Abstract
Stress is a well-known risk factor for psychopathology and rodent models of social defeat have strong face, etiological, construct and predictive validity for these conditions. Syrian hamsters are highly aggressive and territorial, but after an acute social defeat experience they become submissive and no longer defend their home territory, even from a smaller, non-aggressive intruder. This defeat-induced change in social behavior is called conditioned defeat (CD). We have shown that dominant hamsters show increased neural activity in the ventromedial prefrontal cortex (vmPFC) following social defeat stress and exhibit a reduced CD response at social interaction testing compared to subordinates. Although the vmPFC can inhibit the neuroendocrine stress response, it is unknown whether dominants and subordinates differ in stress-induced activity of the extended hypothalamic-pituitary-adrenal (HPA) axis. Here, we show that, following acute social defeat, dominants exhibit decreased submissive and defensive behavior compared to subordinates but do not differ from subordinates or social status controls (SSCs) in defeat-induced cortisol concentrations. Furthermore, both dominants and SSCs show greater corticotropin-releasing hormone (CRH) mRNA expression in the basolateral/central amygdala compared to subordinates, while there was no effect of social status on CRH mRNA expression in the paraventricular nucleus of the hypothalamus or bed nucleus of the stria terminalis. Overall, status-dependent differences in the CD response do not appear linked to changes in stress-induced cortisol concentrations or CRH gene expression, which is consistent with the view that stress resilience is not a lack of a physiological stress response but the addition of stress coping mechanisms.
Keywords: Cortisol, corticotropin-releasing hormone, resilience, vulnerability, social dominance, social defeat
Introduction
Stress is a contributing factor in the etiology of several mood and anxiety disorders, including post-traumatic stress disorder (PTSD) (Meewisse, Reitsma, de Vries, Gersons, & Olff, 2007). Psychosocial stress is a particularly salient form of trauma (Björkqvist, 2001), and people exposed to interpersonal violence are at a greater risk for developing PTSD than those exposed to nonsocial trauma (Charuvastra & Cloitrere, 2008). Rodent social defeat models are an ethologically relevant form of psychosocial stress used to investigate the biological basis of stress-related mental illness. In Syrian hamsters, a single social defeat experience leads to social avoidance and a complete loss of species-typical territorial aggression in a subsequent social interaction test; this defeat-induced change in social behavior is called conditioned defeat (CD). Corticotropin-releasing hormone (CRH) is a neuropeptide that initiates the neuroendocrine response to stress and also acts outside the paraventricular nucleus (PVN) of the hypothalamus to coordinate behavioral responses to stressful events. CRH has a well-established role in fear-related behaviors (Gilman, DaMert, Meduri, & Jasnow, 2015), stress-induced anxiety (Walker, Miles, & Davis, 2009), and behavioral responses following social defeat (Heinrichs, Pich, Miczek, Britton, & Koob, 1992). CRH acts within neuronal projections from the central amygdala (CeA) to the bed nucleus of the stria terminalis (BNST) to promote the CD response (Jasnow, Davis, & Huhman, 2004). In addition, activation of CRH type-2 receptors (CRH-R2) in the BNST is essential for the CD response because intra-BNST blockade of CRH-R2 reduces the CD response, whereas systemic blockade of CRH-R1 does not (Cooper & Huhmanan, 2005; Jasnow, Banks, Owens, & Huhman, 1999).
Several environmental factors modulate how individuals respond to stress, including social status (Melhorn, Elfers, Scott, & Sakai, 2017). We have shown that, following two weeks of daily, dyadic encounters, dominant hamsters exhibit a reduced CD response compared to subordinates (Morrison, Swallows, & Cooper, 2011). Dominant hamsters also exhibit increased neural activity in the ventral medial prefrontal cortex (vmPFC) compared to their subordinate counterparts, which is necessary for their reduced CD response (Morrison, Bader, McLaughlin, & Cooper, 2013; Morrison et al., 2014). Although neural activity in the vmPFC can inhibit stress-induced HPA axis activity (Radley & Sawchenkoko, 2011), it is unknown whether dominant and subordinate hamsters differ in their neuroendocrine stress response or in CRH activity outside the PVN. Although status-dependent differences have been shown for stress-induced glucocorticoid responses (Melhorn et al., 2017), the effect of social status on CRH activity is less well known. In this study, we predicted that dominant hamsters would display reduced defeat-induced plasma cortisol concentrations and CRH mRNA expression in the PVN and extended amygdala compared to subordinates.
Materials and methods
Subjects
Subjects were male Syrian hamsters (3–4 months old, 120–180 g) obtained from our breeding colony (Experiment 1) or purchased directly from Charles River Laboratories (Wilmington, MA) (Experiment 2). Animals were housed as described previously (Morrison et al., 2014). All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Dominant-subordinate encounters
To allow animals to establish social status, subjects were weight-matched into resident-intruder dyads and paired in daily social encounters for 14 days as described previously (Morrison et al., 2014). Subjects were randomly assigned as a resident or intruder, and all social encounters occurred in the resident’s home cage. Encounters were 10 min in duration prior to establishing a dominance relationship, while all subsequent encounters were 5 min. Dominant and subordinate animals were identified by the unidirectional display of agonistic behavior within each dyad. Some animals did not receive daily dyadic encounters and, therefore, did not maintain a dominance relationship with a partner. These animals were singly housed during this time period and are called social status controls (SSCs) or no defeat controls depending on whether they did or did not receive social defeat stress, respectively.
Social defeat stress
Social defeat stress occurred 24 h after the final dominance encounter and consisted of subjects being placed in the home cages of a larger, unfamiliar animal, called a resident aggressors. Briefly, subjects were exposed to three separate resident aggressors in consecutive 5-min aggressive encounters, with 5-min inter-trial intervals in their own home cage. In Experiment 1, no defeat controls were placed in the empty home cages of three separate resident aggressors for three 5-min exposures. In Experiment 2, empty cage exposure altered CRH mRNA in a few animals producing a great deal of variability in mRNA expression. Therefore, the no defeat control group was modified to receive gentle human handling only. Social defeat trials were digitally recorded and the duration of aggression received and the number of attack received were quantified as described previously (Morrison et al., 2014).
Conditioned defeat testing
Conditioned defeat testing occurred 24 h after social defeat stress, as described previously (Morrison et al., 2014). Briefly, CD testing consisted of a 5-min social interaction test, during which a smaller non-aggressive animal was placed into the subject’s home cage and allowed to freely explore. All testing was digitally recorded and the behavior of the subject was quantified using Noldus Observer. Videos were scored by a researcher blind to treatment conditions. To ensure that behavioral quantification was consistent with previous studies, inter-rater reliability was established as 90% agreement on the duration of submissive and defensive behavior in a subset of videos.
Blood collection and enzyme immunoassay
In Experiment 1, blood was collected under 4% isoflurane anesthesia via retro-orbital eye bleed at two time points: immediately (0 min) and 60 min after social defeat stress. We used cortisol ELISA Kits (Cayman Chemical, Ann Arbor, MI) to assay plasma cortisol, which is the primary glucocorticoid in Syrian hamsters. Samples from each time point were run in a single assay, thereby eliminating inter-assay variability. The intra-assay coefficient of variation was less than 10% for both assays.
qRT-PCR
In Experiment 2, brains were extracted 60 min after social defeat stress. Brains were sectioned coronally at 1 mm thickness and 1 mm diameter tissue punches were collected from the basolateral (BLA)/CeA, anterior BNST, and PVN. Tissue was immediately placed in a lysis buffer (Qiagen, Hilden, Germany) and flash frozen with liquid nitrogen. RNA was extracted using the Qiagen RNeasy Lipid Tissue Mini Kit with on-column DNase I (Qiagen, Hilden, Germany) digestion according to the manufacturer’s instructions. The quantity and quality of RNA were determined by using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA). An aliquot of each sample was then reverse transcribed using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and stored at −20 °C. We performed qRT-PCR to detect the levels of CRH mRNA using a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) with fluorescent SYBR Green technology (Bio-Rad, Hercules, CA). The expression of tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA served as an internal control. As gene specific primers for Syrian hamsters were not available at the time of this study (but see McCann, Sinkiewicz, Norvelle, & Huhman, 2017), we used the National Center for Biotechnology Information (NCBI) GenBank databases to perform BLAST analyses with known mouse gene sequences and predicted Syrian hamster sequences (NCBI Syrian hamster reference sequences: CRH: XM_005066742.2; YWHAZ: XM_021228739.1; GAPDH: XM_013124485.1). BLAST analyses revealed a significant overlap in mouse and hamster gene sequences (CRH: 88%; YWHAZ: 94%; GAPDH: 92%), therefore mouse gene specific primers were used (Bio-Rad, Hercules, CA; Table 1). Primer specificity was verified using melt-curve analysis. Samples were assayed in triplicate, and mean cycles to threshold (Ct) values were used for analysis. Analysis of qRT-PCR results was performed using the 2−ΔΔCT method.
Table 1.
Primer amplicon context sequences.
| Gene | MIQE context sequence |
|---|---|
| CRH | TCTTCTTTTTTTCTTTTGCCTTTTCCCTTTCTCTTCAGTCTCTCAACGTACTTGGGCTCTGAGTTTCTCCACACCAGAGCCTGGAGTGAGATTTTATAATGTCGACCCT CTTCAGAAAGCACGCAGCATTTGC |
| YWHAZ | CTGCTCAGTGACAGACTTCATGCAGGCTGCCATGTCATCATATCGCTCTGCCTGCTCGGCCAGCTTGGCCTTCTGCACCAGCTCATTTTT |
| GAPDH | TGGGAGTTGCTGTTGAAGTCGCAGGAGACAACCTGGTCCTCAGTGTAGCCCAAGATGCCCTTCAGTGGGCCCTCAGATGCCTGCTTCACCACCTTCTTGATGTCA |
Data analysis
Data were tested for homogeneity of variance using a Bartlett’s test or Mauchly’s sphericity test. Cortisol data were analyzed using a two-way repeated measures ANOVA, and one-way ANOVAs with LSD post hoc were also used for planned comparisons. For behavioral and CRH mRNA data, we used one-way ANOVAs followed by LSD post hoc; however, when homogeneity of variance was violated, we used Kruskal–Wallis’s tests followed by Dunn’s tests. Pearson’s correlation coefficients (r) were calculated to measure the strength of the linear relationship between defeat intensity and plasma cortisol levels, as well as defeat intensity and relative CRH mRNA levels. In addition, chi-square tests were used to analyze the proportion of animals that fought back during social defeat. Statistical significance was set at p <.05. Data are reported as mean ± SEM, except where noted.
Results
Experiment 1
On average, dominance relationships were established on day 1.86 (SD = 1.29) and the direction of aggression remained consistent for two weeks. There were no significant difference between dominant, subordinate, and SSC hamsters in the number of attacks received or the duration of aggressive received during social defeat stress (F(2, 29) = 0.069, p=.933, F(2, 29) = 0.192, p = .826, respectively). We also found that dominant animals (4/11) were more likely to fight back against the resident aggressor during the first social defeat episode compared to subordinates (0/12) (χ2 = 5.282, df = 1, p = .022) but not SSCs (1/9) (χ2 = 1.684, df = 1, p = .194).
There were significant main effects of both time (F(1, 39) = 28.27, p <.0001) and status (F(3, 39) = 6.534, p = .001) for cortisol concentrations, as well as a significant time × status interaction (F(3, 39) = 3.257, p = .032; Figure 1). Immediately after social defeat (0 min), no defeat controls had significantly lower cortisol concentrations compared to dominants (p <.001), subordinates (p = .002), and SSCs (p = .004), while dominants, subordinates, and SSCs did not differ from each other (p >.05). One hour after social defeat stress (60 min), subordinates displayed significantly higher cortisol concentrations compared to no defeat controls (p = .006) and SSCs (p = .046), while dominants and SSCs did not differ from no defeat controls (p = .051 and p >.05, respectively). The duration of aggressive behavior received during social defeat did not correlate with plasma cortisol at 0 min (r(29) = 0.147, p = .431) or plasma cortisol at 60 min (r(29) = 0.350, p = .054). However, the number of attacks received during defeat correlated with plasma cortisol at 0 min (r(29) = 0.577, p = .001) and 60 min (r(29) = 0.400, p = .026).
Figure 1.
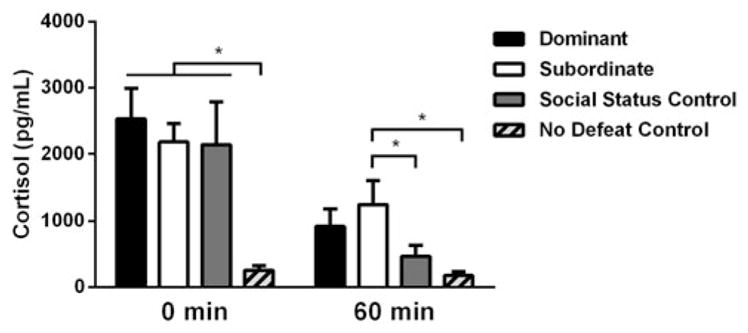
Effects of acute social defeat stress and dominance status on plasma cortisol levels. Immediately (0 min) post-defeat, dominants (n = 12), subordinates (n = 12), and social status controls (n = 9) all display significantly greater levels of cortisol compared to no defeat controls (n =10). At 60 min post-defeat, subordinates remain significantly higher than social status and no defeat controls, while dominants and social status controls are not different from no defeat controls. Data are shown as mean ± SEM. An asterisk indicates a significant difference between groups as determined by an LSD post hoc test (*p <.05).
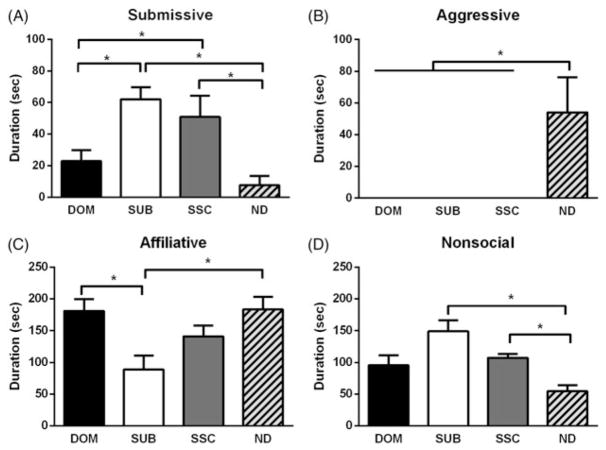
Social status altered the CD response as demonstrated by significant differences in submissive and defensive (F(3, 38) = 8.858, p <.001), aggressive (Kruskal–Wallis’s test, H(3) = 21.70, p <.001), affiliative (F(3, 38) = 5.416, p = .003), and nonsocial behavior (Kruskal–Wallis’s test, H(3) = 15.36, p = .002). Dominants displayed less submissive behavior than subordinates (p = .028), and subordinates and SSCs displayed more submissive behavior than no defeat controls (p <.001 and p = .002, respectively; Figure 2(A)). No defeat controls displayed greater levels of aggression compared to dominants, subordinates, and SSCs (p <.001, p <.001, and p <.001, respectively; Figure 2(B)). Subordinates displayed less affiliative behavior compared to dominants (p = .001) and no defeat controls (p = .001; Figure 3(C)). Affiliative behavior was defined as social investigation and approach behaviors. No defeat controls displayed less nonsocial behavior compared to subordinates and SSCs (p <.001 and p = .008, respectively; Figure 2(D)). Nonsocial behavior included locomotion, self-grooming, nesting, and feeding behaviors. All behaviors (affiliative, nonsocial, aggressive, and submissive) were scored in accordance with a previously published ethogram (Morrison et al., 2014).
Figure 2.
Effects of acute social defeat stress and dominance status on CD behavior in a social interaction test. (A) Subordinate (SUB, n = 12) and social status controls (SSC, n = 9) display a significantly greater duration of submissive behavior compared to no defeat controls (ND, n =10), while dominant (DOM, n = 11) hamsters display significantly less submissive and defensive behavior compared to subordinates. (B) No defeat controls display significantly more aggressive behavior compared to dominants, subordinates, and social status controls. (C) Both dominants and no defeat controls display a significantly greater duration of affiliative behavior compared to subordinates. (D) Subordinates and social status controls display a significantly greater duration of nonsocial behavior compared to no defeat controls, while dominants do not. Data are shown as mean ± SEM. An asterisk indicates a significant difference between groups as determined by an LSD post hoc test or Dunn’s test (*p <.05).
Figure 3.
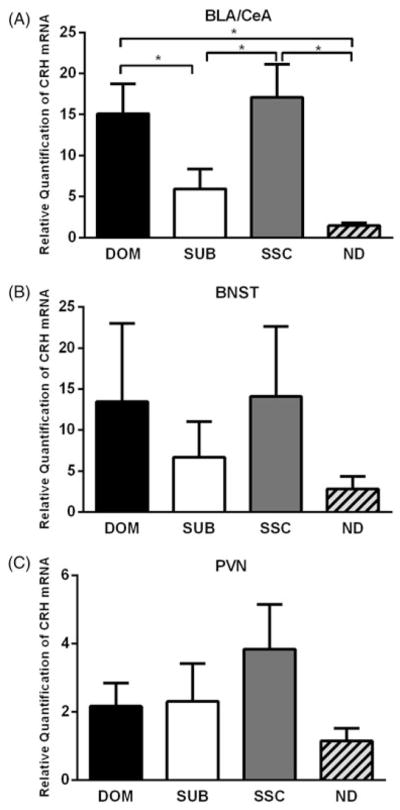
Effects of acute social defeat stress and dominance status on the relative quantification of CRH mRNA expression. (A) In the BLA/CeA, dominants (DOM, n = 8) had a significantly greater expression of CRH mRNA compared to subordinates (SUB, n = 9) and no defeat controls (ND, n = 8), social status controls (SSC, n = 8) were significantly greater than subordinates and handled controls, while dominants and social status controls did not differ. (B) In the anterior BNST, no significant differences were found, although the pattern was similar to the BLA/CeA data, albeit with greater variability (DOM, n = 7; SUB, n = 8; SSC, n = 7; ND, n =6). (C) No significant differences were observed in the PVN (DOM, n =8; SUB, n = 8; SSC, n = 9; ND, n = 7). Data are shown as mean ± SEM. An asterisk indicates a significant difference between groups as determined by a Dunn’s test (*p <.05).
Experiment 2
On average, dominance relationships were established on day 1.2 (SD = 0.41). There was no significant effect of social status on the number of attacks received or the duration of aggression received during social defeat stress (F(2, 27) = 1.453, p = .252, F(2, 27) = 0.566, p = .575, respectively). We also found that 4/10 dominants, 0/10 subordinates, and 3/10 SSCs fought back against the resident aggressor during the first social defeat, indicating that dominant hamsters fought back significantly more often than subordinates (χ2 = 5.00, df = 1, p = .025).
In the BLA/CeA, we found status-dependent differences in the expression of CRH mRNA (Kruskal–Wallis’s test, H(3) = 14.63, p = .002). Both dominants and SSCs had a significantly greater expression of CRH mRNA compared to subordinates (p = .036 and p = .044, respectively) and no defeat controls (p = .001 and p = .002, respectively) (Figure 3(A)). CRH mRNA expression did not significantly differ in either the anterior BNST (Kruskal–Wallis’s test, H(3) = 0.508, p = .917; Figure 3(B)) or PVN (Kruskal–Wallis’s test, H(3) = 2.259, p = .521; Figure 3(C)). In addition, the duration of aggressive behavior received during social defeat did not correlate with CRH mRNA levels in the BLA/CeA (r(22) = −0.308, p = .143), BNST (r(20) = 0.133, p = .556), or PVN (r(23) = −0.005, p = .982). The number of attacks received during defeat was negatively correlated with CRH mRNA in the BLA/CeA (r(22) = −0.462, p = .023), but similar correlations were non-significant in the BNST (r(20) = −0.066, p = .77) and PVN (r(23) = −0.202, p = .332).
Discussion
We found that while dominance status in Syrian hamsters does not alter the maximal cortisol response immediately after social defeat, subordinate hamsters do show elevated cortisol levels one hour after stress. Although other endpoints, such as adrenocorticotropic hormone (ACTH), and additional time points would be helpful in definitively characterizing extinction of HPA axis activity in subordinate hamsters, these findings suggest that subordinates have a slow extinction of the neuroendocrine stress response. This finding is consistent with the disrupted glucocorticoid negative feedback shown for subordinate rhesus macaque females (Michopoulos, Reding, Wilson, & Toufexis, 2012) and the sensitization of HPA axis activity shown in socially defeated male rats (Bhatnagar & Vininging, 2003). Interestingly, the number of attacks received during social defeat was positively correlated with plasma cortisol, although the amount of aggression received was not associated with either dominance status or subsequent CD response. The neuroendocrine effects of social stress can be extremely variable in primate groups and depend on the amount of aggression received and opportunities for coping (Abbott et al., 2003). Also, social defeat parameters can modulate the neuroendocrine stress response in rats (Koolhaas, De Boer, De Rutter, Meerlo, & Sgoifo, 1997). In hamsters, the behavioral consequences of social defeat appear independent of the amount of aggression received (Solomon et al., 2009). Similarly, the CD response is not altered by blocking plasma ACTH production (Jasnow et al., 1999) or glucocorticoid synthesis (Cooper & Huhmanman, 2010), suggesting that glucocorticoid feedback does not modulate subsequent changes in agonistic behavior in Syrian hamsters. Others have proposed a role for glucocorticoid feedback in ongoing and future agonistic behavior and this view has gained traction since the characterization of non-genomic glucocorticoid action (Haller, 2014; Leshner & Politchtch, 1979). While glucocorticoid feedback may alter future agonistic behavior in some cases, its effect likely depends on context. For instance, in green anole lizards glucocorticoids increase aggressive behavior in dominant animals and increase submissive behavior in subordinates (Summers et al., 2005).
We found that SSCs show elevated CRH mRNA in the BLA/ CeA complex compared to non-defeated animals, which is consistent with previous research showing that CRH acts within a CeA-to-BNST circuit to promote the CD response (Jasnow et al., 2004). Interestingly, subordinates show less CRH mRNA expression compared to both dominants and SSCs. The maintenance of dominance relationships in Syrian hamsters appears to result in habituation to stress-induced transcriptional activation of CRH, which is consistent with the effects of repeated restraint in rats (Girotti et al., 2006). Similarly, subordinate olive baboons are hyporesponsive to a CRH challenge compared to subordinates (Sapolsky, 1989). Although CRH mRNA expression may not account for stress vulnerability in subordinate hamsters, other models suggest changes in the CRH system are associated with coping abilities. Subordinate male rats living in a visual burrow system exhibit depression-like behavior and higher CRH mRNA expression in the CeA compared to dominants (McEwen, McKittrick, Tamashiro, & Sakai, 2015). Also, rats with an active coping strategy show reduced CRH mRNA in the PVN compared to passive coping animals (Wood, Walker, Valentino, & Bhatnagar, 2010). Interestingly, active coping rats also show a shift in CRH signaling in the dorsal raphe nucleus from CRH-R1 to CRH-R2 (Wood et al., 2013). However, in Syrian hamsters, dominant and subordinate animals do not show differences in the density of CRH-R1 and CRH-R2 in several limbic regions (Faruzzi, 2006; McCann, Faruzzi, Markham, & Huhman, 2013).
While dominant hamsters show a reduced CD response compared to subordinates and animals without social status, they do not differ from SSCs in extinction of stress-induced plasma cortisol and transcription of CRH mRNA. In contrast, dominant hamsters do differ from subordinates in stress-induced neural activity within the vmPFC, medial amygdala (MeA), and lateral portions of the ventromedial hypothalamus (Morrison et al., 2014), as well as the density of androgen receptors in the MeA (Clinard, Barnes, Adler, & Cooper, 2016). The vmPFC is a key neural substrate regulating status-dependent differences in stress-related behavior because pharmacological inhibition of the vmPFC reinstates a robust CD response in dominant hamsters (Morrison et al., 2013). These findings suggest that reduced CD in dominants does not arise from a reduction in neuroendocrine factors that promote stress responses, but rather activation of limbic substrates that promote coping abilities.
LAY SUMMARY.
Dominant hamsters show resistance to the behavioral effects of acute social defeat compared to subordinates, but it is unclear whether social status modulates the neuroendocrine stress response in Syrian hamsters. This study indicates that dominant social status does not alter stress-induced activity of the extended hypothalamic-pituitary-adrenal (HPA) axis, which suggests that the ability of dominants to cope with social defeat stress is not associated with changes in their neuroendocrine stress response.
Acknowledgments
We would like to thank our team of undergraduate students for their daily support and technical assistance, most notably Ashley V. Campbell and Samuel G. Adler.
Funding
This research was supported by National Institutes of Health grants MH59839 to SD and MH107007 to MAC.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, … Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43:67–82. doi: 10.1016/S0018-506X(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic–pituitary–adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Hormones and Behavior. 2003;43:158–165. doi: 10.1016/S0018-506X(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Björkqvist K. Social defeat as a stressor in humans. Physiology & Behavior. 2001;73:435–442. doi: 10.1016/S0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Charuvastra A, Cloitre M. Social bonds and posttraumatic stress disorder. Annual Review of Psychology. 2008;59:301–328. doi: 10.1146/annurev.psych.58.110405.085650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard CT, Barnes AK, Adler SG, Cooper MA. Winning agonistic encounters increases testosterone and androgen receptor expression in Syrian hamsters. Hormones and Behavior. 2016;86:27–35. doi: 10.1016/j.yhbeh.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behavioral Neuroscience. 2005;119:1042. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Blocking corticotropin-releasing factor-2 receptors, but not corticotropin-releasing factor-1 receptors or glucocorticoid feedback, disrupts the development of conditioned defeat. Physiology & Behavior. 2010;101:527–532. doi: 10.1016/j.physbeh.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruzzi AN. Dissertation. Georgia State University; 2006. Corticotropin releasing factor receptors and agonistic behavior in Syrian hamsters. [Google Scholar]
- Gilman TL, DaMert JP, Meduri JD, Jasnow AM. Grin1 deletion in CRF neurons sex-dependently enhances fear, sociability, and social stress responsivity. Psychoneuroendocrinology. 2015;58:33–45. doi: 10.1016/j.psyneuen.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace T, Gaylord R, Rubin B, Herman J, Spencer R. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Haller J. The glucocorticoid/aggression relationship in animals and humans: An analysis sensitive to behavioral characteristics, gluco-corticoid secretion patterns, and neural mechanisms. Neuroscience of Aggression. 2014;17:73–109. doi: 10.1007/7854_2014_284. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Research. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-H. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Banks MC, Owens EC, Huhman KL. Differential effects of two corticotropin-releasing factor antagonists on conditioned defeat in male Syrian hamsters (Mesocricetus auratus) Brain Research. 1999;846:122–128. doi: 10.1016/S0006-8993(99)02007-7. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral Neuroscience. 2004;118:1052. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Koolhaas J, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiologica Scandinavica Supplementum. 1997;640:69–72. [PubMed] [Google Scholar]
- Leshner AI, Politch JA. Hormonal control of submissiveness in mice: Irrelevance of the androgens and relevance of the pituitary–adrenal hormones. Physiology & Behavior. 1979;22:531–534. doi: 10.1016/0031-9384(79)90021-0. [DOI] [PubMed] [Google Scholar]
- McCann KE, Faruzzi AN, Markham CM, Huhman KL. Effect of social defeat on corticotropin-releasing factor R1 and R2 receptor binding in Syrian hamster brain. Poster presentation at Society for Behavioral Neuroendocrinology; Atlanta, GA. 2013. [Google Scholar]
- McCann KE, Sinkiewicz DM, Norvelle A, Huhman KL. De novo assembly, annotation, and characterization of the whole brain transcriptome of male and female Syrian hamsters. Scientific Reports. 2017;7:40472. doi: 10.1038/srep40472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, McKittrick CR, Tamashiro KL, Sakai RR. The brain on stress: Insight from studies using the Visible Burrow System. Physiology & Behavior. 2015;146:47–56. doi: 10.1016/j.physbeh.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: Systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Melhorn SJ, Elfers CT, Scott KA, Sakai RR. A closer look at the subordinate population within the visible burrow system. Physiology & Behavior. 2017;178:110–116. doi: 10.1016/j.physbeh.2017.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic–pituitary–adrenal function in female rhesus monkeys. Hormones and Behavior. 2012;62:389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Bader LR, Clinard CT, Gerhard DM, Gross SE, Cooper MA. Maintenance of dominance status is necessary for resistance to social defeat stress in Syrian hamsters. Behavioural Brain Research. 2014;270:277–286. doi: 10.1016/j.bbr.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Bader LR, McLaughlin CN, Cooper MA. Defeat-induced activation of the ventral medial prefrontal cortex is necessary for resistance to conditioned defeat. Behavioural Brain Research. 2013;243:158–164. doi: 10.1016/j.bbr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Swallows CL, Cooper MA. Effects of dominance status on conditioned defeat and expression of 5-HT1A and 5-HT2A receptors. Physiology & Behavior. 2011;104:283–290. doi: 10.1016/j.physbeh.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. Journal of Neuroscience. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Hypercortisolism among socially subordinate wild baboons originates at the CNS level. Archives of General Psychiatry. 1989;46:1047–1051. doi: 10.1001/archpsyc.1989.01810110089012. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Norvelle A, Markham CA, Erwin WD, Huhman KL. Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Hormones and Behavior. 2009;56:423–428. doi: 10.1016/j.yhbeh.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Watt MJ, Ling TL, Forster GL, Carpenter RE, Korzan WJ, … Øverli Ø. Glucocorticoid interaction with aggression in non-mammalian vertebrates: Reciprocal action. European Journal of Pharmacology. 2005;526:21–35. doi: 10.1016/j.ejphar.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Walker D, Miles L, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: Role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biological Psychiatry. 2013;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]