit 2. The ECG was performed with participants laying supine with arms relaxed at both sides, after participants had abstained at least 1 hour from smoking and caffeine. We only included participants in sinus rhythm at the time of their ECG. We used baseline RHR (visit 2) for the analysis of cognitive change from visit 2 through visit 5.
Cognitive function was assessed with three tests: Delayed Word Recall (DWR)7, Digit Symbol Substitution (DSS)8, and Word Fluency (WF)9 at visits 2, 4, and 5. In DWR, a test for verbal learning and recent memory, participants learned 10 words and used them in 1 or 2 sentences. After a 5-minute delay, they were given 60 seconds to recall the 10 words, and the score was the number of words correctly recalled. In DSS, a test of executive function and processing speed, participants translated numbers to symbols using a key. The score was the number of numbers correctly translated in 90 seconds. In WF, a test of executive function and language, individuals had to spontaneously generate words beginning with a particular letter, excluding proper nouns. Three letters were used, and the score was the number of words created across the three trials. Our primary outcome was a global Z-score, calculated as the average of these 3 individual Z-scores at each study visit and standardized using the visit 2 global Z-score mean and standard deviation (SD). In other words, a Z score of −1 indicates a cognitive performance 1 SD below the mean visit 2 score. This global Z-score has been used in many other ARIC cognitive analyses.10-13
Additionally, at the ARIC Neurocognitive Study (ARIC-NCS) visit, which took place at visit 5 (2011-2013), participants underwent more comprehensive neuropsychological testing and an adjudicated diagnoses of incident dementia was made by an expert panel as previously described.2 Briefly, the diagnosis of dementia was made based on the longitudinal cognitive results from visits 2, 4, and 5, the more extensive neuropsychological tests at visit 5, informant interviews, and from prior discharge records using ICD-9 codes or death certificate recodes for dementia.
Included covariates were obtained from questionnaires, medication inventory, physical exam, and laboratory data obtained from visit 2 (unless otherwise noted), using standardized protocols.6 In our analysis, we included demographic variables of age (years), age2, sex (women, men), and race/center (Minnesota-whites; Maryland-whites; North Carolina-whites; North Carolina-blacks; Mississippi-blacks); in addition, education (<high school; high school, GED, vocational school; college, graduate or professional school, ascertained at visit 1), body mass index (BMI, <25 kg/m2; 25-<30 kg/m2, ≥30 kg/m2), smoking (never, former, current), alcohol consumption (never, former, current), physical activity (score range 1-5, using modified Baecke Physical Activity Questionnaire14, measured at visit 1). Cardiovascular risk factors such as systolic blood pressure (continuous, mmHg), pulse pressure (continuous, mmHg), cholesterol (total and HDL, continuous, mg/dL), use of antihypertensive and lipid lowering medications (yes/no), presence of diabetes (yes/no; determined by self-report of physician diagnosis, diabetes medication use, fasting blood glucose ≥126 mg/dL, or non-fasting blood glucose ≥200 mg/dL) and coronary heart disease (CHD) (yes/no), and use of AV-nodal blocking medications were also included. APOE genotype (0, 1, or 2 ∈ alleles) was added as a possible predictor.
RHR was categorized into <60 (reference), 60-69, 70-79 and ≥80 bpm, and also evaluated as a continuous variable per increments of 10 bpm. We used multivariable-adjusted linear regression models to assess the cross-sectional and linear mixed effect regression models with random intercepts and slopes to assess the longitudinal associations of RHR with cognitive function (for the global z-score and the 3 individual tests of DWR, DSS, and WF).
As expected, there were key differences in baseline characteristics of participants who did and did not attend visit 5 (which took place approximately 20-years after visit 2) as shown in Supplemental Table 1. Therefore, as per ARIC-NCS recommendations, we addressed this differential attrition and potential selection bias by using multiple imputations by chained equations15 to account for missing data, imputing for both missing CVD risk factors (in model 3) and missing outcomes (for those who did not return for visit 5). This imputation method used in this present analysis has been previously validated using simulations in a previously published manuscript using the same ARIC data.16 Supplemental Table 2 presents the numbers imputed for each variable. We also performed 3 sensitivity analyses regarding imputation as follows: 1) a “complete case” analysis which used all available data without imputation; 2) imputing only for covariates (CVD risk factors) but not outcomes; 3) imputing both covariates (CVD risk factors) and outcomes for only those known to be alive at visit 5.
Additionally, we used Cox proportional hazards regression models to determine hazard ratios and their 95% confidence intervals (CI) for incident dementia by RHR group. We verified that the proportionally hazards assumption was not violated by interacting RHR and log follow-up time; results were not significant.
For all of our analyses, we used three progressively adjusted models. Model 1 adjusted for demographic variables (age, age2, sex, and race/center groups). Model 2 additionally adjusted for socioeconomic and lifestyle variables including education, BMI, smoking, alcohol consumption, and physical activity. Model 3 further adjusted for cardiovascular risk factors (systolic and pulse pressures, HDL and total cholesterol, use of lipid lowering and antihypertensive medications, presence of CHD, diabetes, use of AV-nodal blocking medications, and APOE4 genotype).
Sensitivity analyses were also performed by excluding participants on AV-nodal blocking agents (i.e. drugs which pharmacologically alter heart rates). We also examined for interactions by race/ethnicity and sex. Additionally, we used a demographic-adjusted (Model 1) restricted cubic spline with knots placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles to characterize the association between RHR and incident dementia, centered at RHR of 60 bpm.
A 2-sided p value <0.05 was considered statistically significant, and we performed analyses using Stata Version 15.
Results
Baseline participant characteristics at visit 2 (n=13,720) are found in Table 1. Those with higher RHR were more likely to be women, have higher BMI, have lower physical activity scores, be current smokers, have higher blood pressure and use of antihypertensive medications, and more likely to have diabetes and prevalent CHD.
Table 1.
Baseline Characteristics of Study Participants by Resting Heart Rate Categories, the ARIC Study Visit 2 (1990 -1992)
| Baseline Characteristics | Overall | Resting heart rate (bpm) | |||
|---|---|---|---|---|---|
| <60 | 60–69 | 70–79 | ≥80 | ||
| Number of Participants | 13,720 | 3,797 | 5,490 | 3,189 | 1,244 |
| Resting heart rate (beats per minute), mean (SD) | 66 (10) | 54(4) | 64 (3) | 74 (3) | 86 (6) |
| Cognitive Test Scores, mean (SD) | |||||
| Global Z-Score | 0.0 (1.0) | 0.0 (1.0) | 0.1 (1.0) | 0.0 (1.0) | −0.2 (1.0) |
| Delayed Word Recall Z-Score | 0.0(1.0) | 0.0 (1.0) | 0.1 (1.0) | 0.0 (1.0) | −0.2 (1.1) |
| Delayed Word Recall Raw Score (words) | 6.6(1.5) | 6.6 (1.5) | 6.7 (1.5) | 6.6 (1.5) | 6.4 (1.6) |
| Digit Symbol Substitution Z-Score | 0.0(1.0) | 0.0 (1.0) | 0.1 (1.0) | 0.0 (1.0) | −0.3 (1.0) |
| Digit Symbol Substitution Raw Score (points) | 44.9 (14.2) | 45.2 (14.1) | 45.6 (13.8) | 44.9 (14.4) | 40.6 (14.5) |
| Word Fluency Z-Score | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | 0.0 (1.0) | −0.1 (1.0) |
| Word Fluency Raw Score (words) | 33.3 (12.5) | 33.5 (12.5) | 33.5 (12.4) | 33.3 (12.6) | 31.9 (12.5) |
| Age (years), mean (SD) | 57.5 (5.7) | 57.6 (5.7) | 57.4 (5.7) | 57.2 (5.7) | 57.8 (5.8) |
| Women | 7,665 (55.9%) | 1,720 (45.3%) | 3,154 (57.5%) | 1,997 (62.6%) | 794 (63.8%) |
| Race/Center | |||||
| Minneapolis, MN Whites | 3,696 (26.9%) | 1,177 (31.0%) | 1,496 (27.3%) | 793 (24.9%) | 230 (18.5%) |
| Washington County, MD Whites | 3,528 (25.7%) | 931 (24.5%) | 1,439 (26.2%) | 840 (26.3%) | 318 (25.6%) |
| Forsyth County, NC Whites | 3,184 (23.2%) | 874 (23.0%) | 1,306 (23.8%) | 750 (23.5%) | 254 (20.4%) |
| Forsyth County, NC Blacks | 360 (2.6%) | 105 (2.8%) | 137 (2.5%) | 78 (2.5%) | 40 (3.2%) |
| Jackson, MS Blacks | 2,952 (21.5%) | 710 (18.7%) | 1,112 (20.3%) | 728 (22.8%) | 402 (32.3%) |
| Education† | |||||
| <High School | 2,926 (21.3%) | 793 (20.9%) | 1,080 (19.7%) | 712 (22.3%) | 341 (27.4%) |
| High School, GED, or Vocational School | 5,716 (41.7%) | 1,525 (40.2%) | 2,332 (42.5%) | 1,348 (42.3%) | 511 (41.1%) |
| College, Graduate, or Professional School | 5,078 (37.0%) | 1,479 (39.0%) | 2,078 (37.9%) | 1,129 (35.4%) | 392 (31.5%) |
| Body Mass Index (kg/m2) | |||||
| <25 | 4,267 (31.1%) | 1,318 (34.7%) | 1,703 (31.0%) | 916 (28.7%) | 330 (26.5%) |
| 25-29 | 5,475 (39.9%) | 1,561 (41.1%) | 2,256 (41.1%) | 1,250 (39.2%) | 408 (32.8%) |
| ≥ 30 | 3,978 (29.0%) | 918 (24.2%) | 1,531 (27.9%) | 1,023 (32.1%) | 506 (40.7%) |
| Physical activity index†, mean (SD) Smoking Status | 2.5 (0.8) | 2.6 (0.8) | 2.5 (0.8) | 2.4 (0.8) | 2.3 (0.7) |
| Never | 5,474 (39.9%) | 1,383 (36.4%) | 2,279 (41.5%) | 1302 (40.8%) | 510 (41.0%) |
| Former | 5,193 (37.9%) | 1,600 (42.1%) | 2,037 (37.1%) | 1,141 (35.8%) | 415 (33.4%) |
| Current | 3,053 (22.3%) | 814 (21.4%) | 1,174 (21.4%) | 746 (23.4%) | 319 (25.6%) |
| Alcohol Consumption | |||||
| Never | 3,081 (22.5%) | 742 (19.5%) | 1,223 (22.3%) | 772 (24.2%) | 344 (27.7%) |
| Former | 2,848 (20.8%) | 789 (20.8%) | 1,081 (19.7%) | 682 (21.4%) | 296 (23.8%) |
| Current | 7,791 (56.8%) | 2,266 (59.7%) | 3,186 (58.0%) | 1,735 (54.4%) | 604 (48.6%) |
| Systolic Blood Pressure (mmHg), mean (SD) | 121 (19) | 120 (19) | 121 (18) | 123 (18) | 127 (20) |
| Pulse Pressure (mmHg), mean (SD) | 49 (14) | 49 (14) | 49 (14) | 49 (14) | 52 (16) |
| Use of Hypertension Medications | 4,381 (31.9%) | 1,208 (31.8%) | 1,653 (30.1%) | 1,013 (31.8%) | 507 (40.8%) |
| Use of atrioventricular-nodal Medications | 2,044 (14.9%) | 739 (19.5%) | 725 (13.2%) | 415 (13.0%) | 165 (13.3%) |
| Total Cholesterol (mg/dl), mean (SD) | 210 (39) | 206 (37) | 210 (38) | 212 (40) | 216 (47) |
| HDL Cholesterol (mg/dl), mean (SD) | 50 (17) | 49 (17) | 50 (17) | 49 (16) | 49 (18) |
| Use of Cholesterol Medications | 864 (6.3%) | 246 (6.5%) | 334 (6.1%) | 188 (5.9%) | 96 (7.8%) |
| Diabetes | 1,994 (14.6%) | 351 (9.3%) | 670 (12.3%) | 577 (18.2%) | 396 (32.0%) |
| Prevalent Coronary Heart Disease | 722 (5.3%) | 234 (6.2%) | 270 (4.9%) | 136 (4.3%) | 82 (6.6%) |
| Apolipoprotein ∈4 Genotype | |||||
| TT | 9,186 (69.2%) | 2,528 (68.6%) | 3,694 (69.5%) | 2,132 (69.1%) | 832 (69.7%) |
| CT | 3,741 (28.2%) | 1,048 (28.5%) | 1,490 (28.0%) | 876 (28.4%) | 327 (27.4%) |
| CC | 356 (2.7%) | 107 (2.9%) | 135 (2.5%) | 79 (2.6%) | 35 2.9%) |
Measured at ARIC Visit 1 (1987-1989).
Supplemental Table 3 shows the cross-sectional associations of RHR with cognitive performance at visit 2. After adjusting for CVD risk factors in the fully adjusted model 3, compared to those with those with RHR <60 bpm, those with RHR ≥80 bpm had lower baseline global cognitive Z-score [average difference −0.08 (95% CI −0.13, −0.03)]. This remained essentially unchanged in sensitivity analyses excluding those on AV-nodal blocking medications (Supplemental Table 4).
Over a mean follow-up of 20-years, participants in each RHR group exhibited cognitive decline (Table 2, Part A). However, there was relatively greater global cognitive decline for those with RHR 70-79 and ≥80 bpm compared to <60 bpm (Table 2, Part B). In the fully adjusted model 3, the average difference in global cognitive decline was −0.07 (95% CI −0.13, −0.004) for participants with RHR 70-79 bpm and −0.12 (−0.21, −0.03) for ≥80 bpm, compared to participants with RHR <60 bpm. Results were generally consistent when excluding participants on AV-nodal blockade medications (Supplemental Table 5).
Table 2.
Average Adjusted Decline and Difference in Decline over 20-years for the Global Cognitive Z-Score By Resting Heart Rate Groups:* the ARIC Study 1990-1992 to 2011-2013.Part A: 20-year Change in Global Cognitive Domain Part B: Relative Difference in the 20-yr by Resting Heart Rate Groups Change of Global Cognitive Z-Scores for Higher Resting Heart Rate Groups Compared to Lowest (Reference)
| Resting Heart rate (bpm) |
<60 | 60-69 | 70-79 | ≥80 | Difference 60-69 vs <60 |
Difference 70-79 vs <60 |
Difference ≥80 vs <60 |
|---|---|---|---|---|---|---|---|
| Model 1† |
−0.88(−0.92, −0.83) | −0.92(−0.97, −0.88) | −0.97(−1.03, −0.91) | −1.05(−1.14, −0.96) | −0.05(−0.09,0.00) | −0.10(−0.16, −0.04) | −0.17(−0.27, −0.08) |
| Model 2‡ |
−0.88(−0.93, −0.83) | −0.93(−0.97, −0.88) | −0.97(−1.03, −0.91) | −1.05(−1.14, −0.95) | −0.04(−0.09,0.00) | −0.09(−0.15, −0.03) | −0.16(−0.26, −0.07) |
| Model 3∥ |
−0.90(−0.94, −0.85) | −0.93(−0.98, −0.89) | −0.96(−1.03, −0.90) | −1.01(−1.10, −0.92) | −0.04(−0.09,0.01) | −0.07(−0.13, −0.004) | −0.12(−0.21, −0.03) |
Results are presented in β coefficients (95% CI) from adjusted linear mixed effect models. Missing covariates and cognitive scores were imputed. Results in bold are statistically significant differences between resting heart rate groups (p <0.05).
Model 1: adjusted for age, age2, sex, race/center, and interactions between each of these variables and time
Model 2: Model 1 + education, body mass index, smoking status, alcohol, physical activity, and interactions between each of these variables and time
Model 3: Model 2 + systolic blood pressure, pulse pressure, use of hypertension medication, diabetes, HDL cholesterol, total cholesterol, cholesterol lowering medications, history of prevalent coronary heart disease, use of AV-nodal blocking medications,
APOE4 genotype, and interactions between each of these variables and time
When change in performance on each individualized cognitive test over 20-years was evaluated by RHR groups, the differential cognitive decline in RHR ≥80 bpm vs. <60 bpm was seen for the DSS and WF tests but not for the DWR (Supplemental Table 6). Results were again consistent when excluding those on AV-nodal blocking agents (not shown).
We performed a series of sensitivity analyses to determine the robustness of our findings. First, in complete-case analysis without imputation, findings remained similar to main results (Supplemental Table 7), as they did in imputation only for covariates (but not outcomes) and for imputation for covariates and outcomes only for those who were alive at visit 5 (Supplemental Table 8). Finally, we looked for evidence of effect modification by sex and race. There was no significant interaction of RHR with 20-year global cognitive decline by sex (p interaction=0.95). However, there was an interaction by race (p interaction=0.004). There was a greater cognitive decline over 20-years associated with higher RHR in whites (Supplemental Tables 9) than blacks (Supplemental Tables 10). The adjusted difference in the 20-year change of global cognitive z-score, for those with RHR ≥80 compared to those <60 bpm, was −0.16 (−0.26, −0.06) in whites and 0.01 (−0.16, 0.18) in blacks (model 3).
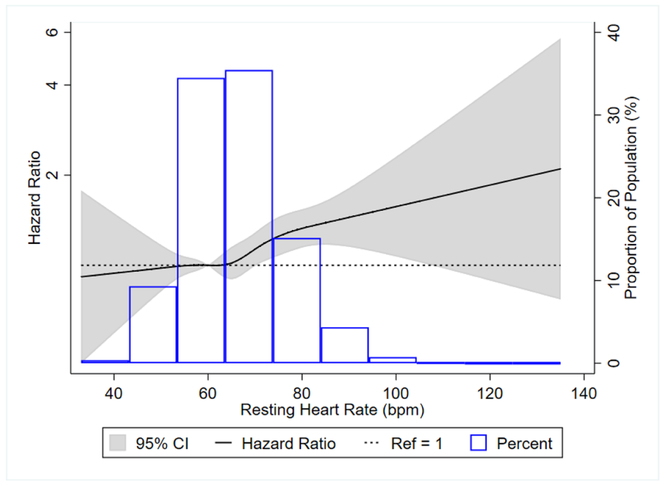
We also examined the association of RHR at visit 2 with incident dementia. We observed 1,350 cases of incident dementia after 248,437 person-years of follow up. In the fully adjusted model (model 3), the hazard ratios (95% CI) for incident dementia was 1.28 (1.04, 1.57) for those with RHR ≥80 bpm, compared to <60 bpm, and 1.08 (1.02, 1.14) for every 10 bpm increment in RHR (Table 3). There were no interactions by age, sex, and race/center for the outcome of incident dementia (all p>0.05). Excluding participants on AV-nodal blocking medications resulted in weaker associations for incident dementia with hazard ratios (95% CI) of 1.19 (0.94, 1.49) when comparing ≥80 bpm to <60 bpm and 1.06 (0.99, 1.12) for every 10 bpm increment in RHR (Supplemental Table 11). Restricted cubic spline modeling indicated that the association of RHR with incident dementia was approximately linear (Figure 2).
Table 3.
Associations of resting heart rate measured at Visit 2 (1990-1992) with incident dementia through 2013: the ARIC Study
| Resting heart rate (bpm) | Per 10 bpm increment | ||||
|---|---|---|---|---|---|
| <60 | 60 – 69 | 70 - 79 | ≥80 | ||
| Number of participants | 3,795 | 5,487 | 3,188 | 1,244 | 13,714 |
| Case | 370 (9.8%) | 534 (9.7%) | 309 (9.7%) | 137 (11.0%) | 1,350 (9.8%) |
| Incidence rate (95% CI)* | 5.26 (4.75, 5.83) | 5.28 (4.85, 5.75) | 5.42 (4.85, 6.06) | 6.83 (5.78, 8.07) | 5.43 (5.15, 5.73) |
| Hazard Ratios (95% CI) | |||||
| Model 1† | Reference (1) |
1.04 (0.91, 1.19) | 1.16 (0.99, 1.35) | 1.44 (1.18, 1.75) | 1.12 (1.06, 1.18) |
| Model 2‡ | Reference (1) |
1.05 (0.92, 1.20) | 1.15 (0.99, 1.34) | 1.42 (1.17,1.74) | 1.11 (1.05, 1.17) |
| Model 3∥ | Reference (1) |
1.08 (0.95, 1.24) | 1.14 (0.97, 1.33) | 1.28 (1.04, 1.57) | 1.08 (1.02, 1.14) |
Incidence Rates are per 1,000 person-years
Model 1: Adjusted for age, age2, sex, and race/center
Model 2: Model 1 plus education, body mass index, smoking status, alcohol, and physical activity
Model 3: Model 2 plus systolic blood pressure, pulse pressure, use of hypertension medication, diabetes, HDL cholesterol, total cholesterol, cholesterol lowering medications, history of prevalent coronary heart disease, use of AV-nodal blocking medications, and APOE4 genotype
Figure 2:
Associations* of resting heart rate with incident dementia in ARIC, 1990-2013.
*Figure is a restricted cubic spline showing association of resting heart rate with incident dementia in ARIC, 1990-2013. We used resting heart rate of 60 bpm as reference in a Cox proportional hazards model adjusted for age, age2, sex, and race. The knots were placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles.
Discussion
Using data from ARIC, a large mostly biracial community-based study, we found an independent association of RHR and cognitive decline over 20-years. Compared to lower RHR, higher RHR at baseline was associated with more cardio-metabolic comorbidities at baseline, suggesting RHR is associated with a poorer health status. However even after adjusting for physical activity and CVD risk factors, a higher RHR was independently associated with a lower cognitive performance at baseline, with greater cognitive decline over 20-years, and with increased risk of incident dementia. Findings remained robust in multiple sensitivity analyses and the relative effect of a higher RHR on cognitive decline appeared greater in whites than in blacks. Our findings are in line with the one study investigating RHR and cognitive decline after an ischemic stroke,5 but to our knowledge, there have been no prior studies directly looking at RHR and cognitive decline in the general population.
There are many possible explanations for the observed association between RHR and cognitive decline. First, RHR is thought to reflect a balance of sympathetic and parasympathetic nervous systems. In resting states, the parasympathetic system predominates. Thus, elevated RHR can be a sign of decreased parasympathetic and increased sympathetic activity, which has been seen in patients with prevalent cognitive impairment17,18 and dementia.19 Higher RHR may be associated with increased blood pressure through increased sympathetic activity, and patients with mild cognitive impairment and dementia have higher arterial stiffness and pulse pressure.20 Additionally, higher RHR was independently associated with incident hypertension, even among younger individuals.21 However, we were able to demonstrate that RHR measured in mid-life was associated with decline in cognitive function over 20-years even after taking into account baseline systolic blood pressure, pulse pressure, and use of antihypertensive medication.
An elevated RHR may also stimulate activation of inflammatory pathways. Higher RHR has been associated with higher levels of inflammatory markers such as hs-CRP, IL-6, and fibrinogen.22 Vagal (parasympathetic) stimulation decreases the release of inflammatory cytokines in stress states.23 Inflammation has been associated with cognitive decline, and thus it is possible that an inflammatory effect conferred by sympathetic stimulation (or withdrawal of parasympathetic stimulation), as reflected in RHR, could also extend to neurodegeneration.
We also found an interaction of race in the association of RHR and cognitive decline, with the decline more evident in whites than blacks. The reason for this is uncertain, and in the absence of any a priori hypothesis to this regard, this should be considered exploratory only and warrants confirmation in other cohorts. Similarly, we are uncertain why we found RHR to be associated with change in DSS and WF but not DWR – findings which also should be considered with caution in the absence of any hypotheses about why RHR would influence certain cognitive domains over others.
Our study has many strengths including the prospective design utilizing data from the well-characterized ARIC study, which allowed us to rigorously adjust for numerous potentially confounding demographic, lifestyle, and CVD risk factors, the large sample size, and the prolonged time course over 20-years to study the long-term associations between RHR and cognition. However our findings should be interpreted in the context of several limitations. First, it is an observational study and residual confounding may explain the associations seen. An elevated RHR may be just a very good surrogate marker for a poorer health state. Second, there was attrition over 20-years, with less than half of the initial cohort from visit 2 still present at visit 5. Attrition is a common problem in studies on cognitive decline. As is standard in studies using ARIC-NCS data, we used imputation models to attempt to account for any potential bias related to attrition. This imputation method has been previously validated in the same ARIC cohort.16 Additionally, our findings remained robust in various sensitivity analyses including a complete case analysis. As an additional limitation, our analysis focused on baseline RHR and we were not able to analyze if changes in RHR over time were associated with differential cognitive decline. However, previous ARIC analyses have also highlighted the importance of other vascular risk factors measured in mid-life with future risk of both incident dementia2 and brain amyloid deposition in late-life.24
In summary, we found that elevated RHR was independently linked to worsening cognition over time. RHR can be readily measured at office clinic visits or by the use of personal fitness trackers; however, it remains underutilized for the purpose of CVD risk assessments. Further studies are needed to determine whether the associations we found between RHR and cognitive decline are causal or secondary to another underlying process. If causal, future interventional studies are needed to determine whether modification of RHR can reduce cognitive decline.
Data availability statement
The ARIC cohort participates in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository (BioLINCC). The ARIC data are available upon request, including data from visits 1 to 5, follow-up data, and ancillary data. Requests for data can be made through the following website: https://biolincc.nhlbi.nih.gov/studies/aric/.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding
Drs. Michos and Zhao are supported by the Blumenthal Scholars Fund for Preventive Cardiology research. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The Atherosclerosis Risk in Communities – Neurocognitive Study was funded by U01 HL096812, HL096814, HL096899, HL096902, and HL096917, with additional support from the National Institute of Neurological Disorders and Stroke. Additionally, Dr. Schneider is supported by the National Institute of Neurological Disorders and Stroke through an administrative supplement to award R25NS065729. Dr. Gottesman is supported by K24 AG052573.
Footnotes
DISCLOSURES:
The authors do not report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naqvi R, Liberman D, Rosenberg J, Alston J, Straus S. Preventing cognitive decline in healthy older adults. CMAJ 2013; 185:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 2017;74:1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Aladin, Whelton SP, Al-Mallah MH, Blaha MJ, Keteyian SJ, Juraschek SP, Rubin J, Brawner CA, Michos ED Relation of resting heart rate to risk for all-cause mortality by gender after considering exercise capacity (the Henry Ford exercise testing project). The American journal of cardiology 2014; 114:1701–1706. [DOI] [PubMed] [Google Scholar]
- 4.Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart 2013;99:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohm M, Cotton D, Foster L, Custodis F, Laufs U, Sacco R, Bath PM, Yusuf S, Diener HC. Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J 2012;33:2804–2812. [DOI] [PubMed] [Google Scholar]
- 6.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 7.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 8.Wechsler D Wechsler Adult Intelligence Scale-Revised (WAIS-R) Manual. New York: Psychological Corp, 1987. [Google Scholar]
- 9.Benton AL, Hamsher K. Multilingual Aphasia Examination, 2nd Edition. Iowa City: AJA Associates, 1989. [Google Scholar]
- 10.Kim SM, Zhao D, Schneider ALC, Korada SK, Lutsey PL, Guallar E, Alonso A, Windham BG, Gottesman RF, Michos ED. Association of parathyroid hormone with 20-year cognitive decline: The ARIC study Neurology 2017;89:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen-Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, Heiss G, Knopman DS, Patel MD, Penman AD, Power MC, Selnes OA, Schneider AL, Wagenknecht LE, Windham BG, Wruck LM, Mosley TH. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol 2014;179:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawlings AM, Sharrett AR, Schneider AL, Coresh J, Albert M, Couper D, Griswold M, Gottesman RF, Wagenknecht LE, Windham BG, Selvin E. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014;161:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, Loehr LR, Folsom AR, Coresh J, Alonso A. Association of Atrial Fibrillation With Cognitive Decline and Dementia Over 20 Years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 15.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011. ;30:377–399. [DOI] [PubMed] [Google Scholar]
- 16.Rawlings AM, Sang Y, Sharrett AR, Coresh J, Griswold M, Kucharska-Newton AM, Palta P, Wruck LM, Gross AL, Deal JA, Power MC, Bandeen-Roche KJ. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol 2017;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging 2012;33:2324–2333. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Lipsitz LA, Ferrucci L, Varadhan R, Guralnik JM, Carlson MC, Fleisher LA, Fried LP, Chaves PH. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women's Health and Aging Study I. J Am Geriatr Soc 2006;54:1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aharon-Peretz J, Harel T, Revach M, Ben-Haim SA. Increased sympathetic and decreased parasympathetic cardiac innervation in patients with Alzheimer's disease. Arch Neurol 1992;49:919–922. [DOI] [PubMed] [Google Scholar]
- 20.Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS, Griswold ME, Mosley TH, Heiss G. Association of Central Arterial Stiffness and Pressure Pulsatility with Mild Cognitive Impairment and Dementia: The Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J Alzheimers Dis 2017;57:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aladin AI, Al Rifai M, Rasool SH, Keteyian SJ, Brawner CA, Michos ED, Blaha MJ, Al-Mallah MH, McEvoy JW. The Association of Resting Heart Rate and Incident Hypertension: The Henry Ford Hospital Exercise Testing (FIT) Project. Am J Hypertens 2015. [DOI] [PubMed] [Google Scholar]
- 22.Whelton SP, Narla V, Blaha MJ, Nasir K, Blumenthal RS, Jenny NS, Al-Mallah MH, Michos ED. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2014;113:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–462. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ARIC cohort participates in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository (BioLINCC). The ARIC data are available upon request, including data from visits 1 to 5, follow-up data, and ancillary data. Requests for data can be made through the following website: https://biolincc.nhlbi.nih.gov/studies/aric/.