Some observational studies have suggested associations of higher prenatal fish intake with protection against offspring asthma, attributing associations to greater exposure to marine n-3 long-chain polyunsaturated fatty acids (n-3 LCPUFAs). Two recent randomized clinical trials have tested that hypothesis. Bisgaard and colleagues1 found that supplementation with n-3 LCPUFAs reduced the risk of persistent wheeze or asthma and lower respiratory tract infections, but not allergic sensitization or eczema, in offspring followed for the first 3 years of life. Their report, which included a succinct review of the literature, was accompanied by an interactive pro-con debate about the merits of prenatal fatty acid supplementation.2 Ramaswami cited an essentially negative trial by Palmer, Best, and colleagues,3 suggesting that after 6 years of follow-up evidence supporting maternal n-3 LCPUFAs supplementation to protect against either allergic disease or asthma in the offspring remained weak. Levy countered that the Bisgaard findings made biologic sense: the n-3 LCPUFAs, mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are substrates for mediators that resolve inflammation and infection (resolvins and protectives), specifically decreasing airway inflammation, mucus metaplasia, and hyperreactivity, while promoting host defense against respiratory infection, acting along pathways that are not directly related to allergic sensitization.
Allergic sensitization evolves throughout childhood and only some wheeze manifested in infancy persists after age 3. In Project Viva, an observational cohort followed from early pregnancy, we evaluated associations of maternal fish consumption as well as prenatal and cord blood n-3 and n-6 fatty acid (FA) levels with allergic sensitization, eczema/atopic dermatitis, wheeze and asthma in early childhood (median 3.3 years) as well as by mid-childhood (median 7.7 years). Because some n-6 as well as many n-3 FAs have anti-inflammatory properties, we hypothesized that specific n-6 as well as n-3 FAs might protect against sensitization and wheeze in children observed by mid-childhood. This hypothesis was supported by our prior observation in this cohort that higher n-3 EPA and n-6 AA levels were each correlated with lower cord blood lymphocyte proliferation and IFN-gamma levels in response to cockroach and dust mite allergen stimulation, suggesting a dampened response to allergens.4 Finally, we evaluated whether greater fish intake in our Massachusetts cohort could account for protective associations of prenatal maternal fatty acids with either allergic sensitization or asthma, knowing that the quantity and type of fish consumption may differ between cultures.
We described details about the analysis cohort (n=1356), exposures, outcomes, and statistical analysis in the Supplementary File. Main exposures were individual n-3 (ALA, EPA, DHA) and n-6 (LA, AA) long-chain polyunsaturated FA concentrations in maternal second trimester (n=1201) and cord (n=762) plasma and prenatal fish and shellfish intake (n=1356). We calculated multivariable odds ratios for allergic sensitization, atopic dermatitis,5 and asthma/wheeze in early and mid-childhood. We categorized all exposures into quartiles and adjusted all models for maternal and paternal history of atopy and child sex and age.
Caracteristics of the 1356 mother-child pairs and exposure distributions are shown in Supplementary Tables 1 and 2. In early childhood, 40% had allergen sensitization and 21% had parental-reported ever asthma or wheeze diagnosis. In mid-childhood, 53% had allergen sensitization and 18% had current asthma.
While cord blood fatty acids were not associated with early childhood allergic sensitization or atopic dermatitis, n-3 and n-6 FAs were inversely associated with early childhood wheeze symptoms (Supplementary Table 3). In mid-childhood, but not in early childhood, the odds of allergic sensitization and of current asthma tended to be reduced with higher levels of maternal second trimester n-3 and n-6 FAs (Figure 1). For example, comparing the highest vs. lowest quartile of EPA, the adjusted OR (95% CI) was 0.56 (0.32, 0.97) for allergic sensitization and 0.55 (0.31, 0.98) for current asthma.
Figure.
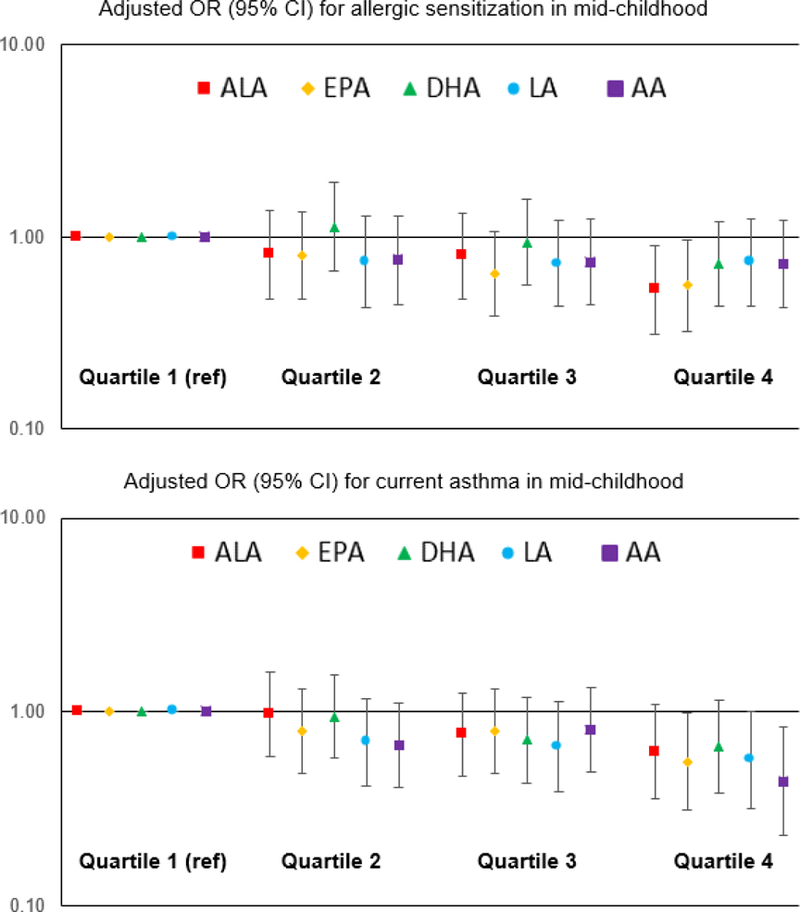
Multivariable associations1 of fatty acids in second trimester maternal plasma (Quartiles 2–4 v. Quartile 1) with allergic sensitization2 and current asthma in mid-childhood3
n-2 FA: ALA, EPA, DHA; n-6 FA: LA, AA
In contrast, the odds of childhood sensitization were increased with higher maternal consumption of fish or shellfish (Supplementary Table 4). Mean (SD) fish and shellfish consumption was 1.7 (1.4) servings/week. Comparing the highest vs. the lowest quartile of fish and shellfish consumption, the adjusted OR (95% CI) for allergen sensitization in mid-childhood was 1.65 (1.07, 2.52). Fish or shellfish consumption had no association with wheeze or asthma, however shellfish was associated with more atopic dermatitis (36%) in early childhood (OR 1.47; 95% CI 1.02, 2,12).
Thus, our observational data suggest potentially protective effects of prenatal maternal LCPUFAs on long-term allergic sensitization and asthma, but not on atopic dermatitis. Our study limitations include limited allergic sensitization measures in early childhood, the absence of shellfish specific IgE measures as well as correlation amongst the n-3 and n-6 FAs making it difficult to distinguish independent effects. We await the follow-up of the children in the Bisgaard trial to see whether the protective associations of supplementation with n-3 LCPUFAs with asthma endure into mid-childhood, whether new protective associations with allergic sensitization or allergic disease emerge, or whether, as in the Best and Palmer trial, the n-3 LCPUFAs supplements appear to have no long-term protective effects on either allergic disease or asthma.
Our data and some other observational mechanistic studies also suggest that potentially beneficial enduring effects may come from specific n-6 as well as n-3 LCPUFAs.6–9 Arachidonic acid, for example, is a precursor to various prostaglandins that have complex relationships with lipoxin, and anti-inflammatory resolvins effects. Protective therapies with specific n-3 and n-6 LCPUFAs may in practice have to be personalized to effect maximal benefits, since maternal, placental and fetal metabolism and utilization of supplements may vary by phenotype or genotype.
For optimal brain development, current FDA guidelines recommend that pregnant women consume a minimum of two to three servings of low-mercury fish every week.10 Most US women consume less fish, including Project Viva mothers. Further evaluation is warranted to assess whether shellfish, which may be the main source of fish for many pregnant mothers, may contribute to the risk of allergic sensitization for some offspring. However, given that thinking has come full circle, suggesting potential benefits of higher maternal ingestion of other foods that contain allergens, such studies should be conducted thoughtfully without premature advice that might contradict current policy.
Supplementary Material
Acknowledgement
We appreciate the assistance from the laboratory of Dr. Joseph R. Hibbeln in performing fatty acid assays for Project Viva and Dr. Hannia Campos for her constructive advice and input on the analysis. We also appreciate the ongoing participation of the wonderful mothers and children of Project Viva.
Sources of financial support: Project Viva is supported by grants from the US National Institutes of Health (K24 HD069408, R01 HD 034568, R01 HL075504, R01AI102960, UG3OD023286, R01 ES016314). EM was funded by a fellowship from the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Clinical trial registration identifier: NCT02820402
Abbreviations used:
- FA
fatty acids
- ALA
α-linolenic acid
- EPA
eicosapentaenoic acid
- DHA
docosapentaenoic acid
- LA
linoleic acid
- AA
arachidonic acid
- FFQ
food frequency questionnaire
- OR
odds ratio
Footnotes
Models adjusted for maternal and paternal history of atopy and child age and sex. Allergic sensitization additionally adjusted for parity.
Allergen sensitization defined as ≥1 detectable allergen-specific IgE or total IgE ≥100 IU/mL.
Current asthma defined as parental report of ever doctor diagnosis of asthma plus wheeze symptoms or use of asthma medication use in the past 12 months. Comparison group includes participants with no asthma, no medication, and no current wheeze.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
References
- 1.Bisgaard H, Stokholm J, Chawes BL, et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med 2016;375:2530–2539. [DOI] [PubMed] [Google Scholar]
- 2.Ramaswami R, Serhan CN, Levy BD, Makrides M. Fish Oil Supplementation in Pregnancy. N Engl J Med 2016;375:2599–2601. [DOI] [PubMed] [Google Scholar]
- 3.Best KP, Sullivan T, Palmer D, et al. Prenatal Fish Oil Supplementation and Allergy: 6-Year Follow-up of a Randomized Controlled Trial. Pediatrics 2016. June;137(6). [DOI] [PubMed] [Google Scholar]
- 4.Gold DR, Willwerth BM, Tantisira KG, et al. Associations of cord blood fatty acids with lymphocyte proliferation, IL-13, and IFN-gamma. J Allergy Clin Immunol 2006. April;117(4):931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. [DOI] [PubMed] [Google Scholar]
- 6.Rucci E, den Dekker HT, de Jongste JC, et al. Maternal fatty acid levels during pregnancy, childhood lung function and atopic diseases. The Generation R Study. Clin Exp Allergy 2016. March;46(3):461–71. [DOI] [PubMed] [Google Scholar]
- 7.Sala-Vila A, Miles EA, Calder PC. Fatty acid composition abnormalities in atopic disease: evidence explored and role in the disease process examined. Clin Exp Allergy 2008. September;38(9):1432–50. [DOI] [PubMed] [Google Scholar]
- 8.Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J Allergy Clin Immunol 2014. May;133(5):1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike KC, Calder PC, Inskip HM et al. Maternal plasma phosphatidylcholine fatty acids and atopy and wheeze in the offspring at age of 6 years. Clin Dev Immunol 2012; 2012: 474613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eating Fish: What Pregnant Women and Parents Should Know U.S. Food and Drug Administration and the U.S. Environmental Protection Agency. Available at: https://www.fda.gov/Food/ResourcesForYou/Consumers/ucm393070.htm Accessed July 20, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


