Abstract
Background:
Pediatric anxiety disorders are among the most common psychiatric mental illnesses in children and adolescents, and are associated with abnormal cognitive control in emotional, particularly threat, contexts. In a series of studies using eye movement saccade tasks, we reported anxiety-related alterations in the interplay of inhibitory control with incentives, or with emotional distractors. The present study extends these findings to working memory (WM), and queries the interaction of spatial WM with emotional stimuli in pediatric clinical anxiety.
Methods:
Participants were 33 children/adolescents diagnosed with an anxiety disorder, and 22 age-matched healthy comparison youths. Participants completed a novel eye movement task, an affective variant of the memory-guided saccade task. This task assessed the influence of incidental threat on spatial WM processes during high and low cognitive load.
Results:
Healthy but not anxious children/adolescents showed slowed saccade latencies during incidental threat in low-load but not high-load WM conditions. No other group effects emerged on saccade latency or accuracy.
Conclusions:
The current data suggest a differential pattern of how emotion interacts with cognitive control in healthy youth relative to anxious youth. These findings extend data from inhibitory processes, reported previously, to spatial WM in pediatric anxiety. Depression and Anxiety 32:289–295, 2015.
Keywords: cognitive load, development, children/adolescents, emotion, saccade, eye movement, cognitive resources
INTRODUCTION
Anxiety disorders in youths are highly prevalent (lifetime prevalence of 28%),[1] interfere with academic and social skill learning, and predict adverse outcomes extending into adulthood.[2–4] Despite these deleterious effects and the critical role of pediatric anxiety in the prevalence of anxiety and mood disorders in adulthood, neurocognitive research in pediatric anxiety is lagging behind research in adult anxiety.
A decade ago, we initiated a series of studies aimed at understanding the influence of anxiety on inhibitory control in pediatric patients, in various conditions of emotional contexts.[5–7] After identifying anxiety-related deficits in the interaction of inhibitory control with incentives, and then with threat stimuli,[5–7] we extended this work to another form of cognitive control, working memory (WM), using the same saccade eye movement platform. One reason for this shift was based on the notion that different cognitive control processes (e.g., set-shifting, inhibition, WM) develop at different rates. Specifically, while inhibition already appears to be developed around 14 years of age, WM is not fully mature until age 19,[8] suggesting that WM might be particularly sensitive to emotional disruption relative to earlier maturing executive functions.
The interplay of cognitive control with emotional stimuli in anxiety has been the object of substantial work in adults, but has to date received relatively little attention in children and adolescents (for review see[9]). Specifically, this adult work, together with research on cognitive control, has generated a number of cognitive theories applicable to the influence of anxiety on cognitive performance, in a way that is particularly relevant to WM.[10–13] These theories are based on two fundamental premises. First, anxiety is characterized by a cognitive component supporting the cardinal symptom of worry, which uses WM. Second, cognitive functioning operates in the context of limited resources that necessitates a prioritization of processes. These theories vary in the way resources are distributed across cognitive and affective information processing. The most parsimonious model predicts that anxiety reduces the cognitive resource pool that in turn affects the distribution of resources, particularly when WM tasks compete with the processing of emotional stimuli. The key question rests on how cognitive resources are redistributed, and hence which processes suffer from this redistribution. Here, we based our predictions on a recent work dedicated to this question.[13]
In this previous work, Vytal et al. examined in healthy adults the effects of threat induced anxiety on WM at various levels of difficulty.[13] Findings revealed disruptive effects of induced anxiety at low-difficulty cognitive level (one-back WM), but not at high-difficulty cognitive level (three-back WM). This result was consistent with a redistribution of resources that permitted, at low level of cognitive difficulty, to process both the threat stimuli and the cognitive task. The processing of the threat stimuli during the low-load WM task led to the coding of anxiety, which, in turn, interfered with task performance. On the other hand, the high-load (three-back WM) task mobilized all available resources, preventing the threat stimuli from being processed, and therefore from interfering with the high-load WM performance. Taken together, these findings suggested that, in healthy adults, processing threat impaired WM at low load. Testing these affective/cognitive interactions in anxious individuals might reveal deviant mechanisms pointing to potential targets of therapeutic intervention.
The present study differed in three ways from Vytal et al.’s. First, we tested adolescents rather than adults; second, we used an external threat stimulus (fearful face stimuli) rather than endogenous threat stimuli (worry thoughts, associated with threat of shock); and third, we employed saccade eye movements rather than manual responses. Some of these differences could affect the strength of the interference effects. Specifically, interference effects might be stronger in adolescents versus adults, but weaker with an external versus internal threat distractor. The reasons for the former are the ongoing maturation of cognitive processes (e.g.,[8]), together with an amplified emotional responsivity during adolescence (e.g.,[14, 15]), resulting in weaker cognitive control and stronger emotional influence, respectively. Regarding the threat stimuli, the internal threat stimuli (worry thoughts) might interact directly with WM processes because they themselves consume WM resources (e.g.,[16]), whereas the external threat stimuli might just divert attention or/and use cognitive resources to be processed, which would compete with WM capacity.[11] Finally, saccadic eye movement responses provide exquisite measures of attention and performance, which have also been shown to be sensitive to pediatric psychopathology (e.g.,[17]), and thus might enhance our power to detect influences on performance. Collectively, these task differences between the previous work and the current study were not expected to modify the basic normative results, as described above.
In sum, this study compared healthy adolescents with clinically anxious adolescents on a low load versus high load WM task (cognitive process) using an emotional (threat-related) versus neutral distractor background (emotional process). We expected to find, similarly to Vytal et al.’s study,[13] impaired performance on the low-load WM task in the presence of the threat stimuli in healthy participants. In contrast, the presence of the threat stimuli in patients with an anxiety disorder would be expected to take priority over task performance given the salience of threat in anxiety. Accordingly, we would then expect alteration of performance on both low- and high-load WM tasks based on prioritization of threat processing. In addition, given that worry consumes WM resources, we might anticipate anxious patients to perform worse on WM than healthy comparisons based on reduced WM capacity, although WM in general is not reported to differ significantly between anxious children and nonanxious children.[18]
METHODS
PARTICIPANTS
Twenty-two typically developing healthy adolescents (13 females, mean age 13.42 years SD 2.15 years) were compared to 33 clinically anxious adolescents (18 females, mean age 12.44 years SD 2.92 years). The study was approved by the National Institute of Mental Health Institutional Review Board. Participants were recruited through local newspaper advertisements and word of mouth. The parents of all participants provided written informed consent, whereas the adolescents signed written assent.
Inclusion criteria for anxious adolescents included (1) primary diagnosis of an anxiety disorder based on a semistructured diagnostic interview by experienced clinicians, and demonstrated excellent interrater reliability for each diagnosis (k > 0.75; schedule for affective disorders and schizophrenia for school-age children—present and life-time version (KSAD-PL);[19] (2) Children’s Global Assessment Scale’s score < 60 (CGAS);[20] (3) Pediatric Anxiety Rating Scale score > 9;[21] and (4) age between 8 and 18 years. All participants were seeking outpatient treatment. Inclusion criteria for healthy adolescents consisted of (1) absence of acute or chronic medical problems; (2) absence of current or past psychiatric mental illness; (3) age range 8–18 years. Exclusion criteria for all participants were (1) current use of any psychoactive substance; (2) current Tourette’s syndrome, obsessive–compulsive disorder, posttraumatic stress disorder, conduct disorder, exposure to extreme trauma, or suicidal ideation; (3) lifetime history of mania, psychosis, or pervasive developmental disorder; or (4) IQ < 70. Individual anxiety and depression levels were assessed with the Screen for Childhood Anxiety Related Emotional Disorders (SCARED),[22] and the Child Depression Inventory (CDI),[23] respectively.
Overall, healthy adolescents did not differ significantly from clinically anxious children in age (t(53) = 1.31, P = .20), IQ (t(53) = 1.74, P = .09), socioeconomic status (SES, t(46) = 0.11, P = .92), or sex distribution (x2(1) = 0.11, P = .74; Table 1). As expected, anxiety ratings (SCARED) on self-reports (t(44.86) = 11.29, P <.001) and parental reports (t(43.92) = 11.49, P <.001), as well as self-rated depression (CDI: t(49) = 4.92, P <.001) were higher in the anxious than the comparison group. Of the anxious adolescents, 20 participants had generalized anxiety disorder (GAD), 21 had social phobia, nine had separation anxiety (SAD), one had panic disorder, and four suffered from comorbid depression, while two additional subjects had comorbid attention deficit hyperactivity disorder (ADHD; see Table 1). All participants were medication-free and treatment-seeking for their anxiety disorder at the time of testing.
TABLE 1.
Demographics for the healthy and anxious groups
| Healthy (n = 22) |
Anxious (n = 33) |
Significance | |
|---|---|---|---|
| Sex (n/female) | 13 | 18 | ns |
| Age | 13.42 (2.15) | 12.47 (2.96) | ns |
| IQ total score | 115.76 (13.51) | 109.12 (14.29) | ns |
| Vocabularya | 60.30 (9.29) | 55.88 (10.29) | ns |
| Matrix reasoninga | 58.60 (6.81) | 55.84 (10.59) | ns |
| SES | 36.89 (14.66) | 36.40 (16.01) | ns |
| SCARED child | 6.82 (5.49) | 33.43 (11.11) | P < .001 |
| SCARED parent | 2.86 (5.11) | 30.75 (11.91) | P < .001 |
| CDI | 40.23 (7.94) | 52.72 (9.71) | P < .001 |
| Diagnosesb: N (%) | |||
| GAD | - | 20 (60.60) | |
| SP | - | 21 (63.63) | |
| SAD | - | 9 (27.27) | |
| Panic | - | 1 (3.03) | |
| MDD | - | 4 (12.12) | |
| ADHD | - | 2 (6.06) |
T score.
SES, socioeconomic status; SCARED, Screen for Childhood Anxiety-Related Emotional Disorders; CDI, Child Depression Inventory; GAD, generalized anxiety disorder; SP, social phobia; SAD, separation anxiety disorder; MDD, major depressive disorder; ADHD, attention deficit hyperactivity disorder.
several co-morbid diagnoses were possible.
SACCADIC WM TASK (SWMT)
In this study, we used saccadic eye movements to probe the interplay of emotional and WM processes. We developed an emotional variant of the memory-guided saccade task. In the standard version of this task, participants are required to maintain fixation to a central point, whereas a target is flashed briefly in the periphery. After a variable delay, the central fixation extinguishes and the participant is required to saccade to the correct location where the target appeared. This task has been successfully used in pediatric patient populations (e.g., ADHD;[24] schizophrenia[25]). We modified the standard version in two ways, first by manipulating the emotional background, and second by manipulating the memory load (Fig. 1).
Figure 1.
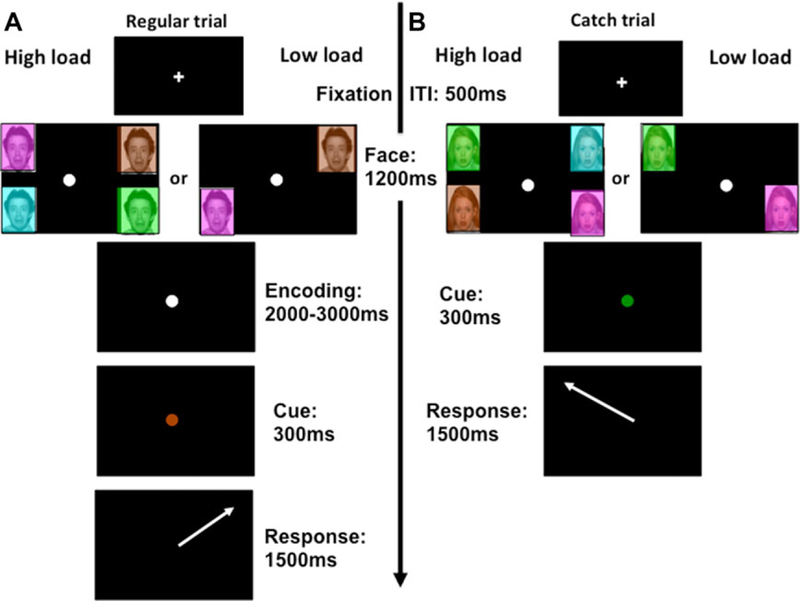
Typical trial. (A) Regular high-load (left half of panel A) and low-load (right half of panel A) trials. (B) Catch trials for high load (left half of panel B) and low load (right half of panel B). The only difference between the trial types is the absence of an encoding phase in the catch trials. Timings are otherwise identical. The white arrow only serves to indicate the correct direction where the response should have been made to for the viewer, but this arrow did not appear in the task.
Each trial started with a 500 ms white central fixation cross (2 mm × 2 mm) on a black background. The fixation cross changed into a white circle to indicate trial onset, and target stimuli were presented in the periphery, for 1200 ms, whereas participants maintained central fixation. Target stimuli consisted of 7.2 mm × 6.2 mm colored squares (cyan, brown, green, and magenta) in two (top left and bottom right or top right and bottom left = low-load trial) or all four screen corners (high-load trial). The colored squares contained a face depicting either a fearful or neutral expression. The peripheral stimuli then disappeared, while participants continued to maintain fixation on central cross (jittered between 2000 and 3000 ms). Finally, the color of the central circle changed from white into one of the target colors for 300 ms (imperative cue; Fig. 1). Participants were instructed to make their eye movement into the quadrant previously occupied by the depicted color and to maintain this fixation until the onset of the next fixation cross that denoted the start of the next trial. To make the task more motivating and encourage compliance, children were told this was a game where they could hone their “spy skills.” They were told they had to observe and memorize objects (in the present case colored rectangles) in the periphery without directly looking at them (just like spies observe people without looking at them).
Emotional and neutral face stimuli consisted of 16 different actors (eight male, eight female) selected from the NIMSTIM dataset (http://www.macbrain.org/resources.htm)[26] Each actor was presented twice, once bearing a neutral expression, and another time a fearful expression. This generated four trial types: low- and high-load trials of either fearful or neutral valence.
To ensure further that participants maintained central fixation during peripheral presentation of the target colors until the imperative cue, 28 catch trials were included in the task. These trials were identical to the high- and low-load trials, but did not include the encoding phase. During the catch trials, the white central circle was immediately replaced with a colored cue for 300 ms, skipping the delay/encoding phase of the other two trial types. During these randomly occurring catch trials, gazing away from central fixation resulted in inability to perform the trial correctly and finding the correct quadrant. Thus, catch trials encouraged participants to maintain central fixation in order to perform the task correctly. Given that participants could not distinguish between catch trials and regular trials until the disappearance of the peripheral faces, they were encouraged to maintain central fixation at all times during all trials. The amount of catch trials was equally split between high-load (50%) and low-load (50%) trials.
The task consisted of 176 trials in total, comprising 74 high-load trials (37 for each emotion), 74 low-load trials (37 for each emotion), and 28 catch trials (14 high load/14 low load). All conditions were randomly presented, and participants were trained on the task prior to study participation. The training task included face stimuli that were not used in the experimental task.
EYE MOVEMENT RECORDING
Eye movements were tracked and recorded with an ASL Model 504 (Applied Science Laboratories, Boston, MA) at 240 Hz temporal resolution and 0.25° spatial resolution. Saccades were defined as movements greater than 30°/s that lasted at least 25 ms. To determine correct and incorrect movements, only the first saccade following the onset of the central imperative cue was considered. Saccade accuracy was indexed as the percent of saccades directed to the correct corner. Saccade latency for correct trials was the time between the onset of the cue and the initiation of a saccade to one of the four target corners. To exclude anticipatory saccades or saccades in response to a preceding trial, saccade latencies faster than 80 ms or slower than 2000 ms were excluded.
DATA ANALYSIS
To obtain a more robust measure of latencies and to account for outliers and skewness in RT distribution, we used the mean of median RT for latency analyses (for each subject, a median for each condition was calculated and submitted to the analyses across subjects; the resulting means are reported, e.g.,[27]). Accuracy data were calculated as percent error. Latency and accuracy data were separately subjected to a 2 × 2 × 2 repeated measures analysis of covariance (rANCOVA) with emotion (fear vs. neutral) and WM load (high vs. low) as the within-subject variables and group (healthy adolescents vs. anxious adolescents) as the between-subject variable. Despite absence of differences between groups on demographic variables, such as age and IQ, these two variables were included as covariates of no interest in the main analysis to factor out the variance associated with these variables.[28] However, to further assess any potential impact of IQ, correlations were performed between IQ (total score and the subscores of matrix reasoning and vocabulary) and WM performance for the low- and high-load trials, in each group separately. Finally, given that catch trials were few in number and only served to ensure fixation, these trials were not analyzed. However, a basic manipulation check revealed that healthy adolescents (21.31% ± SEM 4.04) and anxious adolescents (20.72% ± SEM 3.26) did not differ in the amount of incorrectly executed catch trials (F(1,53) = 0.01, P = .91) and were not analyzed further. Importantly, given that only one of five catch trials were executed incorrectly suggests that participants were aiming to maintain central fixation. Statistical significance was defined using α = 0.05, two-tailed.
RESULTS
LATENCIES
The critical three-way interaction between group, emotion and load was significant (F(1,51) = 6.02, p = .02, η2 = .11; Fig. 2). The main effects of group (F(1,51) = 0.41, P = .53), load (F(1,51) = .007, P = .93), or emotion (F(1,51)<.01, P = .99) were not significant.
Figure 2.
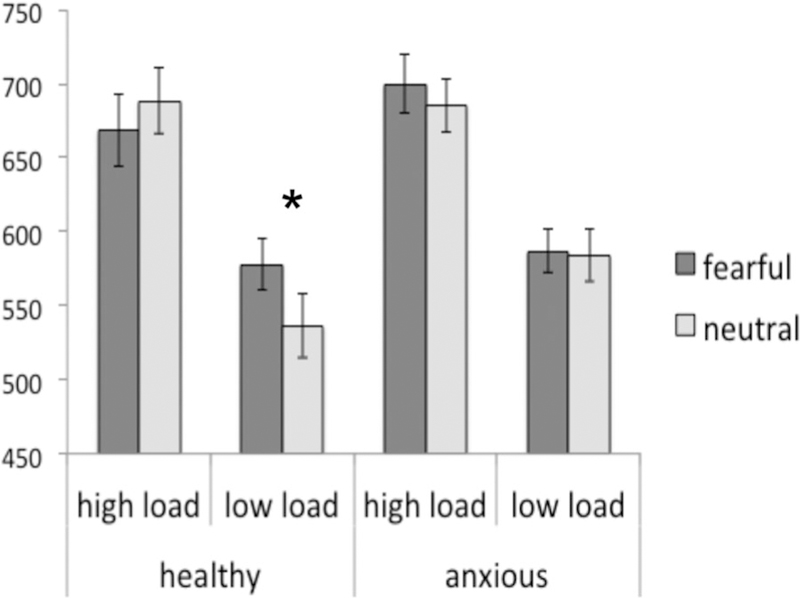
Latencies in milliseconds showing the significantly reduced latencies to neutral stimuli relative to fearful stimuli on low-load trials in the comparison group. This effect is absent in the anxious group. *P < .05.
To follow-up the significant three-way interaction, we conducted an emotion by load analysis of variance (ANOVA) in each group separately. In the healthy group, the emotion by load interaction was significant (F(1,21) = 10.29, P = .004, η 2 = .33). Post hoc paired t-tests indicated that, in the low-load condition, the latencies were significantly slower for fearful than neutral faces (t(21) = 2.19, P = .04). However, in the high-load condition, latencies did not differ between fearful and neutral faces (t(21) = 1.22, P = .24). In the anxious youths, the emotion by load interaction was not significant (F(1,32) = 0.26, P = .62, η2 = .008). Finally, the main effect of load was significant in both groups (healthy: F(1,21) = 57.91, P <.001, η 2 = .73; anxious: F(1,32) = 44.52, P <.001, η2 = .58). In summary, the main group difference in the latencies was that healthy but not anxious participants showed an interference effect of threat during low but not high cognitive load on saccade latency.
ACCURACY
ANOVA on accuracy rates (percent error) only revealed a main effect of load (F(1,51) = 7.51, P = .008, η2 = .13), indicating larger error rates during high (mean = 31.00%, SD = 13.30%) relative to low (mean = 16.79%, SD = 11.74%) cognitive load. In addition, the main effect of age was also significant (F(1,51) = 9.33, P <.01, η2 = .16), revealing an expected greater accuracy with older age. No other effects were significant.
ADDITIONAL ANALYSES OF DIAGNOSTIC COMORBIDITY, INTELLIGENCE, AND SEVERITY
To account for a potential impact of comorbid depression, we reran analyses after removing the four patients with comorbid major depressive disorder (MDD). The three-way emotion by load by group interaction in the latencies remained significant (F(1,49) = 8.83, P = .005), suggesting no impact of comorbid depression. In addition, to assess measures of severity, scores from the SCARED child were correlated with WM performance (latencies and errors) for the low- and high-load trials. This analysis did not yield any significant effects, neither when the groups were combined (all r < .27, all P > .06) or analyzed separately (all r < .20, all P > .30). Finally, we also examined the issue of IQ for each group separately, but none of the correlations between the IQ total score or the IQ subscores (matrix reasoning, vocabulary) and performance to high- or low-load trials reached statistical significance (all r < .32, all P > .17).
DISCUSSION
This study examined the interplay of cognitive and emotional processes in healthy and anxiety disordered adolescents. Our ultimate goal was to shed light on the potential mechanisms underlying the deleterious consequences of clinical anxiety on emotional interferences with cognitive performance, so critical to the pediatric period.
From the perspective of limited resources theories,[29, 30] we expected that healthy adolescents would exhibit cognitive interference by emotional stimuli only at low-load WM, but that anxious adolescents would show such interference at both low- and high-load WM. Findings confirmed predictions for healthy adolescents (longer latencies to fearful vs. neutral trials), but not for anxious adolescents. Anxious adolescents showed no influence of the emotional manipulation on cognitive performance at either low or high load. In addition, overall, WM performance (RT and accuracy) did not differ between the anxiety and the comparison groups, at either low- or high-load WM.
Results in the healthy group support previous findings[13] that cohere with the limited resources theories (e.g.,[29, 30]). Specifically, threat-related distractors impaired WM performance, but only when cognitive resources were left available to process the threat stimuli. These data suggest that high-load cognitive processes might minimize the impact of threat-related stimuli. However, contrary to expectations, this pattern was not evident, let alone amplified, in the clinically anxious group. Thus, the critical question is why incidental threat failed to affect WM performance in anxious patients. We propose two possible interpretations.
The first one relies on the overall effect of chronic worry on WM capacity. Specifically, worry would hijack WM resources such that remaining resources would be just large enough to process the task at hand. In this case, incidental threat stimuli would not be processed, even at low-load WM, and, in turn, would not interfere with performance. Unfortunately, measures of amount of worry were not collected during the study. However, given that these anxious adolescents carried a diagnosis of anxiety, severe enough to lead them to seek treatment, might imply that worry was chronically present in this sample. This interpretation also would imply that the processing of threat stimuli was not prioritized over cognitive processing in this experiment. This order of priority might be reasonable given the mild degree of threat that our incidental stimuli carry. Stronger induced emotions could tip this balance, leading to an engagement of resources that prioritizes emotion over cognitive (WM) processes in anxious adolescents. However, since stronger and more intense emotional pictures may not be ethically age-appropriate, another alternative would be to evoke sensory responses to aversive noise that have also been used in youths.[31]
The second possible interpretation relies on the threat bias for ambiguous stimuli, which has been described in anxiety. Specifically, anxious participants tend to perceive neutral faces as ambiguous or negative.[32] It is currently unclear as to whether anxious children generalized the fearful attribute of fearful faces to neutral faces across both load conditions, or whether they simply blocked out the emotional valence and ignored the faces altogether. However, supporting the first notion, generalization of perceptually similar stimuli, such as faces, is consistent with some accounts of fear learning.[33] Moreover, the idea that the anxious adolescents might have processed the neutral faces as threat stimuli, similarly to the fearful faces, is consistent with the trend that latencies to neutral faces seemed comparatively slower in the anxious group than in the comparison group (see Fig. 2). The inclusion of happy faces or stronger emotional stimuli, as alluded to above, would have been helpful to examine this argument further.
The absence of overall differences in WM performance between the anxious and comparison adolescents is interesting. This highlights the complexity of the relationship between anxiety and WM, which depends on multiple factors, including the level of cognitive engagement and difficulty, the intensity of threat stimuli, the state of worry of the individuals, and the motivation to do well (particularly intense in anxious individuals). The present work provides some preliminary evidence that the interactions between threat stimuli and task difficulty differ between anxious patients and healthy individuals in adolescents. The present findings should be followed by experiments that manipulate wider ranges of cognitive difficulty and incidental threat intensity, while recording levels of worry and motivation in the participants.
Finally, our findings are consistent with body of work that has used the sensitivity of eye movement tasks to examine pathology-related deficits in inhibitory control and WM in children and adolescents (e.g.,[17, 24, 25, 34]). A novel extension of the current study was the addition of two levels of cognitive load and an affective dimension to the classic saccadic WM task. Although the current findings support the utility of using emotional variants of the saccadic WM task to accurately assess the impact of emotion on spatial WM processes, some caution is warranted. A modulation of WM was found in the healthy but not in the anxious patients, who, in addition, failed to show any modulation of the latency findings with measures of IQ or anxiety severity. Therefore, more work is required to further link anxious symptoms to WM deficits. Yet, future work can build on these findings as suggested above (e.g., by including happy faces).
Some limitations should be addressed. First, the relatively small sample size limits generalization. However, although the sample of healthy controls was somewhat smaller than the patient group, a modulating effect of emotion was found in the former group, suggesting that the lack of finding in the patients was unlikely to be due to insufficient statistical power. A second limitation concerns the heterogeneity of the anxious sample in terms of diagnoses of anxiety disorders. Although such heterogeneity can reduce the statistical power to detect differences, it is representative of the phenotypical profile of pediatric anxiety disorders.[35] In addition, we did not expect that the fundamental mechanism associated with limited resources would be disorder specific, a presumption that would need to be tested. Third, given that our stimuli were taken from an established dataset of faces[26], we did not collect stimulus ratings that could have been helpful in assessing the level of threat generalization across fearful and neutral faces. Fourth, and finally, following previous work,[36] we pseudo-randomly interspersed catch trials to motivate participants and encourage task compliance. Although it appeared that participants did indeed follow instruction given the relatively low error rates on these trials, to fully assess their effect, one would have to rerun the task using blocks with and blocks without catch trials to estimate their impact.
In summary, this is the first study to demonstrate differences in WM performance during incidental threat between anxious and comparison adolescents using a memory-guided saccade task. In particular, while threat differentially impacted response latencies in healthy youths, no such effect was seen in the anxious group. These findings in adolescents are important to better understand how clinical anxiety can impact cognitive control processes in adolescents, and, reciprocally, how cognitive activity can modulate anxiety.
Supplementary Material
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62(6):593–602. [DOI] [PubMed] [Google Scholar]
- 2.Mazzone L, Ducci F, Scoto MC, et al. The role of anxiety symptoms in school performance in a community sample of children and adolescents. BMC Public Health 2007;7:e347. doi: 10.1186/1471-2458-7-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egger HL, Costello EJ, Angold A. School refusal and psychiatric disorders: a community study. J Am Acad Child Adolesc Psychiatry 2003;42(7):797–807. [DOI] [PubMed] [Google Scholar]
- 4.Pine DS, Cohen P, Gurley D, et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998;55(1):56–64. [DOI] [PubMed] [Google Scholar]
- 5.Jazbec S, McClure E, Hardin M, et al. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry 2005;58(8):632–639. [DOI] [PubMed] [Google Scholar]
- 6.Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J Child Psychol Psychiatry 2007;48(5):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardin MG, Mandell D, Mueller SC, et al. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. J Child Psychol Psychiatry 2009;50(12):1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luna B, Garver KE, Urban TA, et al. Maturation of cognitive processes from late childhood to adulthood. Child Dev 2004;75(5):1357–1372. [DOI] [PubMed] [Google Scholar]
- 9.Mueller SC. The influence of emotion on cognitive control: relevance for development and adolescent psychopathology. Front Psychol 2011;2:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion 2007;7(2):336–353. [DOI] [PubMed] [Google Scholar]
- 11.Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen 2004;133(3):339–354. [DOI] [PubMed] [Google Scholar]
- 12.Pessoa L How do emotion and motivation direct executive control? Trends Cogn Sci 2009;13(4):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vytal K, Cornwell B, Arkin N, Grillon C. Describing the interplay between anxiety and cognition: from impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology 2012;49(6):842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities—keynote address. Ann N Y Acad Sci; 2004:1–22. [DOI] [PubMed]
- 15.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med 2006;36(3):299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayes S, Hirsch C, Mathews A. Restriction of working memory capacity during worry. J Abnorm Psychol 2008;117(3):712–717. [DOI] [PubMed] [Google Scholar]
- 17.Rommelse NNJ, Van der Stigchel S, Sergeant JA. A review on eye movement studies in childhood and adolescent psychiatry. Brain Cogn 2008;68(3):391–414. [DOI] [PubMed] [Google Scholar]
- 18.Ladouceur CD, Dahl RE, Williamson DE, et al. Altered emotional processing in pediatric anxiety, depression, and comor-bid anxiety-depression. J Abnorm Child Psychol 2005;33(2):165– 177. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry 1983;40(11):1228– 1231. [DOI] [PubMed] [Google Scholar]
- 21.Rupp. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The research unit on Pediatric Psychopharmacology Anxiety Study Group. N Engl J Med 2001;344(17):1279–1285. [DOI] [PubMed] [Google Scholar]
- 22.Birmaher B, Khetarpal S, Brent D, et al. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 1997;36(4):545–553. [DOI] [PubMed] [Google Scholar]
- 23.Helsel WJ, Matson JL. The assessment of depression in children: the internal structure of the Child Depression Inventory (CDI). Behav Res Ther 1984;22(3):289–298. [DOI] [PubMed] [Google Scholar]
- 24.Loe IM, Feldman HM, Yasuni E, Luna B. Oculomotor performance identifies underlying cognitive deficits in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2009;48(8):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross RG, Heinlein S, Zerbe GO, Radant A. Saccadic eye movement task identifies cognitive deficits in children with schizophrenia, but not in unaffected child relatives. J Child Psychol Psychiatry 2005;46(12):1354–1362. [DOI] [PubMed] [Google Scholar]
- 26.Tottenham N, Tanaka JW, Leon AC, et al. NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 2009;168(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller SC, Jackson GM, Dhalla R, et al. Enhanced Cognitive Control in Young People with Tourette’s syndrome. Curr Biol 2006;16(6):570–573. [DOI] [PubMed] [Google Scholar]
- 28.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol 2001;110(1):40–48. [DOI] [PubMed] [Google Scholar]
- 29.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci 2007;11(7):307–316. [DOI] [PubMed] [Google Scholar]
- 30.Pessoa L How do emotion and motivation direct executive control? Trends Cogn Sci 2009;13(4):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncko R, Cui L, Hille J, et al. Startle reactivity in children at risk for migraine. Clin Neurophysiol 2008;119(12):2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci 1998;7(6):177–188. [Google Scholar]
- 33.Dunsmoor JE, Mitroff SR, LaBar KS. Generalization of conditioned fear along a dimension of increasing fear intensity. Learn Mem 2009;16(7):460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller SC, Ng P, Hardin M, et al. Perturbed reward processing in pediatric bipolar disorder: an antisaccade study. J Psychopharmacol 2010;24(12):1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety 2000;12(Suppl 1):69–76. [DOI] [PubMed] [Google Scholar]
- 36.Astle DE, Jackson GM, Swainson R. Fractionating the cognitive control required to bring about a change in task: a dense-sensor event-related potential study. J Cogn Neurosci 2008;20(2):255– 267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


