Abstract
Chalcones containing tertiary amine side-chains have potent activity as acetylcholinesterase (AChE) inhibitors. However, the effects of the location of the tertiary amine groups as well as of other groups on AChE and butyrylcholinesterase (BChE) activity have not been reported. Here, we report the synthesis and testing of 36 new coumarin-chalcone hybrids (5d–7j, 9d–11f, 12k–13m) against AChE and BChE. The nature and position of the chalcone substituents had major effects on inhibitory activity as well as selectivity for AChE over BChE. Compounds with para-substituted chalcone fragments in which the substituents were choline-like had potent activity against AChE and poor activity against BChE, while ortho-substituted analogs exhibited an opposite effect. Replacement of the terminal amine groups by amide, alkyl or alkenyl groups abrogated activity. Compound 5e showed potent inhibitory activity (IC50 = 0.15 ± 0.01 μmol/L) and good selectivity for AChE over BChE (ratio: 27.4) and a kinetic study showed that 5e exhibited mixed-type inhibition against AChE. Computational docking results indicate that 5e binds to Trp 279, Tyr334 and Trp 84 in AChE, but only to Trp 82 in BChE. Overall, the results show that coumarin-chalcone hybrids with choline-like side-chains have promising activity and selectivity against AChE and be promising therapeutic leads for Alzheimer’s disease.
Keywords: Coumarin, Chalcone, Cholinesterase inhibitors, Structure-activity relationship, Tertiary amine group
Introduction
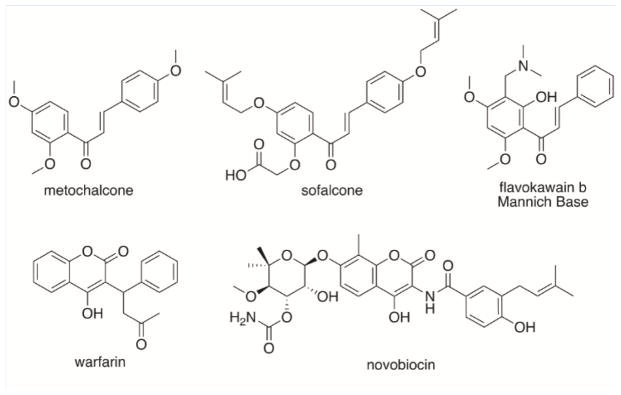
Alzheimer’s disease (AD) is one of most common nervous system diseases and is characterized by impaired memory, cognition, and self-care ability [1]. At present, treatment of AD is mainly provided with acetylcholinesterase (AChE) inhibitors, compounds which increase the level of acetylcholine in the brain. However, since there are frequent adverse side-effects including hepatic injury, diarrhea, or cardiovascular damage [3–5], the discovery of new, selective AChE inhibitors is of interest. In this context, many natural products or their derivatives have been found to have AChE inhibitory activity [6–9]. Chalcones, for example, are promising natural product scaffolds for drug development and exhibit a wide variety of bioactivities including anti-obesity, anti-inflammatory and anti-gout activities. For example, the chalcones metochalcone and sofalcone, Figure 1, have been used in the clinic as choleretic and anti-ulcer agents, respectively [10]. Flavokawain B, first isolated from Piper methysticum Forst [11] is another natural product containing the chalcone scaffold. In our laboratory, we found that flavokawain B Mannich base derivatives had AChE inhibitory effects [12] and, following this discovery, we synthesized a series of tertiary amine chalcone derivatives, some of which had quite potent AChE inhibitory activity(IC50 =0.21~4.68 μmol/L) [13–16]. In our studies, we investigated AChE inhibitory effects of various tertiary amine groups, the nature of the spacer between the chalcone scaffold and the tertiary amine side-chain, as well as the effects of other substituents in the chalcone scaffold, but no investigations into the influence of different substituent positions of tertiary amine groups were reported.
FIGURE 1.
Examples of chalcones and coumarins.
A second interesting class of natural products for scaffold building are coumarins. This class of compound includes anti-coagulants such as warfarin [17], choleretics [18] and even antibiotics such as novobiocin, and several structures are shown in Figure 1. Coumarins are also used extensively as starting materials in drug discovery, and some coumarin derivatives have potent enzyme inhibition activity [19–21]. For example, the hybrids containing coumarin and other moieties have been used as histone deacetylase weinhibitors [22], cyclo-oxygenase inhibitors [23] and monoamine oxidase inhibitor [24]. Coumarin-chalcone hybrids, are reported as anti-parasitics agents against Plasmodium spp. [25], malaria parasites, as well as trypanosomatids. Such hybrids offer multiple opportunities for derivatization on both aromatic rings.
In this work, we synthesized and tested a broad range of coumarin-chalcone hybrids focusing on derivatizing the chalcone ring with both neutral and basic groups. Then we determined IC50 values of these compounds against AChE and BChE, evaluated AChE inhibition kinetics of the most potent inhibitors, and we used computational docking to elucidate AChE/BChE selectivity.
Results and discussion
Chemistry
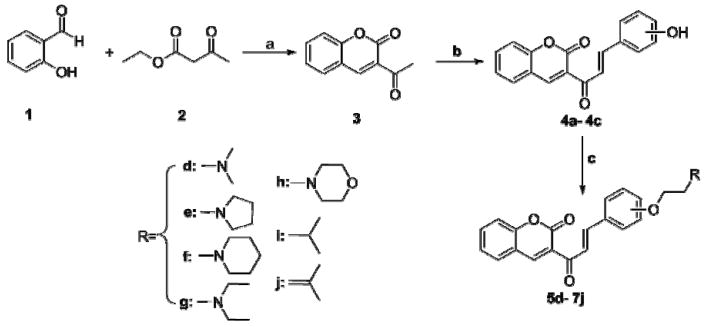
Coumarin-chalcone hybrids were synthesized as follows. Briefly, 3-acetyl coumarin was prepared by the condensation of salicylic acid with ethyl acetoacetate using piperidine as catalyst [26]. Compounds 4a–4c were synthesized from 3-acetyl coumarin and hydroxybenzaldehyde via Claisen–Schmidt condensation in the presence of piperidine [27]. Compounds 5d–7j were generated from compounds 4a–4c with one of the following: chloroethyl dimethylamine hydrochloride, chloroethyl diethylamine hydrochloride, chloroethyl piperidine hydrochloride, chloroethyl pyrrolidine hydrochloride, chloroethyl morpholine hydrochloride, 1-bromo-3-methylbutane, or 3,3-dimethylallyl bromide), all in the presence of K2CO3 and NaI [28].
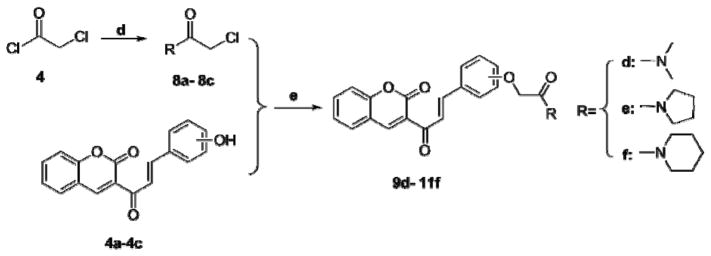
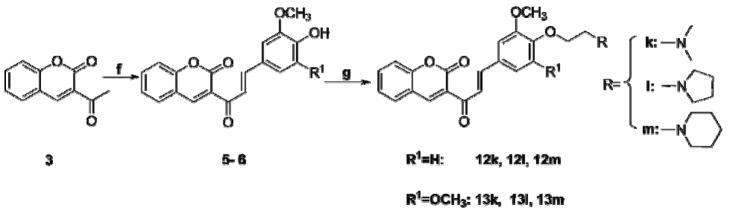
Compounds 9d–11f were synthesized via the routes illustrated in Scheme 2. First, chloroacetyl chloride was converted into compounds 8a–8c using a secondary amine (dimethylamine, pyrrolidine, or piperidine) in the presence of anhydrous sodium acetate [29]. Next, compounds 8a–8c were condensed with compounds 4a–4c (in the presence of K2CO3 and NaI as catalysts) to afford compounds 9d–11f. Compounds 5 and 6 were generated by condensing 3-acetyl coumarin with vanillic aldehyde or syringaldehyde using p-toluenesulfonic acid as catalyst. Then, compounds 12k–13m were synthesized from compound 5 or 6, chloroethyl dimethylamine hydrochloride, chloroethyl piperidine hydrochloride, or chloroethyl pyrrolidine hydrochloride, again in the presence of K2CO3 and NaI (Scheme 3).
Scheme 2.
Synthesis of compounds 9d–11f. Reagents and conditions: (d) HNR1R2, NaAc, DCM, 0 °C to room temperature; (e) K2CO3, NaI, DMF, 56 °C.
Scheme 3.
Synthesis of compounds 12k–13m. Reagents and conditions: (f) Acetic acid, TsOH, 70°C; (g) Cl(CH2)2NR1R2, K2CO3, NaI, acetone, reflux.
The final compounds were purified by silica gel chromatography and structures were confirmed by using nuclear magnetic resonance spectroscopy (1H NMR, 13C NMR) and high-resolution mass spectrometry (HRMS). Representative 1H, 13C NMR and mass spectra are given in the Supplementary Materials.
In vitro inhibition of AChE and BChE
The inhibitory effects of the newly synthesized compounds on AChE and BChE activity were evaluated by using the Ellman method using rivastigmine as positive control. Half-maximal inhibitory concentrations (IC50 values) for AChE and BChE as well as the selectivity for AChE are summarized in Table 1. All assays were conducted in triplicate. These results indicate that many of the compounds show inhibition activity against AChE and BChE, and some of them have far more potent inhibitory activity than that of the control, rivastigmine.
TABLE 1.
Effects of new synthesized compounds on AChE and BChE
| Compounds | Position | R | IC50a (μmol/L)
|
Selectivity for AChEb | |
|---|---|---|---|---|---|
| AChE | BChE | ||||
| 5d | Para |
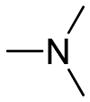
|
0.37 ± 0.03 | >500 | >1340 |
| 6d | Meta | 0.92 ± 0.07 | 15.32 ± 1.16 | 16.64 | |
| 7d | Ortho | 16.40 ± 0.22 | 15.16 ± 0.60 | 0.92 | |
| 9d | Para | 18.61±1.06 | >500 | >26.86 | |
| 10d | Meta | >500 | 44.02±2.37 | <0.09 | |
| 11d | Ortho | >500 | >500 | - | |
| 12k | Para | 1.65±0.09 | 8.48±0.29 | 5.15 | |
| 13k | Para | 7.07 ± 0.46 | 13.22 ± 0.32 | 1.87 | |
|
| |||||
| 5e | Para |
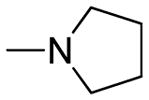
|
0.15 ± 0.01 | 4.11 ± 0.16 | 27.40 |
| 6e | Meta | 3.25 ± 0.14 | 4.62 ±0.16 | 1.42 | |
| 7e | Ortho | 230.34±14.82 | 3.76±0.35 | 0.02 | |
| 9e | Para | >500 | >500 | - | |
| 10e | Meta | >500 | >500 | - | |
| 11e | Ortho | >500 | >500 | - | |
| 12l | Para | 1.67±0.04 | 0.46±0.01 | 0.28 | |
| 13l | Para | 28.95 ± 1.68 | 13.61 ± 0.53 | 0.47 | |
|
| |||||
| 5f | Para |
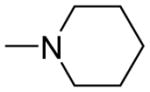
|
0.69 ± 0.06 | 1.03 ± 0.05 | 1.49 |
| 6f | Meta | 0.61 ± 0.02 | 4.01 ± 0.14 | 6.61 | |
| 7f | Ortho | 29.86 ± 0.98 | 4.50 ± 0.22 | 0.15 | |
| 9f | Para | >500 | >500 | - | |
| 10f | Meta | 152.49±12.82 | 222.84±11.54 | 1.46 | |
| 11f | Ortho | >500 | 106.14±3.09 | 0.21 | |
| 12m | Para | 0.37±0.02 | 0.52±0.01 | 1.41 | |
| 13m | Para | 9.58 ± 0.80 | 6.41 ± 0.36 | 0.67 | |
|
| |||||
| 5g | Para |
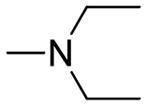
|
4.34 ± 0.29 | 6.21 ±0.19 | 1.43 |
| 6g | Meta | 51.30 ± 1.04 | 1.12 ± 0.11 | 0.02 | |
| 7g | Ortho | 202.70 ± 2.50 | 1.96 ± 0.14 | 0.01 | |
|
| |||||
| 5h | Para |
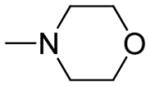
|
>500 | >500 | - |
| 6h | Meta | >500 | >500 | - | |
| 7h | Ortho | 260.95±4.17 | 28.98±1.23 | 0.11 | |
|
| |||||
| 5i | Para |
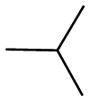
|
>500 | >500 | - |
| 6i | Meta | >500 | >500 | - | |
| 7i | Ortho | >500 | >500 | - | |
|
| |||||
| 5j | Para |
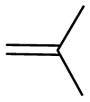
|
>500 | >500 | - |
| 6j | Meta | >500 | >500 | - | |
| 7j | Ortho | >500 | 100.33±6.84 | <0.20 | |
|
| |||||
| Rivastigmine* | 10.54±0.86 | 0.26±0.08 | 0.02 | ||
IC50: 50% inhibitory concentration (means±SD of three assays).
Selectivity for AChE is defined as IC50 (BChE)/IC50 (AChE).
Used for control.
The chemical nature of the substituents as well as their positions in the chalcone benzene ring greatly influenced both activity and selectivity. Compounds 5d, 5e and 5f with IC50 values of 0.37, 0.15, and 0.69 μmol/L, respectively, showed more potent activity than the positive control rivastigmine (IC50 = 10.54 μmol/L, ratio: 0.02) with compound 5e exhibiting the most potent AChE inhibitory activity (IC50 = 0.15 μmol/L) as well as higher selectivity for AChE versus BChE (an IC50 ratio of ratio = 27.4:1) than rivastigmine (a ratio of 0.02:1). In general, the inhibitory potency of the coumarin-chalcone derivatives against AChE was as follows: para-substituted > meta-substituted > ortho-substituted. However, the inhibitory potency against BChE was reversed: ortho-substituted > meta-substituted > para-substituted. In addition, the inhibitory activity against AChE was highly dependent on the nature of the ring substituent. Compounds containing a dimethylamine, pyrrolidine or piperidine group (compounds 5d, 5e, 5f, 6d and 6f) possessed much higher activity than did those compounds containing isopropyl or isopropenyl groups (compounds 5i–7j) (IC50 > 500 μmol/L) (Table 1), while all compounds containing amide groups (9d–11f) showed very weak inhibition activity against both AChE and BChE. It can be observed that the presence of choline-like dimethylethanolamine (5d) or analogous pyrrolidine species (5e) make major contributions to activity.
Since compounds 5d, 5e and 5f had potent activity and high selectivity in inhibiting AChE, we next investigated several analogs of these compounds. Interestingly, compounds 13k, 13l, 13m, containing two methoxyl substituents, had much weaker inhibitory activity against AChE than did the parent compounds, 5d, 5e, 5f. However, compounds 12k, 12l, 12m which contained just a single methoxyl group, had comparable inhibitory activity to that of the parent compounds with compound 12m (IC50 = 0.37±0.02 μmol/L) actually having slightly more potent activity that the parent compound 5f (IC50 = 0.69±0.06 μmol/L). Thus, addition of the second methoxyl group clearly decreases activity, presumably due to a steric repulsion in the AChE active site.
Compound 5e was then selected for a kinetic assay since it had the most potent activity against AChE. A Lineweaver–Burk analysis of the steady-state inhibition data of compound 5e is shown in Supplementary Figure 2 and shows that increasing the concentration of compound 5e results in different slopes and intercepts. The Km values show an increase with increasing concentrations of compound 5e, but the Vmax values exhibit a decrease, indicating a mixed-type inhibition mechanism. As shown in Supplementary Table 2, the competitive inhibition constant (Ki) of compound 5e is 0.11 μmol/L, while the non-competitive constant (Ki′) is 0.19 μmol/L.
Molecular modeling studies
To explore the molecular mechanism of AChE and BChE inhibition, representative compounds (5e, 6e, 7e, 5h) were selected for a molecular docking investigation using MOE2014 (Montreal, Quebec, Canada: Chemical Computing Group Inc. https://www.chemcomp.com/). As shown in Supplementary Figure 3, compound 5e exhibited multiple interactions with AChE (with a total binding energy of −34.6 kJ/mol). In the top of the AChE “gorge”, the phenyl ring bound to the peripheral anionic site (PAS) via a π-π stacking interaction with Trp279 (4.03 Å) and Tyr334 (4.32 Å). In the bottom of the gorge, the protonated nitrogen of the pyrrolidine ring bound to the catalytic active site (CAS) via a cation-π interaction with Trp84 (4.33 Å), while compound 5e bound to BChE (−20.08 kJ/mol) via a π-H+ interaction in the CAS with Trp84 (3.11 Å). Compound 6e exhibited binding to multiple sites in AChE (−35.7 kJ/mol). In the top of the gorge, the phenyl ring bound to the PAS via a π-π stacking interaction with Trp279 (3.44 Å). In the bottom of the gorge, the protonated nitrogen of the pyrrolidine ring bound to the CAS via a cation-π interaction with Trp84 (4.06 Å). Compound 6e bound to BChE (−20.9 kJ/mol) via a π-H interaction with Phe329 (3.77 Å) in the PAS and a cation-π interaction with Trp82 (3.76 Å), in the CAS, providing a partial explanation for the similar inhibition for AChE and BChE. However, compound 7e exhibited multiple binding interactions with BChE (−20.5 kJ/mol). In the bottom of the gorge, the 2-H-chromen-2-one moiety was observed to bind to the CAS via a π-π stacking interaction with Trp82 (3.81 Å), and a π-H interaction with His438 (4.47 Å). Compound 7e bound to AChE (−28.9 kJ/mol) via a π-H+ interaction with Trp84 (3.29 Å) in the CAS. Compound 5h exhibited very weak binding to both AChE and BChE, consistent with its IC50 > 500 μM against both AChE and BChE (see Table 1).
Compound 5e thus has potent inhibitory activity against AChE together with a high selectivity over BChE; compound 6e has moderate inhibitory activity against AChE with low selectivity over BChE, while compound 7e has poor inhibitory activity against AChE as well as poor selectivity over BChE, and compound 5h has almost has no inhibitory activity against either AChE or BChE, indicating that having a hydrophilic morpholine ring abrogates essentially all activity, demonstrating that hydrophobic interactions in this region are essential for activity [35].
Conclusions
In summary, we synthesized a series of coumarin-chalcone hybrids (5d–7j, 9d–11f, 12k–3m) containing amino-alkyl, alkyl, alkenyl and amide side-chains. The nature of the side-chain as well as its location on the chalcone phenyl ring markedly influenced activity in inhibiting AChE and BChE. In general, compounds with para-substituted amino-alkyl groups had the most potent activity and were selective for AChE. Most of compounds with amide, alkyl or alkenyl groups had very poor activity, IC50 values of them against AChE are more than 500 μmol/L. Addition of a single methoxyl group into the benzene ring resulted in activity comparable to that of the parent compounds, but addition of two methoxyl groups again blocked activity. Compound 5e with an IC50 value of 0.15 ± 0.01μmol/L against AChE exhibited the best activity as well as a high selectivity for AChE over BChE and our kinetics results indicate that compound 5e has a mixed-type inhibition against AChE. The molecular docking results show that compound 5e can bind to both the CAS and the PAS of AChE, contributing to its potent activity. In conclusion, compound 5e may serve as a potential lead for the development of novel compounds as AD therapeutics.
Experimental
Chemistry
All chemicals and reagents were of analytical reagent grade and were used without further purification. Melting points were measured on a WRS-lA melting point detector (uncorrected). 1H NMR and 13C NMR spectra were recorded on a Bruker 400 MHz instrument using tetramethylsilane (TMS) as the internal standard ( s = singlet, d = doublet, t=triplet ). Mass spectra were obtained on Waters XEVO-G2XSQTOF Liquid chromatography-mass spectrometry with electrospray ionization (ESI-MS) method. The purity of compounds was checked using a Shimadzu LC-20A high performance liquid chromatography.
Preparation of 3-acetyl-2H-chromen-2-one (3)
2-Hydroxybenzaldehyde (15 mmol, 1.56 mL) and ethyl acetoacetate (15 mmol, 1.89 mL) were added into a flask (25.0 mL) at room temperature. A small amount of piperidine (4 drops) was added as catalyst, and the solution was stirred continuously until the reaction was complete. Than the solvent was removed and the residue was dissolved in ethanol. The target product obtained was recrystallized from ethanol. Pale yellow solid, Yield: 90%, mp: 118 – 119°C.
General procedure for the synthesis of compounds 4a–4c
Compound 3 (5.00 mmol, 0.94 g) was added into absolute ethanol (30 mL) under stirring, followed by the addition of an appropriate hydroxybenzaldehyde (15.00 mmol, 1.83 g). Then, a catalytic amount of piperidine (4 drops) was added into the solution, and the reaction mixture was refluxed for 6 to 8 hours. The mixture was cooled, and the solid obtained by filtration. The crude products were recrystallized from ethanol to afford compounds 4a–4c with yield 82–90 %.
General procedure for the synthesis of compounds 5d–7j
Compounds 4a–4c (1.00 mmol, 0.292 g), together with aminoethyl chloride or aminoethyl bromide (3.00 mmol) and anhydrous potassium carbonate (3 mmol, 0.415 g) in 8 mL DMF were stirred for 20 minutes. Then, a small amount of NaI (0.005 g, 0.02 mmol) was added as catalyst. The reaction mixture was stirred for 10 to 15 hours at 56°C, then cooled to room temperature and filtered. The filtrate was poured into a saturated saline solution and filtered. The solid obtained was purified using column chromatography with dichloromethane:methanol (70:1) to yield compounds 5d–7j, respectively.
(E)-3-(3-(4-(2-(dimethylamino)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (5d)
Yellow solid, yield: 85.6%, mp: 137.5–139.2 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.29 (6H, s, 2×NCH3), 2.70 (2H, t, J = 8 Hz, OCH2CH2), 4.06 (2H, t, J = 8 Hz, OCH2CH2), 6.86 (2H, d, J = 8 Hz, H3,5 phenyl), 7.26–7.34 (2H, m, H6,8 coumarin), 7.55–7.61(4H, m, H2,6 phenyl and H5,7 coumarin), 7.74 (1H, d, J = 16 Hz, Hα), 7.79 (1H, d, J = 16 Hz, Hβ), 8.51(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ: 45.54, 57.46, 65.73, 114.94, 116.68, 118.64, 121.47, 124.91, 125.60, 127.37, 129.95, 130.83, 134.04, 145.23, 147.75, 155.18, 159.36, 161.61, 186.36. HRMS(ESI) m/z: 364.1549 [M+H]+. Purity: 98.9% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (5e)
Yellow solid, yield: 81.5%, mp: 135.9–137.3°C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.88 (4H, m, pyrrolidine -H), 2.77 (4H, m, pyrrolidine -H), 3.02 (2H, t, J = 8 Hz, OCH2CH2), 4.23 (2H, t, J = 8 Hz, OCH2CH2), 6.93–6.95 (2H, d, J = 8 Hz, H3,5 phenyl), 7.34–7.41 (2H, m, H6,8 coumarin), 7.62–7.68(4H, m, H2,6 phenyl and H5,7 coumarin), 7.80–7.89 (2H, m, Hα and Hβ), 8.58(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.50, 54.75, 67.13, 114.99, 116.68, 118.62, 121.63, 124.93, 125.54, 127.65, 129.97, 130.81, 134.08, 145.10, 147.82, 155.18, 159.36, 161.21, 186.30. HRMS(ESI) m/z: 390.1698 [M+H]+. Purity: 98.8% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one(5f)
Brown solid, yield: 85.9%, mp: 144.1–144.9°C; 1H NMR (400 MHz, DCCl3): δ(ppm), 1.18 (5H, m, piperidine-H), 2.61 (4H, m, piperidine-H), 2.89 (2H, m, OCH2CH2), 4.19 (2H, t, J = 8 Hz, OCH2CH2), 6.86 (2H, d, J = 8 Hz, H3,5 phenyl), 7.26–7.34 (2H, m, H6,8 coumarin), 7.55–7.61 (4H, m, H2,6 phenyl and H5,7 coumarin), 7.73–7.81 (2H, m, Hα and Hβ), 8.51(1H, s, H4 coumarin). 13C NMR(100MHz, CDCl3) δ:24.01, 25.28, 54.63, 57.30, 65.34, 114.96, 116.68, 118.62, 121.63, 124.93, 125.54, 127.65, 129.97, 134.18, 145.16, 147.82, 155.18, 159.36, 161.21, 186.34. HRMS(ESI) m/z: 404.1860 [M+H]+. Purity: 99.1% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-(diethylamino)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (5g)
Brown solid, yield: 85.6%, mp: 159.3–160.1 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.14 (6H, t, J = 8 Hz, 2×NCH2CH3), 2.75 (4H, m, 2×NCH2CH3), 2.99 (2H, t, J = 8 Hz, OCH2CH2), 4.17 (2H, t, J = 8 Hz, OCH2CH2), 6.93 (2H, d, J = 8 Hz, H3,5 phenyl), 7.33–7.41 (2H, m, H6,8 coumarin), 7.60–7.68(4H, m, H2,6 phenyl and H5,7 coumarin), 7.81 (1H, d, J = 16 Hz, Hα), 7.86 (1H, d, J=16 Hz, Hβ), 8.58(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:11.34, 47.78, 51.45, 66.13, 114.96, 116.69, 118.62, 121.72, 124.95, 125.52, 127.76, 129.98, 130.83, 134.11, 145.05, 147.85, 155.18, 159.39, 161.00, 186.33. HRMS(ESI) m/z: 392.1855 [M+H]+. Purity: 98.3% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-morpholinoethoxy)phenyl)acryloyl)-2H-chromen-2-one (5h)
Yellow solid, yield: 80.8%, mp: 167.8–169.2 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.63 (4H, s, morpholine-H), 2.87 (2H, s, J = 8 Hz, OCH2CH2), 3.77 (4H, s, morpholine-H), 4.18 (2H, t, J = 8 Hz, OCH2CH2), 6.94 (2H, d, J = 8 Hz, H3,5 phenyl), 7.34–7.41 (2H, m, H6,8 coumarin), 7.63–7.68 (4H, m, H2,6 phenyl and H5,7 coumarin), 7.81–7.89 (2H, m, Hα and Hβ), 8.59 (1H, s, H4 coumarin), 13C NMR(100MHz, CDCl3) δ:54.05, 57.47, 66.76, 114.98, 116.70, 118.62, 121.79, 124.96, 125.50, 127.85, 129.99, 130.82, 134.13, 144.96, 147.89, 155.20, 159.39, 160.99, 186.29. HRMS(ESI) m/z: 406.1660 [M+H]+. Purity: 98.2% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(isopentyloxy)phenyl)acryloyl)-2H-chromen-2-one (5i)
Yellow solid, yield: 80.5%, mp: 161.7–164.3 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 0.97 (6H, d, J = 8 Hz, 2×CHCH3), 1.71 (2H, t, J=8 Hz, OCH2CH2), 1.81–1.88(1H, m, CH(CH3)2), 4.04 (2H, t, J = 12 Hz, OCH2CH2), 6.92 (2H, d, J = 8 Hz, H3,5 phenyl), 7.33–7.41 (2H, m, H6,8 coumarin), 7.62–7.68(4H, m, H2,6 phenyl and H5,7 coumarin), 7.81 (1H, d, J = 16 Hz, Hα), 7.86 (1H, d, J = 16 Hz, Hβ), 8.57 (1H, s, H4 coumarin), 13C NMR(100MHz, CDCl3) δ:22.58, 25.04, 37.85, 66.59, 114.91, 116.68, 118.64, 121.47, 124.91, 125.60, 127.37, 129.95, 130.83, 134.04, 145.23, 147.75, 155.18, 159.36, 161.63, 186.30. HRMS(ESI) m/z: 363.2079 [M+H]+. Purity: 98.5% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(3-methylbut-2-enyloxy)phenyl)-prop-2-enoyl)-2H-chromen-2-one (5j)
Yellow solid, yield,89%, mp,119–121 °C. This is a known compound but without reports regarding inhibiting cholinesterases [27].
(E)-3-(3-(3-(2-(dimethylamino)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (6d)
Yellow solid, yield: 85.5%, mp:127.9–128.7°C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.41 (6H, s, 2×NCH3), 2.83 (2H, t, J = 8 Hz, OCH2CH2,), 4.15 (2H, t, J = 8 Hz, OCH2CH2,), 6.99 (2H, d, J = 8 Hz, H4 phenyl), 7.21 (1H, s, H2 phenyl), 7.24–7.42 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.65–7.69 (2H, m, H5,7 coumarin), 7.82 (1H, d, J =16 Hz, Hα), 7.92 (1H, d, J=16 Hz, Hβ), 8.59 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:45.70, 58.10, 65.75, 114.23, 116.74, 117.38, 118.56, 122.01, 124.20, 125.01, 125.29, 129.92, 130.06, 134.28, 136.15, 144.98, 148.13, 155.26, 159.02, 159.32, 186.49. HRMS(ESI) m/z: 364.1297 [M+H]+. Purity: 98.5% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (6e)
Pale yellow solid, yield: 80.8%, mp: 136.1–138.2 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.91 (4H, m, pyrrolidine-H), 2.85 (4H, t, J = 8 Hz, pyrrolidine-H), 2.97 (2H, t, J = 8 Hz, OCH2CH2), 4.23 (2H, t, J = 8 Hz, OCH2CH2), 7.03 (2H, d, J=8 Hz, H4 phenyl), 7.21 (1H, s, H2 phenyl), 7.29–7.42 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.63–7.69 (2H, m, H5,7 coumarin), 7.82 (1H, d, J = 16 Hz, Hα), 7.91 (1H, d, J = 16 Hz, Hβ), 8.59 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.54, 54.75, 67.05, 114.38, 116.72, 117.34, 118.53, 121.88, 124.18, 125.00, 125.27, 129.90, 130.05, 134.28, 136.13, 144.97, 148.08, 155.23, 159.07, 159.26, 186.43. HRMS(ESI) m/z: 390.1704 [M+H]+. Purity: 98.6% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (6f)
Pale yellow solid, yield: 85.6%, mp: 151.8–153.1 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.47–1.67 (6H, m, piperidine-H), 2.58 (4H, m, piperidine-H), 2.4 (2H, t, J = 8 Hz, OCH2CH2), 4.18 (2H, t, J = 8 Hz, OCH2CH2), 6.97 (2H, d, J = 8 Hz, H4 phenyl), 7.20 (1H, s, H2 phenyl), 7.29–7.41 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.66 (2H, m, H5,7 coumarin), 7.81 (1H, d, J =16 Hz, Hα), 7.90 (1H, d, J = 16 Hz, Hβ), 8.58(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:24.05, 25.73, 55.01, 57.80, 66.85, 114.33, 116.72, 117.34, 118.54, 121.88, 124.18, 125.00, 125.27, 129.90, 130.05, 134.27, 136.13, 144.97, 148.08, 155.23, 158.26, 159.28, 186.49. HRMS(ESI) m/z: 404.1874 [M+H]+. Purity: 99.3% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(2-(diethylamino)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (6g)
Brown viscous liquid, yield: 82.5%; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.14 (6H, t, J = 8 Hz, 2×NCH2CH3), 2.72–2.77 (4H, m, 2×NCH2CH3), 3.00 (2H, t, J = 8 Hz, OCH2CH2,), 4.16 (2H, t, J = 8 Hz, OCH2CH2,), 6.97 (2H, d, J = 8 Hz, H4 phenyl), 7.20 (1H, s, H2 phenyl), 7.29–7.41 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.64–7.67(2H, m, H5,7 coumarin), 7.82 (1H, d, J = 16 Hz, Hα), 7.90 (1H, d, J = 16 Hz, Hβ), 8.57 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:11.85, 47.51, 57.38, 65.90, 114.23, 116.72, 117.34, 118.54, 121.88, 124.18, 125.00, 125.27, 129.90, 130.05, 134.27, 136.13, 144.97, 148.08, 155.23, 159.07, 159.34, 186.42. HRMS(ESI) m/z: 392.1702 [M+H]+. Purity: 98.8% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(2-morpholinoethoxy)phenyl)acryloyl)-2H-chromen-2-one (6h)
Yellow solid, yield: 80.5%, mp: 134.1–135.0 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.62 (4H, t, J = 8 Hz, morpholine-H), 2.85 (2H, t, J = 8 Hz, OCH2CH2), 3.76 (4H, t, J = 8 Hz, morpholine-H), 4.18 (2H, t, J = 8 Hz, OCH2CH2), 6.967 (2H, d, J = 8 Hz, H4 phenyl),7.21 (1H, s, H2 phenyl), 7.28–7.42 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.65–7.69 (2H, m, H5,7 coumarin), 7.82 (1H, d, J = 16 Hz, Hα), 7.92 (1H, d, J = 16 Hz, Hβ), 8.59(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ: 54.13, 57.72, 66.85, 114.35, 116.72, 117.34, 118.54, 121.82, 124.18, 125.00, 125.27, 129.90, 130.05, 134.27, 136.13, 144.97, 148.08, 155.23, 159.07, 159.22, 186.45. HRMS(ESI) m/z: 406.1645 [M+H]+. Purity: 98.9% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(isopentyloxy)phenyl)acryloyl)-2H-chromen-2-one (6i)
Yellow solid, yield: 86.8%, mp: 150.7–152.2 °C;1H NMR (400 MHz, DCCl3): δ(ppm) 0.96 (6H, d, J = 8 Hz, 2×CHCH3), 1.71 (2H, t, J = 8 Hz, OCH2CH2), 1.81–1.89 (1H, m, CH (CH3)2), 4.17 (2H, t, J = 8 Hz, OCH2CH2), 6.98 (2H, d, J = 8 Hz, H4 phenyl), 7.21 (1H, s, H2 phenyl), 7.29–7.42 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.65–7.69 (2H, m, H5,7 coumarin), 7.82 (1H, d, J = 16 Hz, Hα), 7.92 (1H, d, J = 16 Hz, Hβ), 8.52 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:22.91, 25.04, 37.18, 66.75, 114.23, 116.74, 117.38, 118.56, 122.01, 124.20, 125.01, 125.29, 129.92, 130.06, 134.28, 136.15, 144.98, 148.13, 155.26, 159.02, 159.32, 186.51. HRMS(ESI) m/z: 363.1548 [M+H]+. Purity: 98.0% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(3-methylbut-2-enyloxy)phenyl)-prop-2-enoyl)-2H-chromen-2-one (6j)
Pale yellow solid, yield: 88.7%, mp: 144.4–146.3 °C;1H NMR (400 MHz, DCCl3): δ(ppm) 1.77 (3H, s, CHa3), 1.81 (3H, s, CHb3), 4.56 (2H, d, J = 8 Hz, OCH2CH), 5.50 (1H, t, J = 8 Hz, OCH2CH), 6.97 (2H, d, J = 8 Hz, H4 phenyl), 7.20 (1H, s, H2 phenyl), 7.29–7.39 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.63–7.67(2H, m, H5,7 coumarin), 7.82 (1H, d, J = 16 Hz, Hα), 7.91 (1H, d, J = 16 Hz, Hβ), 8.56 (1H, s, H4 coumarin). 13C NMR(100MHz, CDCl3) δ: 54.13, 57.72, 66.85, 114.35, 116.72, 117.34, 118.54, 121.82, 124.18, 125.00, 125.27, 129.90, 130.05, 134.27, 136.13, 144.97, 148.08, 155.23, 159.07, 159.22, 186.45. HRMS(ESI) m/z: 361.3237 [M+H]+. Purity: 98.5% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-(dimethylamino)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (7d)
Yellow solid, yield: 81.8%, mp: 128.7–130.3 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.28 (6H, s, 2×NCH3), 2.89 (2H, t, J = 8 Hz, OCH2CH2), 4.18 (2H, t, J = 8 Hz, OCH2CH2), 6.92–7.00 (2H, m, H3,5 phenyl), 7.32–7.39 (3H, m, H4 phenyl and H6,8 coumarin), 7.62–7.69 (3H, m, H6 phenyl and H5,7 coumarin), 8.00 (1H, d, J = 16 Hz, Hα), 8.20 (1H, d, J = 16 Hz, Hβ), 8.54(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:45.96, 57.98, 66.94, 112.17, 116.64, 118.58, 120.94, 123.90, 124.46, 124.90, 125.65, 129.81, 129.94, 132.17, 134.04, 140.73, 147.66; 155.15, 158.28, 159.18, 186.91. HRMS(ESI) m/z: 364.1552 [M+H]+. Purity: 98.7% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (7e)
Yellow solid, yield: 80.6%, mp: 137.7–138.5 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.82–1.85 (4H, m, pyrrolidine-H), 2.61–2.68 (4H, m, pyrrolidine-H), 2.94 (2H, t, J = 8 Hz, OCH2CH2), 4.16 (2H, t, J = 8 Hz, OCH2CH2), 6.91–7.00 (2H, m, H3,5 phenyl), 7.32–7.39 (3H, m, H4 phenyl and H6,8 coumarin), 7.61–7.69 (3H, m, H6 phenyl and H5,7 coumarin), 8.00 (1H, d, J =16 Hz, Hα), 8.19 (1H, d, J = 16 Hz, Hβ), 8.51 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.53, 54.72, 67.17, 112.25, 116.68, 118.61, 123.89, 124.48, 124.92, 125.75, 129.83, 129.93, 132.19, 134.03, 140.81, 147.85, 155.18, 158.26, 159.23, 186.94. HRMS(ESI) m/z: 390.1707 [M+H]+. Purity: 99.1% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one(7f)
Brown solid, yield: 80.9%, mp: 150.3–151.6 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.45–1.64 (6H, m, piperidine-H), 2.58–2.61 (4H, m, piperidine-H), 2.94 (2H, t, J = 8 Hz, OCH2CH2), 4.24 (2H, t, J = 8 Hz, OCH2CH2), 6.93–7.01(2H, m, H3,5 phenyl), 7.33–7.41 (3H, m, H4 phenyl and H6,8 coumarin), 7.63–7.69 (3H, m, H6 phenyl and H5,7 coumarin), 8.00 (1H, d, J = 16 Hz, Hα), 8.19 (1H, d, J = 16 Hz, Hβ), 8.55 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:24.01, 25.82, 54.97, 57.62, 66.63, 112.24, 116.68, 118.61, 120.92, 123.89, 124.48, 124.92, 125.75, 129.83, 129.93, 132.19, 134.03, 140.81, 147.84, 155.15, 158.26, 159.21, 186.97. HRMS(ESI) m/z: 404.1870 [M+H]+. Purity: 98.7% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-(dimethylamino)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (7g)
Pale red solid, yield: 85.8%, mp: 135.7–136.1 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.13 (6H, t, J = 8 Hz, 2×NCH2CH3), 2.72–2.77 (4H, m, 2×NCH2CH3), 2.99 (2H, t, J = 8 Hz, OCH2CH2), 4.16 (2H, t, J = 8 Hz, OCH2CH2), 6.91–7.00 (2H, m, H3,5 phenyl), 7.32–7.39 (3H, m, H4 phenyl and H6,8 coumarin), 7.61–7.69 (3H, m, H6 phenyl and H5,7 coumarin), 8.00 (1H, d, J =16 Hz, Hα), 8.20 (1H, d, J = 16 Hz, Hβ), 8.54(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:11.36, 47.71, 57.44, 66.16, 112.25, 116.68, 118.61, 123.89, 124.48, 124.92, 125.75, 129.83, 129.93, 132.19, 134.03, 140.81, 147.64; 155.18, 158.29, 159.25, 186.92. HRMS(ESI) m/z:392.4354 [M+H]+. Purity: 98.1% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-morpholinoethoxy)phenyl)acryloyl)-2H-chromen-2-one (7h)
Brown viscous liquid, Yield:85.5%; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.61 (4H, m, morpholine-H), 2.95 (2H, t, J = 8 Hz, OCH2CH2), 3.72–3.74 (4H, m, morpholine-H), 4.24 (2H, t, J = 8 Hz, OCH2CH2), 6.98–7.05 (2H, m, H3,5 phenyl), 7.29–7.39 (3H, m, H4 phenyl and H6,8 coumarin), 7.82–7.86 (3H, m, H6 phenyl and H5,7 coumarin), 8.01 (1H, d, J = 16 Hz, Hα), 8.18 (1H, d, J = 16 Hz, Hβ), 8.55 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ: 54.94, 57.68, 66.68, 67.62, 112.24, 116.68, 118.61, 123.89, 124.48, 124.92, 125.75, 129.83, 129.93, 132.19, 134.03, 140.81, 147.64; 155.18, 158.29, 159.25, 186.99. HRMS(ESI) m/z: 406.1639 [M+H]+. Purity: 98.9% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(isopentyloxy)phenyl)acryloyl)-2H-chromen-2-one (7i)
Yellow solid, yield: 85.6%, mp: 153.4–155.2 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 0.98 (6H, d, J = 8 Hz, 2× CHCH3), 1.71 (2H, t, J = 8 Hz, OCH2CH2), 1.80–1.88 (1H, m, CH(CH3)2), 4.04 (2H, t, J = 8 Hz, OCH2CH2), 6.92–7.01(2H, m, H3,5 phenyl), 7.32–7.41 (3H, m, H4 phenyl and H6,8 coumarin), 7.63–7.69 (3H, m, H6 phenyl and H5,7 coumarin), 8.00 (1H, d, J =16 Hz, Hα), 8.19 (1H, d, J = 16 Hz, Hβ), 8.57 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:22.59, 25.06, 37.87, 66.62, 112.26, 116.68, 118.61, 123.89, 124.48, 124.92, 125.75, 129.83, 129.93, 132.19, 134.03, 140.81, 147.83, 155.18, 158.26, 159.28, 186.93. HRMS(ESI) m/z: 363.1596 [M+H]+. Purity: 98.6% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(3-methylbut-2-enyloxy)phenyl)-prop-2-enoyl)-2H-chromen-2-one (7j)
Yellow solid, yield: 80%, mp: 92–95 °C; This is a known compound but without reports regarding inhibiting cholinesterases[27].
General procedure for the synthesis of compounds 8a–8c
Secondary amine (dimethylamine, pyrrolidine, piperidine, 5.00 mmol) and anhydrous sodium acetate (5.5 mmol, 0.451 g) were sequentially added into CH2Cl2 (10 mL). The reaction mixture stirred for 20 min at 0°C. Chloroacetyl chloride (6.00 mmol, 0.477 mL) was then added into the mixture and the reactions was stirred for 5 to 6 hours at ambient temperature. The resulting mixture was extracted thrice with 60 mL CH2Cl2. The separated organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Then compounds 8a–8c were gained, respectively. Yield: 80–89%.
General procedure for the synthesis of compounds 9d–11f
Compounds 8a–8c (3.00 mmol) were added into a solution containing intermediate compounds 4a–4c (1.00 mmol), K2CO3 (3.00 mmol, 0.43 g,) and NaI (0.05 mmol, 0.012 g) in DMF (6 ml). The reaction mixture was stirred for 10 to 12 hours at 56 °C, then cooled to room temperature and filtered. The filtrate was poured into a saturated saline solution and filtered. The crude product was purified by column chromatography using dichloromethane-methyl alcohol (100:1) as eluent to give the pure product compounds 9d–11f. Yield: 90–95%.
(E)-N,N-dimethyl-2-(4-(3-oxo-3-(2-oxo-2H-chromen-3-yl)prop-1-en-1-yl) phenoxy)acetamide (9d)
Yellow solid, yield: 90.8%, mp: 193.7–195.1 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.99 (3H, s, NCHa3), 3.10 (3H, s, NCHb3), 4.75 (2H, s, OCH2CO), 6.98 (2H, d, J = 8 Hz, H3,5 phenyl), 7.34–7.41 (2H, m, H6,8 coumarin), 7.63–7.68 (4H, m, H2,6 phenyl and H5,7 coumarin), 7.80–7.88 (2H, m, Hα and Hβ), 8.58 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:35.75, 36.58, 67.35, 115.11, 116.70, 118.61, 122.13, 124.95, 125.48, 128.46, 129.99, 130.82, 134.13, 144.76, 147.90, 155.21, 159.36, 160.23, 167.29, 186.30. HRMS(ESI) m/z: 378.2458 [M+H]+. Purity: 98.6% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-oxo-2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (9e)
Yellow solid, yield: 90.3%, mp: 225.7–227.2 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.85–2.01 (4H, m, pyrrolidine-H), 3.53 (4H, m, pyrrolidine -H), 4.68 (2H, s, OCH2CO), 6.98 (2H, d, J = 8 Hz, H3,5 phenyl), 7.33–7.41 (2H, m, H6,8 coumarin), 7.63–7.68 (4H, m, H2,6 phenyl and H5,7 coumarin), 7.79–7.87 (2H, m, Hα and Hβ), 8.58 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.82, 26.28, 46.02, 46.27, 67.82, 115.09, 116.69, 118.60 122.10, 124.96, 125.46, 128.40, 130.00, 130.82, 134.14, 144.76, 147.90, 155.19, 159.35, 160.31, 165.91, 186.32. HRMS(ESI) m/z: 404.1502 [M+H]+. Purity: 98.9% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-oxo-2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (9f)
Yellow solid, yield: 92.5%, mp: 217.4–219.6 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.56–1.65 (6H, m, piperidine-H), 3.48–3.57 (4H, m, piperidine-H), 4.74 (2H, s, OCH2CO), 6.98 (2H, d, J = 8 Hz, H3,5 phenyl), 7.33–7.40 (2H, m, H6,8 coumarin), 7.62–7.68 (4H, m, H2,6 phenyl and H5,7 coumarin), 7.78–7.86 (2H, m, Hα and Hβ), 8.57 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:24.42, 25.53, 26.50, 43.27, 46.41, 67.56, 115.09, 116.67, 118.59 122.08, 124.96, 125.45, 128.35, 130.00, 130.81, 134.14, 144.74, 147.87, 155.17, 159.33, 160.32, 165.57, 186.30. HRMS(ESI) m/z: 418.1854 [M+H]+. Purity: 98.7% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-N,N-dimethyl-2-(3-(3-oxo-3-(2-oxo-2H-chromen-3-yl)prop-1-en-1-yl)phenoxy)acetamide (10d)
Pale yellow solid, yield: 94.6%, mp: 127.8–129.1 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.89 (3H, s, NCHa3), 3.12 (3H, s, NCHb3), 4.78 (2H, s, OCH2CO), 7.03 (1H, d, J = 8 Hz, H4 phenyl), 7.21 (1H, s, H2 phenyl), 7.30–7.40 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.65–7.69 (2H, m, H5,7 coumarin), 7.81 (1H, d, J = 16 Hz, Hα), 7.90 (1H, d, J = 16 Hz, Hβ), 8.54(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:35.66, 36.78, 67.65, 114.29, 116.69, 117.64, 118.55, 121.69, 124.11, 125.28, 130.04, 134.22, 136.10, 145.09, 148.03, 155.24, 158.71, 159.39, 166.25, 186.45. HRMS(ESI) m/z: 378.2412 [M+H]+. Purity: 98.6% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(2-oxo-2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (10e)
Pale yellow solid, yield: 93.2%, mp: 246.7–248.3 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.86–2.02 (4H, m, pyrrolidine-H), 3.55 (4H, t, J = 8 Hz, pyrrolidine -H), 4.68 (2H, s, OCH2CO), 7.03 (2H, d, J = 8 Hz, H4 phenyl), 7.22 (1H, s, H2 phenyl), 7.30–7.41 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.65–7.69 (2H, m, H5,7 coumarin), 7.81 (1H, d, J = 16 Hz, Hα), 7.90 (1H, d, J = 16 Hz, Hβ), 8.58 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.83, 26.28, 46.05, 46.27, 67.84, 114.60, 116.72, 117.24, 118.53, 122.37, 124.41, 125.03, 125.21, 130.08, 134.31, 136.29, 144.71, 148.17, 155.24, 158.41, 159.30, 166.20, 186.48. HRMS(ESI) m/z: 404.2765 [M+H]+. Purity: 98.9% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-(2-oxo-2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (10f)
Yellow solid, yield: 90.7%, mp: 152.6–154.3 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.56–1.65 (6H, m, piperidine-H), 3.50–3.58 (4H, m, piperidine-H), 4.73 (2H, s, OCH2CO), 7.03 (1H, d, J = 8 Hz, H4 phenyl), 7.21 (1H, s, H2 phenyl), 7.30–7.40 (4H, m, H5,6 phenyl and H6,8 coumarin), 7.64–7.69 (2H, m, H5,7 coumarin), 7.81 (1H, d, J = 16 Hz, Hα), 7.90 (1H, d, J = 16 Hz, Hβ), 8.57(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:24.46, 25.55, 26.52, 43.25, 46.43, 67.61, 114.80, 116.70, 117.10, 118.52, 122.25, 124.42, 125.02, 125.22, 130.07, 134.29, 136.28, 144.68, 148.12, 155.23, 158.52, 159.27, 165.78, 186.47. HRMS(ESI) m/z: 418.1599 [M+H]+. Purity: 98.7% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-N,N-dimethyl-2-(2-(3-oxo-3-(2-oxo-2H-chromen-3-yl)prop-1-en-1-yl)phenoxy)acetamide (11d)
Pale yellow solid, yield: 92.3%, mp: 161.7–163.4 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 3.00 (3H, s, NCHa3), 3.15 (3H, s, NCHb3), 4.82 (2H, s, OCH2CO), 6.98–7.06 (2H, d, J = 8 Hz, H3,5 phenyl), 7.33–7.40 (3H, m, H4 phenyl and H6,8 coumarin), 7.64–7.71 (3H, m, H6 phenyl and H5,7 coumarin), 8.04 (1H, d, J = 16 Hz, Hα), 8.19 (1H, d, J = 16 Hz, Hβ), 8.59 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:35.77, 36.91, 68.20, 112.55, 116.68, 118.61, 121.76, 124.06, 124.93, 125.63, 130.00, 130.12, 132.21, 134.10, 140.21, 147.83; 155.20, 157.40, 159.21, 167.55, 186.92. HRMS(ESI) m/z: 378.1331 [M+H]+. Purity: 98.3% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-oxo-2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (11e)
Yellow solid, yield: 92.8%, mp: 288.8–231.0 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.84–1.99 (4H, m, pyrrolidine-H), 3.52–3.59 (4H, m, pyrrolidine -H), 4.76 (2H, s, OCH2CO), 6.98–7.05 (2H, m, H3,5 phenyl), 7.35–7.40 (3H, m, H4 phenyl and H6,8 coumarin), 7.63–7.70 (3H, m, H6 phenyl and H5,7 coumarin), 8.04 (1H, d, J = 16 Hz, Hα), 8.19 (1H, d, J = 16 Hz, Hβ), 8.60 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.77, 26.32, 46.28, 46.33, 68.68, 112.54, 116.66, 118.61, 121.71, 124.01, 124.89, 124.93, 125.61, 130.01, 130.08, 132.22, 134.10, 140.20, 147.83; 155.18, 157.49, 159.18, 166.23, 186.93. HRMS(ESI) m/z: 404.1311 [M+H]+. Purity: 99.1% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(2-(2-oxo-2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (11f)
Pale yellow solid, yield: 90.5%, mp: 148.7–150.3 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.54–1.62 (6H, m, piperidine-H), 3.57 (4H, m, piperidine-H), 4.82 (2H, s, OCH2CO), 7.01–7.05 (2H, m, H3,5 phenyl), 7.35–7.40 (3H, m, H4 phenyl and H6,8 coumarin), 7.63–7.71 (3H, m, H6 phenyl and H5,7 coumarin), 8.02 (1H, d, J = 16 Hz, Hα), 8.20 (1H, d, J = 16 Hz, Hβ), 8.58 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:24.45, 25.58, 26.51, 43.31, 46.62, 68.29, 112.48, 116.66, 118.59, 121.66, 123.92, 124.77, 124.94, 125.57, 129.90, 130.00, 132.27, 134.11, 140.15, 147.82; 155.17, 157.43, 159.20, 165.83, 186.82 HRMS(ESI) m/z: 418.1647 [M+H]+. Purity: 98.5% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
General procedure for the synthesis of compounds 5 and 6
Compound 3 (5.00 mmol, 0.94 g) was added into acetic acid (30 mL) under stirring, followed by the addition of an appropriate vanillic aldehyde or syringaldehyde (5.00 mmol) and p-toluenesulfonic acid (4.5mmol). The reaction was maintained at 70°C for 5 to 7 hours, then cooled to room temperature and filtered. The filtrate was neutralized with a sodium bicarbonate solution, and the resulting mixture was extracted thrice with 90 mL CH2Cl2. The organic layer was separated and dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography using dichloromethane-methyl alcohol (90:1) as eluent to give pure products 5 and 6.
General procedure for the synthesis of compounds 12k–13m
Compounds 5 or 6 (1.00 mmol), togerther with aminoethyl chloride (3.00 mmol) and anhydrous potassium carbonate ( 3.00 mmol, 0.415 g), was dissolved into 15 ml acetone in a flask, and stirred for 20 minutes. Than a little NaI (0.005 g, 0.02 mmol) was added into the reaction solution as a catalyst. The reaction solution kept at 40 °C for 10 to 12 hours, then cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure and the crude product was purified by silica gel column chromatography using dichloromethane-methyl alcohol (40:1) as eluent to give the pure product compounds 12k–13m. Yield: 80–85 %.
(E)-3-(3-(4-(2-(dimethylamino)ethoxy)-3-methoxyphenyl)acryloyl)-2H-chromen-2-one(12k)
Brownish yellow solid, yield: 85.6%, mp: 124.9–126.3 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.62 (6H, s, 2×NCH3), 3.15 (2H, t, J = 8 Hz, OCH2CH2), 3.92 (3H, s, OCH3), 4.34 (2H, t, J = 8 Hz, OCH2CH2), 6.92 (1H, d, J = 8 Hz, H5 phenyl), 7.20(1H, s, H2 phenyl), 7.25 (1H, d, J=8 Hz, H6 phenyl), 7.35–7.42 (2H, m, H6,8 coumarin), 7.65–7.69 (2H, m, H5,7 coumarin), 7.78–7.86 (2H, m, Hα and Hβ), 8.59 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:45.46, 55.98, 57.46, 65.77, 110.75, 112.83, 116.67, 118.59, 122.03, 123.88, 124.99, 125.47, 128.31, 130.00, 134.15, 145.17, 147.85, 149.46, 150.63, 155.15, 159.41, 186.25. HRMS(ESI) m/z: 394.1651 [M+H]+. Purity: 98.5% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-methoxy-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (12l)
Brownish yellow solid, yield: 75.2%, mp: 158.9–160.7 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.22–2.26 (4H, m, pyrrolidine-H), 3.15–3.20 (4H, m, pyrrolidine-H), 3.62 (2H, s, OCH2CH2), 3.92 (3H, s, OCH3), 4.65 (2H, s, OCH2CH2), 6.94 (1H, d, J = 8 Hz, H5 phenyl), 7.20–7.24 (1H, m, H2,6 phenyl), 7.35–7.41 (2H, m, H6,8 coumarin), 7.65–7.70 (2H, m, H5,7 coumarin), 7.81 (2H, m, Hα and Hβ), 8.59 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.29, 53.78, 54.60, 55.98, 64.37, 110.80, 114.18, 116.70, 118.57, 122.74, 123.66, 125.05, 125.34, 129.67, 130.07, 134.25, 144.69, 148.03, 149.06, 149.71, 155.18, 159.44, 186.30. HRMS(ESI) m/z: 420.1808 [M+H]+. Purity: 98.9% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3-methoxy-4-(2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (12m)
Yellow solid, yield: 80.6%, mp: 270.9–272.1 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.26–1.52 (6H, m, piperidine-H), 1.74 (4H, s, piperidine-H), 3.01 (2H, t, J = 8 Hz, OCH2CH2), 3.92 (3H, s, OCH3), 4.31 (2H, t, J = 8 Hz, OCH2CH2), 6.91 (1H, d, J = 8 Hz, H5 phenyl), 7.19 (1H, s, H2 phenyl), 7.25 (1H, d, J = 8 Hz, H6 phenyl), 7.34–7.40 (2H, m, H6,8 coumarin), 7.64–7.69 (2H, m, H5,7 coumarin), 7.77–7.85 (2H, m, Hα and Hβ), 8.57 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:23.55, 25.01, 29.70, 54.68, 55.98, 57.12, 65.77, 110.76, 112.80, 116.67, 118.59, 122.03, 123.88, 124.99, 125.47, 128.31, 130.00, 134.15, 145.17, 147.85, 149.46, 150.60, 155.14, 159.41, 186.27 HRMS(ESI) m/z: 434.1960 [M+H]+. Purity: 98.2% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(4-(2-(dimethylamino)ethoxy)-3,5-dimethoxyphenyl)acryloyl)-2-chromen-2-one (13k)
Yellow solid, yield: 80.2%, mp: 183.9–185.6 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.62 (6H, s, 2×NCH3), 3.14 (2H, s, J = 8 Hz, OCH2CH2), 3.82 (6H, s, 2×OCH3), 4.33 (2H, t, J = 8 Hz, OCH2CH2), 6.83 (2H, s, H2,6 phenyl), 7.36–7.43 (2H, m, H6,8 coumarin), 7.65–7.70 (2H, m, H5,7 coumarin), 7.77 (1H, d, J=16 Hz, Hα), 7.85(1H, d, J = 16 Hz, Hβ), 8.65 (1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:45.67, 52.46, 56.12, 67.93, 105.68, 116.79, 118.54, 123.71, 125.08, 125.87, 128.73, 130.09, 134.35, 139.91, 142.35, 144.78, 148.18, 153.28, 155.29, 159.44, 186.29 HRMS(ESI) m/z: 424.1759 [M+H]+. Purity: 98.8% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3,5-dimethoxy-4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (13l)
Brownish yellow solid, yield: 80.3%, mp: 274.1–276.4 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 2.22–2.26 (4H, m, pyrrolidine-H), 3.12–3.20 (4H, m, pyrrolidine-H), 3.62 (2H, s, J=8 Hz, OCH2CH2), 3.90 (6H, s, 2×OCH3), 4.62 (2H, t, J=8 Hz, OCH2CH2), 6.88 (2H, s, H2,6 phenyl), 7.35–7.41 (2H, m, H6,8 coumarin), 7.65–7.74 (2H, m, H5,7 coumarin), 7.77 (1H, d, J = 16 Hz, Hα), 7.85(1H, d, J = 16 Hz, Hβ), 8.57 (1H, s, H4 coumarin) 13CNMR(100MHz, CDCl3) δ:23.75, 52.46, 56.05, 67.93, 105.35, 116.75, 118.54, 123.71, 125.08, 125.87, 128.75, 130.09, 134.35, 139.91, 142.35, 144.76, 148.18, 153.28, 155.20, 159.35, 186.25 HRMS(ESI) m/z: 450.1915 [M+H]+. Purity: 98.2% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
(E)-3-(3-(3,5-dimethoxy-4-(2-(piperidin-1-yl)ethoxy)phenyl)acryloyl)-2H-chromen-2-one (13m)
Yellow solid, yield: 78.3%, mp: 214.9–216.7 °C; 1H NMR (400 MHz, DCCl3): δ(ppm) 1.26 (6H, s, piperidine-H), 1.63 (4H, s, piperidine-H), 2.33 (2H, s, OCH2CH2), 3.88 (6H, s, 2×OCH3), 4.36 (2H, s, OCH2CH2), 6.87 (2H, s, H2,6 phenyl), 7.35–7.41 (2H, m, H6,8 coumarin), 7.65–7.70 (2H, m, H5,7 coumarin), 7.77(1H, d, J = 16 Hz, Hα), 7.85(1H, d, J = 16 Hz, Hβ), 8.59(1H, s, H4 coumarin) 13C NMR(100MHz, CDCl3) δ:21.33, 23.34, 29.70, 54.00, 56.11, 57.14, 67.93, 105.66, 116.71, 118.54, 123.71, 125.08, 125.87, 128.75, 130.09, 134.35, 139.91, 142.35, 144.76, 148.18, 153.28, 155.20, 159.44, 186.26. HRMS(ESI) m/z: 464.2076 [M+H]+. Purity: 99.1% by HPLC (MeOH/0.1% TEA 85:15 (v/v)).
AChE and BChE inhibition assay
The effects of newly synthesized compounds in AChE or BChE inhibition were measured by using a modified Ellman method[30]. The individual compound was dissolved in Tween 80 (final concentration was 0.06% in each reaction) and diluted with water to different concentrations immediately before use. Five different concentrations were tested for each compound in triplicate. The reaction mixture containing 2.76 mL of Na2HPO4/NaH2PO4 buffer (pH 8.0, 0.1mol/L), 100 μL of the different concentrations of tested compounds, and 100 μL of AChE or BChE (100 μL) was incubated for 30 min (30 °C). Then the reaction was terminated by the addition of 100 μL of 20% sodium dodecylsulfate (SDS), 100 μL 10 mmol/L 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was added as chromogenic agent to generate a yellow solution. The absorbance of each assay solution was measured at 412 nm by UV spectroscopy. The IC50 values were calculated using Bliss method and expressed as mean± SD.
Kinetic studies
Kinetic studies of AChE were performed by using a reported method [31,32]. Compound was added into the assay solution and preincubated with the enzyme at 30 °C, followed by the addition of 100 μL acetylthiocholine iodide including five concentrations. The assay solution contained 100 μL compound, 100 μL DTNB, 2.79 mL 0.1 mol/L Na2HPO4/NaH2PO4 buffer (pH 7.4). Kinetic assay of the hydrolysis of acetylthiocholine iodide catalyzed by AChE was conducted spectrometrically at 412 nm. The parallel control experiment was carried out without compound in the mixture.
Molecular docking
Molecular docking study was carried out with Molecular Operating Environment (MOE) software package (Chemical Computing Group, Montreal, Canada), and structure models of AChE/BChE X-ray crystal structures (PDB ID: 1EVE/1P0I) were gained from protein data bank [33,34]. The 3D structures of the compounds were built with virtue of the builder interface of MOE program, and docked into the active site of the protein after energy being minimized. The Dock scoring in MOE software was done by ASE scoring function.
Supplementary Material
SCHEME 1.
Synthesis of compounds 5d–7j.
Reagents and conditions: (a) Piperidine, room temperature; (b) Piperidine, Hydroxybenzaldehyde, ethanol, reflux; (c) Cl(CH2)2NR/Br(CH2)2R, K2CO3, NaI, DMF, 56 °C.
Acknowledgments
This work was supported by the Natural science foundation of Hu’nan province” (Grants No.2017JJ2050 and No.2018JJ3572) and in part by the United States Public Health Service (NIH grant GM065307).
References
- 1.Association A. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017;13:325–373. doi: 10.1016/j.jalz.2017.02.001. [DOI] [Google Scholar]
- 2.Dey A, Bhattacharya R, Mukherjee A, Pandey DK. Natural products against Alzheimer’s disease: pharmaco-therapeutics and biotechnological interventions. Biotechnol Adv. 2016;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Blanco-Silvente L, Castells X, Saez M, Barceló MA, GarreOlmo J, Vilalta-Franch J, Capellà D. Discontinuation, efficacy, and safety of cholinesterase inhibitors for Alzheimer’s disease: a meta-analysis and meta-regression of 43 randomized clinical trials enrolling 16106 patients. Int J Neuropsychoph. 2017;20:519–528. doi: 10.1093/ijnp/pyx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurbuz AS, Ozturk S, Acar E, Cagan Efe S, Akgun T, Kilicgedik A, Guler A, Kirma C. Acquired long QT syndrome and Torsades de Pointes related to donepezil use in a patient with Alzheimer disease. Egypt Heart J. 2016;68:197–199. doi: 10.1016/j.ehj.2015.07.004. [DOI] [Google Scholar]
- 5.Isik AT, Soysal P, Yay A. Which rivastigmine formula is better for heart in elderly patients with Alzheimer’s disease: oral or patch? Am J Alzheimers Dis Other Demen. 2014;29:735–738. doi: 10.1177/1533317514536598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Li RS, Wang XB, Hu XJ, Kong LY. Design, synthesis and evaluation of flavonoid derivatives as potential multifunctional acetylcholinesterase inhibitors against Alzheimer’s disease. Bioorg Med Chem Lett. 2013;23:2636–2641. doi: 10.1016/j.bmcl.2013.02.095. [DOI] [PubMed] [Google Scholar]
- 8.Asadipour A, Alipour M, Jafari M, Khoobi M, Emami S, Nadri H, Sakhteman A, Moradi A, Sheibani V, Moghadam FH, Shafiee A, Foroumadi A. Novel coumarin-3-carboxamides bearing N-benzylpiperidine moiety as potent acetylcholinesterase inhibitors. Eur J Med Chem. 2013;70:623–630. doi: 10.1016/j.ejmech.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Silva T, Reis J, Teixeira J, Borges F. Alzheimer’s disease, enzyme targets and drug discovery struggles: from natural products to drug prototypes. Ageing Res Rev. 2014;15:116–145. doi: 10.1016/j.arr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Gomes MN, Muratov EN, Pereira M, Peixoto JC, Rosseto LP, Cravo PVL, Andrade CH, Neves BJ. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules. 2017;22:1210. doi: 10.3390/molecules22081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong HJ, Lee CS, Choi JG, Hong YD, Shin SS, Park JS, Lee JH, Lee S, Yoon KD, Ko JY. Flavokawains B and C, melanogenesis inhibitors, isolated from the root of Piper methysticum and synthesis of analogs. Bioorg Med Chem Lett. 2015;25:799–802. doi: 10.1016/j.bmcl.2014.12.082. [DOI] [PubMed] [Google Scholar]
- 12.Liu HR, Huang XQ, Lou DH, Liu XJ, Liu WK, Wang QA. Synthesis and acetylcholinesterase inhibitory activity of Mannich base derivatives flavokawain B. Bioorg Med Chem Lett. 2014;24:4749–4753. doi: 10.1016/j.bmcl.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 13.Liu HR, Liu XJ, Fan HQ, Tang JJ, Gao XH, Liu WK. Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem. 2014;22:6124–6133. doi: 10.1016/j.bmc.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Liu HR, Zhou C, Fan HQ, Tang JJ, Liu LB, Gao XH, Wang QA, Liu WK. Novel potent and selective acetylcholinesterase inhibitors as potential drugs for the treatment of Alzheimer’s disease: synthesis, pharmacological evaluation, and molecular modeling of amino-alkyl-substituted fluoro-chalcones derivatives. Chem Biol Drug Des. 2015;86:517–522. doi: 10.1111/cbdd.12514. [DOI] [PubMed] [Google Scholar]
- 15.Liu HR, Fan HQ, Gao XH, Huang XQ, Liu XJ, Liu LB, Zhou C, Tang JJ, Wang QA, Liu WK. Design, synthesis and preliminary structure-activity relationship investigation of nitrogen-containing chalcone derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors: a further study based on Flavokawain B Mannich base derivatives. J Enzyme Inhib Med Chem. 2016;31:580–589. doi: 10.3109/14756366.2015.1050009. [DOI] [PubMed] [Google Scholar]
- 16.Gao XH, Zhou C, Liu HR, Liu LB, Tang JJ, Xia XH. Tertiary amine derivatives of chlorochalcone as acetylcholinesterase (AChE) and buthylcholinesterase (BChE) inhibitors: the influence of chlorine, alkyl amine side chain and α,β-unsaturated ketone group. J Enzyme Inhib Med Chem. 2017;32:146–152. doi: 10.1080/14756366.2016.1243534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popov Aleksandrov A, Mirkov I, Ninkov M, Mileusnic D, Demenesku J, Subota V, Kataranovski D, Kataranovski M. Effects of warfarin on biological processes other than haemostasis: A review. Food Chem Toxicol. 2018;113:19–32. doi: 10.1016/j.fct.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Wang YW, Li PF, Tang YB, Fawcett JP, Gu JK. Quantitation of Armillarisin A in human plasma by liquid chromatography-electrospray tandem mass spectrometry. J Pharma Biomed Anal. 2007;43:1860–1863. doi: 10.1016/j.jpba.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Revankar HM, Bukhari SNA, Kumar GB, Qin HL. Coumarins scaffolds as COX inhibitors. Bioorg Chem. 2017;71:146–159. doi: 10.1016/j.bioorg.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Anand P, Singh B, Singh N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg Med Chem. 2012;20:1175–1180. doi: 10.1016/j.bmc.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi Y, Nishizono N, Kobayashi D, Yoshimura T, Wada K, Oda K. Evaluation of synthesized coumarin derivatives on aromatase inhibitory activity. Bioorg Med Chem Lett. 2017;27:2645–2649. doi: 10.1016/j.bmcl.2017.01.062. [DOI] [PubMed] [Google Scholar]
- 22.Abdizadeh T, Kalani MR, Abnous K, Tayarani-Najaran Z, Khashyarmanesh BZ, Abdizadeh R, Ghodsi R, Hadizadeh F. Design, synthesis and biological evaluation of novel coumarin-based benzamides as potent histone deacetylase inhibitors and anticancer agents. Eur J Med Chem. 2017;132:42–62. doi: 10.1016/j.ejmech.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Shen FQ, Wang ZC, Wu SY, Ren SZ, Man RJ, Wang BZ, Zhu HL. Synthesis of novel hybrids of pyrazole and coumarin as dual inhibitors of COX-2 and 5-LOX. Bioorg Med Chem Lett. 2017;27:3653–3660. doi: 10.1016/j.bmcl.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Yang HL, Cai P, Liu QH, Yang XL, Li F, Wang J, Wu JJ, Wang XB, Kong LY. Design, synthesis and evaluation of coumarin-pargyline hybrids as novel dual inhibitors of monoamine oxidases and amyloid-β aggregation for the treatment of Alzheimer’s disease. European J Med Chem. 2017;138:715–728. doi: 10.1016/j.ejmech.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Pingaew R, Saekee A, Mandi P, Nantasenamat C, Prachayasittikul S, Ruchirawat S, Prachayasittikul V. Synthesis, biological evaluation and molecular docking of novel chalconeecoumarin hybrids as anticancer and antimalarial agents. Eur J Med Chem. 2014;85:65–76. doi: 10.1016/j.ejmech.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 26.Patil RB, Sawant SD. Synthesis, characterization, molecular docking and evaluation of antimicrobial and antiproliferative properties of 3-substituted chromen-2-one derivatives. Der Pharma Chemica. 2015;7:26–37. http://www.derpharmachemica.com/pharma-chemica/synthesis-characterization-molecular-docking-and-evaluation-of-antimicrobial-and-antiproliferative-properties-of-3substi.pdf. [Google Scholar]
- 27.Moodley T, Momin M, Mocktar C, Kannigadu C, Koorbanally NA. The synthesis, structural elucidation and antimicrobial activity of 2- and 4-substituted-coumarinyl chalconess. Magn Reson Chem. 2016;54:610–617. doi: 10.1002/mrc.4414. [DOI] [PubMed] [Google Scholar]
- 28.Liu HR, Liu LB, Gao XH, Liu YZ, Xu WJ, He W, Jiang H, Tang JJ, Fan HQ, Xia XH. Novel ferulic amide derivatives with tertiary amine side chain as acetylcholinesterase and butyrylcholinesterase inhibitors: the influence of carbon spacer length, alkylamine and aromatic group. Eur J Med Chem. 2016;126:810–822. doi: 10.1016/j.ejmech.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Ji QG, Yang D, Wang X, Chen CY, Deng Q, Ge ZQ, Yuan LJ, Yang XL, Liao F. Design, synthesis and evaluation of novel quinazoline-2,4-dione derivatives as chitin synthase inhibitors and antifungal agents. Bioorg Med Chem. 2014;22:3405–3413. doi: 10.1016/j.bmc.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Ellman GL, Courtney KD, Andres VJ, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 31.Skrzypek A, Matysiak J, Niewiadomy A, Bajda M, Szymański P. Synthesis and biological evaluation of 1,3,4-thiadiazole analogues as novel AchE and BChE inhibitors. Eur J Med Chem. 2013;62:311–319. doi: 10.1016/j.ejmech.2012.12.060. [DOI] [PubMed] [Google Scholar]
- 32.Luo ZH, Sheng JF, Sun Y, Lu CJ, Yan J, Liu AQ, Luo HB, Huang L, Li XS. Synthesis and evaluation of multi-target-directed ligands against Alzheimer’s disease based on the fusion of donepezil and ebselen. J Med Chem. 2013:569089–9099. doi: 10.1021/jm401047q. [DOI] [PubMed]
- 33.Alpan AS, Parlar S, Carlino L, Tarikogullari AH, Alptüzün V, Günesa HS. Synthesis, biological activity and molecular modeling studies on 1H-benzimidazole derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem. 2013;21:4928–4937. doi: 10.1016/j.bmc.2013.06.065. [DOI] [PubMed] [Google Scholar]
- 34.Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with E2020 (Aricept): implications for the design of new anti-Alzheimer drugs. Structure. 1999;7:297–307. doi: 10.1016/S0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 35.Sheng R, Lin X, Li J, Jiang Y, Shang Z, Hu Y. Design, synthesis, and evaluation of 2-phenoxy-indan-1-one derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem Lett. 2005;15:3834–3837. doi: 10.1016/j.bmcl.2005.05.132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.