Abstract
In a September 2018 paper published in Nature Communications, Gong et al. identified the domains through which human PMCA1 and neuroplastin (NPTN) interact. Upon binding, hPMCA1 TM domains separate T110 in TM1 and A370 in TM3 to reveal the Ca2+-binding site. Thus, NPTN is able to directly modulate the accessibility of cytosolic Ca2+ to PMCA.
Graphical Abstract
In recent years, our understanding of the regulation of Ca2+ clearance has expanded beyond calmodulin binding and multimerization to the concept that native PMCAs act as part of a complex requiring other modulators to function in a physiological context. Recent investigations have revealed the extracellular immunoglobulin superfamily members neuroplastin (NPTN) and close paralog basigin (BASI) as key regulators of PMCA complexes. Supporting this, double knockout studies of NPTN and BASI demonstrated decreased Ca2+ clearance1. In a paper by Gong et al. published in Nature Communications in September 2018, a new structure of human PMCA1d (hPMCA1) in complex with the recently identified regulator neuroplastin is presented2. Utilizing cryo-electron microscopy, Gong et al. resolved hPMPCA1/NPTN to 4.1 Å. This revealed that hPMCA1 and NPTN primarily interact via the NPTN TM and hPMCA1 TM10, which is distal from TM1–9, with additional stabilizing interactions along the TM8–9 linker. Additionally, NPTN binding alters PMCA conformation. As hPMCA1 TM7 and TM10 rotate toward NPTN TM, TM1 and TM4 are moved toward TM2, exposing the hPMCA1 cytosolic Ca2+ binding site. Thus, while NPTN interactions may be limited to hPMCA1 TM10, NPTN binding to hPMCA1 induces major structural changes throughout the hPMCA1 TM domain that ultimately affect Ca2+ binding. Finally, hPMCA1 was found in these studies to engage in a gate-locking mechanism between residues T110 in TM1 and A370 in TM3 to control access to the Ca2+ binding site. The studies by Gong et al. not only offer valuable structural information to NPTN/PMCA interactions, but also reveal a novel binding pattern between a P-type ATPase and a partner protein. Furthermore, the degree of homology between NPTN and BASI open the possibility that these structural findings could extend to molecular modeling to predict how BASI interacts with PMCA.
The differences in hPMCA1 and hPMCA1-NPTN raise the question of what differences may exist between purified PMCA structure and in vivo conformation in the presence of endogenous PMCA modulators. The data presented by Gong et al. show purified hPMCA1 required NPTN for ATPase activity in vitro, supporting a model in which PMCA activity is NPTN-dependent2. Data from an in vivo study by Korthals et al. show NPTN co-localized with both PMCA1 and PMCA4 in T cells, suggesting that NPTN may modulate multiple PMCA isoforms in other tissue types3. This extends to evidence of a physiological role for NPTN-mediated regulation, as T cells from Nptn−/− mice displayed elevated resting Ca2+ and Th1 bias. Notably, although Nptn−/− T cells exhibit an elevated baseline and diminished Ca2+ clearance, PMCA expression was downregulated over 70%. Thus, how much NPTN modulates Ca2+ signals by regulating PMCA expression versus as a direct binding partner that induces a conformational change in PMCA remains to be defined. Additionally, PMCA2 expression restores partial Ca2+ clearance, suggesting not every isoform is dependent on the same regulatory partners, and some may not require partners for Ca2+ transporting activity. The host of proteins involved in the regulation of PMCAs may prove as multi-faceted as PMCA splice variants themselves, demonstrating the continued importance of studies such as by Gong et al. to define how physical interactions with regulatory proteins control PMCA conformation and function. Previous investigations by us and others have revealed regulation of PMCA activity by the ER membrane proteins STIM1 and POST4,5. The extent to which these phenomena are co-dependent has not been assessed, however, it is tempting to speculate that changes in PMCA function due to association with proteins like STIM1 or POST might result from changes in PMCA/neuroplastin association, a concept presented in figure 1.
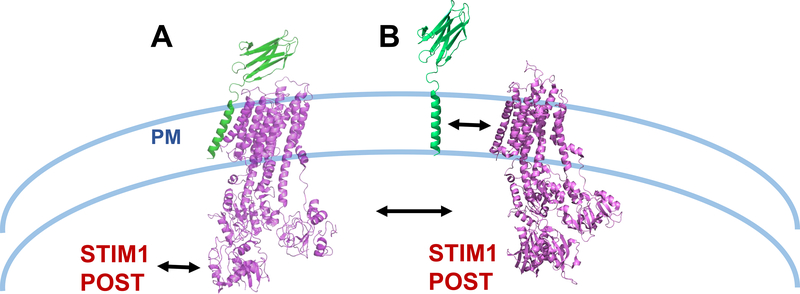
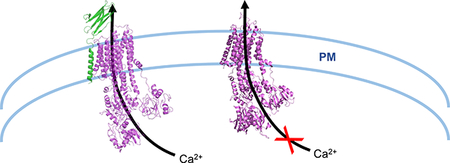
Figure 1. Regulation of PMCA by protein interactions.
(A) As shown in Gong et al (2018)2, PMCA directly associates with neuroplastin via its TM10 domain (PDB: 6A69). STIM1 has also been shown to associate with STIM15 and POST4. (B) The possibility that association between STIM1 and/or POST with PMCA (PDB: 3W5C) may negatively regulate its association with neuroplastin (modified PDB: 6A69) is presented.
NPTN was first defined in neurons, which express different PMCA isoforms between different neuronal populations (reviewed in 6). In the adult rat brain, for example, PMCA1, although ubiquitously expressed, is enriched in the cortex and hippocampus, PMCA2/3 in the cerebellum and forebrain, and PMCA4 in the cortex7. While PMCA2 may be less reliant on NPTN, this could vary across PMCA isoform and therefore across cell type. Therefore, the ability of NPTN to alter basal Ca2+ could have important ramifications for understanding neuronal excitotoxicity, synaptic function, and neurological disease8. The clinical importance of BASI as a marker of resistance and potential therapeutic target in a variety of cancers also continues to gain momentum (reviewed in 9). Others have forayed into defining mechanisms of how BASI contributes to invasiveness, namely through modulation of glycolysis and matrix metalloproteinase expression by tumor cells and stromal cells10,11. Mechanistic studies into whether regulation of PMCA contributes to these processes will be valuable for understanding how BASI/NPTN-mediated signaling may be targeted. Ultimately, future studies may prove critical to define how physical associations between PMCA isoforms and its regulators control PMCA function to integrate control of Ca2+ clearance with other aspects of Ca2+ signal generation.
References
- 1.Schmidt N et al. Neuroplastin and Basigin Are Essential Auxiliary Subunits of Plasma Membrane Ca(2+)-ATPases and Key Regulators of Ca(2+) Clearance. Neuron 96, 827–838.e829, doi: 10.1016/j.neuron.2017.09.038 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Gong D et al. Structure of the human plasma membrane Ca(2+)-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat Commun 9, 3623, doi: 10.1038/s41467-018-06075-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korthals M et al. A complex of Neuroplastin and Plasma Membrane Ca(2+) ATPase controls T cell activation. Scientific reports 7, 8358, doi: 10.1038/s41598-017-08519-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y & Clapham DE POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Natl Acad Sci U S A 108, 19234–19239, doi:1117231108 [pii] 10.1073/pnas.1117231108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritchie MF, Samakai E & Soboloff J STIM1 is required for attenuation of PMCA-mediated Ca2+ clearance during T-cell activation. EMBO J 31, 1123–1133, doi: 10.1038/emboj.2011.495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krebs J The plethora of PMCA isoforms: Alternative splicing and differential expression. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1853, 2018–2024, doi: 10.1016/j.bbamcr.2014.12.020 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Burette A, Rockwood JM, Strehler EE & Weinberg RJ Isoform-specific distribution of the plasma membrane Ca2+ ATPase in the rat brain. 467, 464–476, doi: 10.1002/cne.10933 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Strehler EE & Thayer SA Evidence for a role of plasma membrane calcium pumps in neurodegenerative disease: Recent developments. Neuroscience Letters 663, 39–47, doi: 10.1016/j.neulet.2017.08.035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin X et al. CD147/EMMPRIN overexpression and prognosis in cancer: A systematic review and meta-analysis. Scientific reports 6, doi: 10.1038/srep32804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang P et al. RNA interference targeting CD147 inhibits the proliferation, invasiveness, and metastatic activity of thyroid carcinoma cells by down-regulating glycolysis. International Journal of Clinical and Experimental Pathology 8, 309–318 (2015). [PMC free article] [PubMed] [Google Scholar]
- 11.Grass GD & Toole BP How, with whom and when: an overview of CD147-mediated regulatory networks influencing matrix metalloproteinase activity. Bioscience Reports 36, doi: 10.1042/bsr20150256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]