Abstract
Objective:
To assess whether body mass index (BMI) provides a better assessment of measured adiposity at age 1 month than does weight-for-length (WFL).
Study design:
Participants were healthy, term infants in the Infant Growth and Microbiome (n=146) and the Baby Peas (n=147) studies. Length, weight, and body composition by air displacement plethysmography were measured at 1 month. WHO-based WFL and BMI Z-scores were calculated. Within-cohort Z-scores of percent fat (PF-Z), fat mass (FM-Z), fat mass/length2 (FM/L2-Z), fat mass/length3 (FM/L3-Z), fat-free mass (FFM-Z), and fat-free mass/length2 (FFM/L2-Z) were calculated. Correlation and multiple linear regression (adjusted for birthweight) analyses tested the associations between body composition outcomes and BMI-Z vs. WFL-Z. Quantile regression tested the stability of these associations across the distribution of body composition.
Results:
The sample was 52% female and 56% African American. Accounting for birthweight, both BMI-Z and WFL-Z were strongly associated with FM-Z (coefficients 0.56 and 0.35, respectively), FM/L2-Z (0.73, 0.51), and FM/L3-Z (0.79, 0.58), with stronger associations for BMI-Z versus WFL-Z (P < .05). Even after accounting statistically for birthweight, BMI-Z was persistently more strongly associated than WFL-Z with body composition outcomes across the distribution of body composition outcomes.
Conclusions:
We demonstrate in 2 distinct cohorts that BMI is a better indicator of adiposity in early infancy than WFL. These findings support the preferred use of BMI for growth and nutritional status assessment in infancy.
Keywords: Infant, Infancy, Adiposity, Obesity, Air displacement plethysmography
Rapid weight gain during early infancy is related to later obesity risk.1–4 However, it is unclear whether gains in fat mass (versus lean mass) in early infancy represent the salient component of this risk factor. Human infants increase in adiposity during the first year of life, reaching a peak around 6 to 9 months of age.5 The deposition of adipose tissue is thought to be protective, insulating the body from temperature extremes and providing energy reserves. However, with 8% of U.S. infants and toddlers thought to have excess weight, and an obesity prevalence of 17% among U.S. children and adolescents6, there is increasing interest in body composition changes in early life, as this time may represent an opportunity for early targeted interventions in populations at high risk for future obesity.7 There is no widely accepted definition for infant “obesity”6, and body composition assessment in infants is challenging. However, studies have shown that air displacement plethysmography provides accurate and validated assessments of body fat in early infancy.8–11
Routine measurement of body composition in the clinical setting is not feasible, so it is useful to identify the anthropometric measurement(s) that provides the most accurate proxy for body composition. The World Health Organization (WHO) released body mass index (BMI)-for-age growth charts for children below the age of two years in 200612, but these charts have not been adopted for routine general pediatric use. Although BMI is the anthropometric standard recommended by the American Academy of Pediatrics for assessment of weight status in children over the age of two years, weight-for-length (WFL) is recommended for children younger than 2 years of age.13 We previously showed that BMI-Z in infancy had a significantly higher positive predictive value for early childhood obesity than did WFL-Z.14 The association of these two measures with body composition during infancy is unknown. The aim of this study was to investigate whether BMI-Z or WFL-Z at age 1 month provides a better assessment of body composition by air displacement plethysmography using two independent cohorts.
METHODS
The study sample included healthy, term (≥37 weeks’ gestational age) infants with simultaneous measurement of length, weight, and body composition by air displacement plethysmography at age 1 month ±14 days. Subjects were enrolled in the Infant Growth and Microbiome Study (IGRAM) between 2014–2015 at The Children’s Hospital of Philadelphia (CHOP) or the Baby Peas Study during 2003–2009 at the Oklahoma University Health Sciences Center (OUHSC). Both studies were performed in accordance with the policies and procedures of the institutional review boards of the respective institutions.
IGRAM is a prospective, longitudinal cohort study of infant growth in the first two years of life in babies born to African-American mothers. Inclusion criteria included otherwise healthy African-American mothers (≥ 18 years of age) planning to deliver at the Hospital of the University of Pennsylvania who attended prenatal visits starting before 18 weeks gestation, had pre-pregnancy BMI <25 kg/m2 or >30 kg/m2, and had pregnancies that delivered at term without any maternal or fetal adverse outcomes. Infants were excluded if they were born preterm (<37 weeks), of twin/other multiples status, or if the infant was discovered to have a chromosomal anomaly, intrauterine growth restriction, a significant illness affecting growth and development, or a sibling enrolled in the study.
Baby Peas is a collection of prospective longitudinal growth studies investigating various endpoints in infancy and early life. These studies had the following inclusion criteria: maternal age between 18 – 45 years at the time of delivery; term pregnancy lasting ≥ 37 weeks; singleton birth; and a hospital stay for the infant of less than 3 days following delivery. Exclusion criteria for both included: tobacco use or alcohol consumption (>1 drink per week) during pregnancy; pre-gestational or gestational diabetes; and infants with presumed or known congenital birth defects.
Sex and gestational age were obtained, and exact age at the 1-month visit (days) was calculated. Weight (kg) and length (cm) using length boards were obtained on all subjects using standard procedures15 by trained anthropometrists, and body mass index (BMI, kg/m2) was calculated. Body composition (percent fat (%), fat mass (kg), and fat-free mass (kg)) was determined by air displacement plethysmography (Pea Pod Infant Body Composition System, Cosmed., Concord, California), following manufacturer recommended procedures. In order to express these body tissue compartments relative to skeletal size, indices of fat mass and fat free mass were calculated.16 Fat mass was adjusted for length using length-squared and length-cubed indices: fat mass/length2 and fat mass/length3, respectively. The latter was thought to be the optimal index of fat mass independent of length in a cohort of Irish infants at birth and at 2 months of age.16 Fat-free mass index was calculated using fat-free mass/length2, which has previously been posited to be the optimal index of correction of fat-free mass for length in this age group.16
World Health Organization (WHO) weight-for-length (WFL) Z-scores (WFL-Z) and BMI Zscores (BMI-Z) were calculated using “zanthro” commands in Stata 14.0 (Stata Corporation, College Station, TX), based on published references.17 WFL-Z and BMI-Z were each calculated using sex-specific values. BMI-Z was additionally adjusted for gestational age as the WHO BMI references include this capacity, even among infants with GA ≥37 weeks.
Body composition in early infancy changes rapidly and is significantly associated with age and sex.18 Accordingly, linear regression models were used to adjust all body composition measures for age and sex. The standardized regression residuals were used to calculate Z-scores (percent fat-Z, fat mass-Z, fat mass/length2-Z, fat mass/length3-Z, fat-free mass-Z, and fat-free mass/length2-Z) for infants in the combined IGRAM and Baby Peas cohorts.
Population ancestry (self-reported), birthweight, and birth length were obtained in all subjects where available. Population ancestry was categorized as European, African-American, and other, because the majority of subjects in the Baby Peas cohort were European and all subjects in the IGRAM study, by design, were African-American. Given the significant difference in population ancestry between cohorts, a sensitivity analysis was performed adjusting each body composition variable for cohort (IGRAM vs. Baby Peas) and ancestry in addition to age and sex. Z-scores for birthweight (birthweight-Z) and birth length (birth length-Z) were calculated using the INTERGROWTH-21st newborn size application tool, which included adjustment for sex, and gestational age.19
Statistical analyses
Bivariate analyses (2-sided t-tests and Chi-squared analyses, as appropriate) were used to assess for differences in clinical characteristics between the two cohorts. Pearson correlation analysis was performed to investigate the association between each body composition variable Z-score and BMI-Z or WFL-Z. In order to determine whether a stronger correlation existed with either BMI-Z or WFL-Z and each body composition variable, Fisher r-to-z transformation was used to test for significant differences between the two correlation coefficients using the STATA command “corcor” and the R package “cocor.”20 Sensitivity analyses were performed using body composition Z-scores that were additionally adjusted for age, sex, and cohort along with age, sex, and population ancestry. In order to examine expected associations between size at birth and later body composition and growth,21 Pearson correlation analysis tested the association between birthweight-Z and BMI-Z or WFL-Z at age 1 month, and Fisher r-to-z transformation was used to test for significant differences between the 2 correlation coefficients.
Multivariable linear regression analyses were then performed to test the association of BMI-Z or WFL-Z at age 1 month with each body composition variable Z-score, independent of birthweight-Z. The adjustment for birthweight-Z was done in order to understand the extent to which body composition at age 1 month is simply a reflection of birth size. Quantile regression analysis22, 23 was used to test the association of BMI-Z or WFL-Z at the median and at percentiles above and below the median, of each body composition variable Z-score, independent of birthweight-Z. This analysis was done to understand how BMI-Z and WFL-Z vary across the distribution of each body composition variable Z-score. Post-estimation linear combinations of estimators were performed to examine the effect of the 10th vs the 90th percentile of BMI-Z or WFL-Z at age 1 month on each body composition variable Z-score.
Analyses were performed using STATA version 14.0 and R version 3.0.0 statistical software. For all analyses, two-tailed statistical significance was noted as p-value less than 0.05.
RESULTS
The combined sample consisted of 293 subjects, of whom 146 were from the IGRAM cohort and 147 from the Baby Peas cohort (Table 1). The sample was 52% female, with 56% African-American and 33% European ancestry, and age 30.1±5.8 days. There was a difference in ancestry between the two cohorts: 100% of the IGRAM subjects were African-American, compared with 12% in Baby Peas. IGRAM infants had significantly lower birthweight, birthweight-Z, birth length, and birth length-Z, consistent with previous reports among African-American infants (Table 1).24, 25
Table 1.
Characteristics of the sample.
| Combined Cohort (n=293) | I-GRAM Cohort (n=146) | Baby Peas Cohort (n=147) | Cohort difference (pvalue) | |
|---|---|---|---|---|
| Sample Characteristics at Birth | ||||
| Sex Male Female |
141 (48%) 152 (52%) |
68 (47%) 78 (53%) |
73 (50%) 74 (50%) |
p=0.60 |
| Ancestry European African- American Other |
(n=291) 95 (33%) 164 (56%) 32 (11%) |
0 (0%) 146 (100%) 0 (0%) |
95 (65%) 18 (12%) 32 (22%) |
p<0.001 |
| Gestational age (weeks) | 39.3 (1.1) (n=287) | 39.4 (1.1) | 39.3 (1.1) | p=0.53 |
| Birthweight (kg) | 3.3 (0.4) (n=287) | 3.2 (0.4) | 3.4 (0.4) (n=141) | p<0.001 |
| Birthweight Z-scorea | 0.21 (0.98) (n=286) | −0.11 (0.92) | 0.54 (0.93) (n=140) | p<0.001 |
| Birth length (cm) | 49.8 (2.5) (n=286) | 49.0 (0.02) | 50.6 (0.02) (n=140) | p<0.001 |
| Birth length Z-scorea | 0.41 (1.30) (n=286) | −0.04 (1.12) | 0.88 (1.31) (n=140) | p<0.001 |
| Sample Characteristics at Age 1 Month | ||||
| Age (days) | 30.1 (5.8) | 31.4 (3.3) | 28.7 (7.2) | p<0.001 |
| Weight (kg) | 4.3 (0.6) | 4.3 (0.5) | 4.3 (0.6) | p=0.98 |
| Length (cm) | 53.7 (2.3) | 53.3 (2.1) | 54.0 (2.4) | p=0.005 |
| BMI (kg/m2) | 14.7 (1.3) | 14.9 (1.2) | 14.5 (1.4) | p=0.006 |
| BMI-Z | 0.24 (0.9) | 0.33 (0.9) | 0.17 (1.0) | p=0.15 |
| WFL-Z | 0.1 (1.1) | 0.3 (1.0) | −0.2 (1.2) | p<0.001 |
| Body Composition at Age 1 Month | ||||
| Percent Fat (%) | 18.7 (4.4) | 19.3 (3.9) | 18.1 (4.8) | p=0.018 |
| Fat Mass (kg) | 0.80 (0.3) | 0.83 (0.2) | 0.78 (0.3) | p=0.085 |
| Fat Mass/Length2 (kg/m2) | 2.77 (0.8) | 2.91 (0.7) | 2.64 (0.9) | p=0.005 |
| Fat Mass/Length3 (kg/m3) | 5.17 (1.50) | 5.46 (1.31) | 4.88 (1.63) | p=0.001 |
| Fat-Free Mass (kg) | 3.44 (0.4) | 3.42 (0.4) | 3.46 (0.4) | p=0.386 |
| Fat-Free Mass/ Length2 | 11.94 (0.9) | 12.03 (0.8) | 11.84 (0.9) | p=0.068 |
Outcomes are summarized as mean (SD) for continuous variables or n (%) for categorical variables. P-value indicates difference between IGRAM and Baby Peas cohorts (2-sided t-test for continuous variables and chi-squared test for categorical variables). Birthweight and birth length Z-scores account for sex and gestational age. BMI Z-score accounts for age, sex, and gestational age. WFL Z-score accounts for age and sex. The number of participants with available measurement is as shown if different for the total number in the cohort.
IGRAM infants had significantly higher 1-month BMI than Baby Peas infants (14.9 kg/m2 vs. 14.5 kg/m2, p=0.006). This reflected their significantly shorter length (53.3 cm vs. 54 cm, p=0.005) despite having the same mean weight as Baby Peas infants (Table 1). IGRAM subjects had significantly higher percent fat (19.3% vs. 18.1%, p=0.018), fat mass/length2 (2.91 kg/m2 vs. 2.64 kg/m2, p=0.005), and fat mass/length3 (5.46 kg/m2 vs. 4.88 kg/m2, p=0.001) but no significant differences in fat mass, fat-free mass, or fat-free mass/length2 compared with Baby Peas infants. Of note, the mean age at one month was significantly higher in the IGRAM infants compared with Baby Peas infants (31.4 vs 28.7 days, p<0.001).
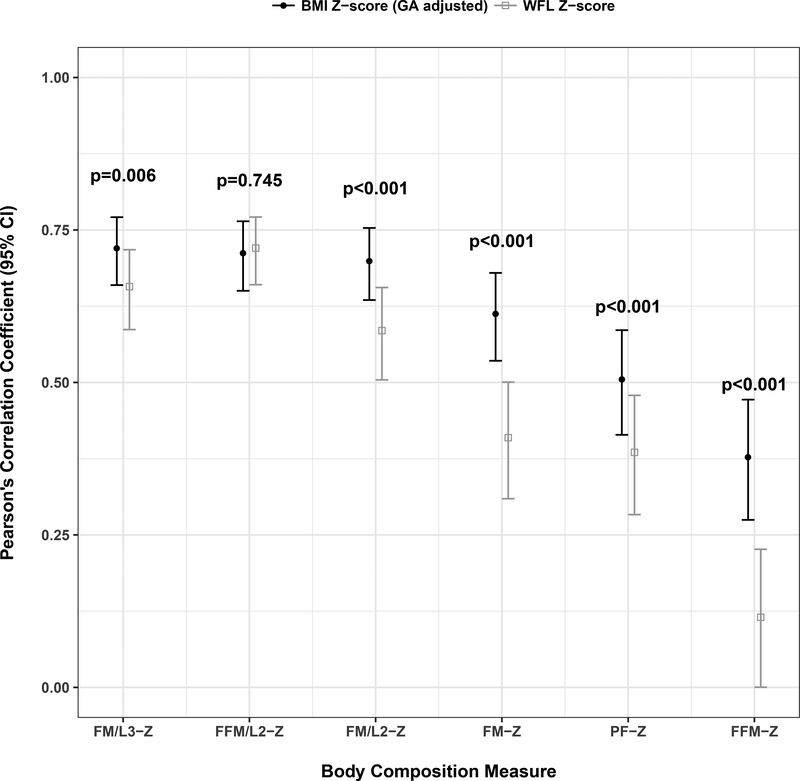
BMI-Z was more strongly correlated than WFL-Z with fat mass-Z (r=0.61 vs. 0.41, p<0.001), fat mass/length2-Z (r=0.70 vs. 0.59, p<0.001), fat mass/length3-Z (r=0.72 vs 0.66, p=0.006), and fat-free mass-Z (r=0.38 vs. 0.11, p<0.001) (Figure 1). There was no significant difference in correlation of fat-free mass/length2-Z (r=0.71 vs. 0.72, p=0.745) with either BMI-Z or WFL-Z. Cohort-stratified analyses demonstrated similar results to that noted in the combined cohort. There were no significant differences in findings with additional adjustment for cohort or ancestry (data not shown); thus, cohort and ancestry were not included in subsequent models.
Figure 1.
Correlation between body composition and BMI-Z vs. WFL-Z at age 1 month. By Pearson correlations, at age 1 month, BMI-Z was more strongly correlated than WFL-Z with fat mass-Z (r=0.62 vs. 0.41, p<0.001), fat mass/length2-Z (r=0.70 vs. 0.59, p=0.022) and fat-free mass-Z (r=0.38 vs. 0.11, p<0.001) after adjusting for age and sex.
Birthweight-Z was more strongly correlated with BMI-Z than with WFL-Z (r=0.36 vs. 0.05, p<0.001), implying that BMI-Z contains more information about weight at birth than does WFL-Z. Similar results were noted in cohort-stratified analyses (data not shown).
The independent association of BMI-Z vs. WFL-Z with each body composition Z-score was investigated, accounting for birthweight-Z (Table 2). For all fat mass and fat-free mass body composition measures, each 1-unit change in BMI-Z (vs. WFL-Z) was independently associated with a greater increase in body composition Z-score; e.g., for fat mass/length3-Z, the coefficient for BMI-Z was 0.79 (95% CI 0.70 – 0.89) vs. for WFL-Z, 0.58 (0.50 – 0.66). (Table 2). Notably, the association of birthweight-Z with each body composition Z-score was higher in all models including WFL-Z (vs. BMI-Z), consistent with previously described results, again demonstrating that BMI-Z intrinsically incorporates more information about birthweight than does WFL-Z. Similar results were noted in cohort-stratified analyses (Table 2).
Table 2.
Association between BMI-Z and WFL-Z at 1 month of age and body composition at 1 month of age, accounting for birthweight-Z.
| Percent Fat-Z | Fat Mass-Z | Fat Mass/Length2Z | Fat Mass/Length3Z | Fat-Free Mass-Z | Fat-Free Mass/ Length2-Z | |
|---|---|---|---|---|---|---|
| Combined Cohort (n=285) | ||||||
|
BMI-Z BW-Z R2 |
0.53 (0.42 – 0.65)*** 0.01 (−0.10 – 0.12) 0.25 |
0.56 (0.46 – 0.66)*** 0.25 (0.15 – 0.35)*** |
0.73 (0.63 – 0.83)*** 0.05 (−0.04 – 0.14) 0.49 |
0.79 (0.70–0.89)*** −0.06 (−0.15–0.03) 0.52 |
0.19 (0.09 – 0.29)*** 0.58 (0.48 – 0.68)*** |
0.74 (0.65 – 0.83)*** 0.04 (−0.05 – 0.13) 0.50 |
|
WFL-Z BW-Z R2 |
0.33 (0.23 – 0.43)*** 0.18 (0.08 – 0.29)** |
0.35 (0.26 – 0.43)*** 0.43 (0.33 – 0.53)*** |
0.51 (0.43 – 0.59)*** 0.28 (0.18 – 0.37)*** |
0.58 (0.50 – 0.66)*** 0.19 (0.10 – 0.28)*** |
0.08 (0.00 – 0.16)# 0.63 (0.53 – 0.72)*** 0.38 |
0.64 (0.57 – 0.71)*** 0.25 (0.17 – 0.33)*** 0.58 |
| IGRAM Cohort (n=146) | ||||||
|
BMI-Z BW-Z R2 |
0.53 (0.37 – 0.69)*** 0.10 (−0.05 – 0.25) 0.28 |
0.57 (0.44 – 0.70)*** 0.31 (0.19 – 0.43)*** |
0.73 (0.60 – 0.86)*** 0.12 (0.00 – 0.24)# 0.53 |
0.78 (0.66 – 0.91)*** 0.01 (−0.10 – 0.13) 0.55 |
0.24 (0.09 – 0.39)** 0.58 (0.44 – 0.72)*** |
0.75 (0.61 – 0.89)*** 0.04 (−0.09 – 0.17) 0.47 |
|
WFL-Z BW-Z R2 |
0.35 (0.21 – 0.48)*** 0.25 (0.10 – 0.39)* 0.20 |
0.37 (0.25 – 0.49)*** 0.47 (0.34 – 0.59)*** |
0.53 (0.42 – 0.64)*** 0.32 (0.20 – 0.44)*** |
0.59 (0.48 – 0.70)*** 0.23 (0.11 – 0.35)*** |
0.16 (0.03 – 0.28)* 0.65 (0.51 – 0.78)*** 0.40 |
0.70 (0.60 – 0.80)*** 0.24 (0.13 – 0.35)*** 0.59 |
| Baby Peas Cohort (n=139) | ||||||
|
BMI-Z BW-Z R2 |
0.56 (0.38 – 0.75)*** −0.10 (−0.30 – 0.10) 0.23 |
0.56 (0.39 – 0.73)*** 0.20 (0.02 – 0.38)* 0.37 |
0.74 (0.58 – 0.89)*** −0.01 (−0.18 – 0.16) 0.45 |
0.81 (0.66 – 0.96)*** −0.13 (−0.29 – 0.04) 0.49 |
0.11 (−0.04 – 0.27) 0.64 (0.48 – 0.81)*** 0.40 |
0.69 (0.55 – 0.83)*** 0.12 (−0.03 – 0.27) 0.52 |
|
WFL-Z BW-Z R2 |
0.33 (0.18 – 0.48)*** 0.12 (−0.07 – 0.30) 0.14 |
0.32 (0.19 – 0.46)*** 0.41 (0.23 – 0.58)*** |
0.50 (0.37 – 0.63)*** 0.24 (0.08 – 0.40)** |
0.58 (0.46 – 0.70)*** 0.14 (−0.01 – 0.29)# 0.43 |
−0.02 (−0.14 – 0.10) 0.69 (0.54 – 0.84)*** 0.38 |
0.57 (0.47 – 0.67)*** 0.30 (0.17 – 0.43)*** 0.56 |
Multivariable linear regression analysis performed to investigate the association between BMI-Z (accounting for age, sex, and gestational age or WFL-Z (account for age and sex) at one month of age on each body composition parameter Z-score (accounting for for age and sex) at one month of age, accounting statistically for birthweight-Z (BW-Z). Results are shown as coefficient (95% CI). Statistical significance is indicated by
p<0.001,
p<0.01,
p<0.05,
p<0.1; results are in bold text if p<0.05.
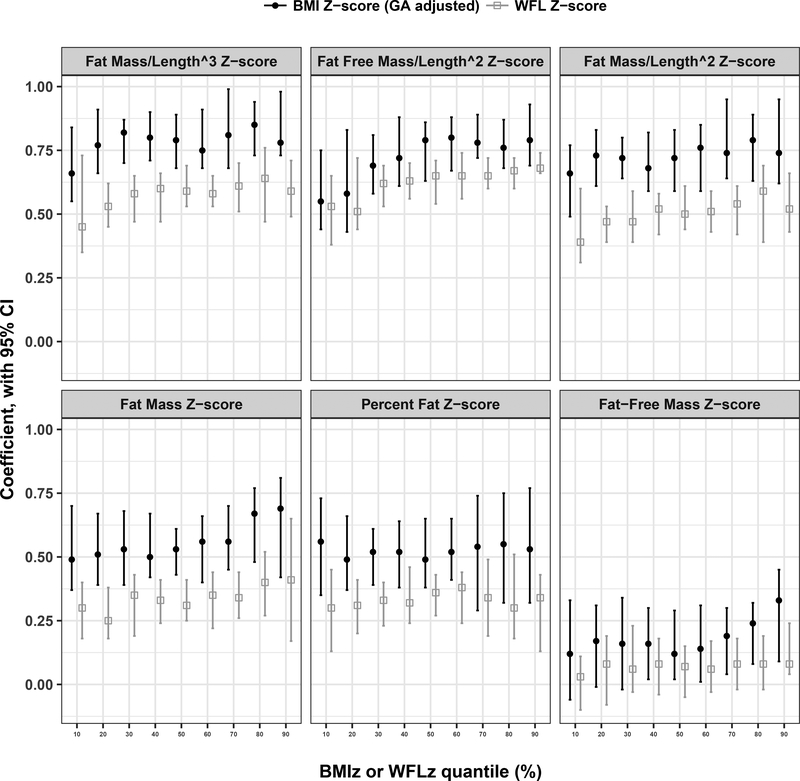
After accounting for birthweight-Z, the associations between BMI-Z and indices of body composition (percent fat-Z, fat mass-Z, fat mass/length2-Z, fat mass/length3-Z, and fat-free mass/length2-Z) were consistently stronger than the associations between WFL-Z and these same indices across the entire distribution of each body composition measure. However, there was no association between either BMI-Z or WFL-Z with fat-free mass Z except a modest positive association with BMI-Z at the highest end of the distribution (Figure 2; Table 3 [available at www.jpeds.com]).
Figure 2.
Variation in BMI-Z and WFL-Z across body composition distributions at age 1 month by quantile regression analysis. Quantile regression analyses assessed for consistency in the association of BMI-Z (blue circles) vs. WFL-Z (red circles) across the distribution of each body composition parameter. Post-estimation linear combination of estimators evaluated the stability of BMI-Z vs. WFL-Z at the tails of each parameter.
Table 3.
Quantile regression analysis of how BMI-Z and WFL-Z vary across the distribution of body composition.
| Quan tile | 0.10 | 0.20 | 0.30 | 0.40 | 0.50 | 0.60 | 0.70 | 0.80 | 0.90 | P-value 0.10 vs 0.90 |
|---|---|---|---|---|---|---|---|---|---|---|
| Percent Fat-Z | ||||||||||
| 0.56*** | 0.49*** | 0.52*** | 0.52*** | 0.49*** | 0.52*** | 0.54*** | 0.55*** | 0.53*** | 0.23 | |
| BM I-Z | (0.35 – | (0.37 – | (0.39 – | (0.38 – | (0.38 – | (0.41 – | (0.29 – | (0.32 – | (0.32 – | |
| 0.73) | 0.66) | 0.61) | 0.64) | 0.65) | 0.65) | 0.74) | 0.75) | 0.77) | ||
| 0.3*** | 0.31*** | 0.33*** | 0.32*** | 0.36*** | 0.38*** | 0.34*** | 0.3** | 0.34*** | 0.65 | |
| WF | (0.13 – | (0.2 – | (0.23 – | (0.24 – | (0.27 – | (0.24 – | (0.19 – | (0.18 – | (0.13 – | |
| L-Z | 0.45) | 0.41) | 0.4) | 0.46) | 0.43) | 0.44) | 0.49) | 0.51) | 0.43) | |
| Fat Mass-Z | ||||||||||
| 0.49*** | 0.51*** | 0.53*** | 0.5*** | 0.53*** | 0.56*** | 0.56*** | 0.67*** | 0.69*** | 0.06 | |
| BM | (0.37 – | (0.39 – | (0.39 – | (0.42 – | (0.43 – | (0.4 – | (0.45 – | (0.48 – | (0.42 – | |
| I-Z | 0.7) | 0.67) | 0.68) | 0.67) | 0.61) | 0.66) | 0.7) | 0.77) | 0.81) | |
| 0.3*** | 0.25*** | 0.35*** | 0.33*** | 0.31*** | 0.35*** | 0.34*** | 0.4*** | 0.41*** | 0.16 | |
| WF | (0.18 – | (0.18 – | (0.19 – | (0.24 – | (0.25 – | (0.22 – | (0.26 – | (0.27 – | (0.17 – | |
| L-Z | 0.4) | 0.38) | 0.43) | 0.41) | 0.41) | 0.44) | 0.44) | 0.52) | 0.65) | |
| Fat Mass/Length2-Z | ||||||||||
| 0.66*** | 0.73*** | 0.72*** | 0.68*** | 0.72*** | 0.76*** | 0.74*** | 0.79*** | 0.74*** | 0.06 | |
| BM | (0.49 – | (0.61 – | (0.64 – | (0.59 – | (0.59 – | (0.59 – | (0.64 – | (0.63 – | (0.62 – | |
| I-Z | 0.77) | 0.83) | 0.8) | 0.82) | 0.83) | 0.85) | 0.95) | 0.89) | 0.95) | |
| 0.39*** | 0.47*** | 0.47*** | 0.52*** | 0.5*** | 0.51*** | 0.54*** | 0.59*** | 0.52*** | 0.16 | |
| WF | (0.31 – | (0.39 – | (0.39 – | (0.42 – | (0.44 – | (0.43 – | (0.42 – | (0.39 – | (0.43 – | |
| L-Z | 0.6) | 0.53) | 0.59) | 0.58) | 0.61) | 0.59) | 0.61) | 0.69) | 0.66) | |
| Fat Mass/Length3-Z | ||||||||||
| 0.66*** | 0.77*** | 0.82*** | 0.8*** | 0.79*** | 0.75*** | 0.81*** | 0.85*** | 0.78*** | 0.04 | |
| BM | (0.55 – | (0.66 – | (0.7 – | (0.71 – | (0.68 – | (0.68 – | (0.68 – | (0.73 – | (0.73 – | |
| I-Z | 0.84) | 0.91) | 0.87) | 0.9) | 0.89) | 0.91) | 0.99) | 0.94) | 0.98) | |
| 0.45*** | 0.53*** | 0.58*** | 0.6*** | 0.59*** | 0.58*** | 0.61*** | 0.64*** | 0.59*** | 0.30 | |
| WF | (0.35 – | (0.45 – | (0.47 – | (0.47 – | (0.53 – | (0.53 – | (0.51 – | (0.47 – | (0.49 – | |
| L-Z | 0.73) | 0.62) | 0.65) | 0.66) | 0.69) | 0.65) | 0.7) | 0.76) | 0.71) | |
| Fat-Free Mass-Z | ||||||||||
| 0.12 (– | 0.17* (– | 0.16 (– | 0.16* | 0.12 | 0.14* | 0.19** | 0.24** | 0.33** | 0.03 | |
| BM | 0.06 – | 0.01 – | 0.02 – | (0.02 – | (0.02 – | (0.01 – | (0.04 – | (0.08 – | (0.09 – | |
| I-Z | 0.33) | 0.31) | 0.34) | 0.3) | 0.29) | 0.31) | 0.3) | 0.32) | 0.45) | |
| 0.03 (−0.1 | 0.08 (– | 0.06 (– | 0.08 (– | 0.07 (– | 0.06 (– | 0.08 (– | 0.08 (– | 0.08 | 0.008 | |
| WF | −0.11) | 0.08 – | 0.03 – | 0.04 – | 0.05 – | 0.03 – | 0.02 – | 0.02 – | (0.04 – | |
| L-Z | 0.19) | 0.23) | 0.18) | 0.15) | 0.17) | 0.18) | 0.19) | 0.24) | ||
| Fat-Free Mass/Length2-Z | ||||||||||
| 0.55*** | 0.58*** | 0.69*** | 0.72*** | 0.79*** | 0.8*** | 0.78*** | 0.76*** | 0.79*** | 0.03 | |
| BM | (0.44 – | (0.43 – | (0.58 – | (0.61 – | (0.63 – | (0.67 – | (0.72 – | (0.68 – | (0.69 – | |
| I-Z | 0.75) | 0.83) | 0.81) | 0.88) | 0.86) | 0.88) | 0.89) | 0.87) | 0.93) | |
| 0.53*** | 0.51*** | 0.62*** | 0.63*** | 0.65*** | 0.65*** | 0.65*** | 0.67*** | 0.68*** | 0.02 | |
| WF | (0.38 – | (0.44 – | (0.53 – | (0.56 – | (0.54 – | (0.56 – | (0.6 – | (0.6 – | (0.66 – | |
| L-Z | 0.65) | 0.72) | 0.69) | 0.7) | 0.71) | 0.74) | 0.72) | 0.72) | 0.74) | |
From quantile regressions, coefficients and 95% confidence intervals (in parentheses) are shown. Statistical significance is indicated by
p<0.05;
p<0.01;
p<0.001. ANOVA was used compare results from 10th versus 90th quantile regression models (last column).
Post-estimation linear combination of estimators was used to evaluate the stability of BMI-Z vs. WFL-Z at the tails (10th and 90th percentiles) of each body composition variable (Figure 2; Table 3). Overall, both BMI-Z and WFL-Z were modestly more strongly associated with body composition parameters at the upper end of the distribution. (Figure 2; Table 3).
DISCUSSION
Detailed assessments of body composition are not feasible in clinical settings, so it is critical to determine which anthropometric measure is the best indicator of infant adiposity. There is no gold standard method for assessing adiposity in infancy. We used air displacement plethysmography, a widely accepted, non-invasive technique for infant body composition measurement.26 We expressed adiposity in several ways because it is unclear which adiposity measure or index best captures nutritional status in very early infancy. We demonstrate, in two distinct cohorts with different ancestral backgrounds, that BMI is a better indicator of adiposity, as reflected by fat mass and fat mass index, than is WFL in early infancy. Although there is no commonly accepted definition of excess adiposity in children less than 2 years6, fat mass accrual in early infancy is related to later childhood obesity.27 Our study suggests that infants with high BMI, even as early as age 1 month, have higher fat mass. This is important given that most, if not all, clinical settings can feasibly measure BMI. In addition, use of BMI in infancy would provide continuity in assessment of excess adiposity throughout the lifecycle.
Previously, we demonstrated discordance between BMI and WFL in young infants, and BMI at 2 months was the better predictor of high BMI at 2 years.14 WFL is currently the recommended anthropometric measure to assess weight status in children less than two years of age both in the United States and worldwide, although BMI is the measure recommended from two years through adulthood.13, 28 We and others have provided evidence that early infant BMI has a significantly higher positive predictive value for early childhood obesity than does WFL.14, 29, 30 The findings of our current study provide further evidence that BMI provides a more accurate reflection than does WFL of adiposity at age 1 month.
The AAP’s recommendation that WFL be used for nutritional assessment in children under 2 years likely dates back to the development of the National Center for Health Statistics (NCHS) growth charts in 1977.31 The NCHS authors acknowledged that in a normal population of healthy infants of the same length, older infants are likely to weigh more. However, they argued that for children less than age 2 years, the relationship between length and weight is “close enough” to being age-independent, and therefore WFL is a useful indicator of nutritional status, especially when age is not reliably known.31 For over 40 years, WFL has been used for this purpose. The empirically observed association between low WFL and subsequent mortality has been cited to justify its ongoing use.32 Importantly, the association between infant BMI and future health outcomes deserves additional consideration. Recent studies suggest overall good agreement between WFL and BMI and note that BMI may actually be more sensitive in identifying wasting in infants.33 As noted above, early infant BMI is a better predictor of early childhood obesity than WFL.14, 29, 30 Our study further supports that BMI may also have the advantage of being more closely associated with body composition, even after accounting for birth size.
There are two key differences between the BMI and WFL growth charts that might account for some of our findings. First, BMI charts allow for a combined measure of weight and length to be plotted according to age. In contrast, WFL charts plot weight and length, but do not account for age; thus, a short infant could be considered to have high WFL because s/he is being compared with younger infants who have not yet gained as much weight. Secondly, because BMI charts take age into account, BMI can be further adjusted for gestational age, whereas WFL charts cannot make such an adjustment.
It has been reported that in children and in adolescents, BMI is a better measure of fat mass for overweight and obese children than in thin and normal weight children.34–36 Accordingly, we examined the stability of both BMI and WFL across the distribution of body composition outcomes during early infancy. We observed that BMI was more highly associated with indices of fat mass across the distributions of body composition in early infancy than WFL. Overall, both indices demonstrated a modestly stronger association with body composition at the higher end of the distribution.
Limitations of this study include that it is cross-sectional, and the implications of these measures on later health outcomes are not known. Future studies should investigate these same associations throughout infancy and into childhood. Additionally, infant feeding data were not consistently available and thus not considered in this study. Previous studies have evaluated the test characteristics of air displacement plethysmography for measuring body composition in infants, and concluded it had adequate accuracy and reproducibility for assessment of fat mass but may have less reproducibility for lean mass.26 This study was not designed to specifically consider lean body mass, and the extent to which BMI-Z vs. WFL-Z are good indicators of this component of nutritional status in at-risk infants cannot be concluded from this study. Furthermore, because there are no clinical definitions of underweight or obesity in very young infants, it was not possible to determine the sensitivity and specificity of WFL vs BMI. As an alternative, we compared the strength of associations between WFL and BMI with body composition outcomes and used quantile regression techniques to evaluate these associations across the range of body composition outcomes. Quantile regression is a non-parametric method and we may be underpowered to detect additional quantile specific associations.
The results of this study, combined with our previous work showing the association of BMI-Z in infancy with obesity in early childhood, add to the accumulating evidence supporting the preferred use of BMI-for-age for growth and nutritional status assessment in infancy.
ACKNOWLEDGEMENTS
We thank the families that participated in this study for the generous contribution of their time and interest, and to the staff of the Center for Human Phenomic Science of the Children’s Hospital of Philadelphia, the Infant Growth and Microbiome study team, and the Baby Peas study team.
S.R. receives grant support from NIDDK (2T32DK063688) and the Endocrine Society. J.M. received grant support from K01HL123612. S.M. received grant support from NIDDK (5K12DK094723 and 1K23DK102659). B.Z. received grant support from R01 DK107565, UL1TR001878, and The Children’s Hospital of Philadelphia Healthy Weight Program.
Abbreviations:
- AAP
American Academy of Pediatrics
- BMI
Body mass index
- BMI-Z
Body mass index Z-score
- CDC
Centers for Disease Control and Prevention
- CHOP
Children’s Hospital of Philadelphia
- FFM-Z
Fat-free mass Z-score
- FFM/L2-Z
Fat-free mass/length2 Z-score
- FM-Z
Fat mass Z-score
- FM/L2-Z
Fat mass/length2 Z-score
- FM/L3-Z
Fat mass/length3 Z-score
- Height-Z
Height for age Z-score
- IGRAM
Infant Growth and Microbiome Study
- NCHS
National Center for Health Statistics
- OUHSC
Oklahoma University Health Sciences Center
- PF-Z
Percent fat Z-score
- Weight-Z
Weight for age Z-score
- WFL
Weight-for-length
- WFL-Z
Weight-for-Length Z-score
- WHO
World Health Organization
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. The American journal of clinical nutrition. 2003;77(6):1374–1378. [DOI] [PubMed] [Google Scholar]
- 2.Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, et al. Association of weight gain in infancy and early childhood with metabolic risk in young adults. The Journal of clinical endocrinology and metabolism. 2007;92(1):98–103. [DOI] [PubMed] [Google Scholar]
- 3.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta paediatrica (Oslo, Norway : 1992). 2006;95(8):904–908. [DOI] [PubMed] [Google Scholar]
- 4.Woo JG, Sucharew H, Su W, Khoury PR, Daniels SR, Kalkwarf HJ. Infant Weight and Length Growth Trajectories Modeled Using Superimposition by Translation and Rotation Are Differentially Associated with Body Composition Components at 3 and 7 Years of Age. The Journal of pediatrics. 2018;196:182–188.e181. [DOI] [PubMed] [Google Scholar]
- 5.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47(5):578–585. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for Childhood Obesity in the First 1,000 Days A Systematic Review. American journal of preventive medicine. 2016;50(6):780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM. Gender- and gestational age-specific body fat percentage at birth. Pediatrics. 2011;128(3):e645–651. [DOI] [PubMed] [Google Scholar]
- 9.Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, et al. Validation of a new pediatric airdisplacement plethysmograph for assessing body composition in infants. The American journal of clinical nutrition. 2004;79(4):653–660. [DOI] [PubMed] [Google Scholar]
- 10.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. The American journal of clinical nutrition. 2007;85(1):90–95. [DOI] [PubMed] [Google Scholar]
- 11.Demerath EW, Fields DA. Body composition assessment in the infant. American journal of human biology : the official journal of the Human Biology Council. 2014;26(3):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Child Growth Standards based on length/height, weight and age. Acta paediatrica (Oslo, Norway : 1992) Supplement. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 13.Daniels SR, Hassink SG. The Role of the Pediatrician in Primary Prevention of Obesity. Pediatrics. 2015;136(1):e275–292. [DOI] [PubMed] [Google Scholar]
- 14.Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, et al. Infant BMI or Weight-for-Length and Obesity Risk in Early Childhood. Pediatrics. 2016;137(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohman T, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 16.Hawkes CP, Zemel BS, Kiely M, Irvine AD, Kenny LC, J OBH, et al. Body Composition within the First 3 Months: Optimized Correction for Length and Correlation with BMI at 2 Years. Horm Res Paediatr. 2016;86(3):178–187. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization; WHO Global Database on Child Growth and Malnutrition. http://whqlibdoc.who.int/hq/1997/WHO_NUT_97.4.pdf. Published 1997. Accessed October 28, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Toro-Ramos T, Paley C, Pi-Sunyer FX, Gallagher D. Body composition during fetal development and infancy through the age of 5 years. European journal of clinical nutrition. 2015;69(12):1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. [DOI] [PubMed] [Google Scholar]
- 20.Hittner JB, May K, Silver NC. A Monte Carlo evaluation of tests for comparing dependent correlations. The Journal of general psychology. 2003;130(2):149–168. [DOI] [PubMed] [Google Scholar]
- 21.Ong KK. Size at birth, postnatal growth and risk of obesity. Hormone research. 2006;65 Suppl 3:65–69. [DOI] [PubMed] [Google Scholar]
- 22.Beyerlein A, Toschke AM, von Kries R. Risk factors for childhood overweight: shift of the mean body mass index and shift of the upper percentiles: results from a cross-sectional study. International journal of obesity (2005). 2010;34(4):642–648. [DOI] [PubMed] [Google Scholar]
- 23.Beyerlein A Quantile regression-opportunities and challenges from a user’s perspective. American journal of epidemiology. 2014;180(3):330–331. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J. US birth weight/gestational agespecific neonatal mortality: 1995–1997 rates for whites, hispanics, and blacks. Pediatrics. 2003;111(1):e61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossen LM, Schoendorf KC. Trends in racial and ethnic disparities in infant mortality rates in the United States, 1989–2006. American journal of public health. 2014;104(8):1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazahery H, von Hurst PR, McKinlay CJD, Cormack BE, Conlon CA. Air displacement plethysmography (pea pod) in full-term and pre-term infants: a comprehensive review of accuracy, reproducibility, and practical challenges. Maternal health, neonatology and perinatology. 2018;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koontz MB, Gunzler DD, Presley L, Catalano PM. Longitudinal changes in infant body composition: association with childhood obesity. Pediatric obesity. 2014;9(6):e141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Onis M, Onyango A, Borghi E, Siyam A, Blossner M, Lutter C. Worldwide implementation of the WHO Child Growth Standards. Public health nutrition. 2012;15(9):1603–1610. [DOI] [PubMed] [Google Scholar]
- 29.Smego A, Woo JG, Klein J, Suh C, Bansal D, Bliss S, et al. High Body Mass Index in Infancy May Predict Severe Obesity in Early Childhood. The Journal of pediatrics. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perng W, Ringham BM, Glueck DH, Sauder KA, Starling AP, Belfort MB, et al. An observational cohort study of weight- and length-derived anthropometric indicators with body composition at birth and 5 mo: the Healthy Start study. The American journal of clinical nutrition. 2017;106(2):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF. NCHS growth curves for children birth-18 years. United States. Vital Health Stat 11. 1977(165):i–iv, 1–74. [PubMed] [Google Scholar]
- 32.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. [DOI] [PubMed] [Google Scholar]
- 33.Furlong KR, Anderson LN, Kang H, Lebovic G, Parkin PC, Maguire JL, et al. BMI-for-Age and Weight-for-Length in Children 0 to 2 Years. Pediatrics. 2016;138(1). [DOI] [PubMed] [Google Scholar]
- 34.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124 Suppl 1:S23–34. [DOI] [PubMed] [Google Scholar]
- 35.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006;117(3):e487–495. [DOI] [PubMed] [Google Scholar]
- 36.Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, et al. Relation of BMI to fat and fat-free mass among children and adolescents. International journal of obesity (2005). 2005;29(1):1–8. [DOI] [PubMed] [Google Scholar]
- 37.Evans EM, Rowe DA, Racette SB, Ross KM, McAuley E. Is the current BMI obesity classification appropriate for black and white postmenopausal women? International journal of obesity (2005). 2006;30(5):837–843. [DOI] [PubMed] [Google Scholar]
- 38.Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by raceethnic group. The American journal of clinical nutrition. 2010;91(4):1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]