Abstract
Background
In patients with coronary disease (CAD), low diastolic blood pressure (DBP) is associated with increased risk of myocardial infarction, but its association with angina is unknown.
Objective:
To examine the association of low DBP and angina in patients with CAD.
Methods:
We assessed the frequency of angina, assessed with the Seattle Angina Questionnaire Angina Frequency score, by DBP in patients with known CAD from 25 US cardiology clinics. Hierarchical logistic regression was used to test the association between DBP and angina, with a spline term for DBP to assess non-linearity.
Results:
Among 1259 outpatients with CAD, 411 (33%) reported angina in the prior month, with higher rates in the lowest DBP quartile (40–64 mmHg: 37%). In the unadjusted model, DBP was associated with angina with a J-shaped relationship (p=0.017, p for nonlinearity=0.027), with a progressive increase in odds of angina as DBP decreased below ~70–80 mmHg. This association remained significant after sequential adjustment for demographics (p=0.002), comorbidities (p=0.002), heart rate (p=0.002), SBP (p=0.046), and antihypertensive antianginal medications (p=0.045).
Conclusion:
In patients with chronic CAD, there appeared to be an association between lower DBP and increased odds of angina. If validated, these findings suggest that clinicians should consider less aggressive blood pressure control in patients with CAD and angina.
Keywords: angina, diastolic blood pressure, coronary artery disease
CONDENSED ABSTRACT
Blood pressure (BP) treatment is an effective strategy for cardiovascular risk reduction. Recent guidelines advocate a more aggressive blood pressure treatment for cardiovascular risk reduction than was previously recommended. However, prior studies suggest that low diastolic BP is associated with the risk of myocardial injury. The present study extends the literature by demonstrating that low diastolic BP is significantly associated with an increased risk of angina in patients with coronary artery disease. This implies that patients with CAD and low diastolic BP may need to consider alternative, non BP-lowering therapies for angina management.
Treatment of hypertension (HTN) through reduction in blood pressure (BP) is a cornerstone for the prevention of cardiovascular events in patients with and without established coronary artery disease (CAD) (1). However, the optimal BP range for patients is unclear, as aggressively reducing BP has the potential risks of symptomatic hypotension, worsening kidney function (2), and an increased risk for cardiovascular events (3). The association of BP with outcomes has generally been observed to have a J-shaped relationship, although there has been much discussion about where the nadir of the J-point lies (4). While much of this research has focused on systolic BP (SBP), studies have also shown that aggressively treating diastolic BP (DBP) is associated with an increased risk of myocardial infarction and cardiovascular death (5).
Despite the extensive literature associating low DBP with cardiac events, there is limited information on its association with angina. Since coronary perfusion occurs during diastole, where the perfusion pressure is the difference between DBP and end-diastolic left ventricular pressure, we hypothesized that excessive reduction in DBP could lead to inadequate coronary perfusion and a greater prevalence of angina in patients with stable CAD (6). This is a particularly relevant clinical question, as many antianginal medications may also lower DBP, and this analysis could provide guidance on the optimal level of BP control among patients with symptomatic CAD. We therefore used a multicenter registry of outpatients with CAD to describe the association of DBP with the presence of angina.
METHODS
Study Population and Procedures.
The Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study was a cross-sectional, observational study designed to quantify and assess angina and quality of life in outpatients with chronic CAD. Consecutive patients with a diagnosis of CAD (defined as stable angina, prior myocardial infarction, or prior coronary revascularization) were enrolled from 25 US cardiology outpatient practices between April 2013 and July 2015. Eligible patients included those ≥18 years of age who had ≥1 prior office visit to the practice. Patients who had dementia, were unable to speak or read English, or were unable or unwilling to participate were excluded. Trained site coordinators recorded patient demographics, comorbidities, medications (prior to and after the clinic visit), and vital signs via chart review at the time of the office visit. Each site obtained Institutional Research Board (IRB) approval for the study, and all patients provided informed consent.
Assessment of Angina.
Prior to meeting their clinician, patients completed the Seattle Angina Questionnaire (SAQ), a reliable and valid 19-item self-administered, disease-specific health status questionnaire with a 4-week recall period that measures clinically relevant dimensions of health in patients with CAD (7,8). The primary outcome for this study was the Angina Frequency (SAQ-AF) scale, which correlates well with daily angina diaries and is associated with mortality, hospitalizations for acute coronary syndromes, and healthcare costs in patients with chronic CAD (9–11). Scores range from 0–100, with higher scores indicating less angina, and were dichotomized into any angina (SAQ-AF score <100) versus no angina (SAQ-AF=100) over the past 4 weeks, congruent with prior work (12).
Statistical Analysis.
As there are no established categories of risk for DBP, we first divided the cohort into quartiles of DBP. We then compared demographic, socio-economic, and clinical characteristics across the DBP quartiles using ANOVA for continuous variables and Chi-square test for categorical variables. We constructed a hierarchical, logistic regression model to examine the association of DBP with the odds of angina, First unadjusted and then sequential adjusted for factors that could confound the association of DBP with angina: 1) sociodemographic variables (age, sex, race, insurance status, education history, avoidance of care due to cost), 2) comorbidities (body mass index, HTN, diabetes, history of stroke or transient ischemic attack, history of myocardial infarction, chronic kidney disease, 3) heart rate, and 4) antihypertensive antianginal medications (calcium channel blockers, beta blockers). Restricted cubic spline terms were included for all continuous variables. For our primary predictor DBP, the number of knots was chosen based on fit statistics and visual inspection of the smoothness of the resulting curve. The overall association with DBP with angina was evaluated by a k – 1 degree of freedom F-test jointly testing all associated model terms. A DBP of 80 mmHg was used as the reference. Site was included in all models as a random effect, to account for patient clustering within sites.
The inclusion of beta blockers and calcium channel blockers in the model is complex, as these medications both reduce angina (through coronary vasodilation, reducing myocardial demand, etc.) and DBP (thereby potentially blunting the antianginal effects). As such, we were over-adjusting by adjusting for these medications, but we felt that their inclusion in the model was important to reduce confounding. As ranolazine does not impact DBP and long-acting nitrates impact DBP little on a chronic basis, we did not adjust for these medications. Finally, despite a strong correlation between SBP and DBP (r=0.5), we constructed a model that additionally adjusted for SBP, as high SPB has been associated with increase myocardial wall stress (13), which can also contribute to angina. All analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, North Carolina) and a p-value of <0.05 was considered statistically significant.
RESULTS
Study Cohort.
APPEAR included 1259 outpatients with CAD who were evaluated by 155 cardiologists from 25 sites located in 19 US states. The mean age of the analytic cohort was 71.1 years, 68.5% were male, 89.7% were white, and 10.1% were current smokers (Table 1). A history of myocardial infarction was noted in 38.3%, coronary stenting in 56.7%, and bypass graft surgery in 37.5%, and 32.7% of patients reported at least 1 episode of angina in the prior month. A diagnosis of HTN was documented in 79.7% of patients, and patients were on an average of 1.3±0.8 antihypertensive medications. Mean SBP was 127.9±17.1, and mean DBP was 72.x±10.2, with a median of 72 mmHg (IQR 65–80 mmHg, range 40–108 mmHg). Patients with lower DBPs were more likely to be older, have chronic kidney disease (CKD), and have lower body mass indices (Table 1). Diuretic, long-acting nitrate, and ranolazine use were higher in patients with lower DBPs.
Table 1:
Patient characteristics by quartile of diastolic blood pressure
| Q1:40–64 n=309 | Q2:65–71 n=316 | Q3: 72–79 n=278 | Q4: 80–108 n=356 | P-Value | |
|---|---|---|---|---|---|
| Age, y | 75.0 ± 10.5 | 72.2 ± 10.1 | 70.6 ± 11.1 | 67.3 ± 11.0 | <0.001 |
| Female, % | 35.3 | 31.0 | 34.9 | 26.1 | 0.030 |
| Race, % | 0.084 | ||||
| White | 87.1 | 91.1 | 91.7 | 89.0 | |
| Black | 3.6 | 3.8 | 3.6 | 5.9 | |
| Other | 9.4 | 5.1 | 4.7 | 5.1 | |
| Avoid care due to cost, % | 4.9 | 6.3 | 6.9 | 6.2 | 0.770 |
| High school education, % | 32.6 | 36.1 | 37.1 | 35.0 | 0.684 |
| Heart rate, bpm | 68.1 ± 11.7 | 69.5 ± 11.7 | 73.2 ± 56.9 | 70.0 ± 11.1 | 0.182 |
| Systolic BP, mmHg | 117.7 ± 16.3 | 124.4 ± 14.6 | 128.8 ± 13.2 | 139.1 ± 16.0 | <0.001 |
| Heart Failure, % | 23.0 | 18.7 | 17.7 | 14.6 | 0.049 |
| Current Smoker, % | 6.6 | 9.6 | 12.1 | 12.0 | 0.077 |
| Hypertension,% | 77.0 | 80.1 | 79.8 | 81.7 | 0.509 |
| Diabetes,% | 35.9 | 39.9 | 33.9 | 32.3 | 0.207 |
| History of stroke/TIA, % | 7.4 | 7.6 | 7.9 | 7.6 | 0.996 |
| History of MI, % | 34.0 | 35.4 | 45.1 | 39.3 | 0.027 |
| Chronic kidney disease, % | 17.8 | 14.2 | 9.0 | 9.0 | <0.001 |
| BMI, kg/m2 | 29.6 ± 6.5 | 30.5 ± 6.4 | 30.6 ± 6.5 | 31.3 ± 6.2 | 0.008 |
| Calcium channel blocker, % | 26.9 | 26.9 | 21.6 | 27.5 | 0.320 |
| Beta blocker, % | 77.3 | 81.0 | 82.7 | 76.4 | 0.166 |
| ACE inhibitor, % | 35.3 | 40.2 | 38.8 | 37.4 | 0.623 |
| ARB, % | 27.2 | 20.9 | 23.7 | 26.1 | 0.259 |
| Diuretic, % | 51.1 | 42.7 | 36.3 | 34.6 | <0.001 |
| Nitrate, % | 58.6 | 52.5 | 52.5 | 46.9 | 0.028 |
| Ranolazine, % | 7.4 | 6.0 | 7.9 | 2.8 | 0.023 |
| Any angina in past month, % | 37.0 | 33.7 | 29.9 | 30.3 | 0.198 |
| SAQ Angina Frequency | 90.0 ±17.5 | 91.1± 15.1 | 92.6± 15.3 | 92.4 ± 15.3 | 0.143 |
Values presented as mean ± SD or %. Continuous variables compared using ANOVA. Categorical variables compared using Chi-square.
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; MI, myocardial infarction; SAQ, Seattle Angina Questionnaire; TIA, transient ischemic attack
Association of DBP with Angina.
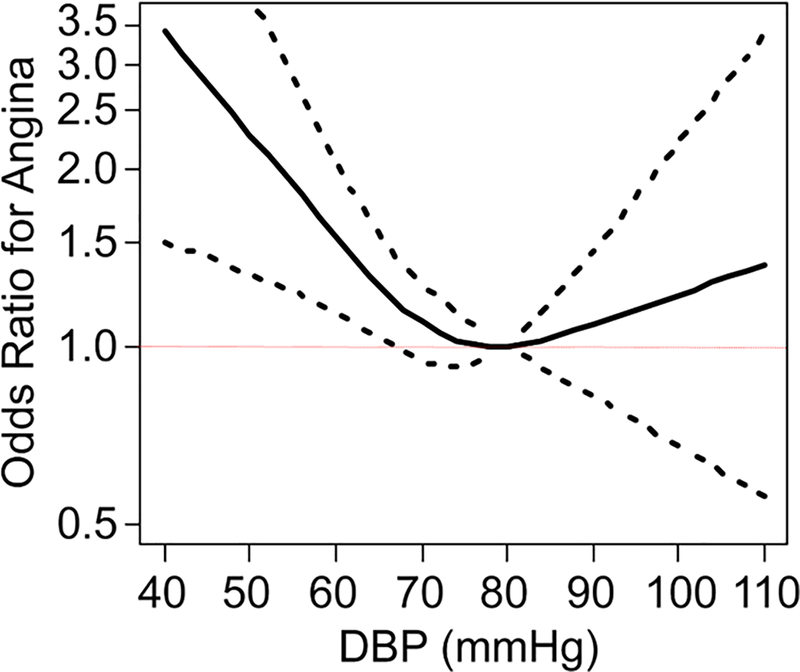
DBP was 71.6 ± 10.8 for those with any angina and 72.7 ± 9.8 for those without angina (p =0.074). In unadjusted analysis, 37.0% of patients in the lowest DBP quartile reported having at least 1 episode of angina in the prior month, as compared with 33.7%, 29.9%, and 30.3% of patients in quartiles 2, 3, and 4, respectively. Mean SAQ-AF scores were similar across DBP quartiles (Table 1). In the unadjusted logistic regression model, DBP was significantly associated with angina (p=0.017) with a J-shaped relationship (p for nonlinearity=0.027; Central Illustration). For example, compared with a patient having a DBP of 80 mmHg, a patient with a DBP of 60 mmHg had 1.37 greater odds of angina (95% CI 1.06–1.77). This association remained significant after sequential adjustment for demographics (DBP p=0.002), comorbidities (DBP p=0.002), heart rate (DBP p=0.002), and antihypertensive antianginal medications (DBP p=0.002). Finally, after additionally adjusting for SBP, the association of DBP with angina remained significant (DBP p=0.045). Supplemental Table 1 shows multivariable adjusted odds of angina by DBP level.
Central Illustration: Unadjusted association between odds of angina and diastolic blood pressure levels.
Dotted lines represent the 95% confidence interval
DISCUSSION
While aggressive BP control is a cornerstone of cardiovascular risk reduction, excessive reduction of diastolic BP could reduce coronary perfusion pressure and be associated with worse outcomes. Adding to prior analyses showing an association between very low DBP and increased risk of myocardial infarction and cardiovascular death (3,5), we found a J-shaped relationship between DBP and angina, with the nadir between ~70 and 80 mmHg. This association persisted after adjusting for patient demographics, comorbidities, SBP, and medications, thereby underscoring the need for caution when aggressively lowering BP in patients with CAD and angina.
Our findings extend prior knowledge about the potential risks of excessive BP reduction and are particularly relevant in light of the new HTN guidelines recommending aggressive BP lowering for cardiovascular risk reduction (14,15). These guidelines were driven to a large degree from the results of the Systolic Blood Pressure Intervention Trial (SPRINT) trial that showed improved mortality with aggressive SBP treatment to a goal of <120 mmHg (2). These findings were in contrast with those seen in ACCORD (Action to Control Cardiovascular Risk in Diabetes), where intensive BP control was not associated with a significant reduction in cardiovascular events compared to the standard BP goal in patients with diabetes and HTN (16). Importantly, the mean DBP in the intensive BP arm of ACCORD was 64 mmHg versus 69 mmHg in SPRINT, which could partially account for the seemingly discordant findings between trials. Treatment-induced diastolic hypotension, which is more common in older patients and those with diabetes, has been associated with an increased risk of adverse cardiovascular events in both observational studies (3,5) and in post-hoc analyses of BP-lowering trials (4,17). It is for this reason that guidelines recommend caution in reducing DBP <60 mmHg in patients with CAD (1,18). By describing an increased prevalence of angina in patients with a DBP less than ~70–80 mmHg, our study identifies an additional potential concern for aggressive DBP lowering and can provide a therapeutic option (de-intensifying BP control) for patients with angina and low DBP.
Our study suggests that in patients with low DBP and CAD, alternative medications for angina, such as ranolazine, ivabradine, or isosorbide (although this does impact BP acutely but likely less long-term effect), may be more beneficial, although head-to-head trials of antianginal medications in patients with low DBP would be needed to definitively support such a strategy. Alternatively, patients with low DBP may benefit more from revascularization or other non-pharmacological antianginal treatment (e.g., enhanced external counter pulsation, coronary sinus reducing device), as opposed to further titration of beta-blockers or calcium channel blockers.
Our study should be considered in the context of several potential limitations. First and foremost, the cross-sectional design of our study means that we cannot establish the direction of causality in the association between low DBP and angina risk. This is evident as patients who have more angina are treated more aggressively with beta-blockers and calcium channel blockers for their angina, which also lowers DBP. While we saw minimal attenuation in the association of DBP with angina after adjustment for these medications, we were unable to examine doses of medications or to monitor patients longitudinally to better understand this association. Future studies are needed to better disentangle this potential source of confounding. Second, the interplay between DBP, SBP, coronary perfusion pressure, coronary flow, and myocardial wall stress is complex and cannot be fully analyzed in a cross sectional study. For example, we cannot account for the effect of coronary autoregulation in our analysis, nor can we account for the effect of myocardial wall stress, which is a determinant of coronary perfusion pressure. Additionally, high SBPs can increase myocardial wall stress, which may precipitate angina. All of these factors impede our ability to make firm conclusions based on our results. Third, as APPEAR was an observational cohort study, office BP measurement was not standardized across participating sites, and as such, we may be under- or over-estimating the association of low DBP with angina. However, we believe the real-world nature of our study represents an important strength and supported the generalizability of our study, as this represented BP assessment in routine clinical practice. Fourth, SAQ AF integrates angina experienced over a 4-week period, while DBP was measured once. It is unclear how results may differ if BP is averaged during the same period as the recall frame of the SAQ. Finally, the overall burden of angina in our cohort was moderate, which limited our ability to assess the relationship between DBP and angina burden. Our study instead explores the association of DBP and the prevalence of angina
In conclusion, in a multicenter cross-sectional analysis of patients with stable CAD, we found that very low DBP was associated with an increased odds of angina. With recent guidelines promoting an aggressive blood pressure lowering paradigm, it will become increasingly important to individualize treatment strategies to minimize adverse events and maximize benefits. Prospective studies are needed to further examine the relationship between DBP and angina and to help guide management of patients with CAD and low DBP.
Supplementary Material
PERSPECTIVES
Competency in Medical Knowledge:
Low diastolic blood pressure may precipitate or worsen angina in patients with coronary artery disease. In patients with coronary artery disease, angina and low diastolic blood pressure, clinicians should consider de-intensifying antihypertensive medications as part of the treatment strategy for angina.
Translational Outlook 1:
Further research is required to prospectively determine the minimum diastolic blood pressure floor for patients with coronary artery disease and angina.
Acknowledgments
Source of Funding: The Angina Prevalence and Provider Evaluation of Angina Relief (APPEAR) study was supported by an investigator-initiated grant from Gilead Sciences. Poghni Peri-okonny and Krishna K. Patel were supported by a T32 training grant from the National Heart Lung and Blood Institute (T32HL110837). All data collection, data analyses, the preparation of the manuscript, and the decision to submit the manuscript for publication were done independently of the study sponsor.
ABBREVIATIONS
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- AF
Angina Frequency
- APPEAR
Angina Prevalence and Provider Evaluation of Angina Relief
- BP
blood pressure
- CAD
coronary artery disease
- DBP
diastolic blood pressure
- HTN
ypertension
- SAQ
Seattle Angina Questionnaire
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Footnotes
Disclosures: JAS: Research grants: NHLBI, PCORI, ACCF, Abbott Vascular; Consultant honoraria: United Healthcare, Bayer, Novartis, Janssen, V-wave, Corvia; Copyright: Seattle Angina Questionnaire. The other authors report no potential conflicts.
REFERENCES
- 1.Rosendorff C, Lackland DT, Allison M et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension (Dallas, Tex : 1979) 2015;65:1372–407. [DOI] [PubMed] [Google Scholar]
- 2.Wright JT Jr., Williamson JD, Whelton PK et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEvoy JW, Chen Y, Rawlings A et al. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. Journal of the American College of Cardiology 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J-curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? Jama 1991;265:489–95. [PubMed] [Google Scholar]
- 5.Vidal-Petiot E, Greenlaw N, Ford I et al. Relationships Between Components of Blood Pressure and Cardiovascular Events in Patients with Stable Coronary Artery Disease and Hypertension. Hypertension (Dallas, Tex : 1979) 2017. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank JM. The role of coronary perfusion pressure. Eur Heart J 1992;13 Suppl D:39–43. [DOI] [PubMed] [Google Scholar]
- 7.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol 1994;74:1240–4. [DOI] [PubMed] [Google Scholar]
- 8.Spertus JA, Winder JA, Dewhurst TA et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 9.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation 2002;106:43–9. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J 2003;146:1015–22. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SV, Morrow DA, Lei Y et al. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circulation Cardiovascular quality and outcomes 2009;2:344–53. [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation 2004;110:3789–94. [DOI] [PubMed] [Google Scholar]
- 13.Burke GL, Arcilla RA, Culpepper WS, Webber LS, Chiang YK, Berenson GS. Blood pressure and echocardiographic measures in children: the Bogalusa Heart Study. Circulation 1987;75:106–14. [DOI] [PubMed] [Google Scholar]
- 14.Beddhu S, Chertow GM, Cheung AK et al. Influence of Baseline Diastolic Blood Pressure on Effects of Intensive Compared to Standard Blood Pressure Control. Circulation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines 2017.
- 16.Cushman WC, Evans GW, Byington RP et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581–4. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta K, Quinn RR, Zarnke KB et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. The Canadian journal of cardiology 2014;30:485–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.