Abstract
As the incidence of chronic kidney disease (CKD) increases and women pursue pregnancy at more advanced ages, the management of renal disease in pregnancy has become increasingly relevant to the practicing nephrologist. Women with renal disorders face several challenges in pregnancy due to increased physiologic demands on the kidney and risk for disease progression, the potential teratogenicity of medications, and the increased risk of complications such as preeclampsia and preterm delivery. Challenges posed by an underlying disease process in pregnancy, such as autoimmune disease or diabetes mellitus (DM), necessitate an interdisciplinary team to ensure good maternal and fetal outcomes. Rates of acute kidney injury (AKI) in pregnancy are generally declining worldwide, but remain a significant public health concern in developing countries. Pregnancy may also be the first time that a woman is diagnosed as having renal disease or hypertension. An understanding of what constitutes normal physiologic changes in pregnancy is critical in a diagnostic evaluation. In this review, we will review the physiologic changes in pregnancy, the causes and management of AKI in pregnancy, hypertensive disorders of pregnancy, and how to care for women with CKD of various etiologies, including the use of anti-hypertensives and immunosuppressants.
Keywords: Pregnancy, renal disease, preeclampsia, renal outcomes, maternal fetal complications, acute kidney injury (AKI), chronic kidney disease (CKD), autoimmune disease, diabetes mellitus, hypertension, immunosuppression, dialysis, kidney transplantation, review
Physiologic Changes in Pregnancy
There are significant hemodynamic and immunologic shifts that occur during the course of healthy pregnancy (Box 1). The major hemodynamic changes in pregnancy include increased blood volume, decreased systemic vascular resistance, and increased cardiac output. There are increased systemic levels of vasodilators, such as nitric oxide and relaxin, and relative resistance to vasoconstrictors, such as angiotensin II. There is typically a fall in systemic blood pressure, usually reaching a nadir by 20 weeks gestation. Glomerular filtration rate (GFR) increases by approximately 50%, resulting in a physiologic reduction in serum creatinine in the setting of hyperfiltration. The normal serum creatinine in pregnancy is in the 0.4–0.6 mg/dL range. The combination of smooth muscle relaxation due to progesterone and mechanical compression by the enlarging uterus can cause physiologic hydronephrosis and retention of urine in the collecting system during pregnancy.
Box 1. Physiologic changes in pregnancy
Increased
Blood volume
Cardiac output
Levels of nitric oxide and relaxin
Relative resistance to vasconstrictors
GFR by 50%
Urine protein excretion
TH2 phenotype
Circulation of Tregs
Decreased
Systemic vascular resistance
Systemic blood pressure
Serum creatinine
TH2, T helper type 2; Tregs, regulatory T cell; GFR, glomerular filtration rate
Urine protein excretion increases over the course of normal pregnancy, from 60–90 mg/d to 180–250 mg/d, as measured by a 24-hour urine collection. As a consequence of this physiologic increase in proteinuria, the threshold for elevated proteinuria in pregnancy has been set at a higher level of 300 mg/d. The increase in proteinuria has been attributed to hyperfiltration, as described above, but may also be due to changes in glomerular permeability. Some studies have demonstrated an increase in tubular proteinuria, reflected as an increase in urinary retinol-binding protein, as opposed to an increase in albuminuria, which would reflect a glomerular source. The use of spot urine protein-creatinine ratio (UPCR) has gained favor in the diagnosis of preeclampsia, which is typically characterized by proteinuria (UCPR > 0.3 g/g). The UPCR is a faster test that has an acceptable sensitivity and specificity. There may be an increased UPCR in the absence of hypertension or renal disease, a phenomenon known as isolated proteinuria, present in as many as 15% of pregnancies.
Lastly, there are several changes in the function of the innate and adaptive immune systems in pregnancy that may have important impacts on the behavior of autoimmune diseases, a common cause of reduced kidney function in young women. Normal pregnancy is characterized by a shift from a TH1 (cell-mediated immunity) to a TH2 (humoral-mediated immunity) phenotype, which is important for tolerance to fetal antigens, trophoblast invasion, and placental formation. In addition, the number of regulatory T cells (Tregs), which promote immune tolerance, is increased in normal pregnancy, further contributing to establishing fetal tolerance. In autoimmune diseases, such as systemic lupus erythematosus (SLE), alterations in the number and function of Tregs may correlate with an increased risk for pregnancy complications, such as preeclampsia, and poor fetal and maternal outcomes.
Additional Readings
Odutayo A, Hladunewich M. Obstetric nephrology: Renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol. 2012;7(12):2073–80
Kattah A, Milic N, White W, Garovic V. Spot urine protein measurements in normotensive pregnancies, pregnancies with isolated proteinuria and preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R418-R424
Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–71.
Hypertension in Pregnancy
Hypertensive disorders of pregnancy are common, occurring in 6–8% of pregnancies. The differential diagnosis of hypertensive events during pregnancy includes chronic hypertension, gestational hypertension, or preeclampsia. Patients who have a known history of hypertension prior to pregnancy or those who are found to have a blood pressure ≥140/90 mm Hg before 20 weeks of gestation are considered to have chronic hypertension. Women with chronic hypertension have an increased risk of superimposed preeclampsia, which can occur in up to 35% of their pregnancies. Some hypertensive patients with unknown histories of hypertension prior to pregnancy may present with blood pressures in the normal range during the first and second trimesters due to the normal, physiologic drop in blood pressure seen during this time, thus masking the diagnosis of pre-existing hypertension. This may lead to the erroneous assumption that the finding of an elevated blood pressure later during the pregnancy is related to gestational hypertension. The correct diagnosis ultimately is confirmed during the post-partum period, as blood pressure should normalize in those with true gestational hypertension. Gestational hypertension is more common during the second half of pregnancy in patients with no prior history of preexisting hypertension, with an incidence of 6–7%.
Preeclampsia is defined as a blood pressure ≥ 140/90 mm Hg in a previously normotensive woman, measured on 2 different occasions, at least 4 hours apart, after 20 weeks of gestation, in the presence of proteinuria ≥300 mg/d, or a protein/creatinine ratio ≥0.3 g/g. A diagnosis of preeclampsia also can be made in the absence of proteinuria in the presence of clinical features of severity (Box 2).
Box 2. Diagnostic criteria of preeclampsia.
Systolic blood pressure* ≥140 mm Hg, or diastolic blood pressure* ≥90 mm Hg, and
Proteinuria ≥300 mg/d, or UPCR ≥0.3 (mg/mg)
- If no proteinuria is present, new onset of any of the following: §
- Platelets < 100 ×103/L
- Scr >1.1 mg/dL or doubling of Scr concentration, in the absence of other kidney disease
- Liver transaminases 2× upper limits of normal
- Pulmonary edema
- Cerebral or visual symptoms (new-onset and persistent headaches, blurred vision, flashing lights)
*measured on 2 occasions, at least 4 hr apart, after 20 weeks of pregnancy in a previously normotensive patient
UPCR, urinary protein-creatinine ratio; Scr, serum creatinine
§Signs of severe preeclampsia
Over the last 2 decades, the heterogeneity of preeclampsia with respect to underlying mechanisms and resultant clinical phenotypes has been increasingly recognized. Major breakthroughs in the pathophysiology of preeclampsia have occurred that attributed impaired angiogenesis in preeclampsia to an imbalance between proangiogenic (serum vascular endothelial growth factor [VEGF] and placental growth factor [PIGF]) and anti-angiogenic factors (soluble fms-like tyrosine kinase 1 [sFlt-1] and soluble endoglin), favoring the latter. However, angiogenic abnormalities seem to be informative for severe and early (< 34 weeks of gestation) forms of preeclampsia, but not for late disease (≥ 34 weeks of gestation). Consequently, screening tests to predict preeclampsia, including the sFlt-1-PIGF ratio, currently are not recommended for clinical practice. The only available screening approach that has a substantial net benefit is serial blood pressure measurements during pregnancy. Similarly, several approaches aiming at preventing preeclampsia have been studied. The only approach with proven benefit is low dose aspirin when prescribed during the late first trimester in patients with moderate or high risk of preeclampsia; patients with a history of preeclampsia and preterm delivery at <34 weeks; and those with a history of preeclampsia in 2 or more pregnancies.
Delivery remains the mainstay of therapy for preeclampsia, particularly for its severe forms and anticipated life threatening complications. Expectant management can be considered for milder forms of preeclampsia, with the goal of extending pregnancy to full term (37 weeks of gestation) to decrease the risks of fetal complications related to prematurity.
Additional readings
American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31
Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–14.
Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia Screening: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317(16):1668–1683
Hypertension Management
The management of hypertension during pregnancy has evolved over time. Uncontrolled hypertension increases the risk of maternal hemorrhagic stroke, while tight blood pressure control has been linked historically to placental hypoperfusion and fetal compromise. In a meta-regression analysis evaluating the effects of anti-hypertensives on fetal growth, a significant association between mean arterial pressure (MAP) and fetal weight was identified, with a 10 mm Hg drop in MAP associated with a 176 g decrease in fetal birth weight. Due to these concerns, an open label, international, multicenter, randomized control trial (The Control of Hypertension in Pregnancy [CHIP] Trial) analyzed 987 pregnant women with preexisting or gestational hypertension who were randomized to less tight blood pressure control (target diastolic blood pressure of 100 mm Hg) versus tight blood pressure control (target diastolic blood pressure, 85 mm Hg) during pregnancy. No significant differences were found between the groups in terms of pregnancy loss, need for high-level neonatal care, or incidence of maternal complications, other than a higher frequency of severe maternal hypertension in the less tight control arm (40.6% vs 27.5%, respectively). It should be noted, however, that the achieved mean separation of blood pressure in the tight vs. less tight blood pressure groups was 4.6 (95% confidence interval (CI), 3.7–5.4) mm Hg diastolic, less than the intended target difference of 15 mm Hg.
Guidance regarding management of hypertension differs slightly depending on the organization. The American College of Obstetricians and Gynecologists recommends starting anti-hypertensive therapy in patients with gestational hypertension or preeclampsia with a persistent blood pressure of ≥160/110 mm Hg. Delivery is recommended for these women at 37 weeks or later, if no severe features of preeclampsia are observed. Initiation of anti-hypertensive therapy is recommended for pregnant women with pre-existing hypertension if the systolic blood pressure is ≥160 mm Hg and/or if the diastolic blood pressure is ≥105 mm Hg, without evidence of end-organ damage. The National Institute for Health and Care Excellence (NICE) in the United Kingdom, in contrast, recommends initiation of treatment in pregnant women with systolic blood pressures ≥150 mm Hg and/or diastolic blood pressures ≥100 mm Hg. Despite these conflicting guidelines, the authors of this review feel it is safe to treat women with pre-existing hypertension and/or renal disease with anti-hypertensive therapy to a target diastolic blood pressure of 85 mm Hg based on the results of the CHIP Trial.
Additional readings
Magee LA, Singer J, von Dadelszen P; CHIP Study Group. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;11;372(24):2367–8
American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31
von Dadelszen, P, L.A. Magee, Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. J Obstet Gynaecol Can, 2002. 24(12): p. 941–5.
Acute Kidney Injury in Pregnancy
Pregnancy-related acute kidney injury (AKI) in young women worldwide is an important cause of maternal and fetal morbidity and mortality. AKI etiology varies geographically and according to the availability of health resources. The main cause of AKI during pregnancy in developing countries is severe sepsis from septic abortions. Other causes of AKI include hypertensive disorders of pregnancy and hemorrhage. In developed countries, causes of AKI also include hypertensive disorders of pregnancy and sepsis, as well as thrombotic microangiopathy, heart failure, acute fatty liver and post-partum hemorrhage. Rates of pregnancy-related AKI overall have decreased in the last several decades, most likely due to improved prenatal care and a decrease in septic abortions. More recent data from Canada, however, show an increasing incidence of pregnancy-related AKI, from 1.66 per 10,000 deliveries in the 2003–2004 era, to 2.68 per 10,000 deliveries during 2009–2010. While these incidence rates are still quite low, the trend is concerning. This could be due to several factors, including the increased use of assisted reproduction technology that allows women to become pregnant at more advanced ages, an increasing incidence of hypertensive pregnancy disorders, and increasing obesity. AKI requiring dialysis in pregnancy or post-partum is even less common, occurring in 1 per 10,000 pregnant women, but it is associated with increased mortality.
Most AKI episodes in pregnancy occur in otherwise healthy women with an isolated pregnancy-related condition. Hyperemesis gravidarum in the first trimester can lead to volume depletion that can require hospitalization and IV fluid replacement. Hemodynamic compromise in the setting of hemorrhage, pulmonary embolism, heart failure, or sepsis can cause a pre-renal AKI, which can lead to ischemic acute tubular necrosis, if the injury is of sufficient severity and duration. Acute tubular necrosis can also be seen in the setting of acute fatty liver of pregnancy, amniotic fluid embolism, or as a secondary injury related to severe preeclampsia, in particular, hemolysis, elevated liver function tests, low platelet count (HELLP) syndrome (Figure 2). Acute cortical necrosis can occur in the setting of severe hypotension and appears to occur more commonly in pregnancy than in other conditions characterized by a similar degree of hemodynamic compromise (Figure 1). The increased risk of cortical necrosis in pregnancy may be due to the hypercoagulable nature of pregnancy.
Figure 2:
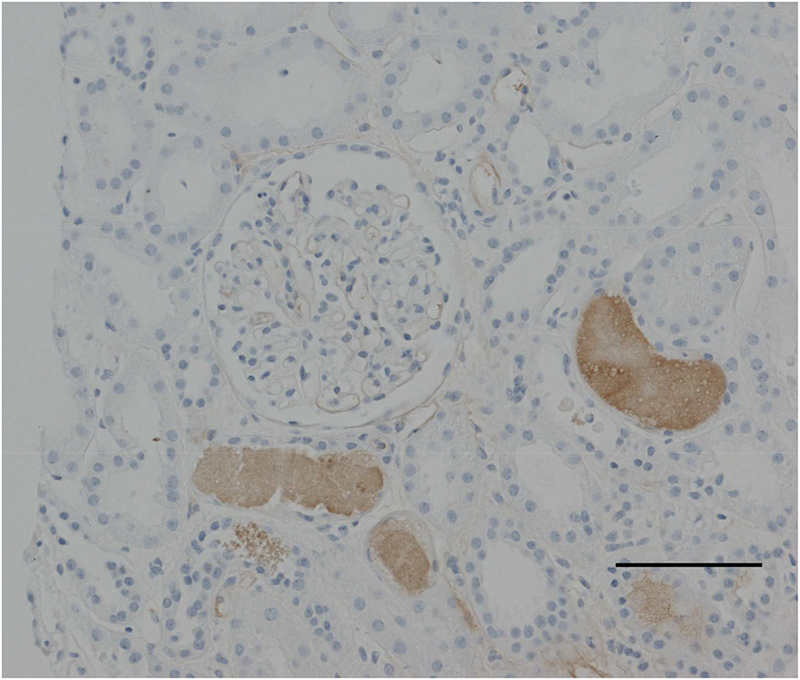
Kidney biopsy image from a 30 yo women with hemolysis, elevated liver enzymes and low platelet (HELLP) syndrome. Kidney biopsy was performed due to acute kidney injury and revealed severe acute tubular necrosis, with hemoglobin casts, likely related to hypoperfusion and hemolysis. (Light microscopy image demonstrating hemoglobin-containing casts in renal tubules and acute tubular necrosis. Scale bar: 100 microns)
Figure 1:
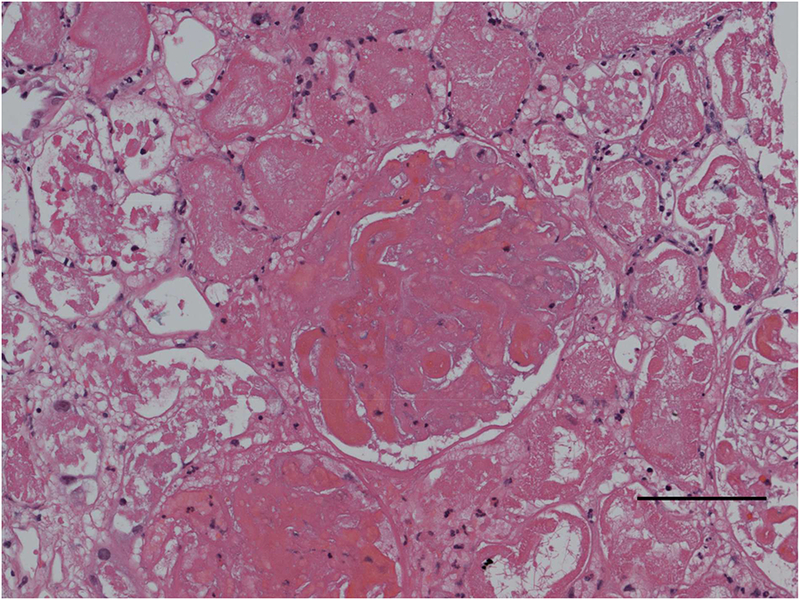
Light microscopy image, hematoxylin and eosin stain, demonstrating acute cortical necrosis in the setting of pregnancy. Scale bar: 100 microns (see Case 1).
The criteria for the diagnosis of AKI in pregnancy have not been standardized. Scr typically is lower in pregnancy due to hyperfiltration, as mentioned previously. An increase in Scr of 0.3 mg/dl, consistent with stage 1 in the AKIN (AKI Network) scheme, may represent a significant kidney injury. There are currently no distinct criteria for the diagnosis of AKI in pregnancy, though it is possible that smaller increases in Scr (less than 0.3 mg/dl) may be more sensitive for picking up early injury. In the absence of data, changes in Scr should be interpreted in each clinical scenario. For example, a rise in Scr by 0.2 mg/dl in a woman with new onset hypertension and low platelets, concerning for HELLP syndrome or atypical HUS is likely to herald renal injury, calling for a close follow up of serial Scr values and relevant serologies. Renal ultrasounds that are performed to rule out post-renal causes of AKI may show hydronephrosis, but this may be physiologic rather than pathologic. Serum complements tend to be elevated in pregnancy due to increased synthesis by the liver, which can complicate making the diagnosis of certain conditions, such as lupus nephritis.
The hemodynamic, inflammatory and immunologic shifts in pregnancy may unmask an underlying renal disease and it can be difficult to diagnose an acute, as opposed to a chronic renal injury. This may be particularly true for glomerular disease. A kidney biopsy should be considered in women in < 32 weeks of gestation, when delivery is not a viable alternative and treatment may result in prolongation of a desired pregnancy. A systematic review of 39 studies provided data on 243 biopsies performed during pregnancy. The main indication for biopsy was to differentiate between glomerulonephritis and preeclampsia; and the results led to changes in therapy in 66% of the cases. Reports of potential complications of kidney biopsies during pregnancy vary from mild to severe, depending on the pregnancy stage and appear to be significantly higher later in pregnancy, with a peak at 25 weeks of gestation (i.e. major bleeding requiring transfusion, embolization, severe obstetric complications, early preterm delivery and, in one case, presumably related fetal death). Therefore, careful consideration of the clinical scenario and counseling of the patient are necessary prior to proceeding with a renal biopsy. If a woman presents with decreased kidney function in the late pre-term period (34 weeks), the provider should consider whether it might be prudent to wait to biopsy after delivery. Biopsy may still be indicated in certain scenarios, but fetal outcomes are generally quite good after 34 weeks and so careful discussion of risks and benefits with the patient, obstetrician and neonatologist are needed prior to proceeding.
Additional Readings
Mehrabadi A, Liu S, Bartholomew S, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ. 2014;349:g4731.
Hildebrand AM, Liu K, Shariff SZ, et al. Characteristics and Outcomes of AKI Treated with Dialysis during Pregnancy and the Postpartum Period. J Am Soc Nephrol. 2015;26(12):3085–91.
Piccoli GB, Daidola G, Attini R, et al. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG. 2013;120(4):412–27
Piccoli GB, Alrukhaimi M, Liu ZH, et al; World Kidney Day Steering Committee. What we do and do not know about women and kidney diseases; questions unanswered and answers unquestioned: reflection on World Kidney Day and International Woman’s Day. BMC Nephrol. 2018;19(1):66
Lupus Nephritis
Systemic Lupus Erythematosus (SLE) disproportionately affects women of childbearing age. Counseling regarding family planning should be discussed early after a SLE diagnosis in order to implement measures to reduce the risks of adverse maternal and fetal outcomes in desired pregnancy. SLE patients should have quiescent disease for at least 6 months prior to attempting to conceive.
Assessment of disease activity through the use of biological markers and assessments of renal function and degree of proteinuria are necessary to diagnose underlying disease flares and to monitor for any potential complications during the pregnancy. Timely identification of high risk patients is important to reduce the risk of possible disease progression and to minimize any potential side effects.
Extra-renal lupus flares are more common during the second and third trimesters, whereas renal disease activity seems to be more common during the post-partum period. Evidence suggests that severe maternal flares occur in 3–5% of pregnancies. Immunological activity at conception (as measured by low C3 levels and anti-DNA antibodies) has been described as the best predictor of renal flares. Low C4 levels and high anti-C1 q antibodies are associated with early flares (encountered during the first or second trimesters of pregnancy). A high body mass index (BMI) has been associated with an increased risk of late flares, defined as those flares encountered during the third trimester or during the post-partum period. The presence of a renal flare does not constitute an absolute contraindication to maintaining a pregnancy.
Several studies have evaluated the risk of maternal complications in women with SLE. A systematic review and meta-analysis of pregnancy outcomes in patients with SLE and lupus nephritis by Smyth et al, showed that lupus flares, hypertension, and preeclampsia were among the main maternal complications. Patients with SLE and preexisting lupus nephritis have a higher risk of a preterm delivery and earlier onset of preeclampsia compared to those women with SLE without nephritis. The overall maternal mortality rate in pregnant patients with SLE and lupus nephritis is estimated to be about 1%. The PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus) study consisted of a prospective cohort of 385 pregnant patients with SLE and evaluated adverse pregnancy outcomes and pregnancy related flare rates. It demonstrated that 19% of patients had an adverse pregnancy outcome: fetal death in 4%, neonatal death 1%, preterm delivery in 9%, and SGA in 10%. Independent predictors of adverse pregnancy outcomes included the presence of a lupus anticoagulant, a physician global assessment of disease activity score >1, anti-hypertensive use, and platelet counts of <100 ×103/μL.
Lupus activity during the last year before conception is a good predictor of fetal outcomes. For example, miscarriages are best predicted by the amount of steroids taken in the year prior to conception, stillbirths are predicted by the number of SLE flares in the year before conception, and preterm births are predicted by the existence of both anti-phospholipid antibody syndrome and anti-double-stranded DNA antibody levels before conception. Complete congenital heart block is a severe manifestation of neonatal lupus, with an incidence of 1–2% after exposure to SSA/Ro and/or SSB/La antibodies. This incidence increases to 20% if there is a maternal history of previous delivery of an infant with neonatal lupus. Therefore, anti-Ro (SSA) and anti-LA (SSB) antibodies should be screened for in pregnant patients with SLE.
Anti-phospholipid antibodies may be present in up to one quarter of SLE pregnancies. Anti-phospholipid antibody syndrome (APS) is associated with pregnancy complications including fetal loss and an increased relative risk of preeclampsia. During the initial pregnancy evaluation in women with SLE, screening is recommended to determine if these antibodies are present. The mainstay of APS management is anticoagulation using either unfractionated heparin or low molecular weight heparin during pregnancy. The risk of thrombotic events is increased in pregnancy, both because pregnancy is a hypercoagulable state, and because of obstruction of venous return by the enlarged uterus. In addition, women with glomerular disease may experience worsening proteinuria and may commonly reach nephrotic range values, which have been associated with increased risks for thrombotic events. Therefore, anticoagulation is indicated for all SLE patients who have anti-phospholipid antibodies and a history of thrombotic event(s), and for those lacking a history of thrombotic event(s), but who meet the obstetric criteria for APS, such as three or more pregnancy losses, or a late pregnancy loss.
The safety principles of immunosuppression during pregnancy, including steroid use, are presented in Table 1. Hydroxychloroquine should be continued throughout pregnancy to maintain quiescence of lupus nephritis and decrease the risk of systemic flares. Discontinuation of hydroxychloroquine has been associated with increased lupus activity and flares during pregnancy, requiring higher doses of steroids to control symptoms.
Table 1.
Immunosuppression therapy in pregnancy
| Drug | Adverse effects during pregnancy |
|---|---|
| Safe | |
| Hydroxychloroquine | No known risk of teratogenicity; withdrawal may cause flare |
| Glucocorticoids | Risk of gestational diabetes; risk of cleft lip and palate; risk of premature rupture of membranes |
| Azathioprine | No known risk of teratogenicity |
| Cyclosporine | Increased risk of cholestasis |
| Tacrolimus | Risk of gestational diabetes and hypertension |
| Hazardous | |
| Cyclophosphamide | Fetal malformations, higher rates of pregnancy loss |
| Mycophenolate mofetil | Teratogenic (lip, palate, ear abnormalities), higher rates of pregnancy loss |
| Unknown | |
| Rituximab | Transient fetal B cell depletion |
Additional Readings
Kim MY, Buyon JP, Guerra MM, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol. 2016;214(1):108
Smyth A, Oliveira GH, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010 Nov;5(11):2060–8
Moroni G, Doria A, Giglio E, et al. Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J Autoimmun. 2016;74:6–12
Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):844S-86S.
Atypical Hemolytic Uremic Syndrome
Atypical hemolytic uremic syndrome (aHUS) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and decreased kidney function as a result of uncontrolled complement activation. Unlike classical HUS, which is triggered by diarrheal illness, aHUS may be triggered by any process that activates the alternative complement pathway. Pregnancy is a classic example of a condition that can trigger aHUS, and many women may be diagnosed with this condition, especially in the postpartum period. A retrospective cohort study of 100 patients with aHUS reported that 21 women developed aHUS in association with pregnancy, with complement abnormalities detected in 85.7% (n=18). These patients were at an elevated risk of fetal loss and preeclampsia, and 76% had reached ESRD by last follow up.
The diagnosis of pregnancy-associated aHUS may be difficult. Several conditions may be associated with acute kidney injury, microangiopathic hemolytic anemia, and thrombocytopenia, including aHUS, thrombotic thrombocytopenic purpura (TTP), and severe pre-eclampsia with HELLP syndrome. TTP presents most commonly in the third trimester, while aHUS presents in the postpartum period. When one of these conditions is suspected, plasma exchange should be started while awaiting ADAMTS13 activity results (<10% is associated with TTP). If the ADAMTS13 activity is normal and aHUS is suspected, eculizumab should be initiated. Prognosis of this condition prior to the use of eculizumab was poor and associated with high morbidity and mortality rates. There are only few reports about the use of eculizumab in pregnancy-associated aHUS, and all of them show promising results. A recent retrospective cohort of 22 patients with pregnancy-associated aHUS found that 16 patients presented during their first pregnancies and 9 patients required dialysis at the time of presentation. Seventeen patients were treated with plasmapheresis, with a renal response in only 3, whereas all of the 10 women who received eculizumab had a favorable outcome. Eculizumab is therefore becoming the preferred therapy for pregnancy-associated aHUS.
Based on recent work, it appears that mutations in the genes encoding for complement regulatory proteins may enhance the risk for preeclampsia in other disease entities, such as SLE and/or anti-phospholipid antibodies. Considering the overlap in disease processes and the lack of a single diagnostic test to clearly identify one process from another, studying complement disorders in these different groups is challenging. One small study (n=11 women) suggested that about 36% of patients who develop HELLP syndrome during pregnancy may have complement abnormalities. Research is ongoing in this area.
Additional Readings
Fakhouri F, Roumenina L, Provot F, et al. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. JASN. 2010;21(5):859–67.
Huerta A, Arjona E, Portoles J, et al. A retrospective study of pregnancy-associated atypical hemolytic uremic syndrome. Kidney Int. 2018;93(2):450–459.
Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8(3):e1001013.
Fakhouri F, Jablonski M, Lepercq J, et al. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008;112(12):4542–5.
IgA Nephropathy
IgA nephropathy is often diagnosed in the second and third decades of life and thus affects many women of child-bearing age. There are several studies that have evaluated pregnancy outcomes in women with IgA nephropathy, and the overall body of evidence suggests that pregnancy does not impact long-term renal function, although the majority of the included women had mild disease (CKD stage 1 and 2). A systematic review and meta-analysis included 4 studies that evaluated pregnancy outcomes in IgA nephropathy and found that pregnancy did not increase the risk of adverse renal events (defined as doubling of Scr, a 50% decline in estimated GFR, or ESRD). There was, however, a high risk of pregnancy complications, including pregnancy loss (12.2%; 95% CI, 7.4%−19.4%), preterm delivery (8.5%; 95% CI, 5.9%−12.1%), low birth weight (9.5%; 95% CI, 6.7%−13.3%), and preeclampsia/eclampsia (7.3%; 95% CI, 4.9%−10.6%). A recent study showed that in women with IgA nephropathy, pregnancy did not increase the risk of adverse renal events. However, women with hypertension, a baseline eGFR < 60 ml/min/1.73 m2, or > 1 g/d of proteinuria had significantly higher risks of renal disease progression.
Most patients with mild, stable, or slowly progressive IgA nephropathy do not receive immunosuppressive treatment. In most cases, ACEi should be discontinued before to pregnancy and immunosuppressive agents should be reviewed and switched to pregnancy-safe alternatives, ideally before conception, or at the first sign of pregnancy, in order to minimize risk to the fetus. Steroids can be used in pregnancy, if immunosuppression is needed for more active disease.
Additional Readings
Limardo M, Imbasciati E, Ravani P, et al. Pregnancy and progression of IgA nephropathy: results of an Italian multicenter study. Am J Kidney Dis. 2010;56(3):506–12.
Park S, Yoo KD, Park JS, et al. Pregnancy in women with immunoglobulin A nephropathy: are obstetrical complications associated with renal prognosis?. Nephrol Dial Transplant. 2018 Mar 1;33(3):459–465.
Liu Y, Ma X, Zheng J, et al. A Systematic Review and Meta-Analysis of Kidney and Pregnancy Outcomes in IgA Nephropathy. Am J Nephrol. 2016;44(3):187–93.
Diabetic Nephropathy
Diabetic nephropathy is characterized by a slowly progressive course, with the gradual development of hypertension, albuminuria, and loss of GFR. Diabetic nephropathy is present in 6% of pregnant women with type 1 DM. Type 2 DM and associated nephropathy is less common among women of child-bearing age. Similar to other glomerular diseases, the risk of pregnancy complications in young women with type 1 DM is related to the degree of pre-pregnancy decrease in kidney function. The risk for deterioration in renal function and progression to ESRD as a consequence of pregnancy is highest in women with a Scr > 1.4 mg/dl.
ACE inhibitors and ARBs are contraindicated in pregnancy, but 3–6 months of therapy prior to conceiving may have renal protective effects. In the absence of reninangiotensin-aldosterone system blockade and under the physiologic conditions of pregnancy, urinary albumin excretion may increase substantially over the course of pregnancy in women with diabetic nephropathy. In one study of 12 women with pregestational moderately increased albuminuria, defined as urinary albumin excretion between 30–300 mg/d, urinary albumin excretion increased on average 7-fold, with several woman exceeding 3 g/d. All women returned to their prior baseline by 12 weeks post-partum. This increase in albuminuria, as noted previously, can complicate the diagnosis of preeclampsia in the absence of other severe features (extreme elevations of blood pressure, thrombocytopenia, neurologic symptoms, etc.). Women with type 1 DM are at an increased risk of preeclampsia, irrespective of their baseline proteinuria, with as many as two-thirds of women with diabetic nephropathy developing preeclampsia in some studies. Available evidence does not show that aspirin decreases the risk of preeclampsia specifically in women with type 1 DM, though it may be reasonable as preeclampsia prophylaxis given the theoretical benefit and overall low risk of harm.
In addition to the risk of preeclampsia, women with diabetes are at increased risk for other pregnancy complications, such as miscarriage, congenital malformations, preterm delivery, macrosomia, and perinatal mortality. Pre-pregnancy counseling is critical so that woman can understand the risks and optimize their chances of having a successful pregnancy. Tighter glycemic control for a period of at least 6 months prior to conceiving is associated with improved outcomes, and the American Diabetes Association recommends targeting a HbA1c goal of <6.5%, while watching carefully for hypoglycemia. Insulin is the mainstay of therapy, though oral hypoglycemics, such as metformin and glyburide, may be continued in some women with pre-gestational type 2 diabetes and excellent glycemic control.
Some women with diabetic retinopathy may have worsening in the setting of pregnancy, as well. A baseline ophthalmic exam prior to pregnancy is needed in all women with diabetes. Women with moderate to severe retinopathy at the time of conception may need more careful monitoring and even intervention in pregnancy. This is particularly relevant for women with high HbA1c in the beginning of pregnancy, as rapid improvement in glycemic control in early pregnancy has been associated with worsening of retinopathy.
Given the complexities of care in women with diabetes, a multidisciplinary approach with endocrinology, nephrology, and obstetrics is likely to achieve the best pregnancy outcomes.
Additional Readings
Reece EA, Leguizamon G, Homko C. Pregnancy performance and outcomes associated with diabetic nephropathy. Am J Perinatol. 1998;15(7):413–21.
Mackie AD, Doddridge MC, Gamsu HR, et al. Outcome of pregnancy in patients with insulin-dependent diabetes mellitus and nephropathy with moderate renal impairment. Diabet Med. 1996;13(1):90–6.
Hod M, van Dijk DJ, Karp M, et al. Diabetic nephropathy and pregnancy: the effect of ACE inhibitors prior to pregnancy on fetomaternal outcome. Nephrol Dial Transplant. 1995;10(12):2328–33.
Chew EY, Mills JL, Metzger BE, et al. Metabolic control and progression of retinopathy. The Diabetes in Early Pregnancy Study. National Institute of Child Health and Human Development Diabetes in Early Pregnancy Study. Diabetes Care. 1995;18(5):631.
Nephrotic Syndrome in Pregnancy
It can be difficult to differentiate intrinsic renal disease causing nephrotic syndrome from preeclampsia, particularly after 20 weeks of gestation. Preeclampsia may cause heavy proteinuria and edema, and hypoalbuminemia is often present. If one is considering the new diagnosis of nephrotic syndrome in pregnancy, particularly before 20 weeks gestation, a kidney biopsy can be safely performed to determine whether immunosuppression is needed. A recent case series of 19 women with nephrotic syndrome in pregnancy found that the average gestational age at presentation was 18 weeks. Kidney biopsies were performed in 8 women, and resulted in a change of management in 6 cases. Of the 26 pregnancies in the cohort, 7 were complicated by preeclampsia, 6 by AKI, 2 by premature rupture of membranes, and 3 by cellulitis. There were 14 infants with low birth weight (<2500 g) and 8 required a neonatal ICU admission.
In the setting of massive proteinuria and hypoalbuminemia (albumin < 2 mg/dl), women may be at risk for thrombosis and anti-coagulation should be strongly initiated (Figure 3).
Figure 3:
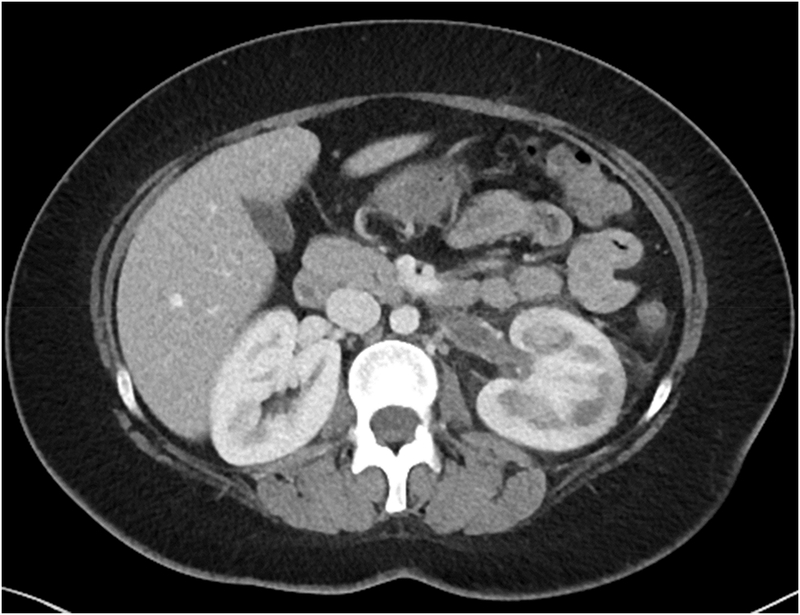
Computed tomography (CT) images from a young woman with a history of HIV-associated nephropathy who developed heavy proteinuria (11 g/d) at the time of delivery and developed an acute left-sided renal vein thrombosis 5 weeks post-partum.
Focal segmental glomerulosclerosis (FSGS) is a pathologic pattern that can be due to either primary or secondary causes. Primary FSGS is typically characterized by diffuse foot process effacement (> 80%), while secondary FSGS will have moderate foot process effacement. Primary FSGS will present with acute or subacute onset of nephrotic syndrome, as opposed to secondary FSGS, which often presents with non-nephrotic range proteinuria and decreased kidney function. Glucocorticoids and/or calcineurin inhibitors can be safely used for primary FSGS in pregnancy.
Minimal change disease is another common cause of nephrotic syndrome, but it is rare in pregnancy. The management of minimal change disease in pregnancy should include anti-hypertensive therapy and glucocorticoids. Duiretics can be used judiciously. There is a theoretical concern of causing intravascular volume depletion with excessive diuretic use that can worsen and/or contribute to systemic vasoconstriction and placental hypoperfusion in preeclampsia. While salt restriction does not seem to protect against preeclampsia, the authors recommend that women with nephrotic syndrome limit their fluid intake and adhere to a low salt diet. Similar to FSGS, women with a pre-existing diagnosis of minimal change disease should be in clinical remission prior to conceiving. Patients with relapsing disease desiring to become pregnant can be safely treated with AZA or calcineurin inhibitors.
Membranous nephropathy in women of child-bearing age is most often secondary to other diseases, such as SLE, drug exposure (in particular NSAIDs or biologic agents), hepatitis B or hepatitis C virus infection, syphilis, or less commonly, malignancy, but can also be primary in some cases. Pregnancy presents therapeutic challenges for this disease as ACE inhibitors, lipid-lowering therapy, warfarin, and cyclophosphamide are contraindicated.
Additional Readings
De Castro I, Easterling TR, Bansal N, Jefferson JA. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int. 2017;91(6):1464–1472.
Nagai Y, Arai H, Washizawa Y, et al. FSGS-like lesions in pre-eclampsia. Clin Nephrol. 1991;36(3):134–40.
Chronic Kidney Disease in Pregnancy
Women with CKD, compared to pregnant women with no CKD, are at an increased risk of adverse maternal and fetal events, including preeclampsia, preterm delivery, low birthweight and an increase in overall mortality (Box 3). Women with advanced CKD may also have a deterioration of renal function. In a classic study by Jones et al of 67 women (with 82 pregnancies) with Scr of at least 1.4 mg/dl in pregnancy, 51% of women had no change in GFR as a result of pregnancy, but 31% had a decline in renal function that persisted 6 months post-partum. Women with antepartum Scr of > 2.0 mg/dl were at particularly high risk of losing renal function as a consequence of pregnancy.
Box 3. Adverse perinatal outcomes in women with CKD
Maternal adverse events
Deterioration in renal function
Flare of underlying disease
Preeclampsia
HELLP* syndrome
Complications from immunosuppression
Preterm delivery
Fetal adverse events
Miscarriages
Stillbirths
Neonatal death
Preterm births
Small for gestational age
Low birth weight
*Defined as: microangiopathic hemolytic anemia with schistocytes, elevated bilirubin ≥1.2 mg/dL, elevated AST twice the upper limit of normal, and a low platelet count of <100 ×103/µL
HELLP, hemolysis, elevated liver enzymes and low platelet; CKD, chronic kidney disease.
A meta-analysis published in 2015 evaluated both the effect of kidney disease on pregnancy outcomes and the effect of pregnancy on kidney outcomes. The authors reviewed 23 observational studies, which included data on 506,340 pregnancies in women with CKD, excluding women with systemic lupus erythematosus, hereditary renal diseases, kidney transplants, ESRD, AKI, or a solitary kidney. They found that women with CKD had increased odds of developing preeclampsia (OR, 10.36; 95% CI, 6.28 – 17.09), premature delivery (OR, 5.72; 95% CI, 3.26–10.03), small for gestational age (SGA) infants (OR, 4.85; 95% CI, 3.03–7.76) and failure of pregnancy, including stillbirth, fetal, and neonatal deaths (OR, 1.80; 95% CI, 1.03–3.13). The second aim of this study was to evaluate the effect of pregnancy on CKD progression. There were a total of 216 renal events (defined as a doubling of Scr, a 50% decrease in eGFR or creatinine clearance, or ESRD) in 1268 participants included in the various studies analyzed in this meta-analysis; however, most of the women had only mild CKD with near to normal serum creatinine with moderately increased albuminuria.
Women with CKD who are contemplating pregnancy, given the above risks, should be evaluated by a high-risk obstetrician and nephrologist prior to pursuing pregnancy, whenever possible. They should receive extensive counseling regarding the risks particular to their disease processes and renal function. Women with milder CKD (Scr <1.4 mg/dL) may expect to have good maternal and fetal outcomes, while women with advanced disease (Scr 1.4–2.9 mg/dL), are at a high risk for pregnancy complications. Women with Scr values ≥ 3.0 mg/dL may permanently lose renal function with pregnancy. The underlying disease, such as diabetes or lupus nephritis, may impose additional, disease-specific risks, as is discussed in more detail in the preceding sections. While the risks of growth restriction, preterm delivery, and SGA infants, still exist, infant survival has improved over the last two decades, likely owing to advances in neonatal care.
Additional Readings
Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6(11):2587–98.
-
Zhang JJ, Ma XX, Hao L, et al. A Systematic Review and Meta-Analysis of Outcomes of Pregnancy in CKD and CKD Outcomes in Pregnancy. Clin J Am Soc Nephrol. 2015;10(11):1964–78.
Jones DC and Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1996;335(4):226.
End-stage Renal Disease in Pregnancy
By the time a woman requires dialysis, her fertility is significantly diminished and she may have erratic and/or absent menstrual cycles. However, pregnancy still occurs, particularly in women during their first year of dialysis and in those receiving intensive therapy, such as daily nocturnal dialysis. The diagnosis of pregnancy may be delayed in dialysis patients due to irregular menstrual periods. Serum hCG levels can be used to diagnose pregnancy in patients with minimal urinary output. Mild elevations of hCG can be seen in the absence of pregnancy in ESRD patients, but significant elevations with appropriate doubling of values every 48–72 hours is indicative of true pregnancy. Ultrasound should be used as a confirmatory test.
Recent data have suggested that dialysis patients can have positive maternal and fetal outcomes if intensive dialysis is instituted in an effort to maintain a near normal serum urea nitogen (SUN). Although a goal of 36 hours per week is ideal, in practice this may be difficult to accomplish. Therefore, greater than 20 hours per week, with a SUN target of < 50 mg/dl, is a more viable goal. Providers may also take into account the amount of residual kidney function when writing a dialysis prescription for a pregnant patient. A recent systematic review and meta-analysis from studies published between 2000 and 2008 evaluated 574 pregnancies in 543 patients on dialysis. Results showed that maternal perinatal mortality was very low (0.4%). A trend towards better outcomes with an increased frequency and length of dialysis sessions was observed.
Fetal outcomes have also improved over time, with an increase in fetal survival in patients receiving high efficiency dialysis treatments. Some studies, however, have shown that early mortality in the perinatal period remains high, while the incidence of preterm delivery can reach 80%. It has been shown that a successful pregnancy is possible in those undergoing peritoneal dialysis or hemodialysis, but the prevalence of SGA babies has been shown to be higher in mothers on peritoneal dialysis compared to those on hemodialysis (66 % versus 31%, respectively).
Additional Readings
Hladunewich MA, Hou S, Odutayo A, et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol. 2014;25(5):1103–9
Piccoli GB, Minelli F, Versino E, et al. Pregnancy in dialysis patients in the new millennium: a systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol Dial Transplant. 2016;31(11):1915–1934
Renal Transplant Recipients/Donors and Pregnancy
Women with advanced kidney disease are often encouraged to wait until after a successful kidney transplant to pursue pregnancy. The rationale behind this recommendation is that fertility is improved after transplantation and the risk of pregnancy complications, such as preterm delivery and preeclampsia, are much lower. Pregnancy is also a sensitizing event, which can result in the formation of anti-HLA antibodies that may make finding a future suitable donor more difficult. The recommendations from the current KDIGO (Kidney Disease: Improving Global Outcomes) guideline state that women should wait for 1 year post-transplantation before pursuing pregnancy, provided renal function is stable; however, a more recent study suggested that waiting 2 years may be prudent to reduce the risk of allograft failure.
There have been several single center studies that have evaluated the impact of pregnancy on graft survival, but it is has been difficult to draw firm conclusions from these results given methodologic differences, including different eras, study populations, immunosuppressive agents, and control groups. A meta-analysis published in 2011 combined the results of 50 different studies that reported pregnancy-related outcomes in renal transplant recipients. They had over 4700 pregnancies in 3570 renal transplant recipients. They found that the live birth rate was similar to the general population (73.5% vs. 66.7%), but that the rates of preeclampsia (27% vs. 3.8%), gestational diabetes (8% vs. 3.9%) and preterm delivery (45.6% vs. 12.5%) were much higher. The risk of graft loss in the cohorts was low (5.8% at 1 year and 6.9% at 5 years).
Women with renal transplants require close monitoring in pregnancy by both obstetricians and nephrologists. The immunosuppressive regimen needs to be modified to medications that are safe in pregnancy, usually a combination of azathioprine, tacrolimus/cyclosporine, and prednisone. Tacrolimus doses often need to be increased substantially in pregnancy, though recent pharmacologic studies have shown that whole blood measurements of tacrolimus do not accurately reflect free tacrolimus levels in the setting of pregnancy, meaning women may experience toxicity with seemingly therapeutic levels.
Kidney donors may also have need for more careful monitoring in pregnancy, given an increased risk of preeclampsia that has been reported in several observational studies. A study by Garg et al published in 2015 suggested that kidney donors had a 2.4 times increased odds of having preeclampsia or gestational hypertension (11% vs. 5%), but they did not have an increased risk of preterm delivery or low birthweight.
Additional Readings
Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant, 2009. 9 Suppl 3: p. S1–155.
Deshpande, N.A., et al., Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant, 2011. 11(11): p. 2388–404.
Zheng, S., et al., Pharmacokinetics of Tacrolimus during pregnancy. Ther Drug Monit, 2012. 34(6); p. 660–670.
Garg, A.X., et al., Gestational Hypertension and Preeclampsia in Living Kidney Donors. N Engl J Med, 2015. 372; p. 124–133.
Medications in Pregnancy
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) should be discontinued before pregnancy because of their teratogenicity in favor of safe alternative(s) such as methyldopa, labetalol, or nifedipine.
Anti-coagulation may be required in the setting of severe nephrotic syndrome, anti-phospholipid syndrome (APS) or other thrombophilic conditions. Warfarin is contraindicated in pregnancy due to its teratogenicity, but low molecular weight heparin or unfractionated heparin given as a subcutaneous injection can safely be given. Novel oral anticoagulants (NOACs) are increasingly used. They are known to cross placenta, however, available data about maternal and fetal outcomes is insufficient to conclude on the risk associated to their use during pregnancy, mainly due to limited number of reported outcomes after exposure.
Glucocorticoids remain the mainstay of immunosuppressive therapy for many glomerulonephritides (Table 1). Prednisone is considered safe during pregnancy due to placental metabolism, with less than 10% of the maternal dose found in the fetal circulation. Calcineurin inhibitors (i.e. cyclosporine, tacrolimus) also are known to induce hypertension and gestational diabetes. The majority of the data regarding their side effects have been obtained from transplant registries, with just a few small case series evaluating their side effects in the context of lupus nephritis. The calcineurin inhibitors have not been associated with teratogenic effects. Cyclophosphamide and mycophenolate mofetil (MMF) are teratogenic and should be discontinued before conception when possible, or immediately after a pregnancy diagnosis is made in the event of an unexpected pregnancy. Azathioprine (AZA) is the drug of choice for pregnant patients previously on MMF who require continuation of immunosuppressive therapy. AZA ideally should be started 3 months prior to conception. Despite being considered safe to use during pregnancy, calcineurin inhibitors and AZA have been associated with SGA infants and preterm deliveries. Rituximab may be used during pregnancy as part of a chemotherapy regimen for treatment of incident or recurrent malignancies, or severe non-malignant hematologic diseases. While it is considered safe to administer during the first trimester, neonatal B cell depletion has been seen in those who have been exposed in utero during the third trimester of pregnancy. Long term outcomes of rituximab use during pregnancy are not known.
Additional Readings
Bullo M, Tschumi S, Bucher BS, et al. Pregnancy outcome following exposure to angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists: a systematic review. Hypertension. 2012;60(2):444–50.
Brown CM, Garovic VD. Drug treatment of hypertension in pregnancy. Drugs. 2014;74(3):283–96
Levy RA, Vilela VS, Cataldo MJ et al. Hydroxychloroquine in lupus pregnancy: double-blind and placebo-controlled study. Lupus 2001;10: 401
Ostensen M, Lockshin M, Doria A et al. Update on safety during pregnancy of biological agents and some immunosuppressive antirheumatic drugs. Rheumatology 2008; 47: iii28
Conclusions
Exacerbations of preexisting kidney disease or an incident diagnosis of nephropathy during pregnancy are relatively common. It is important to be familiar with the diagnoses and management of renal disorders during pregnancy. Close monitoring and awareness of possible complications will allow for earlier intervention with the aim of improving maternal and fetal outcomes.
FEATURE EDITOR:
Asghar Rastegar
ADVISORY BOARD:
Ursula C. Brewster
Michael Choi
Ann O’Hare
Manoocher Soleimani
The Core Curriculum aims to give trainees in nephrology a strong knowledge base in core topics in the specialty by providing an overview of the topic and citing key references, including the foundational literature that led to current clinical approaches.
Case 1: A 36 year old woman, G1P0, with a history of chronic hypertension, type 2 diabetes presented at 35 weeks of gestation to the emergency department and was found to have BPs in the 200s/110s mmHg on arrival. She began having seizures. She received IV magnesium sulfate and lorazepam. Following cessation of seizure activity, her blood pressures were in the 160s/100s mm Hg. She had an emergent surgical delivery. She remained hypertensive after delivery, despite anti-hypertensive medication therapy, which included labetalol and hydralazine. Her baseline creatinine was not known. Her serum creatinine (Scr) upon arrival to the hospital was 2.6 mg/dl and it peaked at 3.2 mg/dl during her hospitalization. Her hemoglobin was 9.3 mg/dl, with no signs of hemolysis on peripheral smear, and platelets were 92 ×103/μL (once month prior, platelet count was 160 ×103/μL). Urinalysis was negative for proteinuria and hematuria. Renal ultrasound showed normal size kidneys, with no signs of CKD.
Question 1: What is the most likely diagnosis for this patient?
a) Severe preeclampsia
b) Acute cortical necrosis
c) Hypertensive emergency
d) HELLP syndrome
For answer, see Appendix,
Case 2: A 42 yo woman with a history of poorly controlled hypertension presented at 29 weeks of gestation with vaginal bleeding and abdominal cramping. She had not received any prenatal care and had stopped her anti-hypertensives. In the emergency room, she was found to have intrauterine fetal demise and placental abruption. Her laboratory studies were notable for hemoglobin of 9.5 mg/dl, platelet count 75 ×103/μL (baseline platelet count was 140 ×103/μL), serum creatinine of 1.8 mg/dl, and elevated liver enzymes. She also had low fibrinogen (110 mg/dl; reference range, 200–393) and prolonged protime, consistent with disseminated intravascular coagulation. She had severe elevations in blood pressure, with systolic blood pressure (BP) close to 200 mm Hg. She was taken for urgent delivery and had significant blood loss requiring multiple transfusions. She became hypotensive with systolic BPs in the 80–90 mm Hg for at least 30 minutes. The next day she was anuric and she was started on dialysis, which she required for 4 weeks, with gradual recovery of renal function. A kidney biopsy was performed (Figure 1).
Question 2: What is the pathologic diagnosis?
a) Glomerulonephritis
b) Hypertensive nephrosclerosis
c) Thrombotic microangiopathy
d) Acute cortical necrosis
For answer, see Appendix.
Case 3: A 25 year old pregnant woman presented with new onset proteinuria, up to 7 g/d, and hypertension at the time of delivery. Post-partum, her proteinuria persisted, and at 6 months after delivery, it was quantified at over 2 g/d. Her serum albumin and lipid profile were within reference ranges, she had a negative serologic evaluation. She had a normal BMI, was a non-smoker, and had previously been normotensive. A kidney biopsy was performed.
Question 3: Which one of the following renal lesions has been most commonly associated with a prior history of preeclampsia?
a) Minimal change disease
b) Membranous nephropathy
c) Focal segmental glomerulosclerosis
d) Amyloidosis
For answer, see Appendix.
Acknowledgments
Support: VDG is supported by award number P50AG44170 from the National Institute on Aging.
Appendix
Answer to Question 1: (a) This patient presented with severe hypertension and at least one of the features of severe preeclampsia: platelets of 92 ×103/L. Her Scr was also elevated, but there was no baseline for comparison. She did not present with proteinuria. During the next few days of her hospitalization, her thrombocytopenia worsened to a platelet count of 80 ×103/L, her liver function tests remained unremarkable, and she did not develop hemolysis. Patient was not planning to breastfeed, so a diuretic was safely added to her regimen. In addition, she required a calcium channel blocker for blood pressure control. Her blood pressure was under control and her Scr had improved to 2.1 mg/dl at her follow up visit.
Answer to Question 2: (d) This patient presented with severe preeclampsia in the setting of chronic hypertension. She had kidney injury with an elevated Scr upon presentation, likely due to severe preeclampsia. HELLP syndrome, a severe form of preeclampsia, is commonly associated with kidney injury and, on kidney biopsy, frequently demonstrates glomerular endotheliosis and thrombotic microangiopathy. This patient developed disseminated intravascular coagulation in the setting of placental abruption and became acutely hypotensive due to blood loss. She subsequent developed acute cortical necrosis, shown here on light microscopy (Figure 1). Pregnant women are particularly susceptible to acute cortical necrosis in the setting of hypotension.
Answer to Question 3: (c) Patient’s kidney biopsy revealed FSGS, with perihilar variant, and mild to moderate glomerulomegaly, consistent with a secondary FSGS. While preeclampsia is classically characterized by glomerular endotheliosis and thrombotic microangiopathy in the acute setting, several studies have shown FSGS lesions in women with histories of preeclampsia and proteinuria that persists months to years postpartum an association between preeclampsia and FSGS lesions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.


