Abstract
Recent studies have shown that sex and sex steroids influence the composition of the gut microbiome. These studies also indicate that steroid regulation of the gut microbiome may play a role in pathological situations of hormonal excess, such as PCOS. Indeed, studies demonstrated that PCOS is associated with decreased alpha diversity and changes in specific Bacteroidetes and Firmicutes, previously associated with metabolic dysregulation. These studies suggest that androgens may regulate the gut microbiome in females and that hyperandrogenism may be linked with a gut ‘dysbiosis in PCOS. Future mechanistic studies will be required to elucidate how sex steroids regulate the composition and function of the gut microbial community and what the consequences of this regulation are for the host.
Keywords: Sex steroid, polycystic ovary syndrome, gut microbiome, 16s rRNA gene sequencing
Sex-Dependent Interactions with the Gut Microbiome
The microbial community in the mammalian gastrointestinal tract (gut), comprised of bacteria, archaea, fungi, and viruses, rapidly diversifies after birth until it reaches a stable state in adulthood [1] (Box 1). Numerous host and environmental factors are associated with variation in the gut microbiome, including diet, host genetics, and hormones [14,15]. An intriguing example of how hormones interact with the gut microbiome came from investigating the effects of sex on the development of type 1 diabetes in a non-obese diabetic mouse model. Studies showed that exposure of female mice to androgens or the gut microbiome of male mice conferred protection from type 1 diabetes [16–18], indicating that sex-dependent interactions with the gut microbiome can influence the development of pathological states. Given the vast potential for crosstalk among hormones and gut microbes, it is worth reviewing what is currently known about the interactions of sex and sex steroids with the gut microbiome and whether these interactions have implications for disorders with altered steroid levels, such as polycystic ovary syndrome (PCOS) (Box 2).
Box 1. Introduction to the Gut Microbiome.
Coevolution of mammals and the gut microbial community resulted in microbial regulation of many processes critical for the host, including energy biogenesis due to the fermentation of dietary fiber into short-chain fatty acids; synthesis of specific vitamins such as folic acid and B12; metabolism of bile acids, neurotransmitters, and hormones; elimination of specific toxins; defense against pathogens; and modulation of immunity and metabolism [2]. Studies showed that a diverse gut microbiome develops after birth and is influenced by mode of delivery (vaginal versus C-section), breast-feeding, and weaning [3,4]. While the gut microbiome of breast-fed infants is dominated by bifidobacteria, the community shifts to one composed predominantly of bacteria from the Bacteroidetes and Firmicutes phyla after the introduction of solid food.
Over the past 15 years, many studies have investigated the relationship between the gut microbiome and host metabolism. Studies demonstrated that the gut microbiome of individuals with metabolic disorders, such as obesity and type 2 diabetes, differed from healthy individuals [5–8]. In addition, mouse models of obesity, such as leptin-deficient ob/ob mice and mice fed a high-fat diet, were associated with changes in gut microbes [9,10]. Meta-analyses of the human obesity studies demonstrated that the alpha diversity of the gut microbiome was consistently lower in obese individuals compared with controls but did not observe consistent changes in the Bacteroidetes:Firmicutes ratio or specific bacterial abundances [11]. Studies showed that fecal microbiome transplantation from obese humans into germ-free mice resulted in an obese phenotype [12,13], indicating a potential causative role of the gut microbiome in the development of metabolic disorders.
Box 2. The Pathoetiology of PCOS.
PCOS is a heterogeneous disorder with an estimated world-wide prevalence of 5%–15%. PCOS is diagnosed using the Rotterdam Consensus criteria that require at least two of the following: hyperandrogenism, oligo- or amenorrhea, and polycystic ovaries [19] (Figure 2). Studies have shown that PCOS can result in profound, long-term health consequences [20]. PCOS is the leading cause of anovulatory infertility and women diagnosed with PCOS also have an increased risk of miscarriage and pregnancy complications. In addition, a majority of women with PCOS have metabolic abnormalities such as obesity, hyperinsulinemia, insulin resistance, and dyslipidemia that increase their risk of developing type 2 diabetes, hypertension, and non-alcoholic fatty liver disease [21–25].
The etiology of PCOS is poorly understood, although PCOS is considered to be a polygenic complex genetic trait [26]. Familial studies found a high rate of PCOS in the mothers and sisters of women diagnosed with PCOS [27]. A large study with monozygotic and dizygotic twins identified the heritability of PCOS as ~70% [28]. Moreover, genome-wide association studies have reported multiple susceptibility loci associated with an increased risk of developing PCOS [29]. In addition to genetics, environmental factors such as prenatal exposure to androgens may also play a role in the etiology of PCOS [30].
Despite the association of PCOS with obesity, there is little evidence to suggest that dietary factors or obesity predispose women to PCOS [31]. However, metabolic dysfunction occurs predominantly in women with PCOS diagnosed with hyperandrogenism and ovulatory dysfunction, irrespective of body mass index [32,33]. Although these studies suggest that hyperandrogenism is correlated with metabolic dysregulation, it is not understood why women with PCOS become insulin resistant and why they have a higher incidence of insulin resistance than predicted by their body mass index. Insulin resistance and hyperinsulinemia may contribute to metabolic dysregulation by upregulating ovarian androgen production and increasing androgen bioactivity through decreased sex hormone-binding globulin production [34,35]. In addition, obesity exacerbates insulin resistance, hyperinsulinemia, and androgen production in women with PCOS [36], resulting in a vicious cycle of hyperandrogenism and insulin resistance.
While initial 16S rRNA gene sequencing (see Glossary) studies did not identify significant sex-dependent differences in the gut microbiome [37], subsequent studies reported an important influence of sex on the taxonomic composition of gut microbes [38,39]. Compared with men, women were associated with greater alpha diversity of the gut microbiome [39] (see Figure 1 for an explanation of alpha and beta diversity). Studies also reported that men had a higher relative abundance of Bacteroidetes (including Prevotella and Bacteroides thetaiotaomicron) than women [38,40,41]. In addition, men had a lower abundance of Clostridia, Methanobrevibacter, and Desulfovibrio compared with women [39].
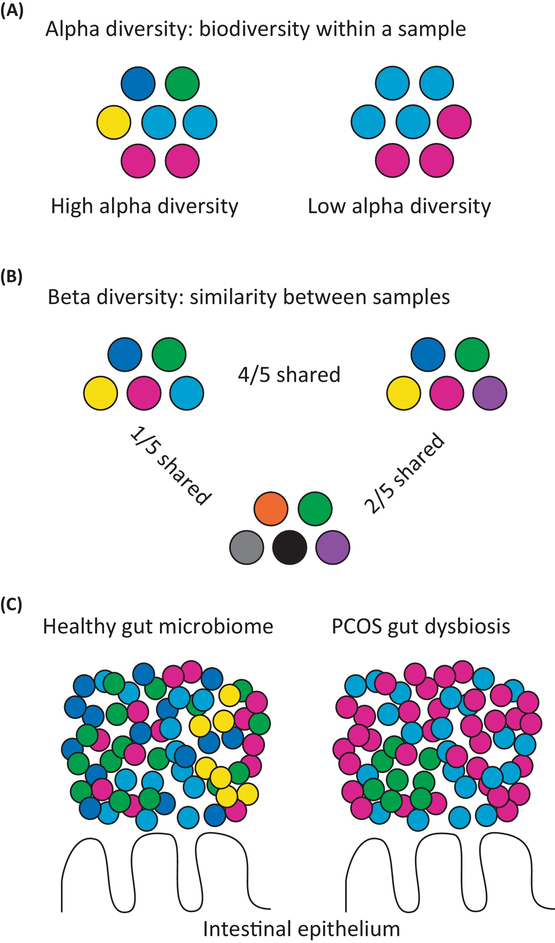
Figure 1.
Polycystic Ovary Syndrome (PCOS) Is Associated with Changes in the Gut Microbiome. Comparisons of alpha and beta diversity between hypothetical, gut microbial communities. Different bacterial taxa are represented by circles with different colors. (A) Alpha diversity represents the biodiversity (species richness) within a specific community or individual sample. The sample on the left has high alpha diversity (five bacterial taxa), while the sample on the right has low alpha diversity (two bacterial taxa). (B) Beta diversity represents how similar one community or individual sample is to another. The samples on the left and right are similar to each other (4/5 shared bacterial taxa), while the sample on the left is not very similar to the sample in the middle (1/5 shared bacterial taxa). (C) PCOS is associated with a microbial imbalance or dysbiosis compared with the healthy state including lower alpha diversity and changes in the relative abundance of bacteria from the Bacteroidaceae, Clostridiaceae, Erysipelotrichidae, Lachnospiraceae, Lactobacillaceae, Porphyromonadaceae, Prevotellaceae, Ruminococcaceae, and S24–7 families.
Sex differences were also observed in the composition of the rodent gut microbiome. Similar to humans, female mice had greater alpha diversity than male mice and male mice were reported to have a higher abundance of Bacteroidetes compared with female mice [18,42]. Several other bacterial taxa were reported to differ between the sexes but these results were not consistent among the studies. For instance, one study found that, at the phyla level, the relative abundances of Actinobacteria and Tenericutes were higher in male mice than in females [43]. Moreover, Allobaculum, Anaeroplasma, and Erwinia were more abundant in males compared with females, while SMB53, Dorea, Coprococcus, and Ruminococcus were less abundant [43]. In contrast, another study indicated that Ruminococcaceae and Anaerostipes were more abundant in males than females, while Peptostreptococcaceae were less abundant [42]. Altogether, these studies in humans and rodents demonstrated that there are sex-dependent differences in the gut microbiome. Future studies are needed to characterize the comprehensive effects of sex on the composition of gut microbes, including bacteria, archaea, fungi, and viruses. In addition, the mechanisms involved in sex-dependent effects on gut microbes and the functional consequences for the gut microbial community and the host need to be elucidated.
Influence of Sex Steroids on the Gut Microbiome
Differences associated with sex in the gut microbiome could be attributable to genetic (sex chromosomes) or hormonal mechanisms. If sex steroid hormones are linked with changes in the gut microbiome, one would expect the gut microbiome to be similar prior to puberty and then diverge after puberty. Multiple studies in humans and rodents support this idea. For instance, a significant difference was found in the composition of the gut microbiome of fraternal twins of the opposite sex during adolescence compared with twins of the same sex, while no difference was detected between fraternal twins of the same or different sex during infancy [44]. Furthermore, studies in rodents demonstrated that the gut microbiome diverged after puberty in a sex-specific manner. The gut microbiome was found to be similar between male and female mice at 3 weeks of age (prepuberty) but significant differences were observed at 6 weeks of age (postpuberty) [17,18].
Evidence of activational effects of sex steroids on the gut microbiome comes from studies characterizing the effect of gonadectomy on adult rodents. Studies showed that alpha diversity decreased in ovariectomized mice and rats [45,46]. One study in mice reported a decrease in Bacteroidetes and an increase in Firmicutes in ovariectomized mice compared with controls [46]. Specifically, ovariectomy resulted in a decreased abundance of Ruminococcaceae, Rikenellaceae, Clostridia, S24–7, Bacteroides, and Prevotella and an increase in the abundance of Lactobacillus and Bifidobacterium animalis. Conversely, another study reported the opposite trend in ovariectomized rats bred for low aerobic capacity, with an increase in Bacteroidetes such as Bacteroides, Barnesiella, and Prevotella, as well as a decrease in Firmicutes, compared with control rats [47]. With regards to males, it is not clear whether any changes in alpha diversity are associated with gonadectomy although castration was reported to result in changes in the beta diversity of the gut microbiome [43]. Similar to ovariectomy, castration was associated with decreased Bacteroidetes compared with control male mice as measured by quantitative PCR [48] and decreased abundance of Ruminococcaceae [43]. Differences in other bacterial taxa were reported to be mouse strain-specific [43].
Given the inherent variation in the gut microbiome amongst individuals and strains of rodents, additional studies will be required to understand how the composition and function of the gut microbiome are regulated by sex steroids and, potentially, sex chromosomes in males and females. Future studies investigating proximal versus long-term effects of gonadectomy and the effects of gonadectomy with or without steroid replacement in rodents will be informative, as well as studies investigating the effects of gonadectomy in other mammalian species. Studies focusing on whether changes in the gut microbiome are associated with sex steroid levels during different developmental stages, such as puberty and menopause, or in pathological states of hormone insufficiency/excess, should also be investigated.
Changes in the Gut Microbiome Are Associated with PCOS
Since steroid hormone levels are linked with changes in the gut microbiome and many women with PCOS have androgen excess that is associated with metabolic dysregulation, it is possible that a microbial imbalance in the gut may contribute to PCOS. Several recent studies investigated whether there was a link between the gut microbiome and PCOS [49–52]. These studies involved women from China and Europe (Austria, Poland, and Spain) diagnosed with PCOS using the Rotterdam criteria [19]. PCOS was associated with a change in the overall composition of the bacteria in the gut, including a decrease in alpha diversity (species richness and phylogenetic diversity) as well as changes in beta diversity [49–51] (Figure 1). One study did not detect a change in alpha or beta diversity in women with PCOS compared with women without PCOS, potentially due to a small sample size and high interindividual variability in the gut microbiome [52]. Alpha diversity has been proposed to correlate with the health of an ecosystem, as diverse communities may increase the stability and productivity of an ecosystem [53]. Lower alpha diversity of the gut microbiome has been associated with human obesity in several meta-analyses [11,54,55]. Thus, it is possible that decreased bacterial diversity results in changes in gut function that contribute to metabolic dysfunction in women with PCOS. Future studies will be required to understand how changes in alpha diversity influence the gut microbiome and host physiology.
In addition to detecting changes in the overall composition of the gut microbiome, several studies also demonstrated changes in the relative abundances of specific types of gut bacteria of summarized in Table 1. Notably, changes in the abundance of multiple bacteria from the Bacteroidetes and Firmicutes phyla were associated with PCOS. In particular, Bacteroidetes from the Bacteroidaceae, Porphyromonadaceae, and S24–7 families and Firmicutes from the Clostridiaceae, Erysipelotrichidae, Lachnospiraceae, Lactobacillaceae, and Ruminococcaceae families were higher or lower in women with PCOS. Since many of these bacteria produce butyrate and propionate, changes in the abundance of specific Bacteroidetes and Firmicutes in PCOS may result in altered production of short-chain fatty acids that impact metabolism, gut barrier integrity, and immunity [15]. In addition, one study observed a decrease in bacteria from the Tenericutes phylum (order ML615J) [49], a group of bacteria previously associated with obesity and metabolic dysregulation [56]. Another study observed a decrease in Akkermansia from the Verrucomicrobia phylum [50], which has been reported to be decreased in diet-induced obese mice [57].
Table 1.
Changes in Bacteroidetes and Firmicutes Relative Abundance Associated with PCOS
| Study | Cohort characteristics | Bacteroidetes | Firmicutes | Refs |
|---|---|---|---|---|
|
Lindheim, 2017 |
Austria 19 controls 24 PCOS 58% HA Mild IR and not obese |
↓ S24–7 |
[49] | |
|
Liu, 2017 [50] |
China 15 controls (6 obese) 33 PCOS (21 obese) All HA, OA, and PCOS |
↓ Bacteroidaceae Bacteroides |
↓ Lactobacillaceae Lactobacillus ↓ Ruminococcaceae ↓ Ruminococcaceae Clostridium IV ↓ Ruminococcaceae Oscillibacter |
[50] |
|
Torres, 2018 [51] |
Poland 48 controls 73 PCOS 85% HA Average BMI non-obese |
↑ Bacteroidaceae Bacteroides coprophilus ↑ Porphyromonadaceae Porphyromonas ↓ Porphyromonadaceae Odoribacter |
↑ Clostridiaceae Faecalibacterium
prausnitzii ↑ Lachnospiraceae Blautia ↓ Clostridiales XI Anaerococcus ↓ Lachnospiraceae Roseburia ↓ Ruminococcaceae Ruminococcus brotnii |
[51] |
|
Insenser, 2018 [52] |
Spain 16 control (8 obese) 15 PCOS (8 obese) All HA Groups age- and BMI-matched |
↑ Erysipelotrichidae Catenibacterium ↑ Erysipelotrichidae Kandleria ↑ Lachnospiraceae Oribacterium |
[52] | |
|
Kelley, 2016 [65] |
Letrozole-induced mouse model 4-week old, pubertal mice pellet implant, 50 μg/day |
↓ Bacteroidaceae Bacteroides ↓ Porphyromonadaceae Parabacteroides ↓ Rikenellaceae Alistipes ↓ S24–7 |
↑ Erysipelotrichidae Allobaculum ↑ Lachnospiraceae Blautia ↑ Lachnospiraceae Coprococcus ↑ Lachnospiraceae Roseburia ↑ Ruminococcaceae Ruminococcus ↓ Ruminococcaceae |
[65] |
|
Guo, 2016 [66] |
Letrozole-induced rat model 6-week old, pubertal rats 1 mg kg daily oral garage |
↑ Prevotellaceae Prevotella |
↓ Lactobacillaceae Lactobacillus ↓ Ruminococcaceae Clostridium ↓ Ruminococcaceae Ruminococcus |
[66] |
|
Sherman, 2018 [68] |
Prenatal androgenized rat model 5 mg kg testosterone cypionate Gestational day 20 |
↑ Porphyromonadaceae Tannerella ↑ Prevotellaceae Paraprevotella ↑ Rikenellaceae Rikenella ↓ S24–7 ↓ Bacteroidaceae Bacteroides ↓ Porphyromonadaceae Odoribacter ↓ Porphyromonadaceae Parabacteroides |
↑ Clostridiaceae F. prausnitzii ↑ Lachnospiraceae Blautia ↑ Lachnospiraceae Oribacterium ↑ Lachnospiraceae Roseburia ↓ Erysipelotrichidae Allobaculum ↓ Lachnospiraceae Coprococcus ↓ Lachnospiraceae Dorea ↓ Lactobacillaceae Lactobacillus ↓ Ruminococcaceae Ruminococcus |
[68] |
| Moreno-Indias, 2016 [45] | Neonatal androgenized rat model 1250 μg testosterone propionate Postnatal day 1 |
↑ Prevotellaceae Paraprevotella ↑ S24–7 ↓ Bacteroidaceae Bacteroides |
↑ Lachnospiraceae Coprococcus ↑ Ruminococcaceae Ruminococcus ↓ Ruminococcaceae Oscillospira |
[45] |
Bacteria are listed by family, genus, and species (if known); bacteria altered in more than one study are in bold type. Human studies are highlighted in grey.
BMI, Body mass index; HA, hyperandrogenemia; IR, insulin resistance; OA, oligo- or amenorrhea; PCOS, polycystic ovary syndrome.
There are many caveats that need to be considered when comparing studies of the gut microbiome. Factors such as diet, geography, age, prescription drug use, alcohol consumption, and smoking have been reported to influence the composition of the gut microbiome [58,59]. In addition, the use of small sample sizes may limit the ability to detect changes in specific bacteria. Moreover, the use of different methodologies to extract DNA, PCR amplify and sequence 16S rRNA genes, and analyze the resulting data can complicate comparison of gut microbiome studies.
Given the association of hyperandrogenism with metabolic dysregulation in women with PCOS [33], studies also investigated whether hyperandrogenism is linked with changes in the gut microbiome. Torres et al. showed that a decrease in alpha diversity was negatively correlated with total testosterone levels and hirsutism [51]. In addition, hyperandrogenism was strongly associated with changes in the overall bacterial community composition of the gut (beta diversity). These results agreed with studies demonstrating that changes in the abundance of several gut bacteria correlate with total testosterone levels and hirsutism [49,60]. It should be noted that one study detected a positive correlation between alpha diversity and testosterone levels that was opposite to the trend observed in the other studies [52]. While one cannot infer causation from association studies, the accumulating data suggests that androgens may be an important factor in shaping the composition of the gut microbiome and changes in the gut microbiome may influence the development and pathology of PCOS. Future studies should investigate how the composition and function of the gut microbiome is altered in PCOS and whether the gut microbiome of women diagnosed with PCOS using the criteria of oligo menorrhea and polycystic ovaries is distinct from women diagnosed with the other subtypes of PCOS that include hyperandrogenism.
Since many women diagnosed with PCOS are obese, several studies investigated the influence of obesity on the gut microbiome in the context of PCOS. It is important to understand whether obesity influences the gut microbiome in a similar or distinct manner in women with and without PCOS. It would also be informative to determine whether obesity influences the gut microbiome in a similar or distinct manner from hyperandrogenism. Studies in China and Spain recruited four groups of women (non-obese and obese women with and without PCOS) to address these questions [50,52]. Liu et al. demonstrated changes in alpha diversity between non-obese control women and obese women with PCOS, as well as changes in beta diversity between non-obese control women and the other three groups [50]. However, the authors could not differentiate amongst the other groups, making it difficult to conclude whether the patterns that they observed were driven by obesity or PCOS. Insenser et al. did show that there was a difference in beta diversity between non-obese and obese women with PCOS [52]. Given that these changes were not observed in non-obese and obese control women, it is possible that obesity has specific effects on the gut microbiome in the context of PCOS. Further sampling of the gut microbiome in large cohorts of obese women with and without PCOS should provide greater insight into whether metabolic factors such as obesity and insulin resistance influence the composition and function of the gut microbial community in women with PCOS.
Using Rodent Models to Study the Role of Gut Microbiome in PCOS
In order to study mechanistically how changes in the gut microbiome influence the pathology of PCOS, model systems need to be employed. Studies have shown that there are broad similarities between the gut microbial communities in humans and rodents at the phylum level [61], suggesting that rodents may be suitable models for studying the interaction between the gut microbiome and PCOS. Approximately 80% of the gut bacteria in humans and mice are composed of bacteria from the Bacteroidetes and Firmicutes phyla, with ~10% composed of Actinobacteria and Proteobacteria. Other phyla such as Tenericutes and Verrucomicrobia are present at much lower abundances. Different approaches have been used to model PCOS in rodents, including models that induce hyperandrogenism during fetal development, early postnatal development, puberty, or adulthood [62–64]. Two studies employed the nonsteroidal aromatase inhibitor, letrozole, to increase testosterone levels by limiting the conversion of testosterone to estrogen [65,66]. The letrozole model has many PCOS-like characteristics, including hyperandrogenism, acyclicity, polycystic ovaries, and increased LH, as well as a metabolic phenotype that includes weight gain, hyperinsulinemia, and insulin resistance [67]. However, low estradiol levels and hemorrhagic ovarian cysts in the letrozole model are not characteristic of PCOS. Other models used treatment of testosterone during late gestation or during postnatal day 1 to explore organizational effects of androgens on the adult gut microbiome and metabolism [45,68]. A caveat for these models is that treatment with exogenous testosterone may also result in altered estradiol levels, since testosterone can be aromatized to estrogen.
Androgen treatment of rodents resulted in changes in biodiversity and the relative abundance of specific bacterial families that are also altered in women with PCOS, suggesting that these models may be suitable for exploring the effects of androgens on the gut microbiome and host physiology. Alpha diversity decreased in the letrozole-induced PCOS mouse model and the neonatal androgenized rat model compared with controls, while beta diversity changed in three models [45,65,68] (Figure 2). More specifically, Bacteroidaceae, Clostridiaceae, Erysipelotrichidae, Lachnospiraceae, Lactobacillaceae, Porphyromonadaceae, Ruminococcaceae, and S24–7 were altered in rodent PCOS models and in women with PCOS (Table 1). Additionally, the studies in rodents highlighted changes in Prevotellaceae that were not detected in the studies of women with PCOS (Table 1). Interestingly, Prevotella was reported to be more abundant in men than women [40] and a Prevotella-dominated microbiome was associated with altered short-chain fatty acid production [60]. The prenatal androgenized rat model also had a decrease in Akkermansia similar to Chinese women with PCOS [50,68]. Changes in the relative abundances of additional bacteria from the Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria phyla were also reported in the prenatal androgenized rat model, although the significance of these changes remains to be confirmed, since correction for multiple comparisons does not appear to have been employed in this study. Indeed, more studies in rodent models are needed to define the suite of microbes (bacteria, archaea, fungi, and viruses) that are associated with hyperandrogenism in females. In addition, there is a need to explore the temporal patterns of these changes as well as to determine how these changes impact gut microbial function and host physiology.
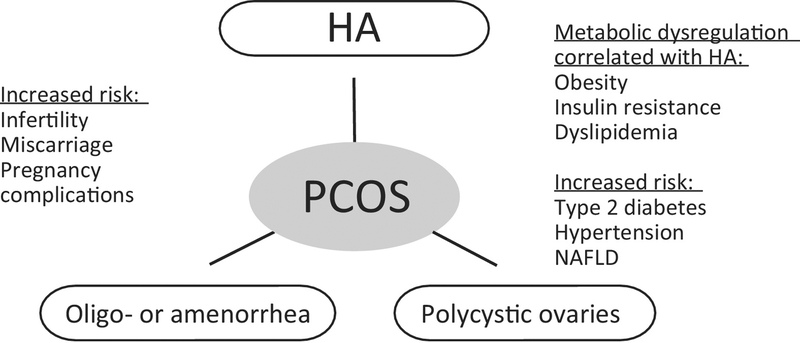
Figure 2.
Polycystic Ovary Syndrome (PCOS) Is Correlated with an Increased Risk of Developing Reproductive and Metabolic Dysregulation. PCOS is diagnosed using the Rotterdam Consensus Criteria that require two out of three of the following criteria: hyperandrogenism (HA), oligo- or amenorrhea, and polycystic ovaries. Women diagnosed with PCOS have an increased risk of infertility, miscarriage, and pregnancy complications. Women with PCOS that have HA also have metabolic dysregulation, including obesity, insulin resistance, and dyslipidemia that increases their risk for type 2 diabetes, hypertension, and non-alcoholic fatty liver disease (NAFLD).
If hyperandrogenism modulates the composition of the gut microbiome, one possibility is that androgen treatment of females results in a microbial community more similar to males than females. As discussed previously, males were reported to have a higher abundance of Bacteroides than females. Since women with PCOS and female mice treated with androgens had a lower abundance of Bacteroides than controls, this suggests that the gut microbiome is not masculinized in PCOS. However, males were reported to have lower abundances of Clostridia and Ruminococcus than women and this decrease was observed in women with PCOS and female mice treated with androgens relative to control females. One study investigated whether the gut microbiome of women with and without PCOS could be distinguished from men [52]. Potentially due to a small sample size, the alpha and beta diversity of the gut microbiome of women with or without PCOS could not be differentiated from each other, making it difficult to evaluate whether the gut microbes of women with PCOS were more similar to men than control women. It is interesting to note that Catenibacterium and Kandleria were observed to be more abundant in women with PCOS and in men compared with women without PCOS. Further studies are needed to clarify whether hyperandrogenism in females results in masculinization of the gut microbiome.
Potential Mechanisms for Sex Steroid Effects on Gut Microbiome
Currently, there is little understanding of the mechanisms involved in sex steroid regulation of the gut microbiome. Studies have reported that the amount of conjugated versus deconjugated estrogens excreted in urine and feces was altered after antibiotic treatment [69,70]. These results make sense in light of the fact that many gut bacteria synthesize beta-glucuronidase enzymes that deconjugate host-derived molecules such as bilirubin, neurotransmitters, and hormones that were previously conjugated in the liver [71]. While conjugation targets the compound for excretion via bile, feces, or urine, deconjugation facilitates reabsorption into enterohepatic circulation. Since deconjugation results in the liberation of a sugar group, glucuronic acid, this process produces energy for the gut bacteria. Thus, it is interesting to speculate that sex steroids could directly affect the composition of the gut microbiome by altering beta-glucuronidase activity and energy production due to changes in substrate levels.
In addition to a potential direct effect on gut microbes, sex steroids may indirectly modulate the gut microbiome through activation of steroid receptors in the host. While steroid hormone signaling in the nervous and immune systems has received significant attention, not much is known about the role of estrogen or androgen receptors in the gastrointestinal system. Estrogen receptor beta is expressed at higher levels in intestinal epithelial cells than estrogen receptor alpha and a knockout of estrogen receptor beta was reported to have an altered gut microbiome compared with wild-type mice [72,73]. Androgen receptor was reported to be expressed in human colon mucosa, while another study observed that the androgen receptor was expressed in colon stromal cells [74,75]. Thus, it is possible that changes in estrogens or androgens could result in altered intestinal functions such as contractility, transit, or enteric hormone secretion by regulating steroid receptor signaling within the gastrointestinal system, the brain, and/or the periphery. Interestingly, stool consistency (which reflects transit time and water availability) has been shown to be an important contributor to individual variation in the human gut microbiome [39].
Since studies have shown that there are sex differences in the immune system and most immune cells express steroid receptors, it is possible that changes in sex steroids could influence the composition of the gut microbiome by modulating systemic or intestinal immunity of the host. Although this is an understudied area, one possible mechanism could involve steroid signaling in dendritic cells, which are antigen-presenting cells important for promoting tolerance of commensal bacteria and bridging innate and adaptive immunity [76]. Studies have shown that estrogen signaling via estrogen receptor alpha is important for the development and function of dendritic cells [77]. However, these studies are complicated by the fact that sex-dependent differences in the gut microbiome may have an effect on immunity. For instance, transplantation of feces from male or female mice into germ-free mice showed that the percentage of specific immune cells in Peyer’s patches and mesenteric lymph nodes in the germ-free recipients differed according to the sex of the donor [78]. It is also possible that changes in sex steroids could alter the immune response indirectly by regulating the integrity of the intestinal barrier. Decreased integrity of the intestinal barrier is associated with infiltration of gram-negative bacteria into circulation and activation of a peripheral inflammatory response by lipopolysaccharide (LPS). Studies have shown that ovariectomy resulted in increased colonic epithelial cell permeability associated with decreased expression of tight junction proteins, occludin, and claudin [79,80]. Studies also demonstrated that markers of intestinal barrier dysfunction, including zonulin, a regulator of tight junctions and LPS binding protein, were increased in the serum of women with PCOS compared with healthy women [49].
Summary
The explosion of data arising from the sequencing of bacterial 16S rRNA genes has begun to provide insight into interactions between sex or sex steroids and the gut microbiome. It is now evident that sex plays a role in the maturation of the gut microbiome after puberty. Furthermore, studies have shown that changes in sex steroid levels can impact the composition of the gut microbial community in models of steroid insufficiency (ovariectomy and castration), suggesting that sex-dependent changes in the gut microbiome are driven primarily by circulating steroid hormone levels in females and males. Metagenomic and metabolomic studies are needed to confirm the associations of sex and sex steroids with the gut microbiome and define the full suite of microbes and microbial metabolites that are regulated by estrogens and androgens. Future mechanistic studies are also needed to understand how sex steroids shape the composition and function of the gut microbiome during normal physiological processes and in pathological situations of hormone insufficiency/excess. Regarding PCOS, studies in germ-free mice will be instrumental in determining whether gut microbes are necessary or sufficient for the development of the PCOS reproductive and metabolic phenotypes. Moreover, further studies are required to determine whether manipulation of the gut microbiome can be used to treat PCOS and to identify potential candidates for pre-/probiotic therapies.
Outstanding Questions
What are the effects of sex and sex steroids on the composition and function of the entire gut microbial community, including bacteria, archaea, fungi, and viruses? In addition to 16S rRNA sequencing, metagenomics and metabolomics could provide exciting insights into hormonal regulation of the gut microbiome.
What are the mechanisms involved in sex-dependent differences in the gut microbiome? How do steroid hormones regulate the gut microbiome in females and males with regards to normal biological functions and in pathological states of hormonal insufficiency or excess? Tissue-specific knockouts of estrogen and androgen receptors in mice could be informative in determining whether steroid receptors in tissues such as the brain, immune cells, intestinal epithelium, or liver are required to mediate sex steroid-dependent differences in the gut microbiome.
What are the consequences of sex and sex steroid-mediated changes in the gut microbiome on host physiology and host–microbe interactions? Do sex-dependent differences in the gut microbiome influence the development and pathology of disease states?
How is the composition and function of the gut microbiome altered in women with PCOS? Are these alterations influenced by androgen excess or obesity? Do women diagnosed with PCOS using different criteria have similar or distinct changes in their gut microbiome? Does hyperandrogenism result in masculinization of the gut microbiome? Can we identify prebiotics, probiotics, or microbial metabolites that could be used to treat PCOS?
Outstanding questions (4–5 statements)
What are the effects of sex and sex steroids on the composition and function of the entire gut microbial community including bacteria, archaea, fungi and viruses? In addition to 16S rRNA sequencing, metagenomics and metabolomics could provide exciting insights into hormonal regulation of the gut microbiome.
What are the mechanisms involved in sex-dependent differences in the gut microbiome? How do steroid hormones regulate the gut microbiome in females and males with regards to normal biological functions and in pathological states of hormonal insufficiency or excess? Tissue-specific knockouts of estrogen and androgen receptors in mice could be informative in determining whether steroid receptors in tissues such as the brain, immune cells, intestinal epithelium or liver are required to mediate sex steroid-dependent differences in the gut microbiome.
What are the consequences of sex and sex steroid-mediated changes in the gut microbiome on host physiology and host-microbe interactions? Do sex-dependent differences in the gut microbiome influence the development and pathology of disease states?
How is the composition and function of the gut microbiome altered in women with PCOS? Are these alterations influenced by androgen excess or obesity? Do women diagnosed with PCOS using different criteria have similar or distinct changes in their gut microbiome? Does hyperandrogenism result in masculinization of the gut microbiome? Can we identify prebiotics, probiotics or microbial metabolites that could be used to treat PCOS?
Highlights.
16S rRNA gene sequencing studies revealed that sex influences the taxonomic composition of gut bacteria in humans and rodents.
The idea that sex-dependent differences in gut microbes are driven by sex steroid levels is supported by studies demonstrating that the gut microbiome diverges after puberty and that gonadectomy results in an altered gut microbiome.
Recent studies demonstrated that changes in the gut microbiome are linked with androgen excess in women with PCOS and in female rodent models of the disorder. s
Studies reported that PCOS was associated with decreased alpha diversity and changes in the relative abundance of specific bacteria from the Bacteroidaceae, Clostridiaceae, Erysipelotrichidae, Lachnospiraceae, Lactobacillaceae, Porphyromonadaceae, Prevotellaceae, Ruminococcaceae, and S24–7 families previously linked with metabolic dysregulation.
Acknowledgements
I thank Scott Kelley for critical reading of the manuscript and editorial comments. V.G.T. was supported by the National Institute of Child Health and Human Development through a cooperative agreement as part of the National Centers for Translational Research in Reproduction and Infertility (P50 HD012303).
Glossary
- Activational effect
steroid hormone signaling which causes transient effects on specific cellular processes that are reversible upon removal of the hormone.
- Alpha diversity
represents the number of different species within a specific community or individual sample.
- Bacteroidetes
gram-negative bacteria that are one of the two predominant phyla present in the mammalian gut.
- Beta diversity
represents how similar one community or individual sample is to another.
- Beta-glucuronidase
enzyme synthesized in gut bacteria that deconjugates host-derived molecules such as bilirubin, neurotransmitters, and hormones that were previously conjugated in the liver.
- Firmicutes
mostly gram-positive bacteria that are one of the two predominant phyla present in the mammalian gut.
- Letrozole
non-steroidal inhibitor of aromatase (the enzyme responsible for the conversion of testosterone to estrogen).
- Lipopolysaccharide (LPS)
also known as endotoxin, LPS is the major component of the outer membrane of gram-negative bacteria. LPS activation of Toll-like receptor 4 results in cytokine production and activation of the innate immune system.
- Organizational effect
exposure to sex steroid hormones during development (e.g., in prenatal or early postnatal periods), which has been shown to result in permanent changes in tissues and organs such as the brain.
- 16S rRNA gene sequencing
universal primers containing distinct barcodes are used to amplify a region of the bacterial 16S ribosomal RNA gene in fecal or cecum samples and the resulting amplicons are pooled, purified, and sequenced.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korpela K and de Vos WM (2018) Early life colonization of the human gut: microbes matter everywhere. Curr. Opin. Microbiol 44, 70–78 [DOI] [PubMed] [Google Scholar]
- 2.Lynch SV and Pedersen O (2016) The human intestinal microbiome in health and disease. N. Engl. J. Med 375, 2369–2379 [DOI] [PubMed] [Google Scholar]
- 3.Backhed F, et al. (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 852. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Bello MG et al. (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. U. S. A 107, 11971–11975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley RE et al. (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ et al. (2009) A core gut microbiome in obese and lean twins. Nature 457, 480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen N et al. (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5, e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin J et al. (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 [DOI] [PubMed] [Google Scholar]
- 9.Ley RE et al. (2005) Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A 102, 11070–11075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cani PD et al. (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 [DOI] [PubMed] [Google Scholar]
- 11.Sze MA and Schloss PD (2016) Looking for a signal in the noise: revisiting obesity and the microbiome. MBio 7, e01018–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 [DOI] [PubMed] [Google Scholar]
- 13.Ridaura VK et al. (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuman H et al. (2015) Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev 39, 509–521 [DOI] [PubMed] [Google Scholar]
- 15.Thursby E and Juge N (2017) Introduction to the human gut microbiota. Biochem. J 474, 1823–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox HS (1992) Androgen treatment prevents diabetes in nonobese diabetic mice. J. Exp. Med 175, 1409–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markle JG et al. (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 [DOI] [PubMed] [Google Scholar]
- 18.Yurkovetskiy L et al. (2013) Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauser BC et al. (2012) Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril 97, 28–38 [DOI] [PubMed] [Google Scholar]
- 20.Teede H et al. (2010) Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azziz R, et al. (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2, 16057. [DOI] [PubMed] [Google Scholar]
- 22.Churchill SJ, et al. (2015) Metabolic consequences of polycystic ovary syndrome. Minerva Ginecol 67, 545–555 [PubMed] [Google Scholar]
- 23.Goodman NF, et al. (2015) American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome-part 2. Endocrine Practice 21, 1415–1426 [DOI] [PubMed] [Google Scholar]
- 24.Vassilatou E (2014) Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J Gastroentero 20, 8351–8363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang R, et al. (2016) Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta-analysis. Reproductive Biology and Endocrinology 14, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mykhalchenko K, et al. (2017) Genetics of polycystic ovary syndrome. Expert Rev Mol Diagn 17, 723–733 [DOI] [PubMed] [Google Scholar]
- 27.Legro RS, et al. (1998) Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A 95, 14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vink JM, et al. (2006) Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab 91, 2100–2104 [DOI] [PubMed] [Google Scholar]
- 29.Jones MR and Goodarzi MO (2016) Genetic determinants of polycystic ovary syndrome: progress and future directions. Fertil Steril 106, 25–32 [DOI] [PubMed] [Google Scholar]
- 30.Abbott DH and Bacha F (2013) Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil Steril 100, 2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkin SS, et al. (2016) Environmental determinants of polycystic ovary syndrome. Fertil Steril 106, 16–24 [DOI] [PubMed] [Google Scholar]
- 32.Barber TM, et al. (2007) Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 66, 513–517 [DOI] [PubMed] [Google Scholar]
- 33.Moghetti P, et al. (2013) Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab 98, E628–E637 [DOI] [PubMed] [Google Scholar]
- 34.Barbieri RL, et al. (1986) Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab 62, 904–910 [DOI] [PubMed] [Google Scholar]
- 35.Plymate SR, et al. (1988) Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67, 460–464 [DOI] [PubMed] [Google Scholar]
- 36.Stepto NK, et al. (2013) Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 28, 777–784 [DOI] [PubMed] [Google Scholar]
- 37.Huttenhower C, et al. (2012) Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominianni C, et al. (2015) Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One 10, e0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falony G, et al. (2016) Population-level analysis of gut microbiome variation. Science 352, 560–564 [DOI] [PubMed] [Google Scholar]
- 40.Mueller S, et al. (2006) Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol 72, 1027–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, et al. (2008) Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A 105, 2117–2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozik AJ, et al. (2017) Age, sex, and TNF associated differences in the gut microbiota of mice and their impact on acute TNBS colitis. Exp Mol Pathol 103, 311–319 [DOI] [PubMed] [Google Scholar]
- 43.Org E, et al. (2016) Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7, 313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yatsunenko T, et al. (2012) Human gut microbiome viewed across age and geography. Nature 486, 222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno-Indias I, et al. (2016) Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology 157, 4888–4898 [DOI] [PubMed] [Google Scholar]
- 46.Choi S, et al. (2017) Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J Microbiol Biotechnol 27, 2228–2236 [DOI] [PubMed] [Google Scholar]
- 47.Cox-York KA, et al. (2015) Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiological Reports 3, e12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harada N, et al. (2016) Castration influences intestinal microflora and induces abdominal obesity in high-fat diet-fed mice. Sci Rep 6, 23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lindheim L, et al. (2017) Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS One 12, e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu R, et al. (2017) Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Frontiers in Microbiology 8, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres PJ, et al. (2018) Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab 103, 1502–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Insenser M, et al. (2018) Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab 103, 2552–2562 [DOI] [PubMed] [Google Scholar]
- 53.Tilman D, et al. (2001) Diversity and productivity in a long-term grassland experiment. Science 294, 843–845 [DOI] [PubMed] [Google Scholar]
- 54.Walters WA, et al. (2014) Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett 588, 4223–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finucane MM, et al. (2014) A taxonomic signature of obesity in the microbiome? Getting to the guts of the matter. PLoS One 9, e84689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim MY, et al. (2017) The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut 66, 1031–1038 [DOI] [PubMed] [Google Scholar]
- 57.Everard A, et al. (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110, 9066–9071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim D, et al. (2017) Optimizing methods and dodging pitfalls in microbiome research. Microbiome 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight R, et al. (2018) Best practices for analysing microbiomes. Nat Rev Microbiol 16, 410–422 [DOI] [PubMed] [Google Scholar]
- 60.Chen T, et al. (2017) Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep 7, 2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clavel T, et al. (2016) The mouse gut microbiome revisited: from complex diversity to model ecosystems. Int J Med Microbiol 306, 316–327 [DOI] [PubMed] [Google Scholar]
- 62.Padmanabhan V and Veiga-Lopez A (2013) Animal models of the polycystic ovary syndrome phenotype. Steroids 78, 734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Houten ELAF and Visser JA (2014) Mouse models to study polycystic ovary syndrome: a possible link between metabolism and ovarian function? Reproductive Biology 14, 32–43 [DOI] [PubMed] [Google Scholar]
- 64.Walters KA (2016) Androgens in polycystic ovary syndrome: lessons from experimental models. Current Opinion in Endocrinology, Diabetes, and Obesity 23, 257–263 [DOI] [PubMed] [Google Scholar]
- 65.Kelley ST, et al. (2016) The gut microbiome is altered in a letrozole-induced mouse model of polycystic ovary syndrome. PLoS One 11, e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo YJ, et al. (2016) Association between polycystic ovary syndrome and gut microbiota. PLoS One 11, 0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skarra DV, et al. (2017) Hyperandrogenemia induced by letrozole treatment of pubertal female mice results in hyperinsulinemia prior to weight gain and insulin resistance. Endocrinology 158, 2988–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherman SB, et al. (2018) Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes, 9, 400–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adlercreutz H, et al. (1984) Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J Steroid Biochem 20, 217–229 [DOI] [PubMed] [Google Scholar]
- 70.Goldin BR and Gorbach SL (1984) Alterations of the intestinal microflora by diet, oral antibiotics, and Lactobacillus: decreased production of free amines from aromatic nitro compounds, azo dyes, and glucuronides. J Natl Cancer Inst 73, 689–695 [PubMed] [Google Scholar]
- 71.Pellock SJ and Redinbo MR (2017) Glucuronides in the gut: sugar-driven symbioses between microbe and host. J Biol Chem 292, 8569–8576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Looijer-van Langen M, et al. (2011) Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 300, G621–G626 [DOI] [PubMed] [Google Scholar]
- 73.Menon R, et al. (2013) Diet complexity and estrogen receptor beta status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol 79, 5763–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Catalano MG, et al. (2000) Altered expression of androgen-receptor isoforms in human colon-cancer tissues. Int J Cancer 86, 325–330 [DOI] [PubMed] [Google Scholar]
- 75.Waliszewski P, et al. (1997) Molecular study of sex steroid receptor gene expression in human colon and in colorectal carcinomas. J Surg Oncol 64, 3–11 [DOI] [PubMed] [Google Scholar]
- 76.Elderman M, et al. (2018) Role of microbiota in sexually dimorphic immunity. Front Immunol 9, 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laffont S, et al. (2017) Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol 8, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fransen F, et al. (2017) The impact of gut microbiota on gender-specific differences in immunity. Front Immunol 8, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braniste V, et al. (2009) Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol 587, 3317–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li JY, et al. (2016) Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest 126, 2049–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]