Abstract
Vascular smooth muscle cells (SMCs) undergo a series of dramatic changes in CADASIL, the most common inherited cause of vascular dementia and stroke. NOTCH3 protein accumulates and aggregates early in CADASIL, followed by loss of mature SMCs from the media of brain arteries and marked intimal proliferation. Similar intimal thickening is seen in peripheral arterial disease, which features pathological intimal cells including proliferative, dedifferentiated, smooth muscle-like cells deficient in SMC markers. Limited studies have been performed to investigate the differentiation state and location of SMCs in brain vascular disorders. Thus, we investigated the distribution of cells expressing SMC markers in a group of genetically characterized, North American CADASIL brains. We quantified brain RNA abundance of these markers in nine genetically verified cases of CADASIL and found that mRNA expression for several mature SMC markers was increased in CADASIL brain compared to age-matched control. Immunohistochemical studies and in situ hybridization localization of mRNA demonstrated loss of SMCs from the arterial media, and SMC marker-expressing cells were instead redistributed into the intima of diseased arteries and around balloon cells of the degenerating media. We conclude that, despite loss of medial smooth muscle cells in diseased arteries, smooth muscle markers are not lost from CADASIL brain, but rather, the localization of cells expressing mature SMC markers changes dramatically.
Keywords: CADASIL, Notch, areteries, smooth muscle, intimal hyperplasia, small vessel disease
1. Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the best-characterized inherited cause of cerebral small vessel disease. Small vessels in CADASIL undergo marked degenerative changes that include loss of vascular smooth muscle cells (SMCs), replacement of arterial media with fibrotic acellular material, accumulation of granular osmiophilic material (GOM) outside the plasma membrane of dying SMCs, and marked thickening of arterial walls in penetrating small arteries of the brain [1–3]. In 1997, Joutel and colleagues identified stereotyped mutations in NOTCH3 in families with CADASIL [4]. The expression of NOTCH3 in vascular smooth muscle suggested that mutant proteins initiate pathological cascades that drive small vessel degeneration in CADASIL. Moreover, there is marked deposition of the NOTCH3 ectodomain surrounding vascular SMCs [5]. Whether this NOTCH3 accumulation alters vascular SMCs or represents compensatory segregation of misfolded protein remains a topic of debate [6, 7].
In addition to the degeneration of vascular SMCs and fibrosis of arterial media in CADASIL, we have previously described the dramatic intimal hyperplasia of cerebral arteries [8]. We have noted strong expression of vimentin and S100 calcium binding protein A4 (S100A4) within the thickened hyperplastic intima of arteries of all sizes in CADASIL brain [8]. In peripheral arterial disease, intimal thickening also features vimentin expression and infiltration of immature, de-differentiated smooth muscle cells that have migrated under the endothelium [9]. Since CADASIL is a result of mutant NOTCH3-mediated SMC dysfunction, it is reasonable to speculate that intimal thickening may result from this same process of SMC de-differentiation and migration. However, studies of the maturation of cerebral intimal cells in CADASIL have not yet been described.
Prior to genetic testing for cysteine-related mutations in NOTCH3, multiple cases were reported that included unusual rounded cells with clear cytoplasm within the degenerating vascular media of leptomeningeal arteries [10, 11]. These cells, described as balloon (or halo) cells, are strikingly distinct from normal vascular smooth muscle. Balloon cells were hypothesized by early investigators to be smooth muscle in origin, based on reactivity with antibodies against desmin, actin, myosin, vimentin, and tenascin [12]. These early studies, however, leave several questions about balloon cells unanswered. For one, early studies were performed before the availability of genetic testing; therefore, balloon cells have yet to be studied in genetically confirmed CADASIL. In addition, it is not known whether balloon cells synthesize smooth muscle markers; an alternative explanation for early findings is that smooth muscle protein released from arteries could be internalized by balloon cells.
Previous studies of SMCs in CADASIL have largely relied on immunoreactivity of smooth muscle actin (SMA) as a marker for SMC differentiation [13]. Histological studies have demonstrated loss of SMA in CADASIL microvessels of the white matter and cerebral cortex, in accordance with medial layer SMC loss [14, 15]. In addition, in these tissues, intimal hyperplasia demonstrated heterogeneous expression of SMA. However, these studies make use of relatively small CADASIL sample size, and not all CADASIL cases in these studies are genetically confirmed [14, 15]. SMA was thought at that time to mark mature SMCs, but SMA expression has more recently been found in a host of other cell types and processes [16] and is now not considered, by itself, an indicator of SMC maturation. Moreover, these previous pathological studies were, at least in part, conducted on brains studied prior to availability of genetic confirmation of Notch3 mutations [10].
Since the initial descriptions of CADASIL vascular pathology, additional vascular smooth muscle markers have been described that differentiate two poles of SMC phenotypes: synthetic and contractile. For example, the markers myosin-11 (MHC or MYH11) [17–19] and smoothelin (SMTN) [20] are thought to be relatively specific for contractile SMCs. Loss of these markers is a common hallmark of phenotypic modulation to synthetic smooth muscle cells. On the other hand, calponin (CNN1) [21], smooth muscle actin (SMA or ACTA2) [22, 23] and transgelin (SM22 or TAGLN) [21, 24] were initially thought to be mature smooth muscle markers, but have now been observed in smooth muscle in multiple developmental and differentiation states [25–27]. Examination of multiple smooth muscle markers could shed light on the fate of SMCs in CADASIL and more accurately phenotype the differentiation state of cells within cerebral intimal hyperplasia.
An expanded analysis of SMC markers in CADASIL has not yet been performed. In this study, we examined in detail arterial expression of five smooth muscle markers in genetically defined CADASIL brains from our North American cohort. We sought to answer two questions. First, we wished to resolve whether expression of mature smooth muscle markers in CADASIL is altered. Second, we wished to characterize the distribution of mature SMC marker expression in the media, intima, and balloon cells of CADASIL arteries. We found that these markers are not lost from CADASIL brain vasculature, but rather redistributed to the hyperplastic intima and balloon cells of diseased arteries.
2. Materials and Methods
Patients and Controls
Nine CADASIL brains from patients with mutations in coding sequences of NOTCH3 that are predicted to result in either gain or loss of cysteine were obtained for analysis by qPCR. The genotypes of these brains are previously described [28]. We included in our analysis an additional brain with the R196C mutation in NOTCH3. The average age at death was 65. All patients died from CADASIL-related complications. Eight brains from age-matched controls were obtained from the Alzheimer’s Disease Center of the University of Michigan. The average age of these non-demented controls at death was 62 years old.
RNA Quantification
RNA from frozen brain tissue was purified using an RNeasy kit, reverse transcribed, and cDNA was quantified by real-time PCR. All experiments were carried out in triplicate. A list of primer sequences used can be found in Table 1. Expression levels shown are relative to 18S ribosomal RNA.
Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| Human 18s | CAGCCACCCGAGATTGAGCA | TAGTAGCGACGGGCGGTGTG |
| Human SMA | CTAAGACGGGAATCCTGTGA | CTTTTCCATGTCGTCCCAGT |
| Human MHC | TTGGAGATCTGGGACCAACA | CTCCTCCTTAATGCTGGCTG |
| Human CNN1 | GAACAAGCTGGCCCAGAAGT | GTTGGCCTCAAAAATGTCGT |
| Human SM22 | TGGTTCCCTAAGAAATCCAAG | CAGAGGTGACAGGACAGGCT |
| Human SMTN | AGGAAGAGAGATGGCAGTGG | TCTTTCTTCTTCTCGGCCTG |
| Human CD31 | TCCGGATCTATGACTCAGGG | ACAGTTGACCCTCACGATCC |
| Human CD34 | AGTTTGCTGCCTTCTGGGTTC | CCATGTTGAGACACAGGGTG |
| Human ABCC1 | AACCAAAACTGCCTTGGGAT | AGAGAGTTGGGCTGACCAGA |
| Human VWF | GACTGTCCAGTGTGCTGAT | CCGTCACTGTATGCTGGAT |
Brain Histology
Frontal cortex samples from the CADASIL brains mentioned above were sectioned and paraffin embedded for immunohistochemical analysis. Formalin-fixed frontal lobe sections from the University of Maryland Brain Bank served as age-matched controls. Control sections were taken from patients with no history of cerebrovascular disease.
Five-micron sections from the frontal cortex were analyzed using chromagenic immunohistochemical staining, counterstained with hematoxylin as previously described [28]. Samples were stained with anti-SMA (1A4; Cell Marque) at 1:1000, anti-MHC (anti-MYH11, Sigma-Aldrich) at 1:300, anti-TAGLN (Sigma-Aldrich) at 1:100, anti-CNN1 (Thermo Scientific) at 1:500, and anti-SMTN (Sigma-Aldrich) at 1:500 dilution. Antibodies to conformational epitopes of NOTCH3 have been previously described [29]. Validation of these antibodies in the Human Protein Atlas (proteinatlas.org) demonstrates expected SMC localization in over three dozen tissues.
Balloon cells were scored as large round cells within the wall of an artery with a central nucleus. Random arteries within each section were scored for the presence of balloon cells and the number of these cells per 100 arteries was manually computed for each patient.
In Situ Hybridization
Localization of SMA and TAGLN mRNA in tissue sections was performed using a system designed by Advanced Cell Diagnostics. The protocol incorporated hybridization of nucleic acid probes, multiple non-enzymatic amplification steps, and probe detection using an alkaline phosphatase-conjugated terminal probe as previously described [28]. Expected distribution of signal in normal smooth muscle was observed for all probes.
Statistical Analysis
All figures display means. Error bars represent standard error of the mean. Statistical analysis was performed using two-tailed, unpaired t-tests and significance was assigned at p<0.05. Tests for statistical significance were conducted using Microsoft Excel and GraphPad Prism 7.
3. Results
Transcripts of mature smooth muscle markers are elevated in CADASIL brain
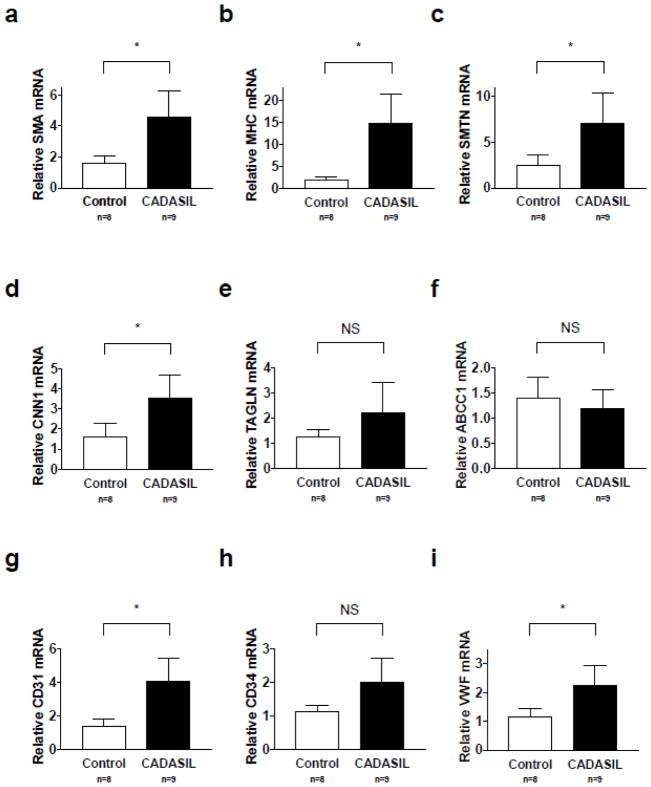
Because of reports that vascular SMCs are lost in CADASIL [14, 15], we determined the relative levels of smooth muscle marker gene expression in brain lysates of CADASIL patients and controls using quantitative RT-PCR. Contrary to expectations, we observed significant increases in the mRNA levels of several smooth muscle marker genes tested, including: SMA (p=0.0100), MHC (p=0.0314), SMTN (p=0.0394), and CNN1 (p=0.0224) (Figure 1a–1d). TAGLN mRNA levels were modestly increased in CADASIL brain lysate, but this difference was not significant (p=0.2094, Figure 1e).
Fig. 1. Transcripts of smooth muscle markers are increased in CADASIL.
Transcripts of endothelial markers are either increased or not significantly altered in CADASIL. Cerebral cortex samples from CADASIL patients and controls were lysed and RNA harvested. SMA (a), MHC (b), SMTN (c), CNN1 (d), TAGLN (e), ABCC1 (f), CD31 (g), CD34 (h), and VWF (i) mRNA levels were assayed using quantitative RT-PCR. SMA, MHC, SMTN, CNN1, CD31, and VWF mRNA levels were significantly elevated in CADASIL patients with p<0.05. There was no significant difference in TAGLN, ABCC1, or CD34 mRNA levels. 9 CADASIL brains and 8 age-matched control brains were analyzed
We also compared the relative levels of endothelial markers in brain lysate from CADASIL patients and controls. ATP binding cassette subfamily C member 1 (ABCC1) levels were not significantly altered in CADASIL brain lysate relative to controls (Figure 1f). Cluster of differentiation 31 (CD31) and von Willebrand factor (vWF) levels were significantly elevated in CADASIL brain lysate (Figure 1g, i) (p=0.0033 and p=0.0255 respectively). Cluster of differentiation 34 (CD34) levels were modestly increased in CADASIL brain lysate, but this difference was not significant (Figure 1h, p=0.0589).
While these studies do not account for potential differences in the amount of total vasculature between CADASIL and age-matched control brain, these results certainly warrant further investigation, as they diverge from previous descriptions of SMC markers in CADASIL brain.
Mature smooth muscle markers disappear from degenerating arterial media but appear in balloon cells and neointima in CADASIL brain
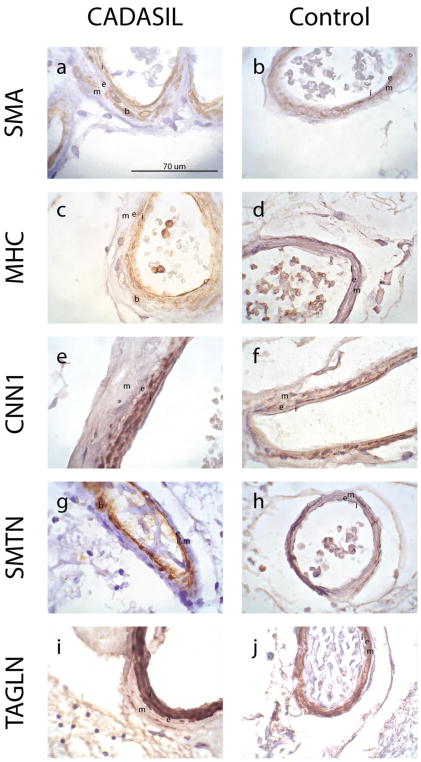
To determine the cell types in the brain that express smooth muscle marker proteins, we performed immunohistochemistry on CADASIL and age-matched control brain tissue samples. Not surprisingly, medial staining was low or absent in CADASIL samples (Figures 2, 3). The media of control vessels appeared healthy and demonstrated expected, medial staining (and little to no intimal staining) for SMA, CNN1, and TAGLN (Figures 2b, 2f, 2j, 3b, 3f, 3j). Control staining for MHC and SMTN in leptomeningeal arteries was modest (Figures 2d, h). The appearance of smooth muscle marker expressing cells was most easily defined in the leptomeningeal arteries of CADASIL (Figure 2a, c, e, g, i), which we have previously been shown exhibit massive intimal proliferation [8]. These leptomeningeal arteries show a clear redistribution of smooth muscle markers from the arterial media to the intimal and balloon cells.
Fig. 2. Expression of smooth muscle proteins in the intima of leptomeningeal vessels in CADASIL brain.
Immunohistochemical analysis of leptomeningeal vessels in frontal lobe sections from genetically confirmed CADASIL patients (a, c, e, g, i) and controls (b, d, f, h, j) were performed on frontal lobe sections with 1A4 (anti-SMA), anti-MHC, anti-CNN1, anti-SMTN, and anti-TAGLN. Leptomeningeal vessels are shown. Noted on the image are the intima (i), internal elastic lamina (e), media (m), and balloon cells (b). Magnification of all images is 1000X. Scale bar in (a) applies to all images in this figure
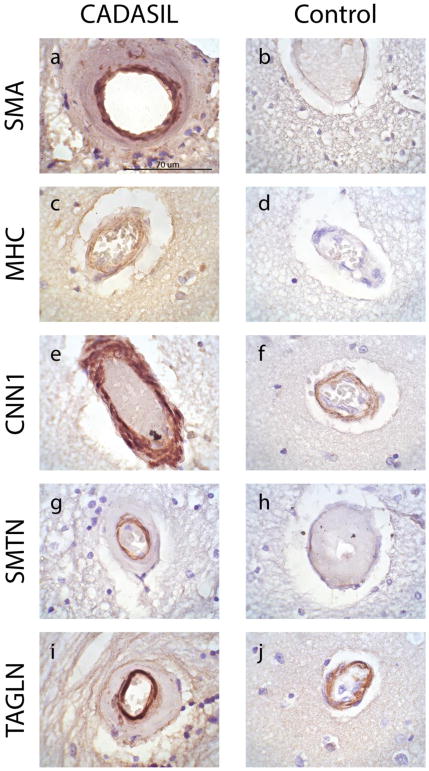
Fig. 3. Expression of smooth muscle proteins in the intima of small penetrating vessels in CADASIL brain.
Immunohistochemical analysis of small penetrating vessels in frontal lobe sections from genetically confirmed CADASIL patients (a, c, e, g, i) and age-matched controls (b, d, f, h, j) were performed on frontal lobe sections with 1A4 (anti-SMA), anti-MHC, anti-CNN1, anti-SMTN, and anti-TAGLN. Small penetrating vessels are shown. Magnification of all images is 1000X. Scale bar in (a) applies to all images in this figure
Staining for all tested smooth muscle markers in CADASIL was very clear in the most inner layer of thickened penetrating arteries, which contain intimal cells resulting from pathological intimal proliferation (Figure 3a, c, e, g, i). The small penetrating arteries in CADASIL cortical sections were noticeably thickened relative to age-matched control, and displayed unequivocal staining for all markers. Age-matched controls displayed modest to no immunoreactivity for SMA, MHC, and SMTN, but displayed staining comparable to that of CADASIL brain for CNN1 and TAGLN (Figure 3). Capillaries did not show appreciable staining for any of the markers tested in either CADASIL or control brain (not shown), further indicating that these markers are primarily expressed in non-endothelial cells.
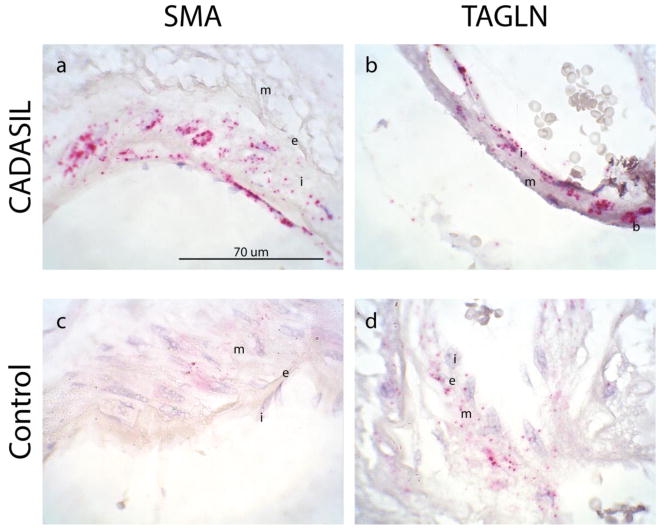
We verified that intimal cells were actively synthesizing these markers by performing in situ hybridization on CADASIL samples to localize SMA and TAGLN mRNA. The intimal cells in CADASIL clearly expressed mRNA encoding both smooth muscle markers (Figure 4a–b). In addition, balloon cells of the media expressed notable amounts of smooth muscle mRNA (Figure 4b). Transcripts of these markers in control arteries were primarily expressed in medial cells, with only some diffuse staining of intimal cells for TAGLN (Figure 4c–d).
Fig. 4. Mature smooth muscle genes are actively transcribed in intimal and balloon cells of CADASIL vessels.
In situ hybridization was performed on cortical sections of brains from CADASIL patients. Leptomeningeal arteries are shown. Distribution of SMA (a, c) and TAGLN (b, d) transcripts is shown in CADASIL (a–b) and control (c–d) vessels. Balloon cells (b), intima (i), media (m) and internal elastic lamina (e) are noted in the image. Presence of mRNA is represented by red punctae. Magnification is 1000X. Scale bar in (a) applies to all images in this figure
In sum, in CADASIL, smooth muscle marker expression was increased due to expression in cells of the intima, despite loss of cells from the arterial media.
Balloon Cells are increased in CADASIL brain vasculature and synthesize molecular markers characteristic of mature smooth muscle
One medial cell type that did not express severely diminished smooth muscle markers was the balloon cell. Balloon cells have been noted in previous pathological studies of CADASIL and were described as large, bloated, degenerating cells of unclear origin [10]. They were proposed to be of smooth muscle origin based on limited immunohistochemical profiling [10]. We found that these cells expressed the screened smooth muscle markers at relatively high levels in leptomeningeal arteries (Figure 2).
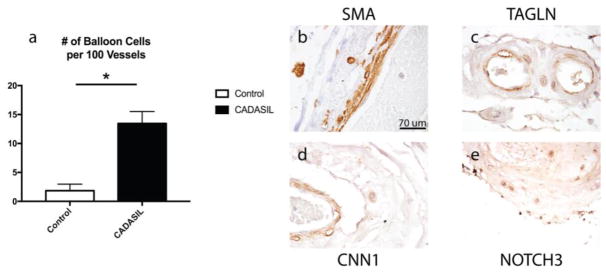
Balloon cells were initially visualized using hematoxylin and eosin staining of frontal lobe brain sections. Figure 5a shows the manually counted number of balloon cells per 100 vessels found in CADASIL compared to control brains. CADASIL leptomeningeal arteries and deep penetrating arteries of the white matter were markedly thickened and displayed profound intimal hyperplasia, relative to control brain vessels, as described earlier [8]. Balloon cells were common in CADASIL leptomeningeal arteries and were principally located in the border between the degenerating medial layer of arteries and the adventitia. Occasional cells in the media of control leptomeningeal arteries showed the same morphological features of balloon cells. Balloon cells were not found in the penetrating arteries of either CADASIL or control brain.
Fig. 5. Balloon cell frequency in CADASIL and expression of smooth muscle marker proteins in balloon cells of CADASIL leptomeningeal arteries.
(a) The number of balloon cells found in control compared to CADASIL arteries is shown. The number of balloon cells per 100 vessels counted was averaged for each patient studied. Significant differences between groups are denoted by an asterisk (p=0.0002). (b–f) Representative balloon cell immunohistochemical staining is shown for leptomeningeal arteries of CADASIL patients. Markers for smooth muscle proteins in CADASIL included: SMA (b), TAGLN (c), CNN1 (d), and CADASIL conformation of NOTCH3 (e; 2079). Leptomeningeal vessels are shown. Magnification is 400X. Scale bar in (b) applies to all histological images in this figure
The location of balloon cells at the edge of the media suggested that they are potentially of vascular smooth muscle origin. To test whether balloon cells shared protein antigens with smooth muscle, we evaluate expression of vascular smooth muscle markers by immunohistochemistry (Figure 5b–5f). Balloon cells were positive for all smooth muscle proteins tested, including CNN1, SMA, TAGLN, MHC and NOTCH3 (Figure 5b–5e).
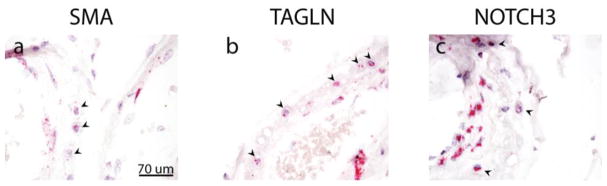
A large amount of smooth muscle protein could be released in the media during cell death in CADASIL. This released protein could be endocytosed by balloon cells. Alternatively, the balloon cells could be the cellular source of these markers. To distinguish between these possibilities, we used in situ hybridization to detect mRNA for vascular smooth muscle genes. Three mRNA species were localized in CADASIL arteries: SMA, TAGLN, and NOTCH3 (Figure 6). Each of these was expressed in both residual smooth muscle cells and intimal cells. Cells lining the lumen were also apparently positive for each of these mRNAs. Virtually all balloon cells examined expressed smooth muscle mRNAs. In situ hybridization was also performed for several endothelial mRNAs. Balloon cells did not express mRNA encoding the endothelial markers CD31, CD34 or vWF (data not shown).
Fig. 6. Vascular smooth muscle mRNA expression in balloon cells of CADASIL.
In situ hybridization for vascular smooth muscle marker mRNA was performed on CADASIL cortical sections. Markers included: SMA (a), TAGLN (b), and NOTCH3 (c). Presence of mRNAs in balloon cells is represented by red punctae. Arrowheads denote balloon cells. Leptomeningeal vessels are shown. Magnification is 400X. Scale bar in (a) applies to all images in this figure
4. Discussion
Mutant NOTCH3 is the initial pathological trigger in CADASIL [7]. Through uncertain molecular mechanisms, mutant NOTCH3 with an altered number of cysteine residues likely adopts an abnormal form that leads to NOTCH3 protein accumulation, culminating in SMC dysfunction and degeneration. Localization of NOTCH3 in mature vascular SMCs in normal arteries suggests a cell autonomous effect of mutant protein resulting in loss of smooth muscle markers due to cell death [30]. Thus, it has been assumed that CADASIL brains feature diminished overall numbers of mature SMCs. To better characterize the fate of SMCs in pathology of cerebral vessels, we examined the expression of mature smooth muscle markers in genetically characterized CADASIL brains from North American patients. Previous studies focused on European samples that were not uniformly genetically confirmed cases. We found that smooth muscle protein expression is maintained in CADASIL, largely due to redistribution of expression from medial to intimal cells, which continue to express mature smooth muscle markers.
Several pathological studies have demonstrated that CADASIL arteries are affected by medial cell loss and concomitant replacement of the medial architecture by matrix proteins such as collagens [31]. Our study provides confirmation of this by demonstrating deficiency of mature smooth muscle protein and mRNA in the arterial media. What is more surprising is that in CADASIL vessels, the intima contains a large number of cells that express mature smooth muscle markers. This contrasts studies of peripheral vessels which suggest that pathological intimal thickening is composed mostly of dedifferentiated SMCs [32, 33]. These results suggest that, in CADASIL, intimal hyperplasia could be driven by processes that are distinct from mechanisms that drive intimal thickening in peripheral tissues. Certainly, clinical studies have suggested that processes that induce intimal hyperplasia in the cerebral circulation, such as stenting, may differ from those occurring in peripheral arterial counterparts [34]. SMCs of the vascular wall display considerable heterogeneity, and their phenotype is subject to modulation at any point along the continuum from synthetic to contractile based on a variety of biochemical and environmental cues [35, 36]. As noted, the process of intimal hyperplasia in peripheral arteries features a notable loss of contractile SMCs and a concordant increase in synthetic SMCs [37].
In the present study, we show that vWF transcripts are significantly elevated in CADASIL brain relative to age-matched control (Figure 1i). However, we have also previously shown that vWF inhibits smooth muscle gene expression in cultured SMCs [38]. There are some key differences between our previous and present studies. First, our previous study examined smooth muscle gene expression in cultured SMCs, while the present investigation examines in vivo expression of these markers in postmortem human brain. Furthermore, while our qPCR data indicate that both vWF and several smooth muscle markers are upregulated in CADASIL brain, it is unclear based on these data if these markers are upregulated in the same brain regions. Finally, our previous study examined the effect of a brief pulse of vWF protein on SMC gene expression. This could differ from a long period of elevated vWF mRNA in the brain.
In 2012, Tikka and colleagues showed that SMA protein levels were not significantly altered in CADASIL SMCs relative to control SMCs [39]. However, our results indicate that SMA mRNA levels are elevated in CADASIL brain lysate (Figure 1a). There could be a few reasons for the divergence between the two studies. First, our CADASIL cohort contained a different group of genotypes compared to the Tikka study. Second, we assayed RNA levels, while the Tikka study analyzed protein levels. Third, our study examined postmortem brain lysate, while the Tikka study generated cell lines from patient samples. Finally, our study analyzed whole-brain lysate, while the Tikka study examined only SMCs in cell culture. These methodological differences could account for differences between the two studies. Furthermore, since our study analyzed RNA levels, and the Tikka study analyzed protein, it is possible that while SMA transcripts are upregulated in CADASIL, those transcripts are not concordantly translated, yielding similar protein yield in CADASIL and control cerebrovascular SMCs.
Our identification of mature smooth muscle markers in the intima of CADASIL arteries calls for us to reconcile these results with existing descriptions of intimal hyperplasia. Several studies of peripheral intimal hyperplasia have suggested that medial SMCs are the origin of neointimal cells after vascular injury [40–43]. This phenotypic transition is marked by a shift from spindle-shaped to epithelioid morphology, increased protein synthesis, cell proliferation, and cell migration from the medial to the intimal layer [36, 44]. For a similar process to occur in CADASIL, one would need to speculate that cerebral SMCs maintain expression of some mature, contractile markers while undergoing a migratory transition. This would suggest SMC transdifferentiation pathways are unique to cerebral vessels.
An alternative mechanism of intimal thickening suggests that neointimal cells originate from circulating pluripotent cells [45–47]. Previous studies have shown the role of PDGF molecules in inducing a synthetic phenotype [48]. Thus, it is possible that circulating progenitor cells permeate the vascular endothelium and burrow into the neointima where they are exposed to biochemical factors, such as PDGFs, and extracellular matrix components, which induce differentiation into synthetic SMCs. If this alternative process is responsible for cerebral intimal hyperplasia, these burrowing cells may be modulated by brain-derived factors that induce (or preserve) expression of mature smooth muscle markers observed in CADASIL intimal hyperplasia. More work in animal models of cerebrovascular intimal hyperplasia needs to be performed to clarify this process.
One can envision yet another alternative mechanism by which resident, non-endothelial, intimal cells are exposed to environmental cues that lead to the proliferation of synthetic SMCs in this layer via a mechanism that leads to concomitant expression of contractile SMC markers. In our current study, we cannot comment on which of these mechanisms is at play. We do, however, present the novel finding that the contractile SMC markers once thought to be lost from CADASIL vessels are expressed in the hyperplastic intima of diseased arteries. Ongoing debates about the origin of neointima in the cerebrovasculature must account for this surprising finding.
This study also investigated remnant cells in the arterial media in CADASIL that have long been described as characteristic of the disease. These cells, so called balloon cells, are a pathological feature of larger cerebral arteries that were identified in pre-genetic cases of CADASIL in European studies [10]. We verify the strong presence of these cells in CADASIL samples from our genetically characterized North American cohort. These data suggest that these cells are, as previously hypothesized, derived from smooth muscle cells. Markers for mature smooth muscle cells, at both the level of protein and mRNA, were found in these cells. The smooth muscle origin of these distinctively abnormal cells supports a cell autonomous role of NOTCH3 in producing CADASIL pathology. Based on these data, balloon cells are not likely to be a distinct cell type, such as an unusual inflammatory cell, but rather could represent a stage of SMC degeneration within the vessel wall.
This investigation calls into question the mechanism by which smooth muscle marker expression is driven in the hyperplastic intima of CADASIL arteries, and what, if any, molecular requirements exist for this upregulation. NOTCH3 ectodomain accumulation is a molecular hallmark of CADASIL arteries [5]. We have also previously described the accumulation of proteoglycans and collagens in the medial extracellular matrix of diseased CADASIL arteries [49–51]. Future studies should investigate what, if any, effect(s) these molecular changes could have on the expression of the in question.
In summary, we report the surprising finding that smooth muscle markers are not lost from CADASIL brain. Rather, these markers are reduced in the degenerating arterial media and redistributed into balloon cells the hyperplastic intima. This result gives pause to the use of total smooth muscle protein as an indicator of brain vascular disease. Lower smooth muscle marker expression does not necessarily mean smooth muscle loss, since smooth muscle marker loss does not seem to correlate with disease progression. We make the novel observation that SMC behavior diverges between vascular diseases in the brain and in the periphery; further studies are needed to determine how arterial intimal growth differs between these distinct vascular beds.
Acknowledgments
The National Institutes of Health (NS099783 and HL108842) and the Department of Veterans Affairs (BX000375 and BX003824) provided funding for these studies. We also appreciate the support of CADASIL Together We Have Hope and members of the international CADASIL community who made donations used in this study.
Funding: This study was funded by the National Institutes of Health (NS099783 and HL108842) and the U.S. Department of Veterans Affairs (BX000375 and BX003824).
Footnotes
Compliance with Ethical Standards
John R. Gatti declares that he has no conflicts of interest.
Xiaojie Zhang declares that she has no conflicts of interest.
Ejona Korcari declares that she has no conflicts of interest.
Soo Jung Lee declares that she has no conflicts of interest.
Nya Greenstone declares that she has no conflicts of interest.
Jon G. Dean declares that he has no conflicts of interest.
Snehaa Maripudi declares that she has no conflicts of interest.
Michael M. Wang declares that he has no conflicts of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Miao Q, Paloneva T, Tuominen S, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14:358–364. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalimo H, Viitanen M, Amberla K, et al. CADASIL: Hereditary disease of arteries causing brain infarcts and dementia. Neuropathol Appl Neurobiol. 1999;25:257–265. doi: 10.1046/j.1365-2990.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 3.Baudrimont M, Dubas F, Joutel A, Tournier-Lasserve E, Bousser M-G. Case Report Autosomal Dominant Leukoencephalopathy and Subcortical Ischemic Stroke. Stroke. 1993;24:122–126. doi: 10.1161/01.str.24.1.122. [DOI] [PubMed] [Google Scholar]
- 4.Joutel A, Vahedi K, Corpechot C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Craggs LJL, Watanabe A, et al. Brain Microvascular Accumulation and Distribution of the NOTCH3 Ectodomain and Granular Osmiophilic Material in CADASIL. J Neuropathol Exp Neurol. 2013;72(5):416–431. doi: 10.1097/NEN.0b013e31829020b5. [DOI] [PubMed] [Google Scholar]
- 6.Ruchoux MM, Domenga V, Brulin P, et al. Transgenic Mice Expressing Mutant Notch3 Develop Vascular Alterations Characteristic of Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. Am J Pathol. 2003;162(1):329–342. doi: 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi K, Adachi K, Yoshizaki K, Kunimoto S, Kalaria RN, Watanabe A. Mutations in NOTCH3 cause the formation and retention of aggregates in the endoplasmic reticulum, leading to impaired cell proliferation. Hum Mol Genet. 2009;19(1):79–89. doi: 10.1093/hmg/ddp468. [DOI] [PubMed] [Google Scholar]
- 8.Dong H, Ding H, Young K, Blaivas M, Christensen PJ, Wang MM. Advanced intimal hyperplasia without luminal narrowing of leptomeningeal arteries in CADASIL. Stroke. 2013;44(5):1456–1458. doi: 10.3174/ajnr.A1650.Side. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varcoe RL, Mikhail M, Guiffre AK, Pennings G, Vicaretti M, Hawthorne WJ. The role of the fibrocyte in intimal hyperplasia. J Thromb Haemost. 2006;4:1125–1133. doi: 10.1111/j.1538-7836.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruchoux M-M, Maurage C-A. CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56(9):947–964. [PubMed] [Google Scholar]
- 11.Gutierrez-Molina M, Caminero Rodriguez A, Martinez Garcia C, Arpa Gutierrez J, Morales Bastos C, Amer G. Small arterial granular degeneration in familial Binswanger’s syndrome. Acta Neuropathol. 1994;87:98–105. doi: 10.1007/BF00386260. [DOI] [PubMed] [Google Scholar]
- 12.Ruchoux M-M, Guerouaou D, Vandenhaute B, Pruvo J-P, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol. 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- 13.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brennan-Krohn T, Salloway S, Correia S, Dong M, De La Monte SM. Glial vascular degeneration in CADASIL. J Alzheimer’s Dis. 2010;21(4):1393–1402. doi: 10.3233/JAD-2010-100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, et al. Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL. Immunohistochemical and ultrastructural studies. Folia Neuropathol. 2007;45(4):192–204. [PubMed] [Google Scholar]
- 16.Owens GK, Kumar MS, Wamhoff BR. Molecular Regulation of Vascular Smooth Muscle Cell Differentiation in Development and Disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 17.Frid MG, Printesva OY, Chiavegato A, et al. Myosin Heavy-Chain Isoform Composition and Distribution in Developing and Adult Human Aortic Smooth Muscle. J Vasc Res. 1993;30:279–292. doi: 10.1159/000159007. [DOI] [PubMed] [Google Scholar]
- 18.Madsen CS, Regan CP, Hungerford JE, White SL, Manabe I, Owens GK. Smooth Muscle Specific Expression of the Smooth Muscle Myosin Heavy Chain Gene in Transgenic Mice Requires 5′-Flanking and First Intronic DNA Sequence. Circ Res. 1998;82:908–917. doi: 10.1161/01.RES.82.8.908. [DOI] [PubMed] [Google Scholar]
- 19.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.RES.75.5.803. [DOI] [PubMed] [Google Scholar]
- 20.van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134(2):401–411. doi: 10.1083/jcb.134.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1–11. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 22.Gabbiani G, Schmid E, Winter S, et al. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens GK, Thompson MM. Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo. Relationship between growth and cytodifferentiation. J Biol Chem. 1986;261(28):13373–13380. [PubMed] [Google Scholar]
- 24.Li L, Miano JM, Cserjesi P, Olson EN. SM22α, a Marker of Adult Smooth Muscle, Is Expressed in Multiple Myogenic Lineages During Embryogenesis. Circ Res. 1996;78(2):188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 25.Han DKM, Liau G. Identification and characterization of developmentally regulated genes in vascular smooth muscle cells. Circ Res. 1992;71(3):711–719. doi: 10.1161/01.res.71.3.711. [DOI] [PubMed] [Google Scholar]
- 26.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 27.Miano JM, Olson EN. Expression of the smooth muscle cell calponin gene marks the early cardiac and smooth muscle cell lineages during mouse embryogenesis [published erratum appears in J Biol Chem 1997 Oct 24;272(43):27492] J Biol Chem. 1996;271(12):7095–7103. doi: 10.1074/jbc.271.12.7095. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Lee SJ, Young MF, Wang MM. The small leucine-rich proteoglycan BGN accumulates in CADASIL and binds to NOTCH3. Transl Stroke Res. 2015;6(2):148–155. doi: 10.1007/978-1-4939-2914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Lee SJ, Young KZ, Josephson DA, Michael D, Wang MM. Latent NOTCH3 epitopes unmasked in CADASIL and regulated by protein redox state. Brain Res. 2014;1583:230–236. doi: 10.1016/j.brainres.2014.08.018.Latent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shawber CJ, Kitajewski J. Notch function in the vasculature: Insights from zebrafish, mouse and man. BioEssays. 2004;26(3):225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- 31.Kalimo H, Ruchoux M-M, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002;12:371–384. doi: 10.1111/j.1750-3639.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aikawa M, Rabkin E, Voglic SJ, et al. Muscle Cells Expressing Smooth Muscle Myosin Heavy. Circ Res. 1998;83(10):1015–1026. doi: 10.1161/01.res.83.10.1015. [DOI] [PubMed] [Google Scholar]
- 34.Yoon NK, Awad A-W, Kalani MYS, Taussky P, Park MS. Stent technology in ischemic stroke. Neurosurg Focus. 2017;42(4):1–9. doi: 10.3171/2017.1.FOCUS16507. [DOI] [PubMed] [Google Scholar]
- 35.Rensen SSM, Doevendans PAFM, van Eys GJJM. Regulation and Characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Hear J. 2007;15:100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao H, Gabbiani G, Bochaton-Piallat ML. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol. 2003;23:1510–1520. doi: 10.1161/01.ATV.0000090130.85752.ED. [DOI] [PubMed] [Google Scholar]
- 37.Beamish JA, He P, Kottke-Marchant K, Marchant RE. Molecular Regulation of Contractile Smooth Muscle Cell Phenotype: Implications for Vascular Tissue Engineering. Tissue Eng Part B Rev. 2010;16(5):467–491. doi: 10.1089/ten.teb.2009.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Meng H, Blaivas M, et al. Von Willebrand Factor permeates small vessels in CADASIL and inhibits smooth muscle gene expression. Transl Stroke Res. 2012;3(1):138–145. doi: 10.1007/s12975-011-0112-2.Von. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tikka S, Ng YP, Di Maio G, et al. CADASIL mutations and shRNA silencing of NOTCH3 affect actin organization in cultured vascular smooth muscle cells. J Cereb Blood Flow Metab. 2013;32:2171–2180. doi: 10.1038/jcbfm.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borgers M, Schaper J, Schaper W. The Origin of Subendothelial Cells in Developing Coronary Collaterals. Virchows Arch Abt A Path Anat. 1973;358:281–294. doi: 10.1007/BF00543269. [DOI] [PubMed] [Google Scholar]
- 41.Imai H, Lee KJ, Lee SK, Lett KT, O’Neal RM, Thomas WA. Ultrastructural features of aortic cells in mitosis in control and cholesterol-fed swine. Lab Investig. 1970;23:401–415. [PubMed] [Google Scholar]
- 42.Yang P, Hong MS, Fu C, et al. Pre-existing smooth muscle cells contribute to neointimal cell repopulation at an incidence varying widely among individual lesions. Surgery. 2016;159(2):602–612. doi: 10.1021/acsnano.5b07425.Molecular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herring B, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6(21) doi: 10.1186/2045-824X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse Contribution of Bone Marrow Cells to Neointimal Hyperplasia After Mechanical Vascular Injuries. Circ Res. 2003;93(8):783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 47.Tang Z, Wang A, Yuan F, et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao H, Ropraz P, Verin V, et al. Heterogeneity of Smooth Muscle Cell Populations Cultured From Pig Coronary Artery. Atheroscler Thromb Vasc Biol. 2002;22:1093–1099. doi: 10.1161/01.ATV.0000022407.91111.E4. [DOI] [PubMed] [Google Scholar]
- 49.Lee SJ, Zhang X, Wang MM. Vascular accumulation of the small leucine rich proteoglycan Decorin in CADASIL. Neuroreport. 2014;25(13):1059–1063. doi: 10.1097/WNR.0000000000000230.Vascular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Lee SJ, Young MF, Wang MM. The small leucine-rich proteoglycan BGN accumulates in CADASIL and binds to NOTCH3. Transl Stroke Res. 2015;6(2):148–155. doi: 10.1007/978-1-4939-2914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong H, Blaivas M, Wang MM. Bidirectional encroachment of collagen into the tunica media in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Res. 2012;1456:64–71. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]