Abstract
Background:
Adequate assessment of control is critical to asthma management. The Asthma Control Questionnaire (ACQ) and the National Asthma Education and Prevention Program (NAEPP) criteria are commonly used measures of asthma control.
Objective:
This study is to examine the relationships between the ACQ and NEAPP criteria and compare the validity in association with lung function, asthma exacerbation and quality of life.
Methods:
The ACQ and the NAEPP criteria were administered to 373 adolescents with asthma, aged 12–20. The two measures correlated with FEV1, asthma exacerbation (oral corticosteroid [OCS] use, hospitalization and emergency department [ED] use) in the past 12 months, and quality of life.
Results:
Agreement between the ACQ and NEAPP criteria was moderate (Kappa=0.40 to 0.61). Neither of the two measures was a reliable predictor of FEV1<80% due to high false positives for ACQ (68%) and low sensitivity for NAEPP (49%). The NAEPP identified more cases of uncontrolled asthma (84.6%) than ACQ (64.6%). The ACQ was a significant predictor of recent OSC use, hospitalization, and ED visits (AUC=0.66, 0.66, 0.64, respectively; p<0.001), as was NAEPP (AUC=0.63, 0.66, 0.61, respectively; p<0.001). Both measures significantly associated with quality of life, and the relationships were particularly strong for ACQ (r=−0.87 for symptom subscale; r=−0.76 for activity subscale; and r=−0.78 for emotional function subscale).
Conclusion:
Neither ACQ nor NAEPP appears to reliably predict lung function, while both measures reasonably associate with acute asthma exacerbation. The ACQ may be the superior measure in gauging the psychosocial impact of asthma control given its particularly strong associations with quality of life.
Keywords: asthma control, ACQ, NAEPP, Adolescents, lung function, asthma exacerbation, quality of life
Introduction
According to a recent national survey, nearly 60% (3.4 million) of children with asthma in the United States (U.S.) had uncontrolled symptoms.1 Uncontrolled asthma in children and adolescents is linked to reduced lung growth2 and increased severity of airway obstruction with age,3 eventually leading to progressive loss of lung function that can last into adulthood.4 Children with poorly controlled asthma had almost 19 times more asthma-related ED visits and 43 times more hospitalizations than their well-controlled counterparts.1
Asthma control is an ultimate goal of asthma management, and assessing asthma control is the first step to patient management.5 Because asthma control is a multidimensional concept that cannot be captured by a single question,5 criteria-based approaches taking account of multiple components of asthma control simultaneously, such as the National Asthma Education and Prevention Program (NAEPP) criteria and the ACQ, have been utilized extensively in clinical practice and research.
NAEPP’s Expert Panel Report 36 recommends periodic monitoring and assessment of asthma control in managing asthma, and offers a classification scheme based on the assessment of impairment. Impairment is concerned with the frequency and intensity of symptoms (i.e., daytime symptoms, night time awakening, and use of short-acting beta agonist (SABA) and functional limitations (daily activity interference) that the patient has experienced in the previous 2–4 weeks.6 Based on the most severe impairment category, NAEPP asthma control is classified into three levels—well controlled, not well controlled and very poorly controlled.
In lieu of or in combination with the NAEPP criteria, a validated psychometric measure such as the Asthma Control Questionnaire (ACQ)7 is often used. The ACQ is the most widely used standardized measure of asthma control in clinical trials.8–11 Of validated measures of asthma control available in the literature, only the ACQ fulfills all measurement characteristics, including validity, responsiveness, stability, internal consistency, and interpretability (minimum important difference and threshold of poor control).5 Unlike the NAEPP’s criteria in which the most severe impairment category determines the level of control, ACQ averages categories and uses two prespecified cut-offs to classify asthma control into either well-controlled or uncontrolled levels. The ACQ aligns well with other standardized measures of asthma control.12 However, little is known about the extent to which the ACQ is in line with the NAEPP guideline based criteria, a routinely used classification system for asthma control the in the U.S.
The NAEPP criteria were developed from expert opinion and use categorical control levels, while the ACQ is a validated scale to quantify the degree of asthma control in a continuum and is used to identify patients with uncontrolled asthma based on an empirically derived cut-off.7,13 Despite the differences, these two measures are similar in that categorical determination of control is based on similar degree of impairment. In their original forms, both methods include an objective criterion or item involving pulmonary function. However, limited use of or access to spirometry in many clinical or research settings and a lack of training for clinicians to interpret spirometric results limits its practical application as a tool to assess asthma control, particularly in pediatric patients.14,15 Hence, NAEPP criteria or the ACQ are often used without spirometric information. Because the NAEPP’s approach determines asthma control based on a single impairment criterion of the highest frequency or intensity, the absence of spirometry information may not be critical, particularly considering the frequent mismatch between spirometry and symptom reports in children.16 A reduced version of the ACQ without spirometry or peak expiratory flow rate testing was also found valid, and showed nearly perfect agreement with the full version ACQ with identical clinical results.9, 17
Despite the wide application of the NAEPP criteria and/or the ACQ for the classification of asthma control, no empirical data are available to show the degree to which these two measures correspond with each other as valid measures of asthma control in adolescents. Without assuming the NAEPP criteria as a gold standard of asthma control, the purpose of this study was to examine the relationships between NAEPP criteria and ACQ concurrently and evaluate the validity of each of these classification approaches separately through widely accepted surrogates of asthma control including pulmonary function, asthma exacerbation and quality of life.1 This study was the secondary analysis of baseline data of a multi-site randomized controlled study of adolescents collected between 2015 and 2017 to evaluate the effects of an asthma self-management intervention (NCT02293499).
Methods
Settings and Sample
Subjects were recruited from three U.S. metropolitan cities located in New York, Maryland, and Tennessee. The majority of the sample were recruited through clinician/school referrals (n=141, 37.8%), followed by word of mouth (n=76, 20%), school or community outreach (n=73, 19.6%), clinic recruitment (n=34, 9%), study flyers (n=32, 8.6%), previous study contact database (n=14, 3.8%), and study website/newspaper ad (n=3, <1%). Eligibility criteria included: 12–20 years of age; physician-diagnosed asthma for at least one year; asthma-related health care utilization in the past 12 months preceding enrollment; reporting current use of a control medication or having persistent asthma based on the NAEPP criteria6; absence of other comorbid conditions requiring daily medication; and capacity to understand spoken and written English.
Data Collection and Study Measurements
The study protocol was reviewed and approved by the Institutional Review Board within each of the participating academic institutions. Prior to data collection, informed written consent from parents and assent from adolescent (<18 years of age) were obtained, while written consent was obtained from older adolescents (≥18 years old) themselves. Questionnaire data were collected during in-person appointments in the project office or in the home, and respiratory therapists at each site performed spirometry.
NAEPP Criteria of Asthma Control (NAEPP-AC):
To measure asthma control based on the NAEPP classification, we devised a survey questionnaire consisting of four items representing impairment criteria including symptoms, nighttime awakenings, activity limitations and short-acting beta-agonist (SABA) use in the past 4 weeks. Each item was originally measured on a 4-point scale of varying degree of frequency and intensity of these impairments to align with the NAEPP classification for severity. This measure was used at screening to determine one of the eligibility criteria, having persistent asthma. To convert the severity measure to the control measure, the 4-point scale was transformed into a 3-point scale by consolidating response options corresponding to moderate and severe-persistent severity (e.g., daily and throughout the day symptoms) into the “very poorly controlled” category for symptoms and SABA use to closely align with the NAEPP control classification. For nighttime awakenings and activity limitations, the responses for mild and moderate persistent severity levels (e.g., “minor limitation” and “some limitation”) were consolidated to correspond with the “not controlled” level. Subsequently, asthma control was classified into three levels (1=well controlled, 2=not-well controlled or 3=very poorly controlled) by the impairment category with the highest frequency or intensity as the guideline indicated. In addition, the original 4-point scale was used to compute total scores ranging from 4 to 16, with the higher scores indicating poorer control. Cronbach alpha of the 4-point scale was .79 in this sample.
Asthma Control Questionnaire (ACQ):
The original survey contains seven items measured on a 7-point scale, from 0 (no impairment) to 6 (extreme impairment), using the past 7 days as a recall period with all items equally weighted. 7 In this study, an item concerning percent predicted forced expiratory volume in one second (FEV1%) was eliminated as obtaining FEV1 prior to SABA use was not consistently achieved in this community-based study. The reduced 6-item version questionnaire has been found to be valid and yielded clinical results comparable to the full version.9, 17 The validity of the questionnaire was established in children and adolescents 6 to 16 years of age.18 The mean score of the six items was computed ranging from 0 to 6, with higher scores indicating a greater degree of uncontrolled asthma. Cronbach alpha of the reduced version in the current sample was .86. The mean score <= 0.75 was classified as “well controlled”, >=1.5 “uncontrolled”, and between these cut-off points “somewhat controlled”.
Asthma Exacerbation:
In line with the recommendation by the expert panel convened at the NIH-organized workshop,19 asthma exacerbation was assessed by the following: (1) oral corticosteroids (OCS) use for asthma for at least 3 days; (2) asthma-specific hospital admission; and (3) asthma-specific emergency department (ED) visits. Adolescents reported whether these events had ever occurred in the 12 months prior to study entry. Each of these dichotomized items was used separately to examine its relationship with asthma control measures.
Pediatric Asthma Quality of Life Questionnaire:
This is a 23-item instrument, consisting of three subscales: activity limitation (5 items), emotional function (8 items), and symptoms (10 items).20 This extensively used scale has proved a valid and reliable measure of asthma-specific quality of life in adolescents.20, 21 The mean score was computed for each subscale, with higher scores indicating higher functioning in each subdomain. Cronbach’s alphas of activity, emotion, and symptom subscales in the current sample were .84, .91 and .94, respectively.
Percent predicted Forced Expiratory Volume in 1 second (FEV1%) was obtained in accordance with the ATS/ERS standardization22 using a portable KoKo® spirometer.
Parents provided sociodemographic (age, sex, SES, race) and asthma-related (medication, age at asthma diagnosis, and family history of asthma) information and adolescents completed measures on asthma control, exacerbation and quality of life.
Data Analysis
Cohen’s Kappa was computed to examine the extent to which the levels of asthma control derived from each of the ACQ and the NAEPP classification methods. Cohen’s Kappa measures the degree to which two different ratings give the same category (match), after adjusting for matching by chance. Pearson correlations were calculated between the mean ACQ score and the NAEPP-AC score with the other continuous measures (FEV1% and QOL subscales). Biserial correlations were calculated between the three dichotomous exacerbation indicators (OCS use, hospitalization, and ED visits) and each of the ACQ and the NAEPP-AC scores. The three ACQ categories and the three NAEPP categories were tested for relation to each of the three dichotomous exacerbation indicators using chi-squared tests. Receiver operating characteristic (ROC) curves were calculated to examine the ability of the mean ACQ scores and the NAEPP criteria to predict low FEV1% (<80%) or asthma exacerbation requiring OCS, hospitalization, or ED visits. ROC curves plot the separation of a sample with a dichotomous outcome (positive and negative) by a single continuous predictor, and the area under the curve (AUC) was calculated at a measure of effect size. When the predictor is unrelated to the categories, we expect a diagonal line with an AUC close to 0.5, meaning that the two categories are equally represented at any threshold for the predictor. AUC values significantly larger than 0.5 show that there are thresholds that separate the sample so that one group has more positive cases than the other. From these figures, an optimal threshold was calculated that best separates the two groups.
Results
Sample Characteristics and Descriptions of Study Variables
A total of 373 adolescents between 12 to 20 years of age (Mean=14.68±1.94) recruited from three urban communities in the U.S. were enrolled in the study. Males and females were equally represented (50% each), and the majority (78.6%) were African American. Early onset of asthma, diagnosed before the age of 6, was reported by 72% of the sample. Three subjects did not provide data after enrollment and were excluded from the subsequent analyses.
Relationships between ACQ and NAEPP-AC
The mean ACQ scores and the NAEPP total scores were significantly correlated with each other (r=0.464, p<0.001). The ACQ prespecified cut-off points (0.75 or lower for well controlled and 1.5 or greater for uncontrolled) and the NAEPP classification generated three levels of asthma control each. Table 1 cross-tabulates two sets of asthma control levels from the two measures in the levels of asthma control. The Cohen’s Kappa for their agreement between three levels of asthma control based on each measure was 0.40 (95%CI 0.31, 0.48). When considering only the two extreme levels of the two measures (well controlled/very poorly controlled by NAEPP vs. well/uncontrolled by ACQ), the Kappa improved to 0.61 (95%CI 0.49–0.74). While the ACQ classified the majority as either well (35.4%) or uncontrolled (43.8%), the NAEPP classification assigned the majority to the not-well controlled (50.3%) or very poorly controlled (34.3%) (Table 1). Forty-six percent (170/370) of participants reported levels of asthma control that were congruent between the two measures, while 54% (200/370) were found to have incongruent control levels by ACQ and NAEPP criteria. Of those determined well controlled by ACQ, 17.6% (23/131) were found to be very poorly controlled by NAEPP.
Table 1:
Cross tabulation between the ACQ criteria and NAEPP criteria
| NAEPP | |||||
|---|---|---|---|---|---|
| Well controlled |
Not well controlled |
Very poorly controlled |
ACQ n (%) | ||
| ACQ | Well controlled | 39 | 69 | 23 | 131 (35.4) |
| Somewhat controlled | 14 | 45 | 18 | 77 (20.8) | |
| Uncontrolled | 4 | 72 | 86 | 162 (43.8) | |
| NAEPP n (%) | 57 (15.4) | 186 (50.3) | 127 (34.3) | 370 (100) | |
ACQ Asthma control Questionnaire; NAEPP National Asthma Education and Prevention Program
Relationships between FEV1% and Asthma Control Measures
A significant negative linear relationship was found between the measures of asthma control and FEV1%, indicating the poorer the asthma control, the lower was the average FEV1%, although NAEPP total score did not reach statistical significance (r=−0.101, p=0.081) while ACQ mean score did (r=−0.115, p=0.047). The proportion of FEV1 <80% did not significantly change with the increased levels of severity by ACQ, despite the increasing trend of the proportions from 17.5% for well controlled to 28.3% for uncontrolled asthma (Figure 1). Meanwhile, Figure 1 illustrates that the proportion of FEV1 <80% is significantly higher for very poorly controlled asthma by NAEPP, 35.1%, compared to well controlled (18%) or not well controlled categories (17.8%).
Figure 1:
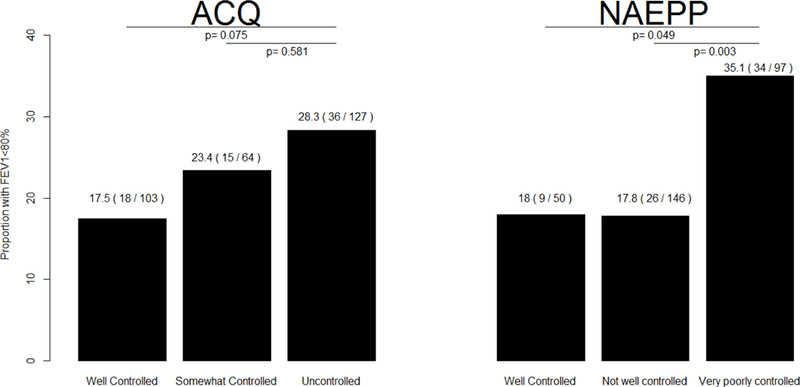
Proportion of the FEV1<80% for each category of the mean ACQ score (left) and NAEPP total score (right). P-values test for a significant difference in the proportion of FEV1<80% (z-test).
Both measures predicted the increased risk of FEV1<80% as illustrated in Figure 2. The difference in AUC between the two measures was not significant (p=0.751). The optimal threshold for ACQ was 0.67, at or above which the likelihood of FEV1 <80% was 86% (sensitivity) and the likelihood of FEV1 ≥ 80% rate was 68% (false positive). The optimal threshold for the NAEPP criteria was 3, equivalent to “very poorly controlled”, at which the likelihood of FEV1 <80% was 49% (sensitivity) and the likelihood of FEV1% ≥80% was 28% (false positive).
Figure 2:
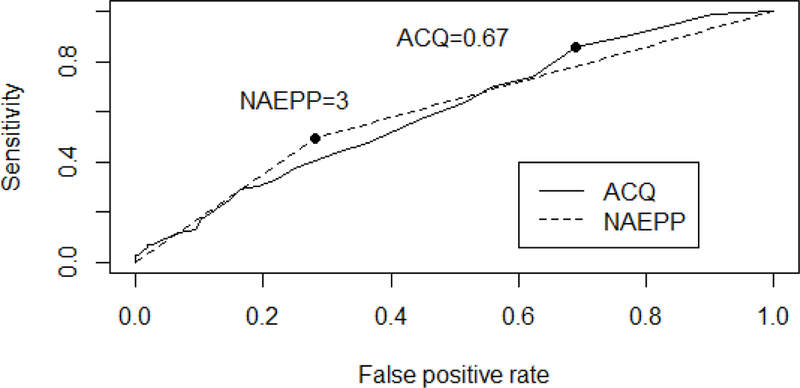
ROC curves for predicting FEV1<80% using either the ACQ (solid, AUC = 0.606, p=0.007) or NAEPP (dashed, AUC= 0.605, p=0.003).
Relationships between Asthma Exacerbation and Asthma Control Measures
The mean ACQ score positively associated with recent OCS use, hospitalization, and ED visits (biserial r=0.33, 0.31, 0.30, respectively with p<0.001 for each). Similarly, the NAEPP total score was also positively related to recent OCS use, hospitalization, and ED visits (biserial r=0.30, 0.33, 0.25, respectively with p<0.001 for each). Table 2 shows the rate of each of the exacerbation indicators in each level of asthma control determined by the ACQ cutoffs and the NAEPP classification. Overall, the rates of asthma exacerbation indicators significantly differed by the control levels. In Table 2, the pairwise p-values (p) comparing the first two levels to the least-controlled level, i.e. uncontrolled by ACQ and very poorly controlled by NAEPP, indicate that the least controlled level had significantly higher rates of OCS use, hospitalization, and ED visits compared to the first better controlled levels. None of the pairwise comparisons between the first two categories were significant (p>0.05 for each pair).
Table 2:
Rates of each exacerbation indicator for ACQ and NAEPP criteria of asthma control.
| OCS use | Hospitalization | ED visit | |||||
|---|---|---|---|---|---|---|---|
| n | n (%) | p | n (%) | p | n (%) | p | |
| ACQ | |||||||
| Well controlled | 131 | 20 (15.2) | <0.001 | 11 ( 8.3) | 0.002 | 23 (17.5) | <0.001 |
| Somewhat controlled | 78 | 21 (26.9) | 0.094 | 7 ( 8.9) | 0.015 | 22 (28.2) | 0.140 |
| Uncontrolled | 162 | 63 (38.8) | 37 (22.8) | 63 (38.8) | |||
| p-value | <0.001 | <0.001 | <0.001 | ||||
| NAEPP | |||||||
| Well controlled | 57 | 8 (14.0) | <0.001 | 4 ( 7.0) | 0.006 | 13 (22.8) | 0.012 |
| Not well controlled | 186 | 43 (23.1) | <0.001 | 18 ( 9.6) | <0.001 | 40 (21.5) | <0.001 |
| Very poorly | 127 | 53 (41.7) | 33 (25.9) | 55 (43.3) | |||
| controlled | |||||||
| p-value | <0.001 | <0.001 | <0.001 | ||||
Each indicator has a p-value for the Chi-square test of whether the rates are independent of the asthma control categories. To the right, the pairwise p-values compare the first two better controlled criteria to the least-controlled criterion.
ACQ Asthma control Questionnaire; NAEPP National Asthma Education and Prevention Program
Both mean ACQ scores and the three levels of NAEPP were significant predictors of exacerbation indicators, as shown by the ROC curves in Figure 3. The AUC for OCS use was 0.66 for ACQ and 0.63 for NAEPP (p<0.001 for each with no significant difference between them, p=0.55). The AUC for recent hospitalization was 0.66 for both ACQ and NAEPP (p<0.001 for each with no significant difference between them, p=0.92). The AUC for recent ED visit was 0.64 for ACQ and 0.61 for NAEPP (p<0.001 for each with no significant difference between them, p=0.47). The threshold in each indicator of exacerbation was chosen from the ROC curve analysis to separate groups with different risks. ACQ had slightly different thresholds for exacerbation indicators with the highest threshold (2.0) for OCS use, followed by hospitalization (1.83) and ED visit (1.0). For the NAEPP criteria, the best thresholds for all three exacerbation indicators were 3 (i.e., very poorly controlled). Table 3 compares the rates of exacerbation indicators between two groups separated by the best threshold for each of OCS use, hospitalization, and ED visit.
Figure 3:
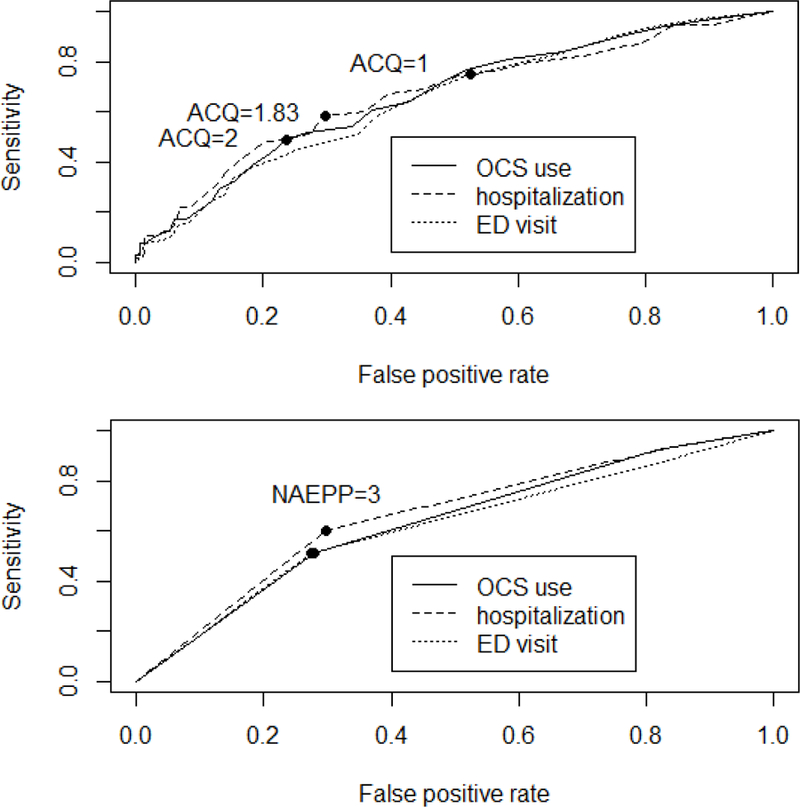
ROC curves for predicting asthma exacerbation determined by oral corticosteroid (OCS) use (solid line), hospitalization (dashed line), or ED visit (dotted line) using either the ACQ (top panel) or NAEPP criteria (lower panel).
Table 3:
Rates of each exacerbation indicator for the levels of asthma control determined by best thresholds based on the ROC curve analyses for the ACQ or NAEPP.
| OCS use | Hospitalization | ED visit | ||||||
|---|---|---|---|---|---|---|---|---|
| Best threshold | n (%) | Best threshold | n (%) | Best threshold | n (%) | |||
| ACQ | ACQ | ACQ | ||||||
| <2 | 53 (20.6) | <1.83 | 23 ( 9.4) | <1 | 27 (17.7) | |||
| ≥2 | 51 (44.7) | ≥1.83 | 32 (25.4) | ≥1 | 81 (36.9) | |||
| NAEPP | NAEPP | NAEPP | ||||||
| <3 | 51 (20.9) | < 3 | 22 ( 9.3) | < 3 | 53 (21.8) | |||
| =3 | 53 (41.7) | =3 | 33 (25.9) | =3 | 55 (43.3) | |||
ACQ Asthma control Questionnaire; NAEPP National Asthma Education and Prevention Program; OCS oral corticosteroid; ED emergency department
Relationship between Quality of Life and Asthma Control Measures
While both mean ACQ scores and NAEPP total scores were negatively associated with all three subscales of the quality of life, the relationships were far stronger for ACQ (Table 4). The strongest association was noted between ACQ and the symptom subscale, as illustrated in Figure 4.
Table 4:
Correlation between the two asthma control measures and subscales of quality of life.
| Quality of Life | |||
|---|---|---|---|
| Symptoms | Activity | Emotional function | |
| ACQ | r =−0.87, p<0.001 | r =−0.76, p<0.001 | r =−0.78, p<0.001 |
| NAEPP-AC | r =−0.45, p<0.001 | r =−0.40, p<0.001 | r =−0.44, p<0.001 |
ACQ Asthma control Questionnaire; NAEPP National Asthma Education and Prevention Program
Figure 4:
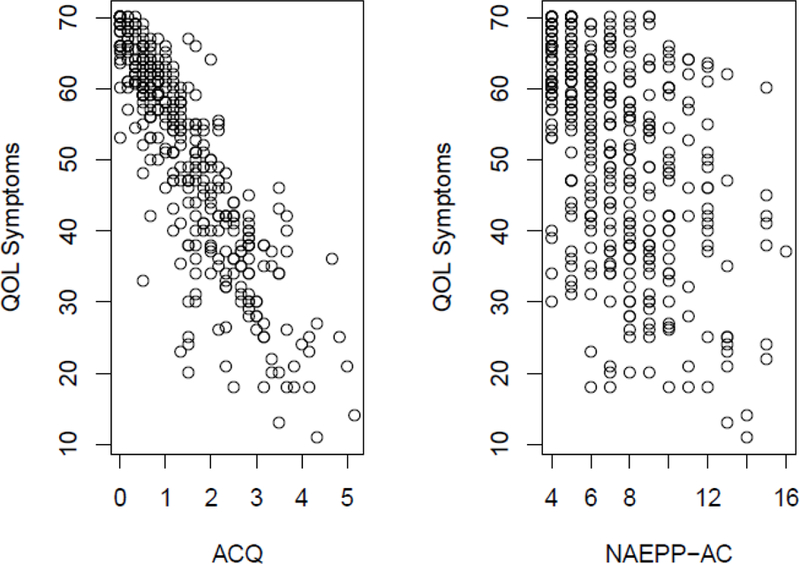
Relationships between the Quality of Life symptoms subscale and each of the mean ACQ score (left) and NAEPP total score (right) (N=370).
Discussion
This study compares the two most commonly used measures of asthma control, the ACQ and NAEPP guideline-based criteria. To our knowledge, this is the first attempt to compare the adequacy of ACQ and the NAEPP criteria in predicting FEV1%, acute exacerbation, and quality of life in adolescents. The degree of agreement between the categories of asthma control generated by each measure seems weak, with Kappas ranging only from 0.40 to 0.61. For 54% of cases, asthma control as classified by the ACQ did not match classification by NAEPP. The NAEPP identified a higher proportion of adolescents with uncontrolled symptoms. It is clinically concerning that nearly 18% of those who were well controlled by ACQ were found to be very poorly controlled according to the NAEPP, whereas only 4 cases qualifying as uncontrolled by ACQ were deemed well-controlled using NAEPP criteria. Other studies in adult populations have reported similarly poor correlations between the ACQ and other guideline-based clinical tools, such as Global Initiatives of Asthma (GINA) criteria.23–25 Perhaps, the NAEPP classification tends to identify more uncontrolled cases because it uses the reference timeframe of four weeks vs. one week in ACQ and/or it requires only one category at the uncontrolled level for asthma to be considered uncontrolled. The lack of strong correlation between theoretically equivalent measures makes it increasingly important to distinguish which is likely to be most useful for research and practice. The relationship of each measure to other key asthma outcomes, such as quality of life, FEV1, and acute exacerbations, may shed light on this issue.
Similar to other reports,24 the ACQ appears to correlate more linearly with FEV1 than NAEPP, although a significantly higher proportion of FEV1<80% was found only in the NAEPP’s poorly controlled asthma category. Nonetheless, neither seems ideal for identifying FEV1<80% adequately due to the high chance of false positives for ACQ (68%) or alternately low sensitivity for NAEPP (49%), which translates to high likelihood of error using either metric as a predictor of lung function. Our findings align with many earlier studies reporting poor correlations between asthma control and spirometry values.8,16,26–29 This has resulted in some skepticism regarding the usefulness of adding spirometry to a self-reported assessment of asthma control control.30 Because asthma is a highly variable disease, symptoms might fluctuate substantially from day to day. Thus, spirometry results obtained at a single encounter may not adequately represent asthma control over an extended period, e.g., 7 days or 4 weeks. Therefore, a symptom-based measure is generally considered more appropriate for identifying uncontrolled asthma in children,31 and is often used without the addition of lung function parameters.
In considering risk of acute exacerbation, we examined the relationship of both ACQ and NAEPP criteria to acute healthcare utilization (i.e., hospitalization and ED visit) and OCS use, which are commonly used proxy measures of asthma control.1 In our data, the risk of exacerbation increased linearly as asthma control moved from well to poorly controlled with both the ACQ and NAEPP criteria. Notably, there were no significant differences in exacerbation between the well-controlled and intermediate controlled subsets (i.e. somewhat/not-well controlled). The findings raise a question regarding the clinical meaningfulness of the middle range control level and echo an earlier concern about the challenge in defining the intermediate level of asthma control by ACQ.32 Significant differences were only seen when cases classified at the poorest of control (ACQ “Uncontrolled” and NAEPP “Very poorly controlled”). Our findings suggest that use of OCS, ED visits, and hospitalizations may be adequate indicators of very poorly controlled asthma, but may not be good markers of moderately uncontrolled asthma in general. Furthermore, while assessing ED visits and hospitalization is undoubtedly essential, our findings suggest that absence of acute healthcare utilization might not be a sufficient indicator of well controlled asthma, particularly in adolescent patients who tend to tolerate high levels of symptoms without reporting or responding.33,34
Subsequently, we sought to establish empirical thresholds/cut-offs for ACQ and NAEPP criteria for acute exacerbation indicators. With the NAEPP classification, our analyses yielded a single threshold corresponding to the “very poorly controlled” level, at which the risk for all exacerbation indicators (ED, hospitalization, OCS use) increased uniformly. This empirical threshold reflects that the NAEPP’s very-poorly-controlled level is associated with an increased risk of exacerbation. With the ACQ, different thresholds were identified for each indicator: 2 for OCS use, 1.83 for hospitalization and 1 for ED visits, with OCS being predictive of the least controlled asthma. It is particularly noteworthy that the threshold for increased risk of ED visits fell under 1.5 on the ACQ—the accepted cut-point for being uncontrolled asthma. This may indicate that ED visits are not always associated with uncontrolled asthma as some low income families in the U.S. may use ED for usual asthma care or management that could have been handled at primary care practices.35,36 It is also worth mentioning the differences in reference timeframes—in the past year for exacerbation indicators vs. previous 4 weeks and 7 days for NAEPP and ACQ respectively, which means that exacerbations have occurred before the measurement of asthma control. Thus, the compelling associations between exacerbation and poorly controlled asthma in this study may suggest inadequate treatment of exacerbation or suboptimal treatment adherence, subsequently leading to poorly controlled asthma. A prospective study is needed to examine the temporal relationships between exacerbation and asthma control, and underlying mechanisms between them.
It is well established that lower quality of life corresponds with poorer asthma control and functional impairment.26,37–40 Consistent with a study of other measures of asthma control,26 both the ACQ and NAEPP scores were significantly associated with asthma-related quality of life. However, correlations were stronger with the ACQ for all domains (e.g. emotional functioning, physical activity, and symptoms). Particularly, the magnitude of its association with the symptom subscale was substantial, suggesting the ACQ’s exceptional capacity to represent the symptom domain of quality of life. This finding must be interpreted with some caution, as the ACQ was validated against quality of life in its development stage.17,41 Thus, it is difficult to determine whether the strong association between ACQ and the quality of life measure indicates exceptional capacity or self-validation. Further study may be needed to understand factors contributing to the differing strength of relationships between ACQ, NAEPP, and quality of life, and to reexamine associations using different measures of functional status.
We acknowledge several limitations pertaining to the study. Most of our data, except for FEV1, were based on self-reports including asthma diagnosis and exacerbation indicators, hence subject to recall bias. However, self-reported medical diagnoses and urgent healthcare services are still of great value given their relatively high accuracy when compared to medical records,42–44 Second, differences in the reference time frames of NAEPP and ACQ, 4 weeks vs. 7 days respectively, may have contributed to the observed incongruences in the levels of asthma control. Finally, despite the relatively large number of participants, this convenience sample of ethnic minority adolescents, predominantly African American, recruited from urban cities in the U.S. inevitably limits the study’s generalization to a larger population.
Overall, the levels of asthma control measured by the ACQ do not always align with those of the NAEPP criteria, and neither measure is a compelling predictor of lung function. Nonetheless, higher levels of uncontrolled asthma determined by either measure associate with asthma exacerbation or poor quality of life reports. Given the strikingly strong association between the ACQ and quality of life, choosing the ACQ over the NEAPP can be justified when gauging the overall impact of asthma control on a patient’s quality of life. Both measures, however, could be used simultaneously to cross-validate a control level, and any incongruences can be further investigated through other objective measures such as spirometry prior to making treatment decisions. Choice of which measure to use may depend on the goals that the measurement is to achieve, either informing clinical decision making or assessing the implications for psychosocial functioning.
Acknowledgments
Funding Source: National Institute of Health/ National Institute for Nursing Research (NIH/NINR R01NR014451).
Abbreviations/Acronyms:
- ACQ
Asthma Control Questionnaire
- NAEPP
National Asthma Education and Prevention Program
- FEV1
Forced Expiratory Volume in one second
- ED
Emergency Department
- OCS
Oral Corticosteroid
- ROC
Receiver Operating Characteristic
- AUC
Area Under the Curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none
References
- 1.Sullivan PW, Ghushchyan V, Navaratnam P, et al. National Prevalence of Poor Asthma Control and Associated Outcomes Among School-Aged Children in the United States. J Allergy Clin Immunol Pract 2018;6(2):536–544.e1. doi: S2213-2198(17)30525-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 2.Lodge CJ, Lowe AJ, Allen KJ, et al. Childhood wheeze phenotypes show less than expected growth in FEV1 across adolescence. Am J Respir Crit Care Med 2014;189(11):1351–1358. doi: 10.1164/rccm.201308-1487OC [doi]. [DOI] [PubMed] [Google Scholar]
- 3.Strunk RC, Weiss ST, Yates KP, et al. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol 2006;118(5):1040–1047. doi: S0091-6749(06)01715-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 4.Anderson WC 3rd, Szefler SJ. New and future strategies to improve asthma control in children. J Allergy Clin Immunol 2015;136(4):848–859. doi: 10.1016/j.jaci.2015.07.007 [doi]. [DOI] [PubMed] [Google Scholar]
- 5.Halbert RJ, Tinkelman DG, Globe DR, Lin SL. Measuring asthma control is the first step to patient management: a literature review. J Asthma 2009;46(7):659–664. doi: 10.1080/02770900902963128 [doi]. [DOI] [PubMed] [Google Scholar]
- 6.National Heart, Lung, and Blood Institute. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma 2007.
- 7.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14(4):902–907. [DOI] [PubMed] [Google Scholar]
- 8.Reddel HK, Taylor DR, Bateman ED, et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and Exacerbations. Am J Respir Crit Care Med 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 9.Schuler M, Faller H, Wittmann M, Schultz K. Asthma Control Test and Asthma Control Questionnaire: factorial validity, reliability and correspondence in assessing status and change in asthma control. J Asthma 2016;53(4):438–445. doi: 10.3109/02770903.2015.1101134 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen JM, Holbrook JT, Wei CY, et al. Validation and psychometric properties of the Asthma Control Questionnaire among children. J Allergy Clin Immunol 2014;133(1):91–7.e1–6. doi: 10.1016/j.jaci.2013.06.029 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallenstein GV, Carranza-Rosenzweig J, Kosinski M, Blaisdell-Gross B, Gajria K, Jhingran P. A psychometric comparison of three patient-based measures of asthma control. Curr Med Res Opin 2007;23(2). [DOI] [PubMed] [Google Scholar]
- 12.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol 2006;117(3):549–556. [DOI] [PubMed] [Google Scholar]
- 13.Juniper EF, Bousquet J, Abetz L, Bateman ED, GOAL Committee. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med 2006;100(4):616–621. doi: S0954-6111(05)00335-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 14.Dombkowski KJ, Hassan F, Wasilevich EA, Clark SJ. Spirometry use among pediatric primary care physicians. Pediatrics 2010;126(4):682–687. doi: 10.1542/peds.2010-0362 [doi]. [DOI] [PubMed] [Google Scholar]
- 15.Banasiak NC. Spirometry in primary care for children with asthma. Pediatr Nurs 2014;40(4):195–198. [PubMed] [Google Scholar]
- 16.Schifano ED, Hollenbach JP, Cloutier MM. Mismatch between asthma symptoms and spirometry: implications for managing asthma in children. J Pediatr 2014;165(5):997–1002. doi: 10.1016/j.jpeds.2014.07.026 [doi]. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99(5):553–558. doi: S0954-6111(04)00392-0 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J 2010;36(6):1410–1416. doi: 10.1183/09031936.00117509 [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012;129(3 Suppl):S34–48. doi: 10.1016/j.jaci.2011.12.983 ; 10.1016/j.jaci.2011.12.983 10.1016/j.jaci.2011.12.983; 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring Quality of Life in Children with Asthma. Quality of Life Research 1996;5(1):35–46. [DOI] [PubMed] [Google Scholar]
- 21.Okelo SO, Wu AW, Krishnann JA, Rand CS, Skinner EA, Diette GB. Emotional quality-of-life and outcomes in adolescents with asthma. J Pediatr 2004;145(4):523–529. [DOI] [PubMed] [Google Scholar]
- 22.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. European Respiratory Journal 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 23.Khalili B, Boggs PB, Shi R, Bahna SL. Discrepancy between clinical asthma control assessment tools and fractional exhaled nitric oxide. Annals of Allergy, Asthma & Immunology 2008;101(2):124–129. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso MN, Chong Neto HJ, Rabelo LM, Riedi CA, Rosario NA. Utility of Asthma Control Questionnaire 7 in the assessment of asthma control. J Bras Pneumol 2014;40(2):171–174. doi: S1806-37132014000200171 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olaguibel JM, Quirce S, Julia B, et al. Measurement of asthma control according to Global Initiative for Asthma guidelines: a comparison with the Asthma Control Questionnaire. Respir Res 2012;13:50–9921-13–50. doi: 10.1186/1465-9921-13-50 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozoh OB, Okubadejo NU, Chukwu CC, Bandele EO, Irusen EM. The ACT and the ATAQ are useful surrogates for asthma control in resource-poor countries with inadequate spirometric facilities. J Asthma 2012;49(10):1086–1091. doi: 10.3109/02770903.2012.729632 [doi]. [DOI] [PubMed] [Google Scholar]
- 27.Tibosch M, de Ridder J, Landstra A, et al. Four of a kind: asthma control, FEV1, FeNO, and psychosocial problems in adolescents. Pediatr Pulmonol 2012;47(10):933–940. doi: 10.1002/ppul.22514 [doi]. [DOI] [PubMed] [Google Scholar]
- 28.Sardon-Prado O, Korta-Murua J, Valverde-Molina J, et al. Association among lung function, exhaled nitric oxide, and the CAN questionnaire to assess asthma control in children. Pediatr Pulmonol 2010;45(5):434–439. doi: 10.1002/ppul.21144 [doi]. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Kao JK, Lee CH, et al. Correlations between pulmonary function and childhood asthma control test results in 5–11-year-old children with asthma. Pediatr Neonatol 2014;55(3):218–224. doi: 10.1016/j.pedneo.2013.10.003 [doi]. [DOI] [PubMed] [Google Scholar]
- 30.Voorend-van Bergen S, Vaessen-Verberne AA, de Jongste JC, Pijnenburg MW. Asthma control questionnaires in the management of asthma in children: A review. Pediatr Pulmonol 2015;50(2):202–208. doi: 10.1002/ppul.23098 [doi]. [DOI] [PubMed] [Google Scholar]
- 31.Leung TF, Ko FW, Sy HY, et al. Identifying uncontrolled asthma in young children: clinical scores or objective variables?. J Asthma 2009;46(2):130–135. doi: 10.1080/02770900802468533 [doi]. [DOI] [PubMed] [Google Scholar]
- 32.Jia CE, Zhang HP, Lv Y, et al. The Asthma Control Test and Asthma Control Questionnaire for assessing asthma control: Systematic review and meta-analysis. J Allergy Clin Immunol 2013;131(3):695–703. doi: 10.1016/j.jaci.2012.08.023 [doi]. [DOI] [PubMed] [Google Scholar]
- 33.Britto MT, Byczkowski TL, Hesse EA, Munafo JK, Vockell AB, Yi MS. Overestimation of Impairment-Related Asthma Control by Adolescents. J Pediatr 2011;158(6):1028–1030.e1. doi: 10.1016/j.jpeds.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Mammen JR, Rhee H, Norton SA, Butz AM. Perceptions and experiences underlying self-management and reporting of symptoms in teens with asthma. J Asthma 2017;54(2):143–152. doi: 10.1080/02770903.2016.1201835 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keet CA, Matsui EC, McCormack MC, Peng RD. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J Allergy Clin Immunol 2017. doi: http://doi.org.ezpminer.urmc.rochester.edu/10.1016/j.jaci.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rand CS, Butz AM, Kolodner K, Huss K, Eggleston P, Malveaux F. Emergency department visits by urban African American children with asthma. J Allergy Clin Immunol 2000;105(1 Pt 1):83–90. doi: S0091674900736724 [pii]. [DOI] [PubMed] [Google Scholar]
- 37.Vollmer WM, Markson LE, O’Connor E, et al. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med 1999;160(5 Pt 1):1647–1652. doi: 10.1164/ajrccm.160.5.9902098 [doi]. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Gould MK, Blanc PD, et al. Asthma control, severity, and quality of life: quantifying the effect of uncontrolled disease. J Allergy Clin Immunol 2007;120(2):396–402. doi: S0091-6749(07)00873-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 39.Alvim CG, Picinin IM, Camargos PM, et al. quality of life in asthmatic adolescents: An overall evaluation of disease control. J Asthma 2009;46:186–190. [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga NY, Ribeiro MA, Saad IA, Morcillo AM, Ribeiro JD, Toro AA. Evaluation of quality of life according to asthma control and asthma severity in children and adolescents. J Bras Pneumol 2015;41(6):502–508. doi: 10.1590/S1806-37562015000000186 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juniper EF, O’Byrne PM, Ferrie PJ, King DR, Roberts JN. Measuring Asthma Control . Clinic Questionnaire or Daily Diary?. Am J Respir Crit Care Med 2000;162(4):1330–1334. [DOI] [PubMed] [Google Scholar]
- 42.Yu S, Chang H, Lin M, Lin Y. Agreement between self-reported and health insurance claims on utilization of health care: A population study. J Clin Epidemiol 2009;62(12):1316–1322. doi: 10.1016/j.jclinepi.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Oksanen T, Kivimäki M, Pentti J, Virtanen M, Klaukka T, Vahtera J. Self-Report as an Indicator of Incident Disease. Ann Epidemiol 2010;20(7):547–554. doi: 10.1016/j.annepidem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. Journal of Clinical Epidemiology 2004;57(10):1096–1103. [DOI] [PubMed] [Google Scholar]


