Summary
To navigate through the world, animals must stabilize their path against disturbances and change direction to avoid obstacles and to search for resources [1,2]. Locomotion is thus guided by sensory cues, but also depends on intrinsic processes, such as motivation and physiological state. Flies, for example, turn with the direction of large-field rotatory motion, an optomotor reflex that is thought to help them fly straight [3-5]. Occasionally, however, they execute fast turns, called body saccades, either spontaneously or in response to patterns of visual motion such as expansion [6-8]. These turns can be measured in tethered flying Drosophila [3,4,9], which facilitates the study of underlying neural mechanisms. Whereas there is evidence for an efference copy input to visual interneurons during saccades [10], the circuits that control spontaneous and visually elicited saccades are not well known. Using 2-photon calcium imaging and electrophysiological recordings in tethered flying Drosophila, we have identified a descending neuron whose activity is correlated with both spontaneous and visually elicited turns during tethered flight. The cell's activity in open- and closed-loop experiments suggests that it does not underlie slower compensatory responses to horizontal motion, but rather controls rapid changes in flight path. The activity of this neuron can explain some of the behavioral variability observed in response to visual motion and appears sufficient for eliciting turns when artificially activated. This work provides an entry point into studying the circuits underlying the control of rapid steering maneuvers in the fly brain.
Keywords: descending neuron, spontaneous behavior, flight, saccades, Drosophila
Results
To find neurons involved in steering behavior, we measured activity of descending neurons that convey signals from the brain to the ventral nerve cord in tethered flying Drosophila melanogaster. The Gal4-line R56G08 targets four to five pairs of descending interneurons that terminate in the ventral nerve cord (VNC) as well as ascending haltere afferents [11] and an assortment of others cells throughout the brain (Fig. 1A, Movie 1). We performed 2-photon calcium imaging from this line using the genetically encoded indicator GCaMP6f [12] while simultaneously monitoring the difference in wing stroke amplitude between the left and right wing (L-R WSA) (Fig. 1B), a measure for intended steering maneuvers. When we imaged from the presumed dendritic region of one of the labeled descending neurons within the posterior slope, we observed that the cell in the right hemisphere exhibited spontaneous activity that was strongly correlated with increases in L-R WSA corresponding to rightward turns (Fig. 1C). We never observed increases in fluorescence within the imaged area when the animal was not flying. Although the driver line labeled other cells in the brain, we were able to specify a region of interest in which a fine process of the cell was well isolated from other neurons (Fig.1A, inset). Because the morphology of the cell suggests that it is an unidentified member of a family of similar descending neurons (A1 through A5) with anterior cell bodies recently described by Shigehiro Namiki at the Janelia Research Campus (pers. com.), we tentatively label it AX until a more permanent nomenclature is established.
Figure 1. Descending neuron activity correlates with spontaneous and looming-elicited changes in L-R WSA.
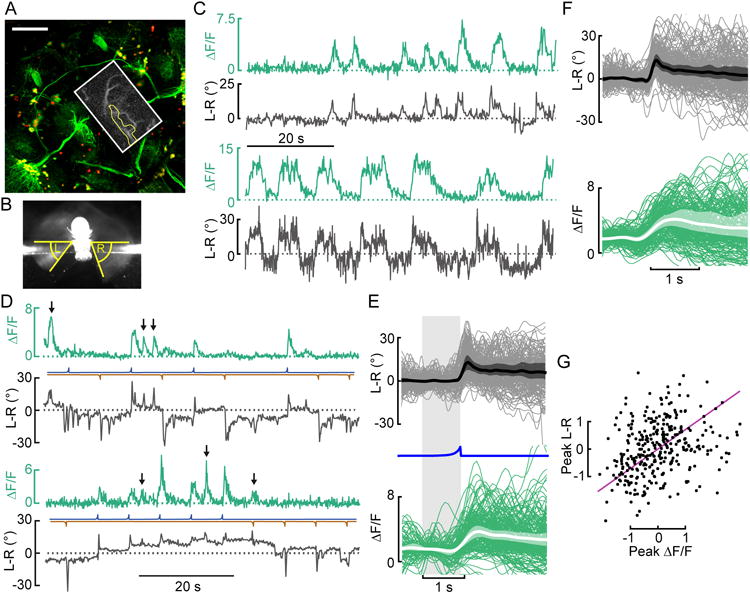
(A) Maximum intensity projection of mCDGFP and stingerRed expression in the whole brain driven by R56G08-Gal4. A 261μm z-stack was taken with the 2-photon microscope (scale bar: 50μm). The approximate imaging area is depicted with a white box. An example image (maximum intensity projection of the tdTomato-signal of one experimental trial) with the region of interest highlighted in yellow is shown as inset. See also Movie S1. (B) Image of a fly taken from below illustrating the measurement of left (L) and right (R) wing stroke amplitude (WSA). (C) Representative traces of spontaneous changes in L-R WSA (L-R) and GCaMP6f fluorescence in AX (ΔF/F) from 2 (out of 19) recorded flies in the absence of visual stimulation. (D) Two example traces of changes in L-R and ΔF/F in AX during presentation of looming stimuli presented either left (blue) or right (brown). Several spontaneous saccades (black arrows) occur in between looming stimuli. (E) Top panel: Baseline-subtracted mean L-R (thick line), boot-strapped 95% CI for the mean of fly means (shaded area), and individual responses (thin lines) to looming stimuli on the left (blue line). Bottom panel: Same as top panel, but baseline-averaged ΔF/F instead of baseline-subtracted traces. N=13. (F) Same as E, with L-R and ΔF/F for spontaneous saccades. (G) For pooled responses to looming stimuli (E) and spontaneous saccades (F), a total least squares regression of fly sample version z-scores (purple line) explained 66.1 % of the variance between peak responses in ΔF/F and L-R (N=13).
Recordings from AX in the absence of any applied visual stimulus indicated that it was correlated with spontaneous turns. To test whether its activity also correlated with visually elicited turns, we subjected flies to a set of moving visual patterns, including looming stimuli that are effective in eliciting rapid evasive turns [14]. When we presented flies with a sequence of dark looming objects from either the left or right, we found that whenever a looming stimulus elicited a turn to the right (i.e. when L-R WSA increased), the right AX cell was transiently active (Fig. 1D, E). Note that the traces show many instances in which the AX cell was briefly active, when no stimulus was presented, and in all of these cases the fly exhibited a turn to the right (Fig. 1F). We observed much variability in the magnitude of the rightward turns, which correlated with the size of the AX response (Fig. 1G). We also recorded failures – i.e. cases in which a loom from the left did not elicit a rightward turn, but in these cases we did not observe a response in AX, suggesting that the cell is better classified as pre-motor than sensory. The failures most often occurred when the fly was – for whatever reason – executing a series of rapid turns to the left. We never observed rapid turns to the right that were not accompanied by transient rises in fluorescence in the right AX. All these results suggest that AX plays an important role in the pathway responsible for both visually elicited and spontaneous turns.
We did not observe any consistent changes in the average GCaMP6f response to other visual stimuli we presented including rightward motion, which evokes the well-characterized optomotor response [3,13]. We did, however, observe substantial trial-by-trial variability in the behavioral as well as the neuronal responses to rightward motion. In most trials, we recorded no changes in the activity of AX during stimulus presentation; however, the trials in which the cell was active tended to be those in which the animal exhibited a particularly large motor response (Fig. S1). To explore this trend in more detail, we divided all trials into 10 equally spaced bins based on the magnitude of the wing motor responses after baseline subtraction. Only during the strongest turns in the direction of visual motion did we observe an increase in fluorescence (Fig. 2A). In some trials, flies responded to the visual stimulus with a ‘contrary’ turn, i.e. opposite to the direction of visual motion. Whenever the fly exhibited a contrary turn, we observed a decrease in fluorescence, suggesting that the cell receives inhibitory input at this time. We speculated that the contralateral AX cell was active during these events. To test this idea, we analyzed activity from the neuron during motion in the opposite direction (i.e. to the left) in the same way (Fig. 2A) and found that the cell on the right side of the brain was indeed active during contrary turns, which in the case of leftward motion stimuli are turns to the right. We also observed a decrease in fluorescence during particularly strong leftward optomotor responses – when the neuron's contralateral partner was presumably active – further suggesting that when one cell is active the contralateral cell is inhibited. In summary, the AX is usually unresponsive to horizontal motion, but when it does respond, its activity is correlated with a large syndirectional turn. When the visual stimulus is towards the ipsilateral side, the changes in L-R WSA are quite large, presumably because the cell's output sums with the output of the optomotor pathway. When active during visual motion toward the contralateral side, the cell's output largely cancels the optomotor response, which results in small changes in L-R WSA (Fig. 2B). The activity of AX can thus partially explain the behavioral variability in responses to horizontal motion.
Figure 2. AX activity is linked to deviations from a straight flight path.
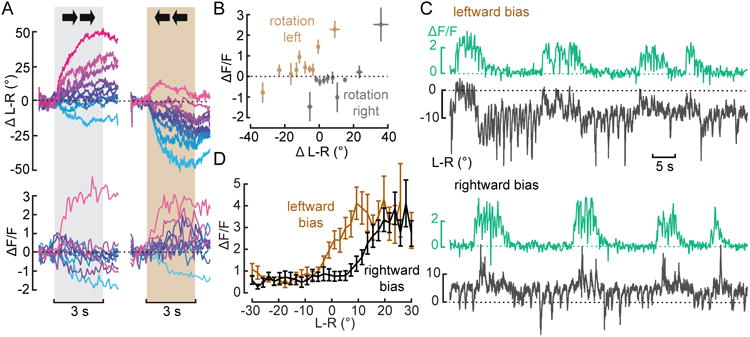
(A) Mean baseline-subtracted changes in L-R WSA and ΔF/F in response to a horizontally moving grating grouped into 10 equally spaced bins based on the magnitude of the behavioral response. Corresponding trials are colored the same. N=9 flies, n=57/65 trials. See also Figure S1. (B) Mean +/- 1 s.e.m. changes in ΔF/F during stimulus presentation plotted against simultaneous changes in L-R for all bins from A. (C) Example traces of simultaneously recorded changes in L-R and ΔF/F from the putative descending neuron during closed loop with a constant bias of left- or rightward motion (temporal frequency: 1.56 Hz.) (D) Mean changes in ΔF/F plotted against L-R for a bias of rightward (black) or leftward (brown) motion. N=10 flies.
The previous experiments suggest that AX mediates deviations from the flight path that are superimposed on the continuous output of the optomotor system. To test this hypothesis further, we created a situation in which the fly had to compensate for a constant visual drift by performing closed-loop experiments in the presence of a steady rotational bias. Flies readily compensate for a rotational bias to either the left or right by shifting the mean level of L-R WSA (Fig. 2C). This shift is not, however, accompanied by tonic changes in the fluorescence signal from AX. Rather, the cell's activity is correlated with transient steering maneuvers superimposed on the new baseline, leading to a shift in the relationship between L-R WSA and fluorescence (Fig. 2D). These results suggest that the pathway that mediates slower optomotor responses and the pathway that mediates spontaneous turns - represented by AX – are parallel and converge linearly on the steering motor system.
As calcium imaging did not allow us to clearly identify the anatomy of the cell and is limited by the slow time constants of the indicator, we attempted whole-cell patch-clamp recordings from the cell bodies of AX. Due to the deep location of the cell body, these were very difficult recordings to achieve in flying animals, but we did successfully record from GFP-labeled descending neurons in eight flies. Three of the recorded cells exhibited a correlation between changes in L-R WSA and membrane potential during flight and thus most likely represent AX. We did not detect action potentials in any of our recordings from this cell and suspect that it conducts information to the VNC via graded potentials. Although action potentials are likely to be heavily filtered in recordings from the cell body, we were able to clearly detect spikes in recordings from a nearby descending neuron with very similar anatomy (not shown).
An example recording of AX performed under closed-loop conditions, in which the L-R WSA signal controlled the horizontal velocity of a vertical grating, is shown in Fig. 3A. The cross-correlation between the two signals (Fig. 3B) as well as the average traces during large increases in L-R WSA (Fig. 3C) indicate that changes in membrane potential clearly precede changes in steering responses. The delay of about 130 ms obtained from the cross-correlation appears long for a premotor neuron, but is within the range of the measured delays in the optomotor response, for which values of up to 220 ms have been reported [15]. These data also suggest that the offset of neuronal activity does not strictly determine the end of a turn, which is likely regulated by other processes, such as sensory feedback from the wings or halteres. We filled the cell with Biocytin during recording and reconstructed its anatomy from a confocal image stack (Fig. 3D, Movie 2, Movie 3), confirming that it was a GFP-positive descending neuron with arborizations in the posterior slope that descends ipsilaterally and terminates dorsally within the wing neuropil of the VNC. Although we acknowledge the anecdotal nature of our whole-cell flight recordings, the results are entirely consistent with our imaging data; AX is descending neuron that appears to be involved in the generation of rapid turns.
Figure 3. Whole-cell recordings enable anatomical identification of AX.
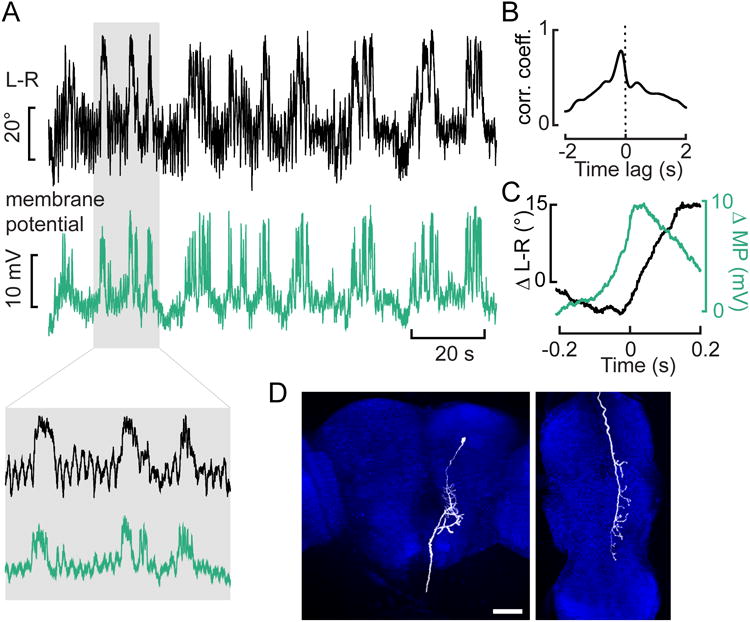
(A) Example traces of the simultaneously recorded L-R and membrane potential (MP) of the descending neuron during closed loop. The mean resting potential of the three cells recorded was -59 mV. The shaded area is expanded on the inset below. (B) Cross-correlation between the traces shown in A. (C) Averages of the large changes in MP that exceed twice the standard deviation (at time zero) and the concomitant changes in L-R from the traces in A. (D) Reconstruction of the biocytin-filled neuron in the brain (left) and ventral nerve cord (right) (maximum intensity projection). The background staining against NC82 is shown in blue. Scale bar: 50μm. See also Movie S2 and Movie S3.
Next, we wanted to test for sufficiency of the AX to elicit behavioral changes during flight. As in two previous studies of descending neurons [16,17], we found that depolarizing the cell by current injection was not feasible due to the long thin neurite connecting the soma and dendrite. Instead, we co-expressed GFP and P2X2, an ATP-gated ion channel, using R56G08 [16,18]. By pressure-injecting ATP locally using a micropipette positioned close to the dendrite of AX, we could reliably elicit changes in L-R WSA (Fig. 4A). Control flies that either did not express P2X2 or were injected with saline lacking ATP showed no reaction to the pulses. Even though the dendrites of the cell reside near the midline, we were able to elicit left or right turns depending upon which hemisphere we injected. The turning reactions involved changes in the motion of both wings, indicating that the cell – which does not cross the midline in the VNC - elicits bilaterally coordinated motor actions (Fig. 4B). Although the Gal4-line is not specific for AX, we believe it is most parsimonious to suspect that its activation is responsible for the elicited behavioral response because it is the only cell in our imaging experiments that exhibited a correlation with changes in wing motion.
Figure 4. ATP-induced activation of P2X2-expressing neurons is sufficient to elicit changes in L-R WSA.
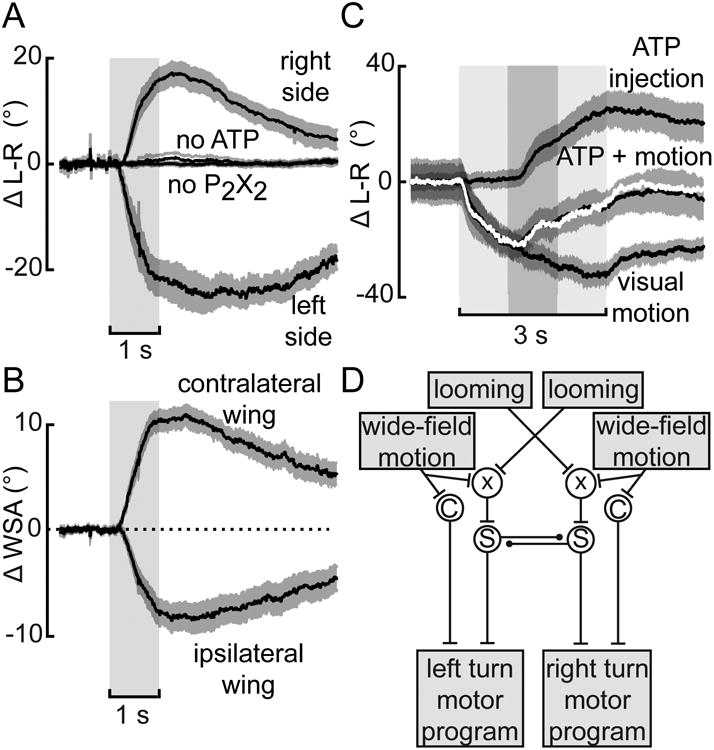
(A) Changes in L-R WSA upon stimulation with ATP/P2X2 driven by R56G08-Gal4 in either the right (N=11 flies) or left hemisphere (N=9) or of control flies (no ATP: N=14, no P2X2: N= 10). (B) Changes in WSA of the ipsi- and contralateral wing for all experimental flies from A. (C) ATP activation (dark gray) in the right hemisphere during stimulation with leftward motion (light gray). The sum of the responses to each manipulation alone is shown in white. Shaded areas represent s.e.m. (D) Qualitative model describing the experimental findings; C stands for the continuous optomotor pathway, S for the pathway mediating saccadic turns represented by AX. An inhibitory pathway connects the two AX cells. Input from the visual system drives AX albeit through a not yet characterized process represented by χ.
To explore the potential interaction between AX and the optomotor pathways, we combined presentation of a large-field leftward motion with ATP injection in P2X2-expressing flies in the right hemisphere. The rationale for combining two manipulations that should result in turns of opposite sign was to avoid saturation of the behavioral response. As controls, we only presented visual motion or only injected ATP within the same flies, which both led to strong changes in L-R WSA of the opposite sign. When we injected ATP during the presentation of motion, we observed a turning response that was almost identical to the sum of the response to either motion or ATP injection alone (Fig. 4C), which is also apparent when looking at individual traces (not shown). This result is further evidence that the optomotor pathway and the AX interact linearly.
Discussion
We have discovered a descending neuron in Drosophila, whose activity is strongly correlated with both spontaneous and visually elicited turns that likely represent the body saccades during free flight. Whereas the cell did respond robustly to looming stimuli and sporadically to large-field motion, it also often exhibited spontaneous events that were correlated with motor responses, but occurred in the absence of any externally applied stimuli. Altogether, our results support the existence of at least two descending pathways for controlling flight direction (Fig. 4D). Via its input from the wide-field system, the optomotor pathway is responsible for trimming the flight motor in response to internal or external perturbations. The pathway mediated by the AX mediates rapid flight responses elicited by loom, but also generates spontaneous turns in the absence of visual input. Our data are consistent with a simple summation of the signals of the optomotor and the AX pathway. A recent study of the steering motor system indicates that direct flight muscles are divided into two groups: tonic muscles that are responsible for maintaining the trim of the flight motor and phasic muscles that are primarily recruited during the largest spontaneous turns [19]. We thus predict that the AX neuron makes particularly strong connections with phasic muscle motor neurons. In addition, we propose that the two AX neurons mutually inhibit each other, albeit in an indirect fashion, because the cells do not cross the midline
A prior statistical analyses of free flight behavior within an enclosed chamber found that while most body saccades exhibited by flies are triggered by visual cues such as expansion, flies also exhibit spontaneous saccades at a rate of ∼0.5 Hz [20]. However, as the exact percentage of saccades driven by sensory stimuli versus intrinsic processes likely depends on the precise structure of the environment, it is hard to compare these prior results with data from tethered conditions. The spontaneous behavioral events correlated with AX activity were somewhat longer than the intended maneuvers that had been described previously and that are thought to represent body saccades [6,10,14]. It is therefore possible that the events represent an unknown class of slower turns, although it is more likely that they represent saccades that are artificially long due to the head-fixed preparation required for imaging.
Recent studies reported evidence for an efference copy of flight saccades that influences the membrane potential of motion-responsive visual interneurons in the lobula plate [10,22], an effect that might explain why flies do not react with an optomotor response to the reafferent visual motion caused by their spontaneous turns. It remains to be shown whether activity of AX is correlated with similar events in visual interneurons or if it is involved in the pathway that provides the efference copy.
Recently, three descending cells, each sensitive to a different axis of rotational visual motion have been characterized in Drosophila [17], two of which appear to be homologs of descending neurons described in larger flies [23-28]. Aside from these cells, little is known of the roughly 1100 cells descending from the brain to the VNC [30]. Two noteworthy exceptions are the so-called “moonwalker neuron”, which elicits backward walking [31] and the giant fiber neuron, which triggers evasive take-offs [16]. Given the number of descending interneurons it is striking that activation of a single cell (or a small group of cells) is sufficient to elicit large turns, which suggests that some descending cells might act as command-like neurons [32]. Although in our experiments we never saw examples of spontaneous turns that were not correlated with the activity of AX, it is still possible that there are other neurons that can elicit similar motor actions.
Premotor neurons that are responsible for turns during search behavior have been described in worms [33,34] and moths [35], suggesting that similar neural architecture might underlie steering maneuvers across species. Here, we describe a previously unknown descending neuron that controls both spontaneous and visually elicited rapid turns in Drosophila. We expect that the further study of AX and its input pathways will uncover the neural circuits in the fly brain that underlie the control of spontaneous turning behavior and could reveal general principles governing course control.
Supplementary Material
Acknowledgments
We would like to thank Ainul Huda and Peter Weir for the image of the R56G08-Gal4 line, Shigehiro Namiki for information about Gal4-lines labeling descending neurons, and Gaby Maimon, Theodore Lindsay and Peter Weir for comments on the manuscript. This work was supported by the Raymond and Beverly Sackler Foundation (B.S.), the Paul G. Allen Family Foundation (M.H.D.) and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award U01NS090514 (M.H.D). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author contributions: Conceptualization, B.S. and M.H.D.; Methodology, B.S. and I.R.; Investigation, B.S. and I.R.; Formal Analysis, B.S. and I.R; Writing – Original Draft, B.S. and M.H.D.; Writing – Review & Editing, B.S., I.R., and M.H.D.; Funding Acquisition, B.S. and M.H.D.; Supervision, M.H.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collett TS, Land MF. Visual control of flight behaviour in the hoverfly Syritta pipiens L. J Comp Physiol. 1975;1:1–66. [Google Scholar]
- 2.Budick SA, O'Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;17:2565–2579. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- 3.Götz KG. Flight control in Drosophila by visual perception of motion. Kybernetik. 1968;6:199–208. doi: 10.1007/BF00272517. [DOI] [PubMed] [Google Scholar]
- 4.Tammero LF, Frye MA, Dickinson MH. Spatial organization of visuomotor reflexes in Drosophila. J Exp Biol. 2004;1:113–122. doi: 10.1242/jeb.00724. [DOI] [PubMed] [Google Scholar]
- 5.Mronz M, Lehmann F. The free-flight response of Drosophila to motion of the visual environment. J Exp Biol. 2008;13:2026–2045. doi: 10.1242/jeb.008268. [DOI] [PubMed] [Google Scholar]
- 6.Heisenberg M, Wolf R. On the fine-structure of yaw torque in visual flight orientation of Drosophila-Melanogaster. J Comp Physiol. 1979;2:113–130. [Google Scholar]
- 7.Tammero LF, Dickinson MH. The influence of visual landscape on the free flight behavior of the fruit fly Drosophila melanogaster. J Exp Biol. 2002;3:327–343. doi: 10.1242/jeb.205.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Bender JA, Dickinson MH. Visual stimulation of saccades in magnetically tethered Drosophila. J Exp Biol. 2006;16:3170–3182. doi: 10.1242/jeb.02369. [DOI] [PubMed] [Google Scholar]
- 9.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;3:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 10.Kim AJ, Fitzgerald JK, Maimon G. Cellular evidence for efference copy in Drosophila visuomotor processing. Nat Neurosci. 2015;9:1247–1255. doi: 10.1038/nn.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenett A, Rubin GM, Ngo TB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Reports. 2012;4:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;7458:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Götz K. Optomotory investigation of the visual-system of some eye mutations of the Drosophila fruit-fly. Kybernetik. 1964;2:77–91. doi: 10.1007/BF00288561. [DOI] [PubMed] [Google Scholar]
- 14.Tammero L, Dickinson M. Collision-avoidance and landing responses are mediated by separate pathways in the fruit fly, Drosophila melanogaster. J Exp Biol. 2002;18:2785–2798. doi: 10.1242/jeb.205.18.2785. [DOI] [PubMed] [Google Scholar]
- 15.Duistermars BJ, Care RA, Frye MA. Binocular interactions underlying the classic optomotor responses of flying flies. Front Behav Neurosci. 2012:6. doi: 10.3389/fnbeh.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, Card GM. A spike-timing mechanism for action selection. Nat Neurosci. 2014;7:962–970. doi: 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- 17.Suver MP, Huda A, Iwasaki N, Safarik S, Dickinson MH. An Array of Descending Visual Interneurons Encoding Self-Motion in Drosophila. J Neurosci. 2016;46:11768–11780. doi: 10.1523/JNEUROSCI.2277-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;1:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay T, Sustar A, Dickinson MH. The Function and Organization of the Motor System Controlling Flight Maneuvers in Flies. Curr Biol. 2017;27:345–358. doi: 10.1016/j.cub.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Muijres FT, Elzinga MJ, Melis JM, Dickinson MH. Flies Evade Looming Targets by Executing Rapid Visually Directed Banked Turns. Science. 2014;6180:172–177. doi: 10.1126/science.1248955. [DOI] [PubMed] [Google Scholar]
- 21.Censi A, Straw AD, Sayaman RW, Murray RM, Dickinson MH. Discriminating External and Internal Causes for Heading Changes in Freely Flying Drosophila. PLoS Comput Biol. 2013;2:e1002891. doi: 10.1371/journal.pcbi.1002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnell B, Weir PT, Roth E, Fairhall AL, Dickinson MH. Cellular mechanisms for integral feedback in visually guided behavior. Proc Natl Acad Sci USA. 2014;15:5700–5705. doi: 10.1073/pnas.1400698111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wertz A, Borst A, Haag J. Nonlinear integration of binocular optic flow by DNOVS2, a descending neuron of the fly. J Neurosci. 2008;12:3131–3140. doi: 10.1523/JNEUROSCI.5460-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wertz A, Gaub B, Plett J, Haag J, Borst A. Robust Coding of Ego-Motion in Descending Neurons of the Fly. J Neurosci. 2009;47:14993–15000. doi: 10.1523/JNEUROSCI.3786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haag J, Wertz A, Borst A. Integration of lobula plate output signals by DNOVS1, an identified premotor descending neuron. J Neurosci. 2007;8:1992–2000. doi: 10.1523/JNEUROSCI.4393-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gronenberg W, Strausfeld NJ. Descending Neurons Supplying the Neck and Flight Motor of Diptera - Physiological and Anatomical Characteristics. J Comp Neurol. 1990;4:973–991. doi: 10.1002/cne.903020420. [DOI] [PubMed] [Google Scholar]
- 27.Gronenberg W, Strausfeld NJ. Premotor Descending Neurons Responding Selectively to Local Visual-Stimuli in Flies. J Comp Neurol. 1992;1:87–103. doi: 10.1002/cne.903160108. [DOI] [PubMed] [Google Scholar]
- 28.Strausfeld NJ, Gronenberg W. Descending Neurons Supplying the Neck and Flight Motor of Diptera - Organization and Neuroanatomical Relationships with Visual Pathways. J Comp Neurol. 1990;4:954–972. doi: 10.1002/cne.903020419. [DOI] [PubMed] [Google Scholar]
- 29.Nolen T, Hoy R. Initiation of behavior by single neurons: the role of behavioral context. Science. 1984;4677:992–994. doi: 10.1126/science.6505681. [DOI] [PubMed] [Google Scholar]
- 30.Hsu CT, Bhandawat V. Organization of descending neurons in Drosophila melanogaster. Scientific Reports. 2016;6:20259. doi: 10.1038/srep20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal Control of Drosophila Walking Direction. Science. 2014;6179:97–101. doi: 10.1126/science.1249964. [DOI] [PubMed] [Google Scholar]
- 32.Kupfermann I, Weiss KR. The command neuron concept. Behav Brain Sci. 1978;01:3–10. [Google Scholar]
- 33.Roberts WM, Augustine SB, Lawton KJ, Lindsay TH, Thiele TR, Izquierdo EJ, Faumont S, Lindsay RA, Britton MC, Pokala N, et al. A stochastic neuronal model predicts random search behaviors at multiple spatial scales in C. elegans. eLife. 2016:e12572. doi: 10.7554/eLife.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piggott B, Liu J, Feng Z, Wescott S, Xu XZ. The Neural Circuits and Synaptic Mechanisms Underlying Motor Initiation in C. elegans. Cell. 2011;4:922–933. doi: 10.1016/j.cell.2011.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olberg RM. Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol. 1983;3:297–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


