Abstract
Interleukin (IL)-18, also known as interferon-gamma (INF-γ)-inducing factor, is involved in Th1 responses and regulation of immunity. Accumulating evidence implicates IL-18 in autoimmune diseases, but little is known of its role in acquired aplastic anemia (AA), immune-mediated destruction of bone marrow (BM) hematopoietic stem and progenitor cells (HSPCs). IL-18 protein levels were significantly elevated in sera of severe AA patients, including both responders and non-responders assayed before treatment, and decreased after treatment. IL-18 receptor (IL-18R) was expressed on HSPCs. Co-culture of human BM CD34+ cells from healthy donors with IL-18 upregulated genes in the helper T cell pathway and Notch signaling pathway, and downregulated genes in cell cycle regulation, telomerase, and IL-6 signaling pathways. In murine models of immune-mediated BM failure, plasma IL-18 levels also were elevated. However, deletion of IL-18 in donor lymph node cells or deletions of either IL-18 or IL-18R in recipients did not attenuate elevations of circulating IFN-γ, tumor necrosis factor-alpha, or IL-6, nor did they alleviate BM failure. In summary, our findings suggest that while increased circulating IL-18 is a feature of severe AA, it may reflect an aberrant immune response but be dispensable to the pathogenesis of AA.
Keywords: Interleukin 18, aplastic anemia, bone marrow failure, murine model, hematopoiesis
1. Introduction
Acquired aplastic anemia (AA) is a bone marrow (BM) failure syndrome characterized by peripheral blood (PB) pancytopenia and BM hypoplasia [1–2]. Success of immunosuppressive therapy (IST), among other clinical and laboratory clues, is compelling evidence of immune pathophysiology of AA [3]. In most cases, AA is an immune-mediated disorder with active destruction of hematopoietic cells by effector T lymphocytes. Increased production of interferon gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin (IL)-2 by patients’ T cells suggests important roles for a type 1 immune response in BM hematopoietic cell destruction [4–6]. Th17 response [7] and impairment of regulatory T cells (Tregs) [8] may also contribute to the pathogenesis of AA. Production of type 1 cytokines by activated T cells or BM cells may contribute to hematopoietic failure development [6–8], as IFN-γ suppresses hematopoiesis [9,10] and facilitates destruction of hematopoietic cells by augmenting apoptosis in the presence of activated cytotoxic T cells (CTLs) in murine BM failure models [11].
IL-18, initially identified as an IFN-γ-inducing factor in T and natural killer (NK) cells, is a member of the IL-1 family [12, 13], and constitutively secreted by several types of cells such as macrophages or dendritic cells (DCs). Biological activity of IL-18 is mainly regulated by its enzymatic processing rather than at a transcription level [14]. IL-18 receptor (IL-18R) is expressed mostly on CTLs. Binding of IL-18 to the IL-18Rα recruits the IL-18Rβ chain and initiates downstream signaling transduction through myeloid differentiation primary response 88 (MyD88), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κb), and activator protein 1 (AP-1) [15–17]. IL-18 cooperates with IL-12 for NK cell activation, induction of IFN-γ production and other type 1 cytokines in response to pathogen products, thus promoting Th1 polarization and CTL responses [18–19]. IL-18 binding protein (IL-18BP), a natural inhibitor of IL-18, has high affinity to mature IL-18 and blocks interactions between IL-18 and IL-18Rα, and subsequent signaling by preventing receptor dimerization [20–21]. Exaggerated IFN-γ production triggered by IL-18 leads to IL-18BP secretion, as a negative-feedback loop to subdue the type 1 immune responses [22–23].
IL-18 has been implicated in the pathogenesis of many inflammatory and autoimmune diseases, including type 1 diabetes, rheumatic arthritis, allergy, asthma, Crohn’s disease, multiple sclerosis, and myasthenia gravis [24–30]. Elevated IL-18 and IL-18BP levels are found in immune thrombocytopenia purpura patients [31]. IFN-γ and IL-18 plasma levels are significantly increased in patients with active AA, compared with healthy controls and AA patients in remission, and the levels of IL-18 were also found to be correlated with disease severity [32]. However, roles of IL-18 in the pathogenesis of AA remain largely unknown.
We have previously developed murine models for immune-mediated BM failure by infusion of allogeneic lymph node (LN) cells into sub-lethally irradiated recipients [33–34], and successfully used these models to study AA pathophysiology [4, 11] and to test new treatments [35, 36]. Our current study was aimed at defining the roles of IL-18 in immune-mediated BM destruction and in the development of clinical pancytopenia. In addition to the analyses of clinical samples collected from AA patients, we also utilized our murine models, with targeted germline deletions of IL-18 and IL-18R, respectively, to assess roles of the IL-18/IL-18R signaling pathway in the development of immune-mediated BM failure.
2. Methods
2.1. Human samples
Serum samples were obtained after informed consent. In Cohort 1, 88 patients with SAA and 20 age- and sex-matched healthy subjects were included: IL-18 levels at baseline (88 SAA patients) and after treatment (23 SAA patients), as well as IL-18BP levels prior to treatment (27 SAA patients) were analyzed. In Cohort 2, another 39 SAA patients and 31 patients with myelodysplastic syndrome (MDS) were included, and only baseline IL-18 levels were analyzed. Standard criteria were used for the diagnosis of SAA, MDS and evaluation of disease severity [37, 38]. SAA patients were enrolled in clinical research protocols (clinicaltrials.gov, NCT00071045, NCT00260689, NCT00922883, NCT01623167, NCT00001397, and NCT00001620). MDS patients were enrolled in clinical research protocols (clinicaltrials.gov, NCT00001397, NCT00001620, NCT00217594 and NCT00961064). BM samples of healthy controls were obtained from donors of the NIH Clinical Center. All human subjects were enrolled in clinical protocols approved by the NHLBI Institutional Review Board. Clinical characteristics of patients in this study are summarized in Table 1.
Table 1.
Patient demographics
| Cohort 1 | Cohort 2 | ||
|---|---|---|---|
| Characteristics | SAA | SAA | MDS |
| No. | 88 | 39 | 31 |
| Median age, years (range) | 33 (2–75) | 31 (6–82) | 60 (23–86) |
| Sex, male/female | 48/40 | 20/19 | 17/14 |
| Disease status | RCUD (7), RCMD (15) | ||
| RARS (4), RAEB-1 (5) | |||
| Treatment | |||
| IST | 41 | 15 | |
| IST+Eltrombopag | 47 | 24 | |
| Time analysis | |||
| Samples at diagnosis | 88 | 39 | 31 |
| Samples 6m after | 23 | 0 | 0 |
| treatment | |||
| Responses to treatment | |||
| Responders | 46 | 29 | |
| Complete response | 21 | 15 | |
| Partial response | 25 | 14 | |
| Non-responders | 37 | 10 | |
| Blood counts, median (range) | |||
| ANC (K/uL) | 0.43 (0.01–9.23) | 0.37 (0.01–3.15) | 1.18 (0.13–4.19) |
| HGB (g/dL) | 8.7 (5.1–13.7) | 8.2 (6.4–11.3) | 9.7 (4.9–12.7) |
| ARC (K/uL) | 24.95 (0–95.7) | 25.7 (5–78.7) | 59.8 (2.3–115.7) |
| PLT (K/uL) | 20 (3–229) | 25 (2–377) | 34 (9–240) |
Abbreviations: SAA, sever aplastic anemia; MDS, myelodysplastic anemia; IST, immunosuppressive therapy; 6m, 6 months; ANC, absolute neutrophil counts; HGB, hemoglobin; ARC, absolute reticulocyte counts; PLT, platelet. RCUD, refractory cytopenia with unilineage dysplasia; RCMD, Refractory cytopenia with multilineage dysplasia; RARS, Refractory anemia with ringed sideroblasts; RAEB-1, Refractory anemia with excess blasts-1.
2.2. Assays of IL-18 and IL-18BP levels
Sera from both patients and controls, and plasma from mice were stored at −80°C until analysis. IL-18 levels in human serum and mouse plasma were measured by ELISA assay (MBL, Woburn, MA) according to manufacturer’s instructions. Samples and standards were assayed in duplicate. Absorbance was read at 450 nm and 620 nm (for background subtraction) using the VICTOR3 1420 Multilabel Plates Counter (PerkinElmer, Waltham, MA). Human IL-18BP was measured with the magnetic Luminex kit (R&D Systems, Minneapolis, MN).
2.3. Co-culture of human CD34+ cells with IL-18
BM-MNCs from three healthy donors were stained with pacific blue-labeled anti-CD3- (Biolegend, San Diego, CA), anti-CD14, and anti-CD19 (Invitrogen, Carlsbad, CA), and anti-CD34-PE (BD Biosciences, Franklin Lakes, NJ). Lineage−CD34+ cells were sorted using the FACSAria II Cell Sorter (BD Biosciences,). Sorted CD34+ cells were cultured in StemSpan SFEM II serum-free medium plus StemSpan CD34+ Expansion Supplement (STEMCELL Technologies Inc., Vancouver, BC, Canada) in the presence or absence of 20 ng/ml recombinant human IL-18 (MBL) for 48 hours, followed by RNA extraction.
2.4. PCR array
Total RNA was isolated from human CD34+ cells using the RNeasy kit (Qiagen, Valencia, CA), digested with RNase-free DNase I (Qiagen), and assessed by using a Nanodrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). First-strand complementary DNA was synthesized by using the RT2 First-Strand kit (Qiagen). Quantitative analysis of messenger RNA expression of hematopoiesis-related genes was performed by using the human polymerase chain reaction array PAHS-054Z (Qiagen), and each sample was run in duplicate.
2.5. Animals and induction of BM failure
Congenic C.B10-H2b/LilMcd (C.B10), inbred FVB/N (FVB), inbred C57BL/6 (B6), and induced mutant B6.129P2-Il18 tm1Aki/J (IL-18−/−) and B6.129P2-Il18r1tm1Aki/J (IL18R−/−) mice were all from the Jackson Laboratory (Bar Harbor, ME), bred, and maintained in the National Institutes of Health Animal Facilities under standard care and nutrition. The genotypes of IL-18−/− and IL18R−/− mice were confirmed by PCR analyses according to producer’s protocols. All animal studies were approved by the Animal Care and Use Committee at the National Heart, Lung, and Blood Institute.
Immune-mediated BM failure was induced as previously reported [33, 35]. In one model, LN cells from IL-18−/− or wild type B6 donor mice were homogenized, washed, filtered, and intravenously injected into sex-matched C.B10 recipients pre-irradiated with 5-Gy of total body irradiation (TBI) 4–6 hours earlier at 5 × 106 cells per recipient [33]. Recipient animals were bled and euthanized 12–18 days later to obtain tissues for histological and cytological assessments. In the other models, the same number of LN cells from FVB donors were infused into IL-18−/−, IL-18R−/− or wild type B6 recipients pre-irradiated with 6.5 Gy TBI 4–6 hours earlier [35]. The recipient animals were bled and analyzed at 9 days later. In all experiments, mice were used at 8–12 weeks of age.
2.6. Blood counts, cell staining, and flow cytometry
Blood was collected from the retro-orbital sinus into Eppendorf tubes with EDTA. Complete blood count was performed using a HemaVet 950 analyzer (Drew Scientific Inc., Waterbury, CT). After mice were euthanized by CO2, BM cells were extracted from tibiae and femurs, filtered through a 95-μM nylon mesh, counted with a Vi-Cell counter (Beckman Coulter, Miami, FL), and stained with antibody mixtures on ice for 30 minutes in RPMI 1640 (Life Technologies, Carlsbad, CA). Samples were subsequently acquired using a BD LSR Fortessa cytometer (BD Biosciences) and post-acquisition analysis was performed using Flowjo software (v.7.6.4; Flowjo LLC, BD Biosciences).
Human monoclonal antibodies used for flow cytometry analyses were CD34 (clone 8G12) from BD Biosciences; and CD38 (clone HIT2), CD45RA (clone HI100), CD90 (Thy1, clone 5E10), and CD18Rα (CD218a, clone H44) from BioLegend. Monoclonal antibodies for murine CD3ε (clone 145–2C11), CD4 (clone GK 1.5), CD8α (clone 53–6.7), CD45R (clone MB4B4), CD11b (clone M1/70), granulocytes (Gr1/Ly6-G, clone RB6–8C5), erythroid cells (clone Ter119), stem cell antigen 1(Sca-1, clone D7), CD117 (c-Kit, clone 2B8), CD48 (clone HM48–1), CD150 (SLAM, clone TC15–12F12.2), and IL18Rα (CD218a, clone BG/IL18RA) were also from BioLegend. Antibodies were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), allophycocyanin 7 (APC-Cy7), phycoerythrincyanin 7 (PE-Cy7), Brilliant Violet (BV) 421, and BV711.
IFN-γ, TNF-α and IL-6 protein levels in mouse plasma were measured using the LEGENDplex Mouse Th1 Panel (5-plex) (BioLegend).
2.7. Data analysis and statistics
Data analysis was performed using Prism (v.7.02; GraphPad software, La Jolla, CA). Results were shown as median ± interquartile range unless stated otherwise. Statistical analysis was performed using two-sided unpaired Mann-Whitney test or t-test for two groups when applicable, or one-way analysis of variance for comparisons (ANOVA) between three or more groups. Correlation analysis was determined using Pearson correlation test for normally distributed data and Spearman’s correlation test for data without normal distribution. PCR array results were analyzed using the Qiagen online tool (https://www.qiagen.com/us/shop/genes-and-pathways/data-analysis-center-overview-page/). Subsequently, scatter plot, volcano plot, and heatmap containing differentially expressed genes were generated. Differentially expressed genes, identified using a threshold p < 0.05 and fold change > 2, were further subjected to pathway analysis by Ingenuity Pathway Analysis software (www.ingenuity.com, v33559992, Qiagen Bioinformatics). p < 0.05 was considered statistically significant.
3. Results
3.1. Serum IL-18 and IL-18BP levels are elevated in treatment-naïve SAA patients
Serum levels of IL-18 in SAA patients (n = 88) and healthy controls (n = 20) were measured using ELISA. Among these samples, IL-18BP levels were also measured in 27 SAA patients and 9 healthy controls. At diagnosis, IL-18 serum levels were significantly increased in SAA patients compared with controls (475.4 ± 44.15 pg/mL vs 192 ± 23.47 pg/mL, p = 0.0001, Fig. 1A). There were no correlations between serum IL-18 levels and blood counts in patients before treatment (data not shown). We also measured levels of IL-18BP (a natural inhibitor of IL-18) in 27 SAA patients and 9 healthy controls, which were significantly higher in SAA patients at diagnosis than in controls (p = 0.0077, Fig. 1B). To investigate if the increased IL-18 levels in AA patients were also exist in MDS-a similar BM failure disease, we measured circulating IL-18 levels in another cohort of 31 MDS patients, 39 SAA patients, and 8 healthy donors. Both SAA and MDS patients showed higher IL-18 levels than did heathy donors, but no difference was observed between SAA and MDS patients (Figure 1C). High IL-18 levels might reflect an inflammation state in both diseases.
Figure 1.
Serum IL-18 and IL-18BP levels in SAA patients. Serum IL-18 (A) and IL-18BP (B) levels were measured in SAA patients (n = 88 and n = 27, respectively) and healthy controls (n = 20 and n = 9, respectively). (C) Serum IL-18 protein levels in another cohort of SAA (n=39) and MDS (n=33) patients, compared with healthy controls (n=8). (D) Serum IL-18 protein levels in SAA patients before (n = 88) and after therapy (n = 23), and in healthy controls (n = 20). (E) Serum IL-18 protein levels in responders and non-responders before (n = 46 and n = 37, respectively) and after therapy (n = 16 and n = 7, respectively). *p < 0.05; **p < 0.01. Abbreviations: SAA, severe aplastic anemia; IL-18, interleukin-18; BP, binding protein; MDS, myelodysplastic syndrome; Pre, pre-treatment; Post, post-treatment.
3.2. Dynamic changes of IL-18 levels with treatment
Twenty-three SAA patients (16 responders and 7 non-responders) in the first cohort were studied for IL-18 levels before and 6 months after treatment. Overall, high serum IL-18 protein levels significantly decreased after treatment (p = 0.0181, Fig. 1D), to the levels similar to those in healthy controls (p = 0.7426). When patients were divided based on their response to treatment at 6-month time point, both responders (p = 0.0486) and non-responders (p < 0.0001) at diagnosis showed significantly higher IL-18 serum levels than did controls, and non-responders displayed higher levels of IL-18 than did responders (p = 0.0052, Figure 1E). The high levels of IL-18 in both responders and non-responders before treatment tended to decrease after treatment, but did not reach a significant difference (p = 0.0696 and p = 0.1354, respectively; Fig. 1E). Both responders and non-responders exhibited identical IL-18 levels to controls after treatment (p = 0.4132 and p = 0.1398, respectively).
3.3. IL-18/IL-18R signaling regulates gene expression in human HSPCs
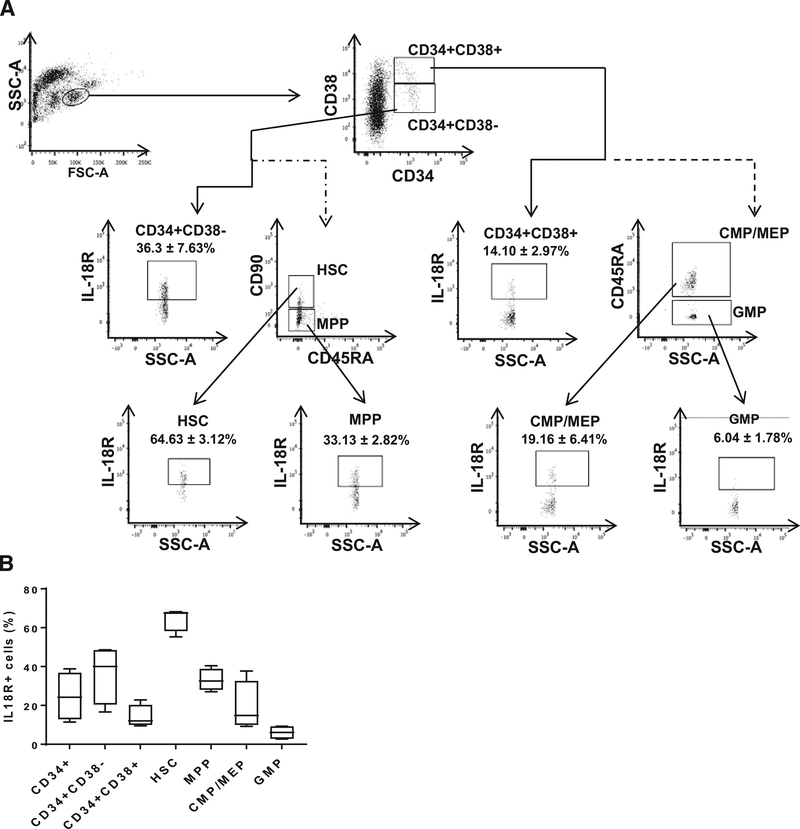
To explore for a possible direct effect of IL-18 on human HSPCs, we first confirmed the existence of IL-18R on human BM-MNCs by flow cytometry. IL18-R was expressed on 24.7 ± 6.25% of CD34+ cells, with 36.3 ± 7.6% of CD34+CD38− cells and 14.10 ± 3.0% of CD34+CD38+ cells. IL-18R was further measured on hematopoietic cell subpopulations [39], including hematopoietic stem cells (HSCs, 64.63 ± 3.12%), multipotent progenitor (MPP, 33.13 ± 2.82%), granulocyte-monocyte progenitor (GMP, 6.04 ± 1.78%), common myeloid progenitor, and megakaryocyte-erythroid progenitor (CMP/MEP, 19.16 ± 6.41%) cells (Fig. 2A, 2B).
Figure 2.
Expression of the IL-18 receptor on human bone marrow hematopoietic stem and progenitor cells. (A) Gating strategy and IL-18 receptor (IL-18R) expression on HSC, MPP, GMP, and CMP/MEP. (B) Bar chart of IL-18R expression in HSC, MPP, CMP/MEP, and GMP cells (n = 4). Abbreviations: HSC, hematopoietic stem cells; MPP, multi-potent progenitors; CMP, common myeloid progenitors; MEP, megakaryocyte-erythrocyte progenitors; GMP, granulocyte-monocyte progenitors.
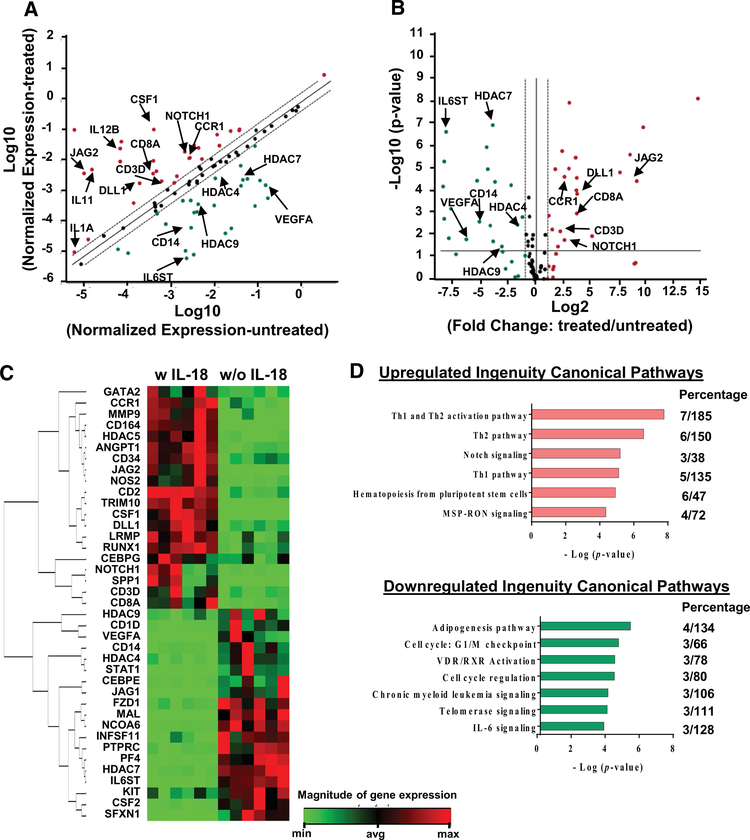
Human BM CD34+ cells were sorted and co-cultured with IL-18 for 48 hours in vitro. PCR-based transcriptome assay focusing on genes in hematopoiesis showed that 20 genes were upregulated and 19 downregulated relative to control CD34+ cells (Fig. 3A–3C). Pathway analysis revealed that upregulated genes were mainly involved in the Th1 and Th2 pathways (CCR1, CD3D, CD8A, DLL1, IL12B, JAG2, and NOTCH1), Notch 1 signaling (DLL1, JAG2, and NOTCH1), and hematopoiesis from pluripotent stem cells (CD3D, CD8A, CSF1, IL11, IL12B, and IL1A). Downregulated genes were involved in cell cycle regulation and telomerase signaling (HDAC4, HDAC7, and HDAC9), and IL-6 signaling (CD14, IL6ST, and VEGFA) (Fig. 3D).
Figure 3.
Direct effects of IL-18 on human CD34+ cells gene expression. (A) Scatter plot of gene expression in IL-18 treated CD34+ cells compared to untreated CD34+ cells. x and y axes indicate Log10 (normalized expression in untreated cells) and y axis indicated Log10 (normalized expression in treated cells), respectively. Upregulated and downregulated genes were labeled in red or green, respectively. (B) Box-plot of gene expression in IL-18 treated CD34+ cells compared to untreated CD34+ cells. x and y axes indicate Log2 (fold change: treated/untreated cells) and Log10 (p-value), respectively. (C) Heatmap of differentially expressed genes in cells treated with and without IL-18. (D) Differentially expressed genes were subjected to Ingenuity Pathway Analysis. -Log (p-value) and aberrant pathways are indicated at x and y axes, respectively. Percentage indicates the number of differentially expressed genes vs. total number of genes in individual pathways. Differentially expressed genes were defined as p < 0.05 and fold change > 2.0.
3.4. Knockout of IL-18 or IL-18R does not alleviate murine immune-mediated BM failure
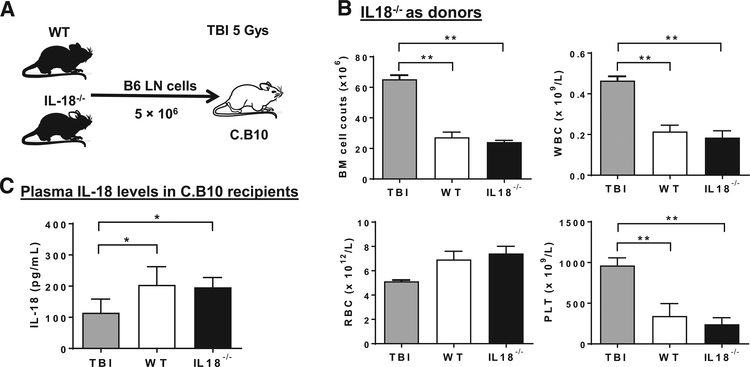
Next, we explored whether disruption of IL-18 signaling could alleviate murine immune-mediated BM failure (Fig. 4A). When LN cells from IL-18−/− or wild type B6 mice were infused into C.B10 recipient mice, both types of LN cells induced similar severity of BM failure, evidenced by similar decreases in BM cell counts, white blood cells (WBC), red blood cells (RBC), and platelet (PLT) in C.B10 recipient mice on day 14 (Fig. 4B), indicating IL-18 in donor T cells was dispensable in induction of murine BM failure. We measured IL-18 levels in C.B10 recipient mice by ELISA, and plasma IL-18 protein levels were significantly increased in recipients with BM failure induced by injection of LN cells from wild type B6 donor mice (Fig. 4C) compared with TBI controls, similar to the finding from SAA patients (Fig. 1A). Yet, recipients which received LN cells from IL-18−/− donor mice exhibited similarly high plasma IL-18 levels (Fig. 4C).
Figure 4.
Infusion of LN cells from IL-18−/− donors into recipient mice induced BM failure. (A) Experimental schematic of mouse experiments with B6 (donors) and C.B10 (recipients) mice. LN cells from IL-18−/− or wild type B6 mice were infused into C.B10 recipient mice (n = 4, respectively) to induce BM failure. C.B10 mice received only TBI were used as controls. (B) BM cell counts, WBC, RBC, and PLT in C.B10 recipients after infused with LN cells from IL-18−/− or wild type B6 donor mice. (C) Plasma IL-18 levels in C.B10 recipients with BM failure induced by injection of LN cells from IL-18−/− or wild type B6 donor mice, compared with TBI controls. *p < 0.05; **p < 0.01. Abbreviations: IL-18, interleukin 18; WT, wild type controls; LN, lymph node; TBI, total body irradiation; B6, C57BL/6 mice; C.B10, C.B10-H2b/LilMcd mice; WBC, white blood cells count; RBC, red blood cells count; PLT, platelets, BM, bone marrow.
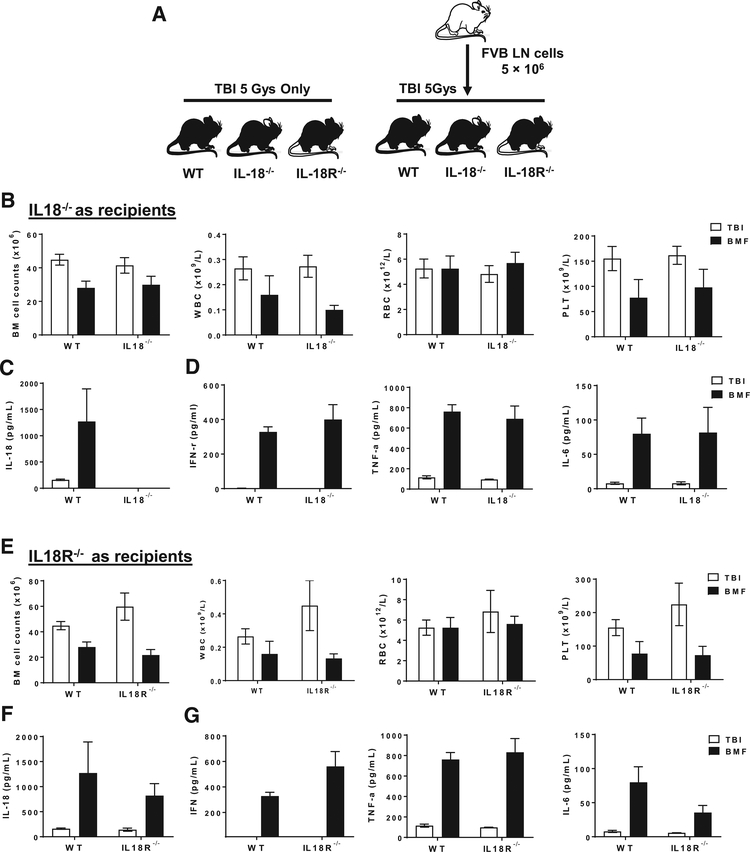
Next, we tested susceptibility of IL-18−/− and IL-18R−/− recipient mice to immune-mediated BM destruction after receiving TBI and lymphocytes injection from FVB donors. Wild-type B6 recipients that had received the same FVB donor LN infusion were used as controls (Fig. 5A). All three genotypes developed BM failure. The severity of BM failure, quantified by decrease of total BM cell counts and peripheral WBC, RBC, and PLT counts, also was not different among IL-18−/− (Fig. 5B), IL-18R−/− recipients (Fig. 5E), and control wild type B6 mice after LN infusion. IL-18 levels were not detectable in IL-18−/− mice with TBI only or BM failure (Fig. 5C), confirming an overall deficiency of IL-18 production in IL-18−/− mice; while wild type and IL-18R−/− mice with BM failure showed more elevated IL-18 levels than did TBI controls (Fig. 5F). We also measured IL-18-related inflammatory cytokines in the plasma of recipients, and found that IFN-γ, TNF-α, and IL-6 were significantly elevated with BM failure, and the levels of these cytokines did not differ between wild type B6 and IL-18−/− (Fig. 5D), or IL-18R−/− recipients (Fig. 5G).
Figure 5.
Bone marrow failure in IL-18−/− and IL-18R−/− recipient mice. (A) Experimental schematic of mouse experiments with FVB (donors) and B6 mice (recipients). LN cells from FVB donor mice were infused into IL-18−/− (n = 5), IL-18R−/− (n = 5) mice, or wild type B6 (n = 6) recipient mice after TBI in order to induce BM failure. Mice received only TBI were used as controls. (B) BM cell, WBC, RBC, and PLT counts in wild type B6 and IL-18−/− mice. IL-18 (C), and IFN-γ, TNF-α and IL-6 (D) protein levels in plasma of wild type B6 and IL-18−/− mice. (E) BM cell, WBC, RBC, and PLT counts in wild type B6 and IL-18R−/− mice. IL-18 (F), and IFN-γ, TNF-α, and IL-6 (G) protein levels in plasma of wild type B6 and IL-18R−/− mice. *p < 0.05; **p < 0.01. Abbreviations: IL-18, interleukin 18; IL-18R, interleukin-18 receptor; WT, wild type; LN, lymph node; TBI, total body irradiation; FVB: FVB/N mice; WBC, white blood cells count; RBC, red blood cells count; PLT, platelets, BM, bone marrow.
4. Discussion
In the current study, we demonstrated increased circulating IL-18 levels in both SAA and MDS patients as well as murine models of immune-mediated BM failure. Furthermore, IL-18 altered the expression of genes critical to hematopoiesis in human CD34+ cells, possibly through the IL-18R signaling pathway, as IL-18R was expressed on human CD34+ HSPCs.
Our observation of increased IL-18 levels in SAA patients was in accordance with a previous report [32] in which serum IL-18 levels in AA patients in remission decreased to similar levels as in healthy controls. In our study, we observed an overall decrease in IL-18 levels post-treatment. However, the trend of decline was observed in both responders and non-responders, indicating that serum IL-18 levels decreased by IST or IST/Eltrombopag, but was not correlated to remission or hematopoietic recovery. The fact that serum IL-18 levels showed no correction with blood counts at a baseline further added complexity to the picture: high serum IL-18 levels might only be a reliable marker of AA at disease onset; and its role in disease progression and remission was very much unclear.
Along with increased levels of IL-18 in AA patients before treatment, we also observed higher levels of IL-18BP in patients than in controls, possibly reflecting negative-feedback to the type 1 immune responses [22–23]. As a natural inhibitor of IL-18’s biologic activity, IL-18BP increases with IL-18 in many pathophysiological settings [31]. In AA, it is possible that the biologic effect of elevated IL-18 was offset by the increased IL-18BP.
IL-18 has been extensively studied in immunity, but its regulatory effect on HSPCs is largely unknown. The IL-18 gene was found to be expressed in proximal rather than distal osteolineage cells in murine BM niche [40]. IL-18R1 is expressed on short-term (ST) progenitors, but not long-term (LT)-HSC. Increased proliferation in ST-HSC and MPP, but not in LT-HSC was observed in IL-18−/− mice. Lin−Scal-1+kit+ (LSK) cells, Lin−Scal-1−kit+ myeloid progenitors, and common lymphoid progenitors (CLP) were better preserved in IL-18−/− mice with fluorouracil (5-FU) treatment, compared to 5-FU-treated WT controls [40]. We observed IL-18R expression on human HSPCs in our current study. Together with the high serum IL-18 levels in SAA patients, our data suggest a direct effect of IL-18 on HSPCs, resulting in suppression of hematopoiesis in AA patients. Indeed, results from our cell culture study in vitro affirmed that IL-18 upregulated the expression of genes associated with the Th1 and Th2 immune pathways and the NOTCH signaling pathway, with downregulation of genes associate with cell cycle, telomerase, and IL-6 signaling. Notch signaling is critical in regulating hematopoiesis. On the one hand, it is still controversial about the role of Notch signaling in regulating the proliferation, self-renewal, and differentiation of HSCs [41]. Activation of the Notch pathway enhances HSC proliferation and self-renewal, while inhibiting especially myeloid differentiation. In contrast, another model proposed that Notch signaling inhibits HSC self-renewal but promotes differentiation. Activation of the Notch pathway directs lymphoid progenitor cells to differentiate toward the T-cell lineage at the expense of B-cell lineage. Telomerase activity may affect telomere length, which is critical to cell replication. Cells will eventually enter either senescence or apoptosis if fail to restore telomere length as they replicate [42]. Impair of telomerase complex functions usually leads to telomere length shortening, which is observed in patients with AA and other bone marrow failure syndromes [42]. As a pleiotropic growth factor with regulatory roles in hematopoiesis and in immune response, IL-6 has been shown to promote HSPCs proliferation and differentiation in vitro in combination with other cytokines such as IL-3, GM-CSF, and SCF [43]. In summary, in line with the mouse study [40], our results suggest direct regulatory roles of IL-18 on human HSPCs. IL-18 may favor helper T cell signaling, and might suppress progenitor cell proliferation and/or myeloid differentiation. These features were in accordance with T cell activation and hematopoiesis suppression in AA.
As human BM failure has been modeled in mice by infusion of LN cells into recipients mismatched at MHC or minor histocompatibility antigen loci, we explored effects of the deficient IL-18/IL-18R signaling pathway in both B6 to CB10 (IL18−/− as donors) and FVB to B6 (IL-18−/− and IL-18R−/− as recipients) models. Both models mimic human AA in hallmark features including severe pancytopenia and BM hypocellularity; oligo T cell expansion, T cell activation, and Fas-Fas ligand expression; altered plasma cytokines including elevated Th1 cytokines; and increased apoptosis and BM cell destruction [33–35]. Moreover, increased levels of plasma IL-18 together with IFN-γ and TNF-α levels were also observed in both murine models of immune-mediated BM failure used in the current work. However, lack of IL-18 in donor LN cells and lack of IL-18 or IL-18R in recipients did not attenuate BM failure. Furthermore, deficiency of IL-18 and IL-18R did not suppress the elevation of inflammatory cytokines, such as IFN-γ, TNF-α, and IL-6, following allogeneic LN cell infusion. Our observations indicated that IL-18/IL-18R signaling played a minor role in the development of immune-mediated BM failure such that disruption of this signaling pathway did not affect the overall destruction of BM cells by allogeneic LN cells. It had been shown that murine BM derived macrophages secrete IFN-γ upon combined IL-12 and IL-18 stimulation, which defines a new pathway of autocrine macrophage activation [44]. The IL-12/IL-18-stimulated IFN-γ production requires STAT4 signaling and is inhibited by IL-4 [,45]. We speculate that the role of IL-18 in immune-mediated BM failure lies on its stimulation of IFN-γ secretion by various cell components. Since the IFN-γ-stimulatory effect can be mediated by many factors other than IL-18, disruption of the IL-18/IL-18R signaling pathway would not affect IFN-γ concentration to alter the level of BM destruction. However, IL-18BP was increased in AA in our and other studies, which might be another aspect of IL-18/IL-18R regulation as revealed by two recent reports in macrophage activation syndrome [46,47], and therefore may be worth further study in BM failure diseases.
Taken together, our current work demonstrated IL-18 as a marker of SAA onset. High levels of IL-18 more likely reflected aberrant immune responses and exaggeration of IFN-γ signaling, and might exhibit fine modifications of hematopoiesis. However, deficiency in IL-18 or IL-18R did not alleviate BM failure as observed in our murine models, thus high levels of IL-18 might play dispensable roles in regulation of immune response or hematopoiesis in AA.
Highlights.
IL-18 and IL-18BP levels were significantly elevated in SAA patients.
IL-18 receptor was expressed on human HSPCs.
Culturing human CD34+ cells with IL-18 perturbed gene expression in hematopoiesis.
Disruption of IL-18/IL-18R signaling pathway did not alleviate murine BM failure.
Acknowledgments
The authors would like to thank Kinneret Broder, Therese Intrater and Janet Valdez for assistance in obtaining samples from healthy volunteers.
Role of funding source
This research was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute.
Clinicaltrials.gov identifiers: NCT00071045, NCT00260689, NCT00922883, NCT01623167, NCT00001397, NCT00001620, NCT00217594, NCT00961064.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Disclosures
The authors declare no competing financial interests.
References
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenaux P, Adès L. How we treat lower-risk myelodysplastic syndromes. Blood 2013;121:4280–4286 [DOI] [PubMed] [Google Scholar]
- 3.Young NS. Hematopoietic cell destruction by immune mechanisms in aquired aplastic anemia. Semin Hematol. 2000;37:3–14. [DOI] [PubMed] [Google Scholar]
- 4.Young NS. Gamma interferon and aplastic anemia. Blood. 1987;70:337–339. [PubMed] [Google Scholar]
- 5.Giannakoulas NC, Karakantza M, Theodorou GL, et al. Clinical relevance of balance between type 1 and type 2 immune responses of lymphocyte subpopulations in aplastic anaemia patients. Br J Haematol. 2004;124:97–105. [DOI] [PubMed] [Google Scholar]
- 6.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100:1185–1191. [DOI] [PubMed] [Google Scholar]
- 7.de Latour RP1, Visconte V, Takaku T, Wu C, Erie AJ, Sarcon AK, Desierto MJ, Scheinberg P, Keyvanfar K, Nunez O, Chen J, Young NS. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116:4175–7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, Huang Z, Zhang J, Nie N, Zheng Y. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012;120:1624–1632. [DOI] [PubMed] [Google Scholar]
- 9.Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87:4149–4157. [PubMed] [Google Scholar]
- 10.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165:538–546. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Feng X, Desierto MJ, Keyvanfar K, Young NS. IFN -γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood. 2015;126:2621–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlanda C, Dinarello CA, Manotovani A The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. [DOI] [PubMed] [Google Scholar]
- 14.Puren AJ1, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci U S A. 1999;96:2256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JK1, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1beta and IL-18. Proc Natl Acad Sci U S A. 2004. June 8;101:8815–8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabbi M1, Carbotti G2, Ferrini S1. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97:665–675. [DOI] [PubMed] [Google Scholar]
- 18.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. [DOI] [PubMed] [Google Scholar]
- 19.Fehniger TA1, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 20.Dinarello CA1, Novick D, Kim S, Kaplanski G Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. [DOI] [PubMed] [Google Scholar]
- 22.Paulukat J1, Bosmann M, Nold M, Garkisch S, Kämpfer H, Frank S, Raedle J, Zeuzem S, Pfeilschifter J, Mühl H Expression and release of IL-18 binding protein in response to IFN-gamma. J Immunol. 2001;167:7038–7043. [DOI] [PubMed] [Google Scholar]
- 23.Mühl H, Kämpfer H, Bosmann M, Frank S, Radeke H, Pfeilschifter J. Interferon-gamma mediates gene expression of IL-18 binding protein in nonleukocytic cells. Biochem Biophys Res Commun. 2000;267:960–963. [DOI] [PubMed] [Google Scholar]
- 24.Katakami N, Kaneto H, Matsuhisa M, Yoshiuchi K, Kato K, Yamamoto K, et al. ,2007. Serum interleukin-18 levels are increased and closely associated with various soluble adhesion molecule levels in type 1 diabetic patients. DiabetesCare. 2007;30:159–161. [DOI] [PubMed] [Google Scholar]
- 25.Nicoletti F, Conget I, Di Marco R, Speciale AM, Morinigo R, Bendtzen K, et al. ,2001b. Serum levels of the interferon-gamma-inducing cytokine interleukin-18are increased in individuals at high risk of developing type I diabetes. Diabetolo-gia. 2001;44:309–311. [DOI] [PubMed] [Google Scholar]
- 26.Volin MV, Koch AE Interleukin-18: a mediator of inflammation and angio-genesis in rheumatoid arthritis. J. Interferon. Cytokine Res. 2011;31:745–751. [DOI] [PubMed] [Google Scholar]
- 27.Cebeci AN, Nuhoglu Y, Arslanoglu I, Erguven M, Agachan N The role of IL-18 in Th1/Th2 balance in children. Allergy Asthma Proc. 2006;27:365–370. [DOI] [PubMed] [Google Scholar]
- 28.Kanai T, Watanabe M, Okazawa A, Sato T, Hibi T Interleukin-18 and Crohn’s disease. Digestion.2001;63 (Suppl 1): 37–42. [DOI] [PubMed] [Google Scholar]
- 29.Nicoletti F, Di Marco R, Mangano K, Patti F, Reggio E, Nicoletti A, et al. Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology. 2001;57:342–344. [DOI] [PubMed] [Google Scholar]
- 30.Jander S, Stoll G Increased serum levels of the interferon-gamma-inducingcytokine interleukin-18 in myasthenia gravis. Neurology. 2002;59:287–289. [DOI] [PubMed] [Google Scholar]
- 31.Shan NN, Zhu XJ, Peng J, Qin P, Zhuang XW, Wang HC, Hou M. Interleukin 18 and interleukin 18 binding protein in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2009;144:755–761. [DOI] [PubMed] [Google Scholar]
- 32.Shan NN, Hu Y, Liu X, Wang X, Yuan D, Li Y. Imbalanced expression of T-bet and T cell immunoglobulin mucin-3 in patients with aplastic anaemia. J Clin Immunol. 2013;33:809–816. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Ellison FM, Eckhaus MA, Smith AL, Keyvanfar K, Calado RT, Young NS. Minor antigen h60-mediated aplastic anemia is ameliorated by immunosuppression and the infusion of regulatory T cells. J Immunol. 2007;178:4159–4168. [DOI] [PubMed] [Google Scholar]
- 34.Bloom ML, Wolk AG, Simon-Stoos KL, Bard JS, Chen J, Young NS. A mouse model of lymphocyte infusion-induced bone marrow failure. Exp Hematol. 2004;32(12):1163–1172. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Desierto MJ, Feng X, Biancotto A, Young NS. Immune-mediated bone marrow failure in C57BL/6 mice. Exp Hematol. 2015;43:256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng X, Lin Z, Sun W, Hollinger MK, Desierto MJ, Keyvanfar K, Malide D, Muranski P, Chen J, Young NS. Rapamycin is highly effective in murine models of immune-mediated bone marrow failure. Haematologica. 2017;102:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camitta BM, Thomas ED, Nathan DG, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood.1976;48:63–70. [PubMed] [Google Scholar]
- 38.Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:60–87. [DOI] [PubMed] [Google Scholar]
- 39.van Galen P, Kreso A, Mbong N, et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510:268–272. [DOI] [PubMed] [Google Scholar]
- 40.Silberstein L, Goncalves KA, Kharchenko PV, et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell. 2016;19:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Sato C, Cerletti M, et al. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. [DOI] [PubMed] [Google Scholar]
- 42.Ly H, Calado RT, Allard P, et al. Functional charaterization of telomerase RNA variants found in patients with hematologic disorders. Blood. 2005;105:2332–2339. [DOI] [PubMed] [Google Scholar]
- 43.Bernad A, Kopf M, Kulbacki R, et al. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994; 1:725–731. [DOI] [PubMed] [Google Scholar]
- 44.Munder M, Mallo M, Eichmann K, et al. Murine macrophages secrete interferon γ upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler H, Lutz MB, Rolinghoff M, et al. The production of IFN-γ by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. [DOI] [PubMed] [Google Scholar]
- 46.Girard-Guyonvarc’h C, Palomo J, Martin P, Rodriguez E, Troccaz S, Palmer G, Gabay C. Unopposed IL-18 signaling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood. 2018;131:1430–1441. [DOI] [PubMed] [Google Scholar]
- 47.Weiss ES, Girard-Guyonvarc’h C, Holzinger D, de Jesus AA, Tariq Z, Picarsic J, Schiffrin EJ, Foell D, Grom AA, Ammann S, Ehl S, Hoshino T, Goldbach-Mansky R, Gabay C, Canna SW. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131:1442–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]