Summary
Epidemiological surveys have revealed that environmental and dietary factors contribute to most of the human cancers. Our earlier studies have shown that resveratrol (RVT), a phytochemical reduced the tumor number, size and incidence of dysplasias induced by benzo(a)pyrene (BaP), an environmental toxicant in the ApcMin/+ mouse model of colon cancer. In this study we investigated to ascertain whether the preventive effects of RVT on BaP-induced colon carcinogenesis is a result of altered BaP biotransformation by RVT. For the first group of mice, 100 μg BaP/kg bw was administered in peanut oil via oral gavage over a 60 day period. For the second group, 45 μg RVT/kg bw was co-administered with BaP. For the third group, RVT was administered for 1 week prior to BaP exposure. Blood, colon and liver were collected from control and BaP/RVT-treated mice at 60 days post-BaP & RVT exposure. We have assayed activities and expression (protein & mRNA) of drug metabolizing enzymes such as cytochrome P4501A1 (CYP1A1), CYP1B1, and glutathione-S-transferase (GST) in colon and liver samples from the treatment groups mentioned above. An increased expression of CYP1A1 in liver and colon and of CYP1B1 in liver of BaP-treated mice was seen, while RVT inhibited the extent of biotransformation mediated by these enzymes in the respective tissue samples. In the case of GST, an increased expression in colon of BaP alone-treated mice was noted when RVT was administered prior to BaP or simultaneously with BaP. However, there is no change in liver GST expression between BaP and RVT treatment groups. The concentrations of BaP aqueous (phase II) metabolites were found to be greater than the organic (phase I) metabolites, suggesting that RVT slows down the phase I metabolism (metabolic activation) of BaP, while enhancing phase II metabolism (detoxification). Additionally, the BaP-DNA adduct concentrations measured in colon and liver of BaP + RVT-treated mice were low relative to their BaP counterparts. Taken together, our findings strongly suggest that RVT alleviates BaP-induced colon carcinogenesis by impairing biotransformation pathways and DNA adduct formation, and therefore holds promise as a chemopreventive agent.
Keywords: Benzo(a)pyrene, resveratrol, colon carcinogenesis, biotransformation, chemoprevention, ApcMin/+ mouse
Introduction
Colorectal cancer is one of the most common cancers in the Western world and 90% of the cases have no familial history of the disease. Sporadic gene damage seems to play an important role in the development of tumors in the colon. Dietary and environmental factors contribute to sporadic gene mutations and therefore are involved in the induction of sporadic colon carcinomas. Benzo(a)pyrene (BaP) is an environmental toxicant that has been linked to dietary intake leading to the development of colon tumors [1,2,3]. When inhaled or ingested through water and diet, BaP becomes activated in biological systems to reactive metabolites that damage cellular macromolecules such as DNA, leading to mutations and as a consequence can lead to the development of cancer [2].
Epidemiological and animal model studies have shown that phytochemical ingredients of diet play a major role in disease prevention [4]. Among the different nutrients, polyphenols have been shown to inhibit the development of tumors induced by carcinogens [5,6,7,8,9]. Resveratrol (RVT; 3,5,4′-trihydroxystilbene), a phytoalexin and a polyphenolic compound present in grapes, peanuts and mulberries [10] has been reported to possess cancer preventive properties [7,8,11,12,13]. Our earlier studies have shown that orally administered RVT caused a decrease in incidence, size and number of adenomas formed in the colon of ApcMin/+ mice exposed to BaP compared to mice exposed to BaP alone [14].
Since most chemopreventive agents mediate their anticarcinogenic effect through altered biotransformation of carcinogens [15,16,17], we attempted to gain a mechanistic insight into how RVT may be reducing BaP-induced colon polyp formation by altering the cytochrome P450 mediated metabolic pathways. In this paper we report the altered protein, mRNA expression, and activities of key enzymes proven to be involved in BaP biotransformation. In addition, the concentrations of BaP metabolites and BaP-DNA adducts in mice exposed to BaP alone and BaP in combination with RVT were also provided.
Materials and methods
Animals
Animal husbandry, BaP and RVT exposure
Five-week-old male ApcMin/+ mice (Jackson Labs, Bar Harbor, ME) weighing approximately 30 g were housed in groups of 2–3 per cage, maintained on a 12/12 hour light/dark cycle and allowed free access to rodent chow (NIH-31 open formula diet) and water. All animals were allowed a seven-day acclimation period prior to being randomly assigned to a control (n = 10 per each time point) or treatment group (n = 10 per each time point). Treatment consisted of a single dose (100μg/kg bw) of BaP (97% pure, Sigma Chemical Co., St. Louis, MO) dissolved in research grade peanut oil (Sigma). Resveratrol (45μg/kg bw; Sigma), dissolved in 10% ethanol and 90% deionized water, was given concurrently with BaP (for 60 days), prior (daily for 1 week) to BaP exposure (for 60 days) or post (daily for 1 week) BaP exposure. The test chemicals (BaP & RVT) were administered through oral gavage (200μL volume). All animal studies carried out in ethical manner and were in conformity with the policies of Institutional Animal Care and Use Committee of Meharry Medical College. The numbers of mice for control and treatment groups were selected after conducting a power analyses. On the basis of our preliminary studies, with 10 samples there is 80% power and a type-1 error of 5% to detect a 20% change in the experimental endpoints (tumors, metabolite concentrations, enzyme activities etc.) among the various experimental groups. As BaP is a potential carcinogen, it was handled in accordance with NIH guidelines [18]. The doses of BaP and RVT used were of dietary relevance to humans [14].
All the mice from control and treatment groups were observed twice a day (including holidays and weekends) for morbidity and mortality. Mice body weight and food consumption were monitored periodically.
At the end of 60 days of exposure, blood was collected through cardiac puncture of mice from both control and experimental groups. Isoflurane was used as an anesthetic (3%) and euthanizing (33%) agent. The target tissues (liver, large intestine, small intestine, stomach) were retrieved. The proximal, middle and distal portions of the colon were cut open and flushed with physiological saline. The tissues were finely diced in a small Petri dish using sharp scissors. The diced tissues were thoroughly mixed and stored at −80°C until processed for biochemical, molecular and analytical studies.
Chemicals
Benzo(a)pyrene (98% pure), peanut oil, endoplasmic reticulum Isolation kit and resveratrol were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Lithium chloride, urea, sodium phosphate (monobasic and dibasic), methanol, chloroform, ethanol and 10% formalin, isopropyl alcohol were purchased from Fisher Scientific Company (Kennesaw, GA). Polyethylenimine-cellulose TLC plates were purchased from Bodman Chemical Company (Aston, PA). DNase and alkaline phosphatase were purchased from Worthington Biochemical Corporation (Feehold, NJ). The CYP1A1, CYP1B1, GST-P, and GAPDH antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). The rabbit anti-goat IgG-HRP and mouse anti-goat IgG-HRP antibodies were purchased from LiCor (Lincoln, NE). The Quick Start Bradford Protein Assay Kit, ethidium bromide, Precision Plus Protein all blue standards, tetramethylethylenediamine (TEMED), EZ load 100 bp molecular ruler, and 2-Mercaptoethanol (βME) were purchased from Bio-Rad Laboratories (Richmond, CA). Easy-DNA kit, RNase/DNase free water and Trizol reagent were purchased from Invitrogen (Carlsbad, CA). The CYP1A1 and 1B1 enzyme assay kits were purchased from Promega (Madison, WI). The GST assay kit was purchased from Biovision Inc. (Mountain View, CA). DNeasy blood and tissue kit was purchased from Qiagen (Valencia, CA). The polyvinylidene difluoride (PVDF) membranes were purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Drug metabolizing enzyme activity assays
Microsomes were isolated from colon and liver tissues using the Endoplasmic Reticulum Isolation Kit (Sigma-Aldrich, St. Louis, MO). The isolated microsomes were then assayed for CYP1A1 and 1B1 enzyme activity in the liver and colon using the P450-Glo assay (Promega, Madison, WI). For analysis of GST activity, isolated proteins from the colon and liver were assayed using the Glutathione S-Transferase Assay Kit (Cayman Chemical, Ann Arbor, MI).
Total RNA isolation
Total RNA was isolated using Trizol total RNA Isolation kit (Promega, Madison, WI). Five hundred milligrams of target tissue were homogenized in 1mL of Trizol reagent. Homogenized samples were then incubated for 5 minutes at 30°C to permit complete dissociation of nucleoprotein complexes. Two hundred microliters of chloroform per mL of Trizol was added to samples, followed by a 15 sec shake, then a 3-minute incubation at 30°C. Samples were then centrifuged at 12,000xg for 15 minutes at 8°C and the upper aqueous phase, containing the RNA, was removed. Five hundred microliters of isopropyl alcohol (per mL of Trizol) was added to samples followed by a 10-minute incubation at 30°C. Samples were then centrifuged at 12,000xg for 10 minutes at 8°C. The supernatant was removed and each pellet was washed once with 75% ethanol and centrifuged at 7,500xg for 5 minutes at 8°C. Each pellet was dried, dissolved in RNase-free water, and RNA concentration was determined by spectrophotometry (by examining the 260/280 ratio).
cDNA Synthesis and Real Time Polymerase Chain Reaction (RT-PCR)
The RNA obtained from the previously described procedure was used for RT-PCR analysis. The cDNA synthesis and amplification of biotransformation enzymes (CYP1A1, 1B1, and glutathione-s-transferase; GST) was performed using the services of Vanderbilt University Genome Science Resource Vantage Core Laboratory.
Western blot analysis
Total protein was resolved by electrophoresis on 10% SDS–PAGE gels followed by electroblotting of suspended proteins to PVDF membranes and hybridization with mouse CYP1A1, CYP1B1, GST, and rabbit GAPDH antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX). Immunodetection was performed with the Odyssey Procedure (Li-Cor Biosciences, Lincoln, NE) using an IRDye800 coupled anti-rabbit IgG secondary antibody and an IRDye680 coupled anti-mouse IgG secondary antibody. Normalization of the signals of drug metabolizing enzymes with GAPDH was performed in order to quantify protein expression.
HPLC analysis of BaP and its metabolites
Sample analyses was conducted on a High-Performance Liquid Chromatograph, (HPLC; Model 1200, Agilent Technologies, Wilmington, DE) equipped with a HP1046 fluorescence detector and a variable wavelength detector as detailed in Ramesh et al. [19,20]. Identification and quantitation of the metabolites was accomplished by comparing the retention times and peak areas of samples with that of standards (National Cancer Institute Chemical Carcinogen Repository, Midwest Research Institute, Kansas City, MO).
HPLC analysis of RVT and its metabolites
The trans-resveratrol content from plasma, liver and colon tissues was analyzed following the method of Katsagonis et al. [21], which is briefly described below: Colon and liver tissue samples (0.5–1.0 gm) were cut individually in ice-cold PBS solution. Plasma samples (200μl) were also shaken in 500μl of ice-cold PBS solution. The samples were extracted with phosphate buffer (pH 6.0; 56.8 mM) and ethyl acetate. The aqueous and organic phases were separated and the organic phase was blown to dryness under nitrogen. The dried extract was reconstituted in 500μL of mobile phase. Fifty microliters of sample extract were injected onto a Waters C18 column (150 × 3.9 mm; 4μm pore size). The sample was eluted with a mobile phase of methanol/phosphate buffer (pH 4.8; 30 mM) at a ratio of 25:75 v/v). The RVT parent compound eluted from the column was detected using a variable wavelength (UV/VIS) detector set 310 nm. The trans-resveratrol (trans-3, 5, 4′-trihydroxystilbene) was used as an internal standard.
Much of the biological activity of RVT has been ascribed to that of its metabolites. Hence these metabolites were assayed to reflect biomarkers of chemopreventive effects observed in our study. Chromatographic separation of conjugated RVT metabolites was achieved using the procedure of Wenzel et al. [22]. Separations were performed using the HPLC (Agilent Technologies, Wilmington, DE) available in the Toxicology Core laboratory at Meharry. Briefly the procedure involves injection of sample extracts using a Phenomenex ODS column (250 × 4.6 mm; 5μm pore size). Gradient separations of analytes were conducted using ammonium formate buffer (10mM; pH 8.2) and methanol as mobile phases. The RVT conjugated metabolites eluting from the column were detected using a variable wavelength (UV/VIS) detector set at 310 nm. Resveratrol metabolite peaks were identified using standards obtained from Toronto Research Chemicals (North York, Ontario, Canada).
Pharmacokinetics
After treatment with RVT and BaP, mice were euthanized, and concentrations of these chemicals were measured in the plasma and their pharmacokinetic behavior was assessed. The pharmacokinetic parameters for BaP and RVT in plasma were analyzed using PK solutions 2.0 (Summit Research Services, Ashland, OH) software. The symbols and units adopted for pharmacokinetic terms were based on the suggestions of Baggot [23]. The biological half-life (t1/2) of BaP was calculated by a linear regression of the log plasma concentration versus the time curve. The area under the curve (AUC) was calculated by measuring the area under the blood BaP concentration-time curve. The mean residence time (MRT) was determined as AUC/AUMC where AUMC is the area under the first moment of curve. The volume of distribution (Vd) was calculated by considering the volume of BaP in the body assuming if present throughout the body, BaP remains at the same concentration as in plasma. The total body clearance (Cl) was computed as the ratio of BaP dose and AUC. The elimination rate constant (Kd) was determined as a ratio of Cl and Vd.
DNA isolation from target tissues
DNA was isolated from target tissues of mice post treatment using a combination of Trizol and Qiagen DNA isolation kits. Five hundred milligrams of target tissues were weighed and homogenized in 1mL of Trizol reagent. Homogenized samples were then incubated for 5 minutes at 30°C to permit complete dissociation of nucleoprotein complexes. Two hundred microliters of chloroform per mL of Trizol was added to samples, followed by a 15s shake, then 3-minute incubation at 30°C. Samples were then centrifuged at 12,000 x g for 15 minutes at 8°C; the lower layer and interphase containing DNA were mixed with 70% ethanol and mixed well. Each sample in its entirety was then transported onto a DNeasy mini-spin column placed in a 2mL collection tube and centrifuged at 6,000xg for 1 minute. Flow through was then discarded and the spin column was placed in new collection tube, followed by addition of 500μl of buffer. Tubes were then spun for 3 minutes at 20,000xg, flow through was discarded, and the spin column was transferred to a new collection tube. DNA was eluted by incubating samples with 200μl of buffer for 1 minute followed by centrifugation for 1 minute at 6,000xg. The concentration of DNA was determined by spectrophotometry.
32P-Postlabeling and Thin Layer Chromatography (TLC)
The methodology of Gupta and Randerath [24] as modified by Ramesh and Knuckles [25] were used for analysis of DNA adducts by 32P-postlabeling and four-directional thin layer chromatography system. Adduct levels were calculated by relative adduct labeling and expressed as fmol/μg DNA.
Identification of BaP-DNA adducts
Experiments were conducted in vitro to confirm which of the BaP-enzymatic pathways was responsible for the in vivo BaP-related adduct patterns. The reactive metabolites generated from the epoxide- [for e.g. BaP 7,8-diol, 9,10-epoxide] and quinone pathways [BaP 3,6-quione, and BaP 6,12-quione] were incubated with 40 μM DNA and subjected to co-chromatography with unknown adduct sample. Those unknowns adduct that exhibit equivalent mobility (co-migration) with that of known standards were mapped and identified.
Assessment of DNA oxidative damage
DNA isolated from treatment groups mentioned above, was used to assess the amount of oxidative DNA damage by using Biovision DNA damage quantification kit. Isolated DNA was incubated with 5μl of Aldehyde Reactive Probe (ARP) solution at 37°C for 1 hour to tag DNA AP sites. Tris and EDTA (TE) buffer and glycogen were then added to incubated samples and mixed well. Samples were then mixed with 70% ethanol for 10 minutes at −20°C and centrifuged at top speed (10,000 rpm) for 10 minutes to precipitate AP-site tagged DNA. Each pellet was washed three times with 0.5ml of 70% ethanol and spun quickly to remove trace amounts of ethanol. The pellet was air dried for 5 minutes and then dissolved in 1ml of TE buffer. Samples were mixed with ARP-DNA standards and DNA binding solution in a 96 well plate and allowed to incubate overnight at room temperature (25°C). The following day, DNA binding solution was discarded and each well was washed with wash buffer 5 times. One hundred microliters of HRP-Streptavidin solution was added to each well and allowed to mix via a rocker for 1 hour at room temperature. Each well was washed with wash buffer. One hundred microliters of HRP developer solution was added to each well and allowed to incubate for 1 hour at 37°C. The absorbance (OD reading) was measured using a spectrometer and the basic AP sites per 105 bp in the DNA samples were calculated using a calibration curve as specified by the manufacturer.
Statistical analysis
A two-way analysis of variance (ANOVA) was used for the determination of statistical differences in BaP metabolite and BaP-DNA adduct concentrations in plasma or tissues at each time point. All pair wise multiple comparisons were conducted by Student-Newman-Keuls method. The Spearman rank order test was used for correlations in adduct levels and metabolite concentrations. The criterion for statistical significance was p<0.05 in all cases.
Results
Pharmacokinetics of BaP and RVT in Apc Min/+ mice
The pharmacokinetic parameters calculated for BaP suggest that a considerable fraction of the compound is present in the body after ingestion. In the case of RVT, the parameters were indicative of a rapid absorption, but when administered simultaneously with BaP, RVTwas shown to impact the time BaP stays at the site of action (target tissues). The results are shown in Table 1.
Table 1.
Pharmacokinetics of resveratrol (RVT) and benzo(a)pyrene (BaP; alone and in the presence of RVT) orally administered to ApcMin/+ male mice.
| Parameter | RVT | BaP | BaP + RVT |
|---|---|---|---|
| Area under curve (AUC; mg x h/ml) | 0.10 ± 0.01 | 0.12 ± 0.005 | 0.07 ± 0.005 |
| Biological half-life (t1/2; hrs) | 1.0 ± 0.011 | 1.8 ± 0.018 | 0.85 ± 0.010* |
| Volume of distribution (Vd; ml/kg) | 0.44 ± 0.050 | 0.58 ± 0.045 | 0.22 ± 0.064 |
| Clearance (Cl; ml/hr/kg) | 0.08 ± 0.006 | 0.10 ± 0.01 | 0.05 ± 0.004* |
| Mean residence time (MRT; hrs) | 1.6 ± 0.018 | 2.4 ± 0.010 | 1.2± 0.004* |
| Elimination rate (Kd; hrs) | 0.18 ± 0.03 | 0.17 ± 0.06 | 0.23 ± 0.004 |
Values represent mean ± standard error (n = 10). Asterisks denote statistical significance (p < 0.05) of toxicokinetic parameter values for BaP and RVT administered together compared to BaP alone administration.
Resveratrol affects the activity, protein and mRNA expression of BaP- induced drug-metabolizing enzymes
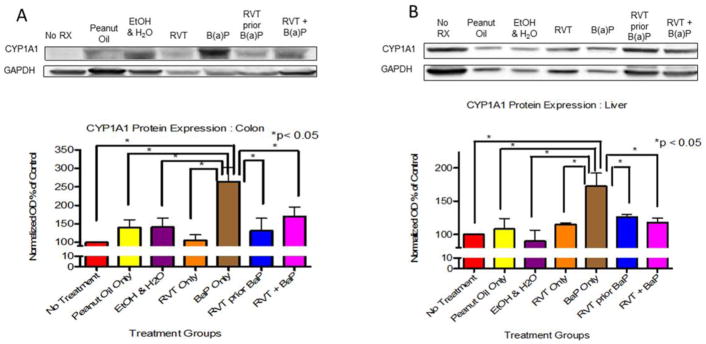
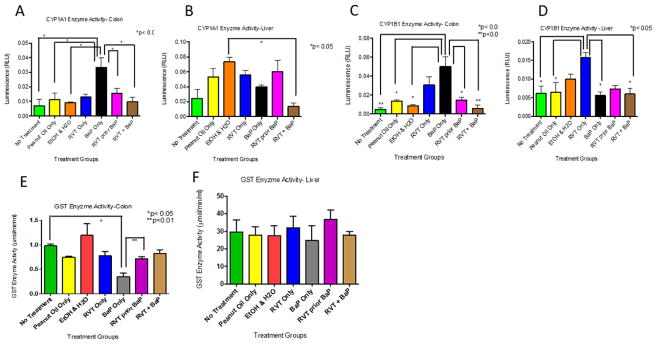
In the colon and liver of mice that received BaP only, CYP1A1 protein expression was significantly increased compared to all other treatment groups (Fig. 1A). However, in the liver, the presence of RVT caused a decrease in expression (Fig. 1B).
Figure 1. Cytochrome P4501A1 (CYP1A1) protein expression in the colon (A) and liver (B) of ApcMin/+ mice.
Mice treated with either BaP only, RVT simultaneously with BaP, or RVT prior to BaP. Values are expressed as mean ± SE. *p < 0.05. n = 10
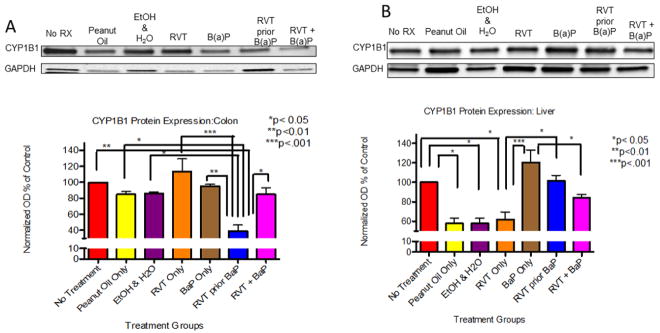
A significant decrease in CYP1B1 protein expression was observed in the colon (Fig. 2A) and liver (Fig. 2B) of mice that received RVT prior to BaP in comparison to no treatment group, as well as in the liver of mice that received RVT simultaneously with BaP.
Figure 2. Cytochrome P4501B1 (CYP1B1) protein expression in the colon (A) and liver (B) of ApcMin/+ mice.
Mice were treated with either BaP only, RVT simultaneously with BaP, or RVT prior to BaP. Values are expressed as mean ± SE. *p < 0.05, **p < 0.01, ***p <.001. n = 10
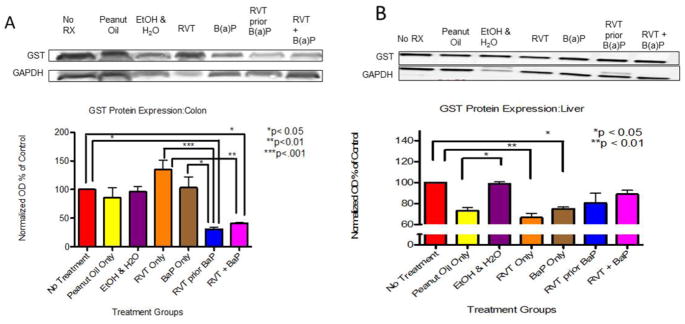
In the colon of treated mice, GST protein expression was increased in the presence of RVT and BaP compared to no treatment group when administered separately. However, this increase was significantly decreased when RVT was administered either simultaneously or prior to BaP treatment (Fig. 3A). Glutathione-S-transferase protein expression was significantly decreased in the liver of mice that received peanut oil only, RVT only, and BaP only in comparison to control mice. Unlike in the colon, glutathione-S-transferase protein expression in liver in RVT-treated mice (regardless of whether RVT administered either simultaneously or prior to BaP) showed no significant change in comparison to BaP treatment (Fig. 3B).
Figure 3. Glutathione-S-transferase protein expression in the colon (A) and liver (B) of ApcMin/+ mice.
Mice were treated with either BaP only, RVT simultaneously with BaP, or RVT prior to BaP. Values are expressed as mean ± SE. *p < 0.05, **p < 0.01, ***p <.001. n = 10.
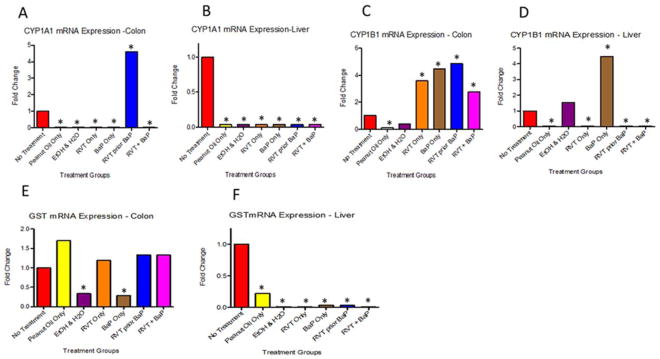
Resveratrol seems to have a profound effect on CYP1A1, 1B1, and GST drug metabolizing enzymes at the transcriptional level. Contrasting results were observed for CYP1A1 mRNA expression in colon (Fig. 4A) and liver (Fig. 4B). While RVT exposure prior to BaP registered a 4-fold expression compared to the no treatment group, the other treatment groups showed a feeble expression in this organ.
Figure 4. mRNA expression levels of Cytochrome P4501A1 (CYP1A1), Cytochrome P4501B1 (CYP1B1), and Glutathione-S-transferase (GST) in the colon and liver of ApcMin/+ mice.
Figs. 4A & 4B correspond to CYP1A1; Figs.4C & 4D correspond to CYP1B1; Figs.4E & 4F correspond to GST. Mice were treated with either BaP only, RVT simultaneously with BaP, or RVT prior to BaP. Values are expressed as fold changes compared to the control (no treatment) group. N = 10. Asterisks indicate statistically significant differences (p < 0.05) in fold change compared to control.
The colonic CYP1B1 mRNA expression in BaP + RVT treatment group was one half of that observed for BaP alone treatment group and RVT prior to BaP treatment group. Cytochrome P4501B1 (CYP1B1) mRNA levels in the colon showed a 3-fold increase in mice treated with BaP and in the RVT prior to BaP group compared to the no treatment category (Fig. 4C). Interestingly, CYP1B1 mRNA expression in liver was greater for BaP alone exposure group. On the other hand, a feeble expression was observed for BaP + RVT, RVT prior to BaP and RVT alone treatment groups (Fig. 4D). Resveratrol treatment simultaneously with BaP caused a 3-fold increase in expression when compared to the no treatment group as well.
In the colon of mice treated with BaP alone, there was a 50% decrease in GST mRNA expression in mice that received BaP alone compared to the no treatment group (Fig. 4E). While the presence of RVT (either simultaneously or prior to BaP treatment) caused an increase in expression in liver compared to the no treatment group (Fig. 4F).
In the colon of mice treated with BaP alone, a significant increase in CYP1A1 enzyme activity was observed when compared to the control groups (Fig. 5A). However, this spike in enzyme activity was decreased when RVT was administered either prior to or simultaneously with BaP treatment. In liver, CYP1A1 activity was also decreased in mice treated with RVT simultaneously with BaP, but not prior to BaP treatment compared to BaP treatment alone (Fig. 5B). In colon, CYP1B1 activity levels were decreased in mice simultaneously treated with RVT and BaP compared to BaP treatment alone and the decrease was statistically significant. No such differences were noted for liver (Fig. 3D). In the colon of mice when RVT was treated prior to BaP, a statistically significant increase in GST activity was noted when compared to mice treated with BaP alone (Fig. 5E), while no significant changes were seen in the liver (Fig. 5F).
Figure 5. Enzyme activities of Cytochrome P4501A1 (CYP1A1), Cytochrome P4501B1 (CYP1B1), and Glutathione-S-transferase (GST) in the colon and liver of ApcMin/+ mice.
Figs. 5A & 5B correspond to CYP1A1; Figs. 5C & 5D correspond to CYP1B1; Figs. 5E & 5F correspond to GST. Mice were treated with either BaP only, RVT simultaneously with BaP, or RVT prior to BaP. Values are expressed as mean ± SE. *p < 0.05. n = 10.
RVT alters BaP metabolism by affecting metabolite formation and phase two conjugate groups
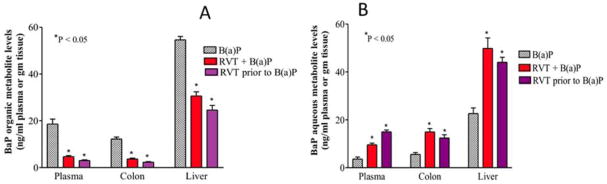
In the presence of RVT (simultaneously or prior to BaP treatment) BaP organic metabolite concentrations in the colon, liver, and plasma were significantly decreased compared to the levels in corresponding organs excised from mice treated with BaP alone (Fig. 6A). Parallel to decreases in BaP organic metabolite levels, increases in BaP aqueous metabolite levels in the same target tissues (Fig. 6B) were observed.
Figure 6. Benzo(a)pyrene (A) organic and (B) aqueous metabolite levels in the plasma, colon, and liver of Apc Min/+ mice.
Mice were treated with either BaP only, RVT simultaneously with BaP, or RVT prior to BaP treatment. Values are expressed as mean ± SE. Asterisks indicate statistical significance between mice that received BaP alone and mice that received BaP + RVT or just the vehicle. *p < 0.05. N = 10.
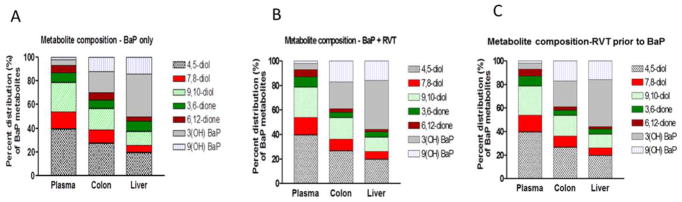
The percent distribution of the BaP organic metabolites in the colon and liver of ApcMin/+ mice are shown in Figs. 7A, B & C. No significant changes in the type of metabolites formed were observed between the colon and liver, but in the presence of RVT (simultaneously or prior to BaP treatment) there were increases in the percentage composition of metabolite groups such as hydroxy, and diols and slight decreases in the composition of some diones.
Figure 7. Percent distribution of BaP organic metabolites in Apc Min/+ mice.
Metabolite levels are from plasma, colon, and liver samples of mice treated with BaP only (A), RVT + BaP (B) and RVT prior to BaP treatment (C).
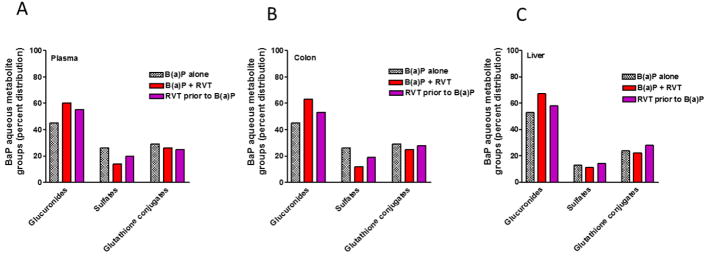
A breakdown analysis of the BaP aqueous metabolites in the liver, plasma, and colon of ApcMin/+ mice, revealed that RVT treatment significantly affected the composition of BaP aqueous metabolite groups. Glucuronide concentrations were greater than sulfates and GSH conjugates in all 3 major treatment groups and in all 3-sample types (Fig. 8A–C). Compared to BaP alone treatment, RVT + BaP and RVT prior to BaP treatment induced the production of more glucuronide metabolites.
Figure 8. Benzo(a)pyrene aqueous metabolite groups in the plasma, liver, and colon of Apc Min/+ mice.
Mice were treated with either BaP only (A), RVT simultaneously with BaP (B), or RVT prior to BaP treatment.
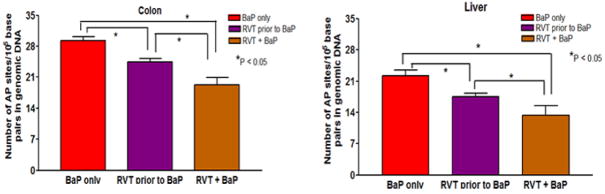
Resveratrol exposure decreases the oxidative DNA damage and formation of BaP-DNA adducts in the colon and liver
The DNA damage was found to be statistically significant and lower in the treatment group that received RVT with BaP in comparison to the groups that received treatment with BaP only and RVT prior to BaP (Fig. 9A). Conversely in the colon, the number of apurinic/apyrimidinic sites in no treatment, respective vehicle [for BaP and RVT] treatment groups were lower when compared to BaP only treatment group. In addition, RVT treatment prior to and simultaneously with BaP revealed a significant reduction in base pair damage as compared to BaP treatment only (Fig. 9B).
Figure 9. Abrogation of BaP-induced DNA base pair damage by RVT in the colon (A) and liver (B) of ApcMin/+ mice.
Mice were treated with BaP only, RVT prior to BaP, and BaP and RVT treated simultaneously. Values are expressed as mean ± SE. *p < 0.05. N = 10.
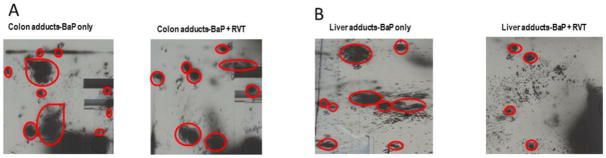
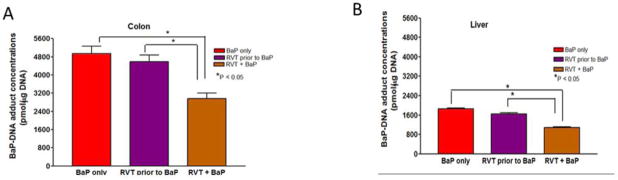
DNA oxidative damage can lead to DNA-base pair damage and if left unrepaired, these damaged sites can lead to the formation of DNA-adducts. To examine the impact RVT has on modifying BaP- induced DNA damage and ultimately BaP-DNA adducts, stable BaP-DNA adduct concentrations were measured. Using TLC, these adducts were presented as dots/blots on the polyethyleneimine-coated TLC plate as shown in Figs. 10A & B. Overall, treatment with RVT reduced stable BaP-DNA adduct concentrations in both the colon (Fig. 11A) and liver (Fig. 11B) of treated mice, with the greatest decrease observed in mice treated with RVT simultaneously with BaP.
Figure 10. Representative pictures of BaP-DNA adduct spots.
Examples are from colon (A) and liver (b) of ApcMin/+ mice treated with BaP only, and BaP and RVT treated simultaneously.
Figure 11. Benzo(a)pyrene-DNA adduct concentrations in liver and colon of ApcMin/+ mice.
Mice were treated with BaP only, RVT prior to BaP, and BaP and RVT treated simultaneously. Values are expressed as mean ± SE. *p < 0.05. N = 10.
The relative distribution of BaP-DNA adduct types in the colon and liver of BaP alone and BaP + RVT-treated mice are shown in Table 2. Among the different adduct types, the proportion of deoxyguanosine (dG) adducts were greater than deoxyadenosine (dA) adducts, deoxycytidine (dC) and deoxythymidine (dT) adducts in all the three treatment groups (BaP alone, RVT prior to BaP and RVT + BaP).
Table 2.
Composition of benzo(a)pyrene-DNA adducts (pmol/μg DNA) in colon and liver of ApcMin male mice treated with resveratrol (RVT) and benzo(a)pyrene (BaP; alone and in the presence of RVT).
| Organ/Adduct type | BaP | RVT prior to BaP | BaP + RVT |
|---|---|---|---|
| Colon | |||
| Deoxyadenosine adduct (dA) | 820 ± 80 | 710± 77 | 446± 40* |
| Deoxyguanosine adduct (dG) | 3640 ± 325 | 3525 ±335 | 2252 ± 212* |
| Deoxycytidine adduct (dC) | 240 ± 25 | 192±18 | 85 ± 8* |
| Deoxythymidine adduct (dT) | 100 ± 12 | 84 ± 9 | 28 ± 2* |
| Liver | |||
| Deoxyadenosine adduct (dA) | 358 ± 17 | 244 ± 12 | 154± 3.5* |
| Deoxyguanosine adduct (dG) | 1202 ± 34 | 1158 ± 24 | 882± 21* |
| Deoxycytidine adduct (dC) | 32 ± 1.2 | 21 ± 1.0 | 18 ± 0.8* |
| Deoxythymidine adduct (dT) | 9 ± 0.45 | 5 ± 0.35 | 4 ± 0.2* |
Values represent mean ± standard error (n = 10). Asterisks denote statistical significance (p < 0.05) of respective adduct types for BaP and RVT administered together compared to BaP alone administration.
Discussion
Insight into the balance between bioactivation and detoxification processes in BaP-treated mice and how RVT alters these key events is a critical step in RVT’s chemopreventive effects. Two very important CYP450s are CYP1A1 and CYP1B1, which are responsible for metabolizing BaP both in the liver and colon [26]. However, RVT significantly decreases CYP1B1 protein expression in both the colon and the liver of BaP-treated mice. Resveratrol also decreases CYP1B1 enzyme activity in the colon but not the liver. According to Halberg et al. [27], elevated CYP1B1 expression is a marker for more aggressive colon tumors. In our studies we see that RVT does not promote a greater expression of CYP1B1, suggesting that RVT does not favor phase I metabolism of BaP. Another group found that RVT exerts its chemopreventive effects by blocking metabolic activation and enhancing the detoxification of various carcinogens [26,28]. This blockage was evident as RVT inhibited CYP1A1 expression in rat primary hepatocytes [29], and CYP1A1, CYP1B1, and CYP1A2 expression in murine hepatoma cells [26].
Arylhydrocarbon receptor (AhR) is involved in various processes such as cell proliferation, differentiation and CYP1A1 induction after xenobiotic exposure. Literature reports have shown that RVT exhibits its action through AhR-dependent pathways [29]. Treatment of HL-60 human leukemia cells with 0–20μM RVT resulted in a concentration-dependent decrease in CYP1B1 mRNA levels [31]. Ciolino et al. [32], Ciolino and Yeh [33] demonstrated that RVT inhibited BaP-induced increase in CYP1A1 expression in MCF-7 human mammary epithelial carcinoma cells, thus preventing an increase in carcinogen bioactivation capacity. Additionally, RVT was reported to reduce the expression of CYP1A1 and 1B1 in BaP-treated A/J mice lung tissues [34].
Our observations of feeble mRNA expression for CYP1A1 and CYP1B1 in liver and CYP1A1 for colon samples suggest differential regulation of these enzymes in BaP and BaP + RVT treatment groups. These results could not be attributed to the probes used or integrity of samples because CYP1B1 mRNA expression in colon was demonstrated employing the same methodology. Since sample processing for RT-PCR studies remained consistent throughout the entire scope of mRNA studies, and extreme care was taken not to compromise sample integrity, mRNA and protein degradation during sample processing can be ruled out. These variations notwithstanding, our findings were consistent with reports of differential regulation of CYP1A1 and 1B1 by toxicants that were AhR agonists in human breast tumor cell lines [35] and also bronchial epithelial cell lines [36]. Interestingly some authors have shown that RVT inhibits CYP1A1 and 1B1 gene expression via an AhR-independent post-transcriptional pathway [37,38]. In the light of these findings, the possibility of RVT exerting its action through several post-transcriptional pathways cannot be ruled out.
For CYP1B1 in colon, the changes in the mRNA expression in BaP and BaP + RVT treatment groups were also reflected at the protein level indicating a functional significance for this isozyme in biotransformation-mediated carcinogenic or anticarcinogenic effects. On the other hand, the lack of concordance between mRNA and protein expression for CYP1A1 could be attributed to post transcriptional regulation and differences in mRNA and protein turnover rates [39,40,41].
Regarding the lack of mRNA expression, doubt may arise whether pharmacologically relevant fraction of RVT reaches the liver and colon. This paradox could be put to rest as our pharmacokinetic studies have clearly shown that both BaP and RVT reaches the target tissues to elicit the effect. Additionally, had these chemicals not reached the target organs, we would not have observed protein expression for the drug metabolizing enzymes in both colon and liver samples. However, one important caveat to be considered is the dose of RVT used. Since we have not conducted differential dose-response studies (choosing more than one dose of BaP and RVT), whether the expression of CYP1A1 and 1B1 are subjected to the same regulatory control at different doses is open for speculation.
Though several isoforms of GST exist, we have chosen GST-Pi as the expression of this isoform has been shown to be increase in gastric and colon tumors compared with adjacent normal tissues [42]. Additionally, GST-Pi gene deleted mice was reported to exhibit an increased susceptibility to PAH-induced tumors [43]. In the presence of RVT, GST protein expression in the colon was significantly decreased when compared to mice that received BaP alone, but it does not cause an increase in enzyme activity. However, in the liver RVT caused an increase in GST protein expression, but no significant changes in enzyme activity, thereby promoting conjugation of BaP metabolites favoring excretion. These findings are consistent with data in the literature that indicates RVT induces phase 2 enzymes [44,45].
Determining the major impact of a drug’s exposure on tissue and its pharmacological activity is tied to that compound’s pharmacokinetic behavior. Considerable accumulation of RVT in mouse intestinal tissues has been reported subsequent to oral administration [46,47] to elicit the presumed beneficial effects. At least 50–60% of the orally administered RVT was found to be absorbed from the GI tract in rats and pigs [48,49,50]. Also, biologically effective concentrations of RVT were shown to result from chronic dosing with this phytochemical as shown in humans [51,52]. Our studies found similar results with the pharmacokinetic properties of RVT indicating its availability at the site of action. Resveratrol-conjugated sulfates and glucuronides were reported to convert back to RVT in target organs [22, 46]. Therefore, it is beyond doubt that biologically active concentrations of RVT could be achieved in ApcMin/+ mice in our subchronic dosing study. Our results also suggest that regardless of the rapid absorption of RVT, this compound could alter the effects of absorbed BaP by interfering with the biotransformation of BaP.
BaP metabolites are critical markers in examining potential DNA-adduct formation and polyp development. Measurement of metabolite formation in target tissues provide an integrated analysis of BaP metabolism and the effect of RVT on that process. Overall, RVT caused an increase in BaP aqueous metabolite concentrations. This increase was also in conjunction with an overall decrease in BaP organic metabolite concentrations and types. These patterns combined with an increase in phase 2 metabolite formation further suggest that RVT favors excretion of BaP by promoting aqueous metabolite generation.
Lastly, this study aimed at studying RVTs effects on BaP-induced oxidative DNA damage and BaP-DNA adduct formation. It is assumed that the pathological changes in target tissues induced by toxicants were associated with production of highly reactive free radicals and initiation of oxidative damage [53]. The presence of RVT in both the liver and colon causes a decrease in the number of DNA base pairs damaged. A critical review of literature by Delmas et al. [54] and Gatz & Wisemiller [55] concluded that RVT modulates DNA damage in affected organs by repairing of damaged DNA. These results suggest that RVT may work to promote the repair of damaged base pairs [53] to off-set adduct formation, and eventual tumor formation and progression. These findings also indicate that RVT may protect mouse liver and colon tissue against DNA damage induced by reactive oxygen species.
A significant decrease in BaP-DNA adducts concentrations in colon and liver in the presence of RVT was observed compared to mice that received BaP alone. Reported studies also show that RVT prevents the binding of BaP ultimate metabolites to DNA and reduce the likelihood of tumor progression and development. For example, co-exposure of human bronchial epithelial cells to both RVT (10–50 μM) and BaP (1 μM) showed inhibition of BaP-DNA adduct formation [36,37]. Similarly, co-treatment of RVT (50 mg/kg bw/wk) and BaP (5mg/kg bw/wk) were shown to inhibit BaP-DNA adduct formation in lung tissues in a Balb-c mouse model [56]. The preponderance of dG adducts relative to those of dA are consistent with the results of studies conducted in our laboratory [27, 57, 58] and those of others [59, 60] using rodent models exposed to BaP. However, not much information is available whether RVT has a role in prevalence of certain nucleotide-specific binding of BaP metabolites, which merits investigation. Taken together, literature reports from other regimens employed, and findings from our study provides definitive evidence that RVT is able to slow down tumor progression via decreasing the rate of BaP-DNA adduct formation in BaP-exposed colon and liver tissues.
Timing of RVT administration appears to be important in eliciting the anticarcinogenic effect. As mentioned elsewhere in this manuscript, we have used RVT concurrently with BaP, and also prior to BaP administration. In order to inhibit tumor growth, RVT must be readily available in target tissues. Since carcinogenesis encompasses initiation, promotion and progression phases, chemopreventive agents like RVT can act at one or more phases to render their protective effect [61]. Given the rapid metabolism of RVT [62], prior treatment of mice with RVT in the present study may not have yielded enough ‘biologically potent fraction of the administered RVT dose’ to be readily available when BaP administration is commenced, and tumor formation is initiated, so that the tumor growth could be inhibited. On the other hand, during concurrent BaP & RVT administration, the biochemical or molecular pathways targeted by BaP could be modulated by RVT as indicated by the drop in tumor counts and tumor size in the present study. We also have investigated whether RVT administration post BaP subchronic exposure could bring down the tumor count and size. Resveratrol failed to reverse the BaP-induced carcinogenic effects (data not shown). These observations are consistent with a previous report where RVT administration post-tumor initiation phase had no effect on the lung tumors induced by BaP in A/J mice [63], which could be attributed to the insufficient bioavailable fraction of RVT at the target site [34] to undo the damage caused by BaP.
Conclusions
This research has provided critical insight into the extent to which resveratrol could prevent environmental and dietary toxicant- induced colon carcinogenesis. Taken together, our findings lend support to the hypothesis that RVT is a promising anticancer agent.
Acknowledgments
Financial support of this study by the National Institutes of Health (NIH) is gratefully appreciated. The authors would like to thank Drs. LaMonica Stewart, Anthony Archibong, and Deacqunita Diggs for helpful suggestions.
Funding
Research reported in this publication was supported by NIH grants 1F31ES019432-01A1 (ACH), 5R01CA142845-04 (AR), 5T32HL007735-12 (ACH, SEA), 5 U54CA163069-04 (SEA, AR), 5U54MD007593-07 (SEA, AR), and 5R25GM059994-13 (ACH), and G12RR003022 (AR, SEA). The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Author’s Contributions
ACH and AR designed the study and applied for Institutional Animal Care & Use Committee approval. ACH, PVR, MSN and AR performed the experiments and collected the data. ACH, PVR and AR analyzed the data and prepared draft figures and tables. ACH, PVR and AR prepared the manuscript draft with intellectual input from SEA. All authors approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
Ashley Huderson declares that she has no conflict of interest. P.V. Rekhadevi declares that she has no conflict of interest. Mohammad Niaz declares that he has no conflict of interest. Samuel Adunyah declares that he has no conflict of interest. Aramandla Ramesh declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
References
- 1.Harris DL, Washington MK, Hood DB, Roberts LJ, II, Ramesh A. Dietary fat-influenced development of colon neoplasia in ApcMin/+ mouse exposed to benzo(a)pyrene. Toxicol Pathol. 2009;37:938–946. doi: 10.1177/0192623309351722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diggs DL, Huderson AC, Harris KL, Myers JN, Banks LD, Rekhadevi PV, Niaz MS, Ramesh A. Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:1–34. doi: 10.1080/10590501.2011.629974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonoda J, Seki Y, Hakura A, Hosokawa S. Time course of the incidence/multiplicity and histopathological features of murine colonic dysplasia, adenoma and adenocarcinoma induced by benzo[a]pyrene and dextran sulfate sodium. J Toxicol Pathol. 2015;28:109–20. doi: 10.1293/tox.2014-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishayee A. Cancer Prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev Res. 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 5.Banks LD, Amoah P, Niaz MS, Washington MK, Adunyah SE, Ramesh A. Olive oil prevents benzo(a)pyrene [BaP]-induced colon carcinogenesis through altered BaP metabolism and decreased oxidative damage in Apc(Min) mouse model. J Nutr Biochem. 2016;28:37–50. doi: 10.1016/j.jnutbio.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Guo Y, Zhang C, Wu R, Yang AY, Gaspar J, Kong AN. Dietary phytochemicals and cancer chemoprevention: A perspective on oxidative stress, inflammation, and epigenetics. Chem Res Toxicol. 2016;29:2071–2095. doi: 10.1021/acs.chemrestox.6b00413. [DOI] [PubMed] [Google Scholar]
- 7.Morris J, Fang Y, De Mukhopdhyay K, Wargovich MJ. Natural agents used in chemoprevention of aerodigestive and GI cancers. Curr Pharmacol Rep. 2016;2:11–20. doi: 10.1007/s40495-016-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddivari L, Charepalli V, Radhakrishnan S, Vadde R, Elias RJ, Lambert JD, Vanamala JK. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complement Altern Med. 2016;16:278. doi: 10.1186/s12906-016-1254-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omidian K, Rafiei H, Bandy B. Polyphenol inhibition of benzo[a]pyrene-induced oxidative stress and neoplastic transformation in an in vitro model of carcinogenesis. Food Chem Toxicol. 2017;106(Pt A):165–174. doi: 10.1016/j.fct.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Pezzuto J. Resveratrol as an inhibitor of carcinogenesis. Pharmaceutical Biology. 2008;46:443–573. [Google Scholar]
- 11.Chung MY, Lim TG, Lee KW. Molecular mechanisms of chemopreventive phytochemicals against gastroenterological cancer development. World J Gastroenterol. 2013;19:984–93. doi: 10.3748/wjg.v19.i7.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer. 2014;21:R209–25. doi: 10.1530/ERC-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazué F, Delmas D, Murillo G, Saleiro D, Limagne E, Latruffe N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014;5:663–70. doi: 10.1039/c3fo60417a. [DOI] [PubMed] [Google Scholar]
- 14.Huderson AC, Myers JN, Niaz MS, Washington MK, Ramesh A. Chemoprevention of benzo(a)pyrene-induced colon polyps in ApcMin/+ mice by resveratrol. J Nutr Biochem. 2013;24:713–24. doi: 10.1016/j.jnutbio.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yates MS, Kensler TW. Detoxication of chemical carcinogens and chemoprevention. In: Penning TM, editor. Chemical Carcinogenesis. Springer; New York: 2011. pp. 159–180. [Google Scholar]
- 16.Wu TY, Khor TO, Lee JH, Cheung KL, Shu L, Chen C, Kong AN. Pharmacogenetics, pharmacogenomics and epigenetics of Nrf2-regulated xenobiotic-metabolizing enzymes and transporters by dietary phytochemical and cancer chemoprevention. Curr Drug Metab. 2013;14:688–94. doi: 10.2174/1389200211314060005. [DOI] [PubMed] [Google Scholar]
- 17.Abel EL, DiGiovanni J. Environmental carcinogenesis. In: Mendelsohn J, Gray JW, Howley PM, Israel MA, Thompson CB, editors. The Molecular Basis of Cancer. Elsevier Publishers; Philadelphia: 2015. pp. 103–128. [Google Scholar]
- 18.NIH guidelines for the laboratory use of chemical carcinogens. U.S. Government Printing Office; Washington, DC: 1981. NIH Publication No. 81–2385. [Google Scholar]
- 19.Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckles ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo(alpha)pyrene in F-344 rats following oral administration. Exp Toxic Pathol. 2001;53:275–90. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh A, Inyang F, Knuckles ME. Modulation of adult rat benzo(a)pyrene (BaP) metabolism and DNA adduct formation by neonatal diethylstilbestrol (DES) exposure. Exp Toxic Pathol. 2004;56:129–138. doi: 10.1016/j.etp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Katsagonis A, Atta-Politou J, Koupparis MA. HPLC method with UV detection for the determination of trans-resveratrol in plasma. J Liq Chromatogr Relat Technol. 2005;28:1393–1405. [Google Scholar]
- 22.Wenzel E, Soldo T, Erbersdobler H, Somoza V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol Nutr Food Res. 2005;49:482–94. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- 23.Baggot JD. Pharmacokinetic terms: symbols and units. J Vet Pharmacol Ther. 2001;24:81–2. doi: 10.1046/j.1365-2885.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 24.Gupta RC, Randerath K. Analysis of DNA adducts by 32P labeling and thin layer chromatography. In: Friedberg EC, Hanawalt PC, editors. DNA Repair. Vol. 3. Marcel Dekker, Inc; 1988. pp. 399–418. [Google Scholar]
- 25.Ramesh A, Knuckles ME. Dose-dependent benzo(a)pyrene [BaP]-DNA adduct levels and persistence in F-344 rats following subchronic dietary exposure to BaP. Cancer Lett. 2006;240:268–278. doi: 10.1016/j.canlet.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Kundu JK, Surh Y-J. Cancer chemo-preventive and therapeutic potential of resveratrol: Mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 27.Halberg RB, Larsen MC, Elmergreen TL, Ko AY, Irving AA, Clipson L, Jefcoate CR. Cyp1b1 exerts opposing effects on intestinal tumorigenesis via exogenous and endogenous substrates. Cancer Res. 2008;68:7394–402. doi: 10.1158/0008-5472.CAN-07-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baur J, Sinclair D. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 29.Andrieux L, Langouet S, Fautrel A, Ezan F, Krauser J, Savouret J, Guengerich F, Baffet G, Guillouzo A. Aryl hydrocarbon receptor activation and cytochrome P450 1A induction by the mitogen-activated protein kinase inhibitor U0126 in hepatocytes. Mol Pharmacol. 2004;65:934–943. doi: 10.1124/mol.65.4.934. [DOI] [PubMed] [Google Scholar]
- 30.Guyot E, Chevallier A, Barouki R, Coumoul X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov Today. 2012 doi: 10.1016/j.drudis.2012.11.014. pii: S1359-6446(12)00403-5. [DOI] [PubMed] [Google Scholar]
- 31.Kang J, Park Y, Choi S, Yang E, Lee W. Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp Mol Med. 2003;35:467–474. doi: 10.1038/emm.2003.61. [DOI] [PubMed] [Google Scholar]
- 32.Ciolino H, Daschner P, Yeh G. Resveratrol Inhibits transcription of CYP1A1 in Vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- 33.Ciolino H, Yeh G. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- 34.Berge G, Øvrebø S, Eilertsen E, Haugen A, Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br J Cancer. 2004b;91:1380–1383. doi: 10.1038/sj.bjc.6602125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coumoul X, Diry M, Robillot C, Barouki R. Differential regulation of cytochrome P450 1A1 and 1B1 by a combination of dioxin and pesticides in the breast tumor cell line MCF-7. Cancer Res. 2001;61:3942–3948. [PubMed] [Google Scholar]
- 36.Tsuji PA, Walle T. Inhibition of benzo[a]pyrene-activating enzymes and DNA binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis. 2006;27:1579–85. doi: 10.1093/carcin/bgi358. [DOI] [PubMed] [Google Scholar]
- 37.Berge G, Øvrebø S, Botnen IV, Hewer A, Phillips DH, Haugen A, Mollerup S. Resveratrol inhibits benzo[a]pyrene-DNA adduct formation in human bronchial epithelial cells. Br J Cancer. 2004;91:333–338. doi: 10.1038/sj.bjc.6601898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beedanagari S, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibitng recruitment of the aryl hydrocarbon receptor and complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110:61–67. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hack CJ. integrated transcriptome and proteome data: the challenges ahead. Brief Funct Genomic Proteomic. 2004;3:212–219. doi: 10.1093/bfgp/3.3.212. [DOI] [PubMed] [Google Scholar]
- 40.Cox B, Kislinger T, Emili A. Integrating gene and protein expression data: pattern analysis and profile mining. Methods. 2005;35:303–314. doi: 10.1016/j.ymeth.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 41.Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 2008;40:426–36. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- 42.Hoensch H, Morgenstern I, Petereit G, Siepmann M, Peters WH, Roelofs HM, Kirch W. Influence of clinical factors, diet, and drugs on the human upper gastrointestinal glutathione system. Gut. 2002;50(2):235–40. doi: 10.1136/gut.50.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR. Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci US A. 1998;95(9):5275–80. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talalay P, Fahey W, Holtzclaw D, Prestera T, Zhang Y. Chemoprevention against cancer by phase 2 enzyme induction. Toxic Lett. 1995;82/83:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 45.Hebbar V, Shen G, Hu R, Kim B, Chen C, Korytko P, Crowell J, Levine B, Kong A. Toxicogenomics of resveratrol in rat liver. Life Sci. 2005;76:2299–2314. doi: 10.1016/j.lfs.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Vitrac X, Desmoulière A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Mérillon JM. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219–33. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 47.Sale S, Tunstall R, Ruparelia K, Potter G, Steward W, Gescher A. Comparison of the effects of the chemo-preventive agent resveratrol and its synthetic analog trans 3,4,5,4-tetramethoxystilbene (DMU-212) on adenoma development in the ApcMin/++ mouse and cyclooxygenase-2 in human derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 48.Andlauer W, Kolb J, Siebert K, Fürst P. Assessment of resveratrol bioavailability in the perfused small intestine of the rat. Drugs Exp Clin Res. 2000;26:47–55. [PubMed] [Google Scholar]
- 49.Soleas GJ, Angelini M, Grass L, Diamandis EP, Goldberg DM. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001;335:145–54. doi: 10.1016/s0076-6879(01)35239-4. [DOI] [PubMed] [Google Scholar]
- 50.Azorín-Ortuño M, Yáñez-Gascón MJ, Vallejo F, Pallarés FJ, Larrosa M, Lucas R, Morales JC, Tomás-Barberán FA, García-Conesa MT, Espín JC. Metabolites and tissue distribution of resveratrol in the pig. Mol Nutr Food Res. 2011;55:1154–68. doi: 10.1002/mnfr.201100140. [DOI] [PubMed] [Google Scholar]
- 51.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–54. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 54.Delmas D, Lançon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 55.Gatz SA, Wiesmüller L. Take a break--resveratrol in action on DNA. Carcinogenesis. 2008;29:321–32. doi: 10.1093/carcin/bgm276. [DOI] [PubMed] [Google Scholar]
- 56.Revel A, Raanani H, Younglai E, Xu J, Rogers I, Han R, Savouret JF, Casper RF. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J Appl Toxicol. 2003;23:255–261. doi: 10.1002/jat.916. [DOI] [PubMed] [Google Scholar]
- 57.Diggs DL, Myers JN, Banks LD, Niaz MS, Hood DB, Roberts LJ, 2nd, Ramesh A. Influence of dietary fat type on benzo(a)pyrene [BaP] biotransformation in a BaP-induced mouse model of colon cancer. J Nutr Biochem. 2013;24(12):2051–63. doi: 10.1016/j.jnutbio.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramesh A, Archibong AE, Niaz MS. Ovarian susceptibility to benzo[a]pyrene: tissue burden of metabolites and DNA adducts in F-344 rats. J Toxicol Environ Health A. 2010;73(23):1611–25. doi: 10.1080/15287394.2010.514225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross J, Nelson G, Kligerman A, Erexson G, Bryant M, Earley K, Gupta RC, Nesnow S. Formation and persistence of novel benzo(a)pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res. 1990;50:5088–5094. [PubMed] [Google Scholar]
- 60.Arif JM, Shappell N, Sikka HC, Kumar S, Gupta RC. 32P-postlabeling analysis of lipophilic DNA adducts resulting from interaction with (+/−)-3-hydroxy-trans-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Chem Biol Interact. 1999;118:87–97. doi: 10.1016/s0009-2797(98)00116-1. [DOI] [PubMed] [Google Scholar]
- 61.Stoner GD, Morse MA, Kelloff GJ. Perspectives in cancer chemoprevention. Environ Health Perspect. 1997;105(Suppl 4):945–954. doi: 10.1289/ehp.97105s4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ndiaye M, Kumar R, Ahmad N. Resveratrol in cancer management: where are we and where we go from here? Ann N Y Acad Sci. 2011;1215:144–149. doi: 10.1111/j.1749-6632.2010.05851.x. [DOI] [PubMed] [Google Scholar]
- 63.Hecht SS, Kenney PM, Wang M, Trushin N, Agarwal S, Rao AV, Upadhyaya P. Evaluation of besylated hydroxyanisole myoinositol, cerceumin, esculation, resveratrol and lycopene as inhibitors of benzo(a)pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-bentanone- induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–130. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]