Abstract
Rationale & Objective:
Randomization to intensive blood pressure lowering (SBP<120 mm Hg) compared to a less intensive BP target (SBP <140 mm Hg) in the ACCORD-BP trial resulted in a more rapid decline in the estimated glomerular filtration rate (eGFR). Whether this reflects hemodynamic effects or intrinsic kidney damage is unknown.
Study Design:
Longitudinal analysis of a sub-group of clinical trial participants.
Settings & Participants:
A subgroup of 529 participants in ACCORD-BP.
Exposures:
Urine biomarkers of tubular injury (kidney injury molecule 1 [KIM-1], interleukin 18 [IL-18]), repair (YKL-40) and inflammation (monocyte chemoattractant protein 1 [MCP-1]) at baseline and year 2.
Outcomes:
Changes in eGFR from baseline to 2 years.
Analytical Approach:
We compared changes in biomarkers and changes in eGFR across participants treated to an intensive vs. less intensive BP goal using analysis of covariance.
Results:
Of the 529 participants, 260 had been randomized to the intensive and 269 to the standard blood pressure arm. Mean age was 62 ± 6.5 and eGFR 90 ml/min/1.73m2. Baseline clinical characteristics, eGFR, urinary albumin-to-creatinine ratio (ACR), and urinary biomarkers were similar across BP treatment groups. Compared to less intensive BP treatment, eGFR was 9.2 ml/min/1.73m2 lower in the intensive BP treatment group at year 2. Despite the eGFR reduction, within this treatment group ACR was 30% lower and 4 urinary biomarkers were unchanged or lower at year 2. Also within this group, participants with largest declines in eGFR had greater reductions in urinary IL-18 and YKL-40. In a subgroup analysis of participants developing incident CKD (sustained 30% decline and eGFR < 60 ml/min/1.73 m2, n=77), neither ACR nor 4 biomarkers increased in the intensive treatment group, whereas one biomarker, IL-18, increased in the less intensive treatment group.
Limitations:
Few participants with advanced baseline CKD. Comparisons across treatment groups do not represent comparisons of treatment arms created solely through randomization.
Conclusions:
Among a subset of ACCORD-BP trial participants, intensive BP control was associated reductions of eGFR but not with an increase in injury markers. These findings support that eGFR decline observed with intensive BP goals in ACCORD participants may predominantly reflect hemodynamic alterations.
Keywords: chronic kidney disease (CKD), hemodynamics, blood pressure (BP), hypertension, urinary biomarkers, CKD progression, intensive BP control, kidney tubule, tubular injury, urine, estimated glomerular filtration rate (eGFR), eGFR decline, renal perfusion
Introduction
The findings from SPRINT (Systolic Blood Pressure Intervention Trial), which demonstrated that randomization to a systolic blood pressure (BP) <120 mm Hg reduced CV events compared to standard treatment to <140 mm Hg, has reinvigorated efforts across health care for aggressive BP control. Aggressive BP control may have unintended consequences, however, including higher risk for acute and chronic declines in kidney function over time. Recent publications have highlighted the increased risk for incident CKD in the intensive vs. standard arm of SPRINT,1 as well as the association between the magnitude of mean arterial pressure reduction in the intensive arm and the risk for incident CKD.2 Whilst intensive BP control increased the risk for apparent incident CKD by 3.5-fold in SPRINT, the CKD was reversible in a large proportion and the intensive treatment group experienced a concomitant 29% reduction in cardiovascular events.1 Thus, as experts have opined, the significance of this type of CKD is unclear, and CV risk reduction outweighs the CKD progression risk.3
Additionally, there has been lack of insight regarding the optimal level of blood pressure control in CKD patients to prevent progression to kidney failure. Although recent large meta-analyses have suggested that randomization to more intensive BP control may reduce mortality in patients with CKD stages 3 to 5,4 the effect of intensive BP control on CKD progression and reaching kidney failure is less clear.5 In addition, diabetes is one of the largest risk factors for CKD progression and kidney failure,6 and there are currently few studies looking at intensive blood pressure control and progression in patients with diabetes. In SPRINT, participants with type 2 diabetes were excluded by design, considering the overall null results in the ACCORD study.1 Thus, whether more intensive BP control in patients in type 2 diabetes leads to intrinsic kidney injury, and thus worse kidney outcomes, is currently an open question.
Urinary biomarkers of kidney injury have been studied extensively to help endophenotype patients with AKI into subgroups with “hemodynamic or prerenal causes” for the acute decrement in GFR vs. those with intrinsic tubular injury or intrinsic AKI.7 Some of the kidney injury biomarkers have subsequently been tested in cohorts of patients with CKD, primarily to serve as a prognostic marker and to ascertain if there is additional value of these markers when added to eGFR and albuminuria.8–10 However, another potential utility for the urinary biomarkers is to assist with further characterization of chronic changes in eGFR.
Thus, in a subcohort of the Action to Control Cardiovascular Risk in Diabetes blood pressure trial (ACCORD BP), we sought to examine the changes in eGFR alongside changes in albuminuria, and 4 urinary biomarkers representing kidney injury (kidney injury molecule 1 [KIM-1] and interleukin 18 [IL-18]), inflammation (monocyte chemoattractant protein 1 [MCP-1]) and fibrosis (YKL-40),11 from randomization to 2 years, in an attempt to disentangle potential treatment-induced changes in eGFR from potential injury and structural changes to the kidneys.12
Methods
The ACCORD Trial
The ACCORD BP Trial was part of the overall ACCORD trial, which was a 2-by-2 factorial design trial of individuals with type 2 diabetes and a hemoglobin A1C (HbA1C) of ≥7.5% between the ages of 40 and 79 years with CV disease or between the ages of 55 and 79 years with anatomic evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for CV disease at 77 clinical centers across the United States and Canada.12 Participants with a systolic blood pressure between 130 and 180 mm Hg who were taking three or fewer antihypertensive medications and who had the equivalent of a 24-hour protein excretion rate of less than 1.0 g were also eligible for the blood-pressure trial. Exclusion criteria included a serum creatinine level of more than 1.5 mg/dL. A total of 4733 patients were randomized to an intensive therapy (systolic BP target <120 mmHg) or standard therapy (systolic BP target <140 mmHg) arm. Total follow-up of patients was 5 years, and urine specimens were collected at the baseline visit and 24 months, and were stored for future research. This study was considered exempt from institutional review board (IRB) approval and informed consent was waived since all of the data was deidentified.
Selection of Subcohort
As part of an ancillary study, we obtained 380 urine samples from ACCORD participants for a nested case-control study of CKD (190 participants with a sustained decline in eGFR ≥40% that were 1:1 matched to controls with ≤10% eGFR decline in a 1:1 fashion on key characteristics (age within 5 years, sex, race, baseline albumin-to-creatinine ratio within 20 μg/mg, and baseline eGFR within 10 ml/min/1.73 m2).13 We randomly selected 710 additional participants from the larger ACCORD population with banked biospecimens, resulting in a total of 1090 participants with biomarker measurements at baseline and 24 months with corresponding eGFR data. Of these, a total of 529 participants were enrollees in the ACCORD BP trial with baseline and 24 month biomarker/ eGFR measurements and included in this current analysis (Figure S1).
Exposure Ascertainment
Our primary exposures of interest were the baseline and 24-month eGFR and urinary biomarker concentrations, respectively.
Biospecimen Storage and Analytes Measurement
The urine samples were stored at −80°C until analysis. Urinary biomarkers were measured once per sample using the four–plex prototype assay on the Mesoscale Platform (Meso Scale Diagnostics, Gaithersburg, MD). The intra- and inter-assay coefficients of variation were 2.2%–6.8% for urinary IL-18 (uIL-18), 5.0%–9.3% for urinary kidney injury molecule-1 (uKIM-1), 3.6%–15.3% for urinary monocyte chemotactic protein-1 (uMCP-1), and 1.6%–12.1% for urinary YKL-40 (uYKL-40), as previously described.12 The average lower limit of detection obtained from multiple runs was 0.09 pg/ml for uIL-18, 0.28 pg/ml for uKIM-1, 0.05 pg/ml for uMCP-1, and 0.16 pg/ml for uYKL-40. The biomarker assays were conducted by V.R. who was blinded to clinical data and the eGFRs.
Statistical Analyses
We expressed descriptive results for the participants’ baseline characteristics and biomarkers via means and standard deviations or medians and interquartile ranges (IQRs) for continues variables, and via proportions for categorical variables. We used two independent sample t-test to compare continuous variables and chi-square tests to compare categorical variables. We applied logarithmic (base 2) transformations to urinary biomarkers KIM-1, IL-18, YKL-40, and MCP-1 at baseline and year 2 to ensure that the distributions within each study arms are normal.
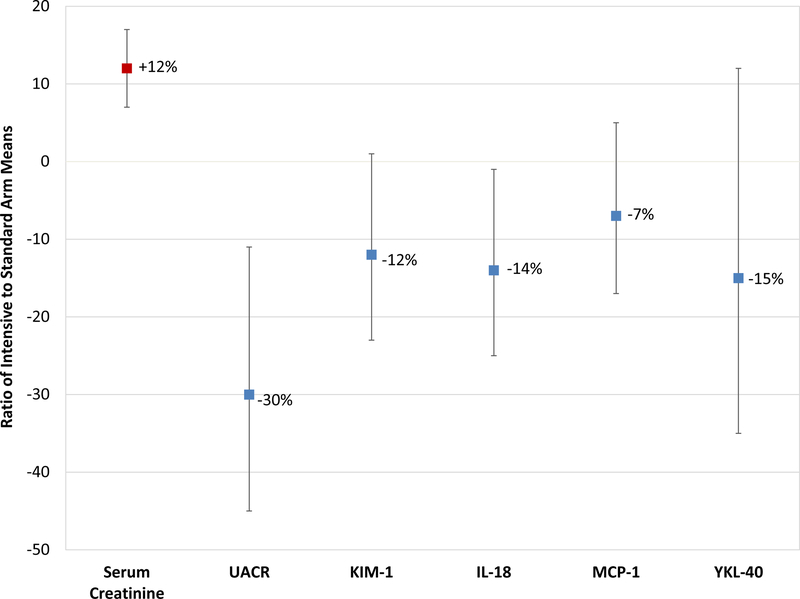
Analysis of covariance (ANCOVA) of log2 transformed values was used to estimate change in eGFR/Serum creatinine and urinary biomarkers over follow-up (baseline to 2 year) period among treatment arms (Table 1 and Figure 1). Geometric least squares mean (GLSM) ratios were calculated by dividing the antilog of the the predicted population means (LSMs) of intensive and standard BP. GLSM ratios were calculated for each follow-up period between treatment arms comparison. An adjustment for log2 transformed linear and quadratic urine creatinine values were included in the model for better fit, except in ACR outcome.
Table 1.
Effects of Intensive vs. Standard Blood Pressure Control on Estimated GFR, ACR, and Urinary Tubular Damage Markers in Participants in ACCORD
| Intensive BP arm | Standard BP arm | Ratio* | P-values | ||||
|---|---|---|---|---|---|---|---|
| eGFR ml/min/1.73m2 | |||||||
| Baseline | n= 260; 85.9 (84.1, 87.7) | n= 269 | 85.4 (83.7, 87.2) | ↑ 1% (↓2%, ↑T4%) | 0.7 | ||
| Year 2 | n= 260 | 70.7 (68.5, 73.0) | n= 269 | 79.7 (77.3, 82.3) | ↓ 11% (↓15%, ↓7%) | <0.001 | |
| ACR (mg/g) | |||||||
| Baseline | n= 259 | 18.4 (15.6, 21.8) | n= 268 | 20.4 (17.3, 24.1) | ↓ 10% (↓29%, ↑14%) | 0.3 | |
| Year 2 | n= 259 | 12.7 (10.7, 15.0) | n= 267 | 18.1 (15.3, 21.3) | ↓ 30% (↓45%, ↓11%) | 0.004 | |
| KIM-1, pg/ml | |||||||
| Baseline | n= 260 | 627.2 (570.0, 690.2) | n= 269 | 680.6 (619.5, 747.7) | ↓ 8% (↓19%, ↑5%) | 0.2 | |
| Year 2 | n= 259 | 560.2 (507.3, 618.8) | n= 269 | 635.0 (576.1, 700.0) | ↓ 12% (↓23%, ↑1%) | 0.08 | |
| IL-18, ng/ml | |||||||
| Baseline | n= 260 | 24.6 (21.9, 27.5) | n= 269 | 22.4 (20.1, 25.1) | ↑ 9% (↓7%, ↑28%) | 0.2 | |
| Year 2 | n= 259 | 23.7 (21.5, 26.1) | n= 269 | 27.5 (24.9, 30.2) | ↓ 14% (↓25%, ↓1%) | 0.04 | |
| MCP-1, pg/ml | |||||||
| Baseline | n= 260 | 135.4 (123.9, 147.0) | n= 269 | 133.5 (122.8, 145.2) | ↑ 1% (↓10%, ↑14%) | 0.8 | |
| Year 2 | n= 259 | 122.5 (112.3, 133.5) | n= 269 | 131.0 (120.6, 142.8) | ↓ 7% (↓17%, ↑5%) | 0.2 | |
| YKL-40, ng/ml | |||||||
| Baseline | n= 260 | 241.3 (199.1, 292.4) | n= 269 | 223.7 (185.2, 270.3) | ↑ 8% (↓18%, ↑41%) | 0.5 | |
| Year 2 | n= 259 | 232.2 (190.9, 282.4) | n= 269 | 272.7 (225.2, 330.3) | ↓ 15% (↓35%, ↑12%) | 0.2 | |
Values for intensive and standard BP arms are given as no. of patients; least-squares mean (95% confidence interval). ACR, KIM-1, IL-18, and MCP-1, YKL-1 levels are urinary concentrations.
Ratio of intensive arm to standard arm least-squares means. Values in parentheses are 95% confidence intervals.
Abbreviations: eGFR; estimated glomerular filtration rate; KIM-1; kidney injury molecule-1, IL-18; interleukin-18, YKL-40; human cartilage glycoprotein-39, MCP-1; monocyte chemoattractant protein 1.
Figure 1. Effects of Intensive Blood Pressure Control vs. Standard Control on Estimated GFR, Albuminuria and Urine Tubular Damage Markers in Participants in ACCORD.
Geometric least squares mean ratios (GLSMRs) were calculated by dividing the antilog of the the predicted population means (LSMs) of intensive and standard BP.
Additionally, we used ANCOVA to evaluate change in urinary biomarkers (baseline to 2 year) and their associations with quintiles of change in eGFR, by treatment arms. As a result, antilog of the least square means for each quintile for given outcome were reported (Table 2). The model was adjusted for linear and quadratic urine creatinine (log2 transformed) values from baseline and 2-year visit. Quintiles of eGFR change were defined based on the observed distribution in the intensive BP arm, and same cut-points were applied to the standard BP arm.
Table 2.
Comparison of 2-Year Changes in Urinary Tubule Damage Biomarkers Stratified by Quintile of Estimated GFR Change among Participants in ACCORD, Stratified by Treatment Arm
| ΔeGFR Q1 (−59.0, −27.3 mL/min/1.73 m2) | ΔeGFR Q2 (−27.3, −17.5 mL/min/1.73 m2) | ΔeGFR Q3 (−17.5, −5.5 mL/min/1.73 m2) | ΔeGFR Q4 (−5.5, +0.02 mL/min/1.73 m2) | ΔeGFR Q5 (+0.03, +30.4 mL/min/1.73 m2) | P-values for Trend | |
|---|---|---|---|---|---|---|
| Intensive BP Arm (2 y:BL) | ||||||
| No of patients (of total n of 260) | 53 | 53 | 52 | 54 | 48 | |
| ACR | ↓33% (↓51%, ↓9%) | ↓28% (↓47%, ↓2%) | ↓48% (↓62%, ↓30%) | ↓19% (↓40%, ↑10%) | ↓23% (↓45%, ↑6%) | 0.2 |
| KIM-1 | ↓20% (↓32%, ↓6%) | ↓1% (↓16%, ↑17%) | ↓18% (↓31%, ↓3%) | ↓5% (↓19%, ↑12%) | ↓5% (↓21%, ↑13%) | 0.2 |
| IL-18 | ↓34% (↓49%, ↓14%) | ↑1% (↓22%, ↑29%) | ↑9% (↓16%, ↑40%) | ↑3% (↓20%, ↑32%) | ↑28% (↓3%, ↑67%) | 0.01 |
| MCP-1 | ↓6% (↓22%, ↑13%) | ↑3% (↓14%, ↑24%) | ↓19% (↓32%, ↓2%) | ↓7% (↓23%, ↑11%) | ↓12% (↓28%, ↑7%) | 0.4 |
| YKL-40 | ↓46% (↓67%, ↓11 %) | ↓13% (↓47%, ↑42%) | ↑24% (↓25%, ↑105%) | ↑19% (↓27%, ↑94%) | ↑39% (↓18%, ↑136%) | 0.07 |
| Standard BP Arm (2 y:BL) | ||||||
| No of patients (of total n of 269) | 24 | 15 | 52 | 82 | 96 | |
| ACR | ↓37% (↓59%, ↓5%) | ↓14% (↓49%, ↑45%) | ↓25% (↓43%, ↓1%) | ↑4% (↓17%, ↑30%) | ↓7% (↓25%, ↑14%) | 0.2 |
| KIM-1 | ↓28% (↓44%, ↓7%) | ↓2% (↓28%, ↑34%) | ↓13% (↓27%, ↑3%) | ↓1% (↓13%, ↑14%) | ↓1% (↓13%, ↑12%) | 0.1 |
| IL-18 | ↑15% (↓22%, ↑70%) | ↑4% (↓37%, ↑71%) | ↑45% (↑11%, ↑90%) | ↑25% (↑1%, ↑55%) | ↑15% (↓6%, ↑40%) | 0.6 |
| MCP-1 | ↓21% (↓42%, ↑9%) | ↑12% (↓25%, ↑68%) | ↔0% (↓19%, ↑24%) | ↑2% (↓15%, ↑21%) | ↓1% (↓6%, ↑16%) | 0.6 |
| YKL-40 | ↓23% (↓63%, ↑61%) | ↓47% (↓79%, ↑35%) | ↑32% (↓20%, ↑118%) | ↑63% (↑9%, ↑144%) | ↑20% (↓17%, ↑73%) | 0.1 |
Unless otherwise indicated, values are ratio (95% confidence interval). ACR, KIM-1, IL-18, and MCP-1, YKL-1 ratios are based in levels in urine.
Abbreviations: eGFR, estimated glomerular filtration rate; ACR, albumin to creatinine ratio; KIM-1, kidney injury molecule 1; IL-18, interleukin 18; YKL-40, human cartilage glycoprotein-39; MCP-1, monocyte chemoattractant protein 1.
Next, we categorized participants as experiencing CKD as a categorical outcome. We defined CKD as a ≥ 30% decline from baseline on two or more values and eGFR <60 ml/min/1.73m2 during the 5-year follow-up. ANCOVA was also used to evaluate change in urinary biomarkers (baseline to 2 year) and their association with CKD, by treatment arms (Table S1). The model was adjusted for covariates including age, gender, race, baseline eGFR, ACEi/ARB, linear and quadratic urine creatinine (log2 transformed) values for each visit. P value was calculated for interaction term between CKD incident and treatment arms. We considered two-tailed P values of less than 0.05 to indicate statistical significance and calculated the 95% confidence intervals for the ratios of least square means. We conducted all analyses using SAS statistical software version 9.4 (Statistical Analyses System Inc, Cary, NC).
Results
The baseline characteristics of the 529 participants by randomization arm were similar (Table 3). Overall, mean eGFR declined by 17% from baseline to 2 years in the intensive arm (from 85.9 to 70.7 ml/min/1.73 m2) vs. 9% in the standard arm (from 85.4 to 79.7 ml/min/1.73 m2; Table 1 and Figure S2). ACR was 30% lower at year 2 in the intensive arm vs. the standard arm (12.7 vs. 18.1 mcg/mg; p =0.004).
Table 3.
Baseline Characteristics of Study Participants by BP Intensity Treatment Group
| Standard BP arm (n = 269) | Intensive BP arm (n = 260) | P-values | |
|---|---|---|---|
| Age, years | 61.8 ± (6.5) | 61.5 ± (5.8) | 0.5 |
| Female sex | 135 (50.2) | 143 (55.0) | 0.2 |
| White | 176 (65.6) | 169 (65.0) | 0.8 |
| Current smoker | 105 (39.0) | 102 (39.2) | 0.9 |
| History of heart disease | 76 (28.2) | 72 (27.7) | 0.8 |
| SBP, mmHg | 139.7 ± (15.5) | 138.4 ± (15.2) | 0.3 |
| DBP, mmHg | 76.6 ± (10.4) | 76.2 ± (9.7) | 0.7 |
| No. of BP medications | 1.6 ± (1.1) | 1.5 ± (1.1) | 0.3 |
| Treated by diuretic | 101 (37.4) | 96 (36.9) | 0.9 |
| Treated by ARB or ACEi | 188 (69.6) | 167 (64.2) | 0.1 |
| Total cholesterol, mg/dL | 186.3 ± (40.5) | 190.6 ± (46.2) | 0.2 |
| HDL cholesterol, mg/dL | 48.2 ± (13.9) | 48.1 ± (13.1) | 0.9 |
| Triglycerides, mg/dL | 164.3 ± (107.2) | 179 ± (132.3) | 0.1 |
| Body mass index, kg/m2 | 32.1 ± (5.5) | 32.7 ± (5.9) | 0.2 |
| eGFR ml/min/1.73m2 | 89.9 [77.3–95.5] | 90.4 [78–96] | 0.6 |
| ACR (mg/g) | 14.8 [6.8–44.2] | 13.8 [6.6–43.6] | 0.2 |
Values for continuous variables are given as mean ± SD or median [IQR] ; those for categorical variables as count (percentage).
Abbreviations: ACR, albumin-creatinine ratio; SD; standard deviation, CVD; cardiovascular disease, eGFR; estimated glomerular filtration rate,; ARB; angiotensin II receptor blockers, ACEi; angiotensin-converting-enzyme inhibitor, HDL; high-density lipoprotein, BP; blood pressure, SBP; systolic blood pressure, DBP; diastolic blood pressure.
Despite the decline in eGFR/increase in serum creatinine, none of the 4 urinary biomarkers were increased in the intensive vs. the standard arm (Figure 1 and Table 1). On the contrary, all 4 tubule markers trended lower (7–15%) in the intensive arm vs. the standard arm at year 2, a finding that reached statistical significance for IL-18 (decrease of 14%, p = 0.04). There were significant trends for larger reduction in IL-18 (p for trend 0.01) and YKL-40 (p for trend 0.07) among participants in the intensive arm who had larger declines in eGFR (Table 2).
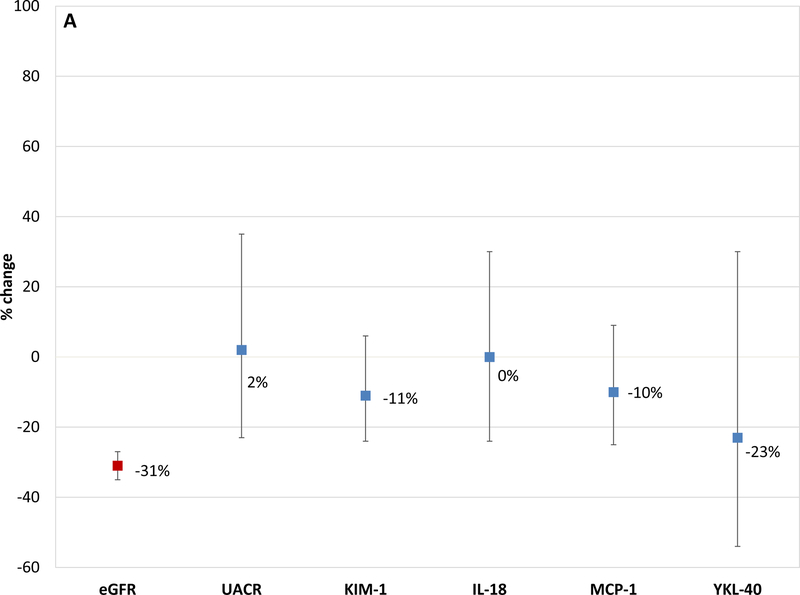
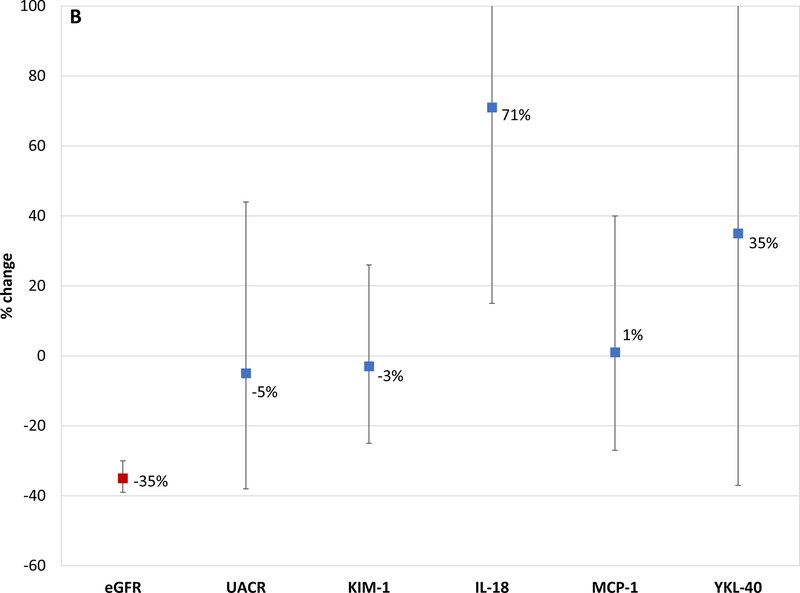
In the 76 participants that experienced incident CKD (sustained 30% decline eGFR and eGFR < 60 ml/min/1.73 m2) in the intensive arm, participants had a mean eGFR decrease of 31% (from 87.3 to 59 ml/min/1.73 m2); none of the 5 urinary biomarkers increased over time despite now meeting the definition for incident CKD (Figure 2a). In the 27 participants that developed incident CKD in the standard arm, participants had a mean eGFR decrease of 35% and one of the 5 biomarkers increased (IL-18 increased by 71%; Figure 2b).
Figure 2a. Changes in estimated GFR and Tubule Damage Biomarkers observed Among Participants who were treated to an intensive BP goal and Developed CKD.
This figure shows the percentage change from baseline to 24 months for eGFR, albuminuria and kidney tubule injury biomarkers (KIM-1; IL-18; MCP-1 and YKL-40) in those participants who developed CKD. CKD was defined as a ≥ 30% decline from baseline on two or more values and eGFR <60 ml/min/1.73m2 during the 5-year follow-up. ANCOVA was also used to evaluate change in urinary biomarkers (baseline to 2 year) and their association with CKD, by treatment arms. The model was adjusted for covariates including age, gender, race, baseline eGFR, ACEi/ARB, linear and quadratic urine creatinine (log2 transformed) values for each visit.
Figure 2b. Changes in estimated GFR and Tubule Damage Biomarkers observed Among Participants who were treated to a less intensive BP goal and Developed CKD.
This figure shows the percentage change from baseline to 24 months for eGFR, albuminuria and kidney tubule injury biomarkers (KIM-1; IL-18; MCP-1 and YKL-40) in those participants who developed CKD. CKD was defined as a ≥ 30% decline from baseline on two or more values and eGFR <60 ml/min/1.73m2 during the 5-year follow-up. ANCOVA was also used to evaluate change in urinary biomarkers (baseline to 2 year) and their association with CKD, by treatment arms. The model was adjusted for covariates including age, gender, race, baseline eGFR, ACEi/ARB, linear and quadratic urine creatinine (log2 transformed) values for each visit.
DISCUSSION
We have shown that there is lack of association between BP treatment-induced changes in eGFR compared with albuminuria and 4 urinary biomarkers of kidney injury, inflammation, and repair. Although we cannot rule out recurrent subclinical episodes of AKI, the decreases in these markers over 24 months suggest that intensive blood pressure lowering effects on eGFR may be hemodynamic in the majority of participants.
We chose these urinary markers since they reflect multidimensional pathways affecting kidney function. Urinary MCP-1, a chemokine promoting recruitment and transformation of monocytes into macrophages,14 is upregulated in kidney diseases as part of ongoing inflammation.15 Urinary levels of MCP-1 at baseline have been associated with a higher odds of kidney disease progression in the ACCORD study.13 Urinary KIM-1, a transmembrane glycoprotein expressed in the apical membrane of proximal tubular cells in response to injury, is an excellent urinary marker of acute kidney injury. Moreover, urinary KIM-1 is associated with proximal tubular injury in CKD,16 is associated with the risk for CKD progression,8,10 and risk for heart failure, cardiovascular events, and death in patients with CKD.17 Urinary IL-18, a proinflammatory cytokine of the IL-1 superfamily, is upregulated after ischemia-reperfusion injury. Studies have shown that urinary IL-18 is a marker of tubular injury and apoptosis in the setting of acute kidney disease. Urinary IL-18 levels also are associated with AKI progression,18 eGFR decline in HIV-infected women,19 and long-term mortality after AKI.20 Finally, urinary YKL-40 is a product of the chitinase 3–like 1 gene and is upregulated in kidney macrophages following ischemia-reperfusion injury. It has been shown to be indicative of fibrosis in both native and donor kidneys.13,21 Thus, we chose biomarkers that together measured the interlinked axes of inflammation, tubular injury, and renal fibrosis, implicated in progression of diabetic kidney disease.
These data may be indicative of a rise in serum creatinine which may not be associated to intrinsic kidney injury, similar to the creatinine rises that occur in prerenal azotemia. This rise in serum creatinine in diabetic patients treated with intensive SBP control can be termed “treatment-induced creatinine elevation” rather than CKD, which implies worsening kidney disease. This paradigm might be analogous to several other therapies that affect peri-glomerular arteriolar hemodynamics. In CKD, RAAS inhibition, through its effect on efferent arteriolar tone, can result in an acute decline in GFR in the short term, but is well known to translate into renoprotective effects in the long term.22 Sodium/glucose co-transporter 2 (SGLT2) inhibitors can result in initial reductions in GFR by constriction of afferent arterioles, presumably through effects on tubuloglomerular feedback, but in the long-term these drugs also appear to be reno-protective.23–25 Finally, in AASK (African American Study of Kidney Disease and Hypertension) and the MDRD (Modification of Diet in Renal Disease) Study, an acute decline in eGFR of 5–19% in the intensive BP arms of the respective trials did not associate with a higher risk of ESRD, whereas a 5–19% decline in the usual BP arms of the trials strongly associated with a higher risk of ESRD.26
Our results should be considered in the light of some limitations. There was only a single measurement of serum creatinine and consequently a single eGFR value, both at baseline and at 24 months, and thus the possibility of misclassification bias cannot be ignored. In addition, since the selection of the cohort was not random, there may be a possibility of selection bias, with patients with increased changes in eGFR being oversampled even though the comparison of the covariates across the selected participants from the intensive versus standard blood pressure arms resulted in equal distribution. We also could not determine whether there were any intercurrent episodes of AKI in the trial participants.
Our findings have important implications. Given similar results in biomarker analyses from SPRINT,27 if other studies show consistent findings in larger populations, then providers may be able to counsel patients that continuation of therapy may still be prudent and beneficial despite worsening eGFR or incident CKD in the setting of aggressive BP treatment. Further workup can be done to ensure the fall in eGFR is consistent with “hemodynamic phenotype” or “treatment-induced creatinine elevation” (low albuminuria, low urinary biomarkers) which would reassure both patient and clinician, and allow intensified blood pressure treatment to continue. Second, based on these findings and those in the SPRINT subgroup,27 researchers designing any clinical trial employing incident CKD as an efficacy or safety endpoint need to recognize and understand the nuances of these potentially different forms of “incident CKD”. At the time of writing, a search of ClinicalTrials.gov showed 67 trials not yet recruiting, enrolling, or active that had an endpoint of CKD. More trials will enter the recruiting phase over the coming years. Efforts should be made by investigators and trialists to distinguish between treatment-induced creatinine elevation and intrinsic kidney injury as they have different prognostic implications.
Supplementary Material
Acknowledgments
Support: This research was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grant no. R01DK096549 to S.G.C.). G.N.N. is supported by a career development award from the National Institutes of Health (NIH) (K23DK107908). C.R.P. is supported by the NIH (K24-DK090203) and P30-DK079310–07 O’Brien Center grant. S.G.C., G.N.N., and C.R.P. are members and are supported in part by the Chronic Kidney Disease Biomarker Consortium (1U01DK106962–01). The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. ACCORD was supported by contracts N01-HC-95178, N01-HC-95179,N01-HC 95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC- 95184, IAA-Y1-HC-9035, and IAA-Y1-HC-1010 from the National Heart, Lung, and Blood Institute; by other components of the NIH, including the NIDDK, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers. The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Beddhu S, Rocco MV, Toto R, et al. Effects of Intensive Systolic Blood Pressure Control on Kidney and Cardiovascular Outcomes in Persons Without Kidney Disease: A Secondary Analysis of a Randomized Trial. Ann Intern Med. 2017;167(6):375–383. doi: 10.7326/M16-2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magriço R, Bigotte Vieira M, Viegas Dias C, Leitão L, Neves JS. BP Reduction, Kidney Function Decline, and Cardiovascular Events in Patients without CKD. Clin J Am Soc Nephrol CJASN. 2018;13(1):73–80. doi: 10.2215/CJN.05510517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamout H, Bakris GL. Consequences of Overinterpreting Serum Creatinine Increases when Achieving BP Reduction: Balancing Risks and Benefits of BP Reduction in Hypertension. Clin J Am Soc Nephrol CJASN. 2018;13(1):9–10. doi: 10.2215/CJN.11811017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhotra R, Nguyen HA, Benavente O, et al. Association Between More Intensive vs Less Intensive Blood Pressure Lowering and Risk of Mortality in Chronic Kidney Disease Stages 3 to 5: A Systematic Review and Meta-analysis. JAMA Intern Med. 2017;177(10):1498–1505. doi: 10.1001/jamainternmed.2017.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai W-C, Wu H-Y, Peng Y-S, et al. Association of Intensive Blood Pressure Control and Kidney Disease Progression in Nondiabetic Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. JAMA Intern Med. 2017;177(6):792–799. doi: 10.1001/jamainternmed.2017.0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis Off J Natl Kidney Found. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 7.Moledina DG, Parikh CR. Phenotyping of Acute Kidney Injury: Beyond Serum Creatinine. Semin Nephrol. 2018;38(1):3–11. doi: 10.1016/j.semnephrol.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C-Y, Xie D, Waikar SS, et al. Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int. 2017;91(1):196–203. doi: 10.1016/j.kint.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fufaa GD, Weil EJ, Nelson RG, et al. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia. 2015;58(1):188–198. doi: 10.1007/s00125-014-3389-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster MC, Coresh J, Bonventre JV, et al. Urinary Biomarkers and Risk of ESRD in the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol. 2015;10(11):1956–1963. doi: 10.2215/CJN.02590315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadkarni GN, Rao V, Ismail-Beigi F, et al. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin J Am Soc Nephrol CJASN. 2016;11(8):1343–1352. doi: 10.2215/CJN.12051115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadkarni GN, Rao V, Ismail-Beigi F, et al. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin J Am Soc Nephrol CJASN. 2016;11(8):1343–52. doi: 10.2215/CJN.12051115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294(4):F697–F701. doi: 10.1152/ajprenal.00016.2008 [DOI] [PubMed] [Google Scholar]
- 15.Rovin BH, Rumancik M, Tan L, Dickerson J. Glomerular expression of monocyte chemoattractant protein-1 in experimental and human glomerulonephritis. Lab Investig J Tech Methods Pathol. 1994;71(4):536–542. [PubMed] [Google Scholar]
- 16.Waikar SS, Sabbisetti V, Ärnlöv J, et al. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2016;31(9):1460–1470. doi: 10.1093/ndt/gfw203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park M, Hsu C-Y, Go AS, et al. Urine Kidney Injury Biomarkers and Risks of Cardiovascular Disease Events and All-Cause Death: The CRIC Study. Clin J Am Soc Nephrol CJASN. 2017;12(5):761–771. doi: 10.2215/CJN.08560816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyner JL, Vaidya VS, Bennett MR, et al. Urinary Biomarkers in the Clinical Prognosis and Early Detection of Acute Kidney Injury. Clin J Am Soc Nephrol CJASN. 2010;5(12):2154–2165. doi: 10.2215/CJN.00740110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlipak MG, Scherzer R, Abraham A, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 1999. 2012;61(5):565–573. doi: 10.1097/QAI.0b013e3182737706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol JASN. 2014;25(5):1063–1071. doi: 10.1681/ASN.2013070742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt IM, Hall IE, Kale S, et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol JASN. 2013;24(2):309–319. doi: 10.1681/ASN.2012060579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 23.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 24.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 25.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 26.Ku E, Bakris G, Johansen KL, et al. Acute Declines in Renal Function during Intensive BP Lowering: Implications for Future ESRD Risk. J Am Soc Nephrol JASN. 2017;28(9):2794–2801. doi: 10.1681/ASN.2017010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra R, Craven T, Ambrosius W, et al. Effects of Intensive Blood Pressure Lowering on Kidney Tubule Injury in CKD: A Longitudinal Subgroup Analysis in SPRINT. Am J Kidney Dis. 2018; ●●(●●):●●●–●●●. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.