Abstract
Purpose:
Loss of colonization resistance within the gastrointestinal microbiome facilitates the expansion of pathogens and has been associated with death and infection in select populations. We tested whether gut microbiome features at the time of intensive care unit (ICU) admission predict death or infection.
Methods:
This was a prospective cohort study of medical ICU adults. Rectal surveillance swabs were performed at admission, selectively cultured for vancomycin-resistant Enterococcus (VRE), and assessed using 16S rRNA gene sequencing. Patients were followed up for 30 days for death or culture-proven bacterial infection.
Results:
Of 301 patients, 123 (41%) developed culture-proven infections and 76 (25%) died. Fecal biodiversity (Shannon index) did not differ based on death or infection (p=0.49). The presence of specific pathogens at ICU admission was associated with subsequent infection with the same organism for Escherichia coli, Pseudomonas spp., Klebsiella spp., and Clostridium difficile, and VRE at admission was associated with subsequent Enterococcus infection. In a multivariable model adjusting for severity of illness, VRE colonization and Enterococcus domination (≥30% 16S reads) were both associated with death or all-cause infection (aHR 1.46, 95% CI 1.06–2.00 and aHR 1.47, 95% CI 1.00–2.19 respectively); among patients without VRE colonization, Enterococcus domination was associated with excess risk of death or infection (aHR 2.13, 95% CI 1.06–4.29).
Conclusions:
Enterococcus status at ICU admission was associated with risk for death or all–cause infection, and rectal carriage of common ICU pathogens predicted specific infections. The gastrointestinal microbiome may have a role in risk stratification and early diagnosis of ICU infections.
Keywords: critical care, nosocomial infection, vancomycin-resistant Enterococcus, microbiome, colonization resistance, mortality
INTRODUCTION
Infection is the leading cause of death in medical intensive care units (ICUs) and accounts for 40% of all ICU expenditures [1]. ICU patients are more likely than ward patients to acquire infections while hospitalized with an attributable mortality up to 25% [2]. Antimicrobials, often given in a probabilistic fashion, are the mainstay of treatment for ICU infections [3]. But antimicrobial resistance is widespread, and there is a need for early diagnostics to facilitate targeted antimicrobial therapy [4].
The gastrointestinal tract is the primary reservoir for the bacterial pathogens that cause most healthcare-associated infections [5]. Yet the majority of patients who are colonized with pathogens never develop infections [6]. It is unknown whether there are features within the gastrointestinal microbiome at the time of ICU admission that predict subsequent infection and how such features may interact with established predictors of ICU morbidity and mortality.
Commensal colonic anaerobes check the expansion of pathogens through a process known as colonization resistance [7]. Fecal biodiversity has been evaluated as a surrogate for colonization resistance and thus as a proxy for the overall health of the gastrointestinal microbiome [8, 9]. In previous studies, low fecal biodiversity has been associated with increased risk for death or infection [10, 11] and domination of the gastrointestinal microbiota by a single organism, often Enterococcus, has been identified as an independent risk factor for adverse outcomes [12–14]. It is uncertain, however, whether measures such as diversity and domination are appropriate predictors for risk for infection in a heterogeneous medical ICU population.
This prospective study sought to evaluate clinically relevant risk factors within the gastrointestinal microbiome by collecting rectal swabs from medical ICU patients at admission and following patients for 30 days for death or culture-proven infection.
METHODS
Population
Unique adult patients were considered for the study if they were admitted to the medical ICU of Columbia University Medical Center (CUMC) between April 1, 2016 and August 31, 2017. Patients were eligible if they were either directly admitted to the ICU (i.e., through the emergency room) or if they were transferred to the ICU from a hospital ward. The study was approved by the institutional review board of CUMC with a waiver of informed consent.
Rectal Swabs
In the participating ICUs, a rectal surveillance swab is routinely performed on all patients at the time of ICU admission to evaluate for VRE colonization status. Flocked rectal swabs (Copan) [15] were collected by nurses with patients turned onto the left lateral decubitus position immediately after transfer into the ICU bed and the swab inserted deeply into the rectal canal and rotated 5 times. Swabs were transported in liquid Amies media for VRE culture and then flash-frozen at −80º C. Additional surveillance swabs were not collected routinely and were not available for the study.
VRE Culture
Swabs were streaked onto plates of chromogenic media impregnated with 6 μg/ml of vancomycin (Remel). Plates were incubated at 33−37º C under aerobic conditions for 24 hours and interpreted categorically according to the manufacturer’s instructions (VRE present versus VRE absent).
16S rRNA Gene Sequencing
Swabs were removed from media, cultured for VRE, and sequenced for the V3/V4 hypervariable regions of the 16S ribosomal RNA gene using the Illumina MiSeq platform with negative controls [16], The QIIME pipeline [17] was used to merge paired–end reads with ≥Q20 quality Phred score and minimum overlap of 25%. Rarefaction was at 6,000 reads based on curve plateaus for alpha diversity. Operational taxonomic units (OTUs) were clustered at 97% sequence similarity and aligned against the Greengenes database with noise filtering [18], Sequencing data is available in the short read archive section of the National Center for Biotechnology Information (accession number SRP130887).
Analysis of 16S Data
Two approaches were used to identify differentially abundant taxa. First, a targeted approach was performed with the most common pathogens classified as present if one or more read was present after rarefaction (≥.017% relative abundance) and otherwise as absent. In this approach, OTU identifiers were assigned to specific pathogens at the lowest possible hierarchical level (species level for E. coli, C. difficile, and S. aureus, and genus level for Pseudomonas, Klebsiella, and Enterococcus). Taxa with median relative abundance ≥1% were further classified based on whether they showed domination using the cut-off of defined by Taur et al. of ≥ 30% 16S reads [12]. Second, an untargeted approach was performed using LEfSe [19] to compare OTU differences between groups after excluding taxa present in <10% of samples; a least discriminant analysis cut-off of ≥2.0 applied. The Shannon index was selected a priori as a measure of within-sample fecal microbial diversity and the Chao index as a measure of fecal microbial richness.
Death and Infection
The primary outcome of the study was a composite of death or culture-proven infection based on cultures obtained during the 30 days after ICU admission. Vital status data were retrieved from the hospital EMR which was cross-referenced with the national social security death index. Culture-proven infection was independently ascertained with blinding to pathogen status by evaluating complete clinical data and using criteria adapted from Sepsis-3 (Supplementary Table 1 for complete criteria) [20]. Culture-proven infections were further classified according to the infecting organism and the site of infection.
Statistical Approach
Clinical and laboratory-based co-variables from ICU admission were derived from the Simplified Acute Physiology Score-3 (SAPS3) and gathered from the electronic medical record using automated queries [21]. A modified SAPS3 score, calculated excluding the Glasgow Coma Scale and surgical site information, was examined both as a linear variable and as an ordinal variable classified into tertiles. For hematocrit and albumin, which have been shown to predict ICU outcomes but are not included in SAPS3 [22], standard laboratory cut-offs were used. Additional non-SAPS3 variables included recent prior hospitalization or surgery, an admission diagnosis of sepsis, immunosuppression, and treatments received at the time of ICU admission including antibiotics, which were further classified as narrow- or broad-spectrum (see supplement). The final multivariable analysis was constructed using a Cox proportional hazards model with patients followed from the time of ICU admission until death, culture-proven infection, or for a maximum of 30 days. The proportional hazards assumption was verified by visual inspection of time-to-event data and by testing for a non-zero slope in the Schoenfeld residuals. Non-SAPS3 variables were assessed in the full model if they had evidence of a relationship with the outcome (p<. 10) and other variables as components of SAPS3. Variables wereretained in the reduced model if they had a significant independent relationship with the composite outcome or if they altered the β-coefficient representing fecal microbial characteristics by at least 10%. All analyses were performed using Stata statistical software (version 14, StataCorp) and were conducted two-sided at the α = .05 level of significance.
RESULTS
A total of 301 patients were included in the study. There were 123 patients (41%) who developed 211 distinct culture-proven infections and 76 patients (25%) who died within the 30 days following ICU admission. Table 1 describes the characteristics of patients at ICU admission stratified by death or culture-proven infection. The SAPS3 score was well calibrated to the outcome (Supplementary Table 2). The most common culture-proven infections were from Staphylococcus aureus (28 patients), Escherichia coli (26), Pseudomonas aeruginosa (23), Enterococcus spp. (22, including 16 VRE infections), Klebsiella pneumoniae (21), and Clostridium difficile (9). The most common infection sites were respiratory (58 patients), blood (27), and urine (19). Supplementary Table 3 describes infections according to organism and infection site.
Table 1.
Demographic, clinical, and laboratory characteristics at the time of ICU admission, stratified by death or infection status during the next 30 days. SAPS3: simplified acute physiology score-3; ICU: intensive care unit; ER: emergency room; AIDS: acquired immunodeficiency syndrome.
| SAPS3 Characteristics at ICU Admission | No Death or Infection N=133 | Death or Infection N=168 | p-value |
|---|---|---|---|
| Age | .11 | ||
| <40 years old | 27 (20%) | 18 (11%) | |
| 40–59 years old | 40 (30%) | 43 (26%) | |
| 60–69 years old | 27 (20%) | 48 (29%) | |
| 70–74 years old | 10 (8%) | 14 (8%) | |
| 75–79 years old | 13 (10%) | 15 (9%) | |
| ≥80 years old | 16 (12%) | 30 (18%) | |
| Hospital ward stay prior to admission | .68 | ||
| ≥28 days | 11 (8%) | 15 (9%) | |
| 15–27 days | 12 (9%) | 14 (8%) | |
| 1–14 days | 47 (35%) | 70 (42%) | |
| None (admission through ER) | 63 (47%) | 69 (41%) | |
| SAPS3 reason for admission | .54 | ||
| Rhythm disturbances | 1 (1%) | 4 (2%) | |
| Hypovolemic shock | 1 (1%) | 4 (2%) | |
| Coma/obtunded | 5 (4%) | 8 (5%) | |
| Septic shock | 5 (4%) | 10 (6%) | |
| Liver failure | 3 (2%) | 6 (4%) | |
| Focal neurological deficit | 0 (0%) | 1 (1%) | |
| Severe pancreatitis | 0 (0%) | 1 (1%) | |
| Other | 118 (89%) | 134 (80%) | |
| SAPS3 preexisting comorbidities | .06 | ||
| Heart failure | 3 (2%) | 13 (8%) | |
| Cirrhosis or AIDS | 5 (4%) | 9 (5%) | |
| Cancer | 7 (5%) | 16 (10%) | |
| None of the above | 118 (89%) | 130 (77%) | |
| Use of vasopressors | 64 (38%) | 104 (62%) | <.01 |
| Temperature >35°C | 3 (2%) | 8 (5%) | .25 |
| Heart rate ≥ 120 bpm | 7 (5%) | 20 (12%) | .05 |
| Systolic blood pressure | .58 | ||
| ≥120 mm Hg | 79 (59%) | 90 (54%) | |
| 70–119 mm Hg | 53 (40%) | 76 (45%) | |
| <70 mm Hg | 1 (1%) | 2 (1%) | |
| PaO2/FIO2 (on mechanical ventilation) | .14 | ||
| <100 mm Hg | 7 (5%) | 9 (5%) | |
| ≥100 mm Hg | 38 (29%) | 66 (39%) | |
| PaO2 <60 mm Hg (not on mechanical ventilation) | 5 (4%) | 8 (5%) | .67 |
| White blood cell count ≥15 × 109/L | 45 (34%) | 62 (37%) | .58 |
| Platelet count (x 103/ml) | .03 | ||
| >100 | 113 (85%) | 131 (78%) | |
| 50–99 | 14(11%) | 19(11%) | |
| 20–49 | 3 (2%) | 17 (10%) | |
| <20 | 3 (2%) | 1 (1%) | |
| pH ≤ 7.25 | 14(11%) | 21 (13%) | .60 |
| Creatinine (μmol/L) | .24 | ||
| >309.4 | 25 (19%) | 43 (26%) | |
| 176.8–309.3 | 22 (17%) | 36 (21%) | |
| 106.1–176.7 | 33 (25%) | 35 (21%) | |
| <106.1 | 53 (40%) | 54 (32%) | |
| Bilirubin (pmol/L) | .06 | ||
| >102.6 | 14(11%) | 28 (17%) | |
| 34.2–102.6 | 11 (8%) | 24 (14%) | |
| <34.2 | 108 (81%) | 116 (69%) | |
| Additional Clinical Characteristics at ICU Admission | |||
| Previous hospital admission within 90 days | 28 (21%) | 59 (35%) | <.01 |
| Major surgery within 30 days | 11 (8%) | 8 (5%) | .21 |
| Sepsis in admitting diagnosis | 22 (17%) | 58 (35%) | <.01 |
| Immunosuppression | 39 (29%) | 43 (26%) | .47 |
| Myeloablative chemotherapy | 7 (5%) | 15 (9%) | .23 |
| Hematocrit <35% | 86 (65%) | 123 (73%) | .11 |
| Albumin <221 μmol/L | 14(11%) | 44 (26%) | .09 |
| Treatments received at admission | |||
| Mechanical ventilation | 45 (34%) | 75 (45%) | .06 |
| Hemodialysis | 22 (17%) | 32 (19%) | .57 |
| Proton pump inhibitors | 39 (29%) | 60 (36%) | .24 |
| Antibiotics | <.01 | ||
| None | 40 (30%) | 24 (14%) | |
| Narrow-spectrum only | 8 (6%) | 13 (8%) | |
| Broad spectrum with or without narrow-spectrum | 85 (64%) | 131 (78%) | |
Microbiome Features at ICU Admission
The presence of potential pathogens at ICU admission was common based on sequencing data: Enterococcus spp. (88%), Klebsiella spp. (61%), S. aureus (29%), Pseudomonas spp. (22%), C. difficile (6%), and E. coli (4%). Among these organisms, only Enterococcus had >1% relative abundance (Supplementary Table 4). Domination by Enterococcus (>30% reads) was present in 44 patients (15%) where it was also the most abundant organism; VRE was cultured in 91 patients (30%). Enterococcus domination was strongly associated with culture showing VRE (77% positive VRE culture in dominated patients vs 22% in other patients, p<.01).
Patient Factors Associated with the Microbiome
Fecal microbial diversity and richness were decreased in patients who were transferred to the ICU from a hospital ward compared to those admitted directly to the ICU (both p<.01). Diversity and richness were inversely correlated with the duration of pre-ICU hospitalization (Spearman p<.01 for both), and were also inversely correlated with VRE colonization and Enterococcus domination (Supplementary Table 5). Enterococcus domination was more common in patients transferred to the ICU (20% vs 8%, p<.01) and also more common with a longer duration of pre-ICU hospitalization (median difference +11 days, p<.01); both of these results were similar when VRE status was assessed by culture. Pathogen status at admission was not clearly associated with transfer to the ICU from a hospital ward for any of the other common pathogens, and no other baseline patient factors were significantly associated with admission microbiome features.
Microbiome Features Associated with Death or Infection
Neither fecal biodiversity (Shannon index) nor richness (Chao) differed between those who died or developed infections compared to those who did not (p=.49 and p=.46 respectively). Principal components analysis did not separate samples based on death or infection (Supplementary Fig.1).
Specific Infections
Enterococcus domination as assessed by 16S sequencing was not associated with subsequent Enterococcus infection (9% domination vs 7% other patients, Fig. 1). However, culture for VRE at ICU admission was associated with subsequent Enterococcus infections (18% vs 3% respectively, p<.01). Patients with E. coli present at ICU admission were more likely to develop subsequent E. coli infection compared to patients without E. coli (p=.05). This was also true for C. difficile (p<.01), P. aeruginosa (p=.02), and K. pneumoniae (p<.01), but not for S. aureus (Fig. 1).
Fig. 1. Rectal carriage of specific bacteria at ICU admission was associated with subsequent infections with the same bacteria.
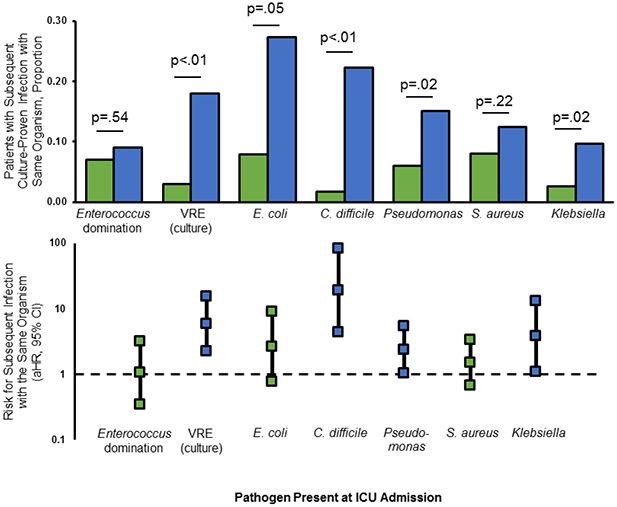
Rectal carriage status for each organism was classified as present versus absent at ICU admission based on selective culture for vancomycin-resistant Enterococcus (VRE) and 16S sequencing for all other organisms. Domination by Enterococcus was classified categorically based on a cut-off of ≥30% 16S reads as per Taur et. al. Patients were then followed for culture-proven infections with these same organisms. These six organisms represent the most common culture-proven infections within the cohort. In the top part of the figure, blue bars = yes/present and green bars = no/absent. In the bottom part of the figure, the same relationships are shown after adjusting for colonization with VRE, SAPS3 score, the presence of sepsis, heart failure, elevated heart rate, and low serum albumin levels (see Table 1 for co-variable definitions). Blue indicates statistical significance whereas green does not.
Death or All-Cause Infection
Colonization with VRE or Enterococcus domination at ICU admission were associated with a 19% and 22% increased risk for death or infection respectively (p<.01 for both). When these outcomes were examined separately, VRE colonization was associated with 30-day mortality (p=.03, Supplementary Fig. 2) and, among those who survived, with all-cause infection (p<.01, Supplementary Fig. 3). Results were similar for Enterococcus domination. VRE colonization and Enterococcus domination were also associated with increased length of ICU stay (median differences +1 and +2 days, p=.04 and p<.01 respectively). Patients who were discordant for Enterococcus domination and VRE had increased risk for death or infection regardless of which test was positive (Fig. 2). Presence of the other common pathogens at ICU admission did not associate with subsequent death or all-cause infection. Alternative cut-points for these organisms were explored but this did not alter the results (Supplementary Table 6). In an untargeted analysis, twenty-two OTUs differed in patients who died or developed infections compared to patients who did not (Supplementary Table 7). Among 14 OTUs that were increased in those who died or developed infections, 7 were assigned to the Enterobacteriaceae or Pseudomonadaceae families.
Fig. 2. Patients who were discordant for Enterococcus domination and VRE had increased risk for death or infection regardless of which test was positive.
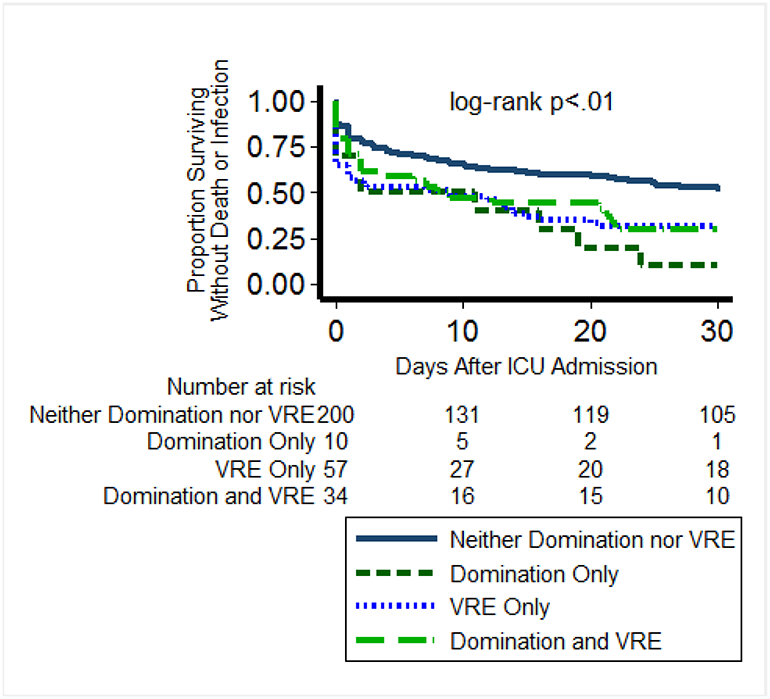
Enterococcus domination (≥30% reads) was assessed by 16S sequencing while VRE status was assessed using culture on selective media. All patients were followed for 30 days for survival or all-cause culture-proven infections. In general, results for Enterococcus domination and VRE in culture were highly concordant. Log-rank test for equality of the survivor functions across the 4 groups. ICU: intensive care unit; VRE: vancomycin-resistant Enterococcus.
Multivariable Model for Death or All-Cause Infection
A Cox proportional hazards regression model was constructed to examine specific pathogens as risk factors for death or all-cause infection after ICU admission (Table 2). Colonization with VRE at ICU admission was a significant predictor of death or infection in the final model and results were similar when Enterococcus domination was entered in the model in lieu of VRE colonization. There was a trend towards increased 30-day mortality associated with VRE and with Enterococcus domination that was not statistically significant (respectively aHR 1.29, 95% CI 0.80–2.07 and aHR 1.24, 95% CI 0.68–2.27). Among those without VRE colonization, Enterococcus domination strongly associated with the combined outcome (aHR 2.13, 95% CI 1.05–4.29). There were no significant changes in these models after excluding 81 subjects who died or developed infections within 72 hours of ICU admission, and also no changes when SAPS3 was classified as a linear variable. Presence of any of the other common bacterial pathogens did not significantly predict death or all-cause infection (Supplementary Table 8). However, the presence of taxa corresponding to C. difficile, Pseudomonas, and Klebsiella all predicted subsequent infections with the same organisms after adjusting for the variables in the final model (Fig. 1). Neither fecal microbial diversity nor richness was significant in the final model (aHR 1.10, 95% CI 0.93 – 1.30 and aHR 1.00, 95% CI 0.99 – 1.01 respectively).
Table 2.
The full and reduced Cox proportional hazards models for death or infection. VRE: vancomycin-resistant Enterococcus; ICU: intensive care unit; CI: confidence interval.
| Risk Factors at ICU Admission | Full Model Hazard Ratio, 95% Cl | Reduced Model Hazard Ratio, 95% Cl |
|---|---|---|
| VRE colonization | 1.43 (1.04–1.97) | 1.46 (1.06–2.00) |
| SAP S3 tertile | ||
| Tertile 1 | Reference | Reference |
| Tertile 2 | 1.84 (1.22–2.79) | 1.93 (1.29–2.88) |
| Tertile 3 | 1.87 (1.23–2.83) | 2.01 (1.36–2.98) |
| Hospitalization within 90 days | 1.43 (1.04–1.98) | 1.42 (1.03–1.96) |
| Mechanical ventilation | 1.19 (0.86–1.64) | --- |
| Receipt of antibiotics | ||
| None | Reference | --- |
| Narrow-spectrum only | 1.70 (0.86–3.37) | |
| Broad-spectrum | 1.31 (0.83–2.08) | |
| Sepsis | 1.69 (1.21–2.36) | 1.72 (1.25–2.39) |
| Albumin <221 μmol/L | 1.60 (1.11–2.29) | 1.60 (1.12–2.28) |
DISCUSSION
This prospective study found that the presence or absence of specific bacteria at ICU admission was associated with death or culture-proven infection during the next 30 days. Taxa corresponding to the common pathogens Enterococcus, Pseudomonas, Klebsiella, and C. difficile were each individually associated with subsequent infection with the same organisms. Enterococcus was unique: domination of the gastrointestinal microbiome by Enterococcus or VRE colonization based on culture predicted death or all-cause infection; even among those who were not VRE colonized, Enterococcus domination remained a strong predictor of death or infection. Overall gastrointestinal diversity and richness, predictors of death or infection in other populations, were not associated with death or infection in this cohort.
Previous studies have demonstrated that pathogens can be cultured from stool or swabs that predict specific infections. Organism-specific colonization has been established as a risk factor for subsequent infection for VRE [13], MRSA [23], C. difficile [24], Enterobacteriaceae [25], and other bacteria. The role of quantitative culture in the ICU has also been investigated, especially for the diagnosis of ventilator-associated pneumonia [26]. This study focused on 16S sequencing rather than culture and found that the presence or absence of certain taxa predicted common infections. These findings highlight the potential for stool testing as a guide to early empiric therapy in the ICU. For Enterococcus, the pathogen that best predicted death or infection, sequencing results were closely comparable to results from VRE cultures. Gain and loss of resistance is common for hospital E. faecalis lineages and domination may be an important marker even in the absence of vancomycin resistance [27].
Microbiome-based interventions such as probiotics have been tested in the ICU to decrease infection, but no currently available therapies are proven to improve gut colonization resistance. Surveillance for gastrointestinal bacterial pathogen colonization usually focuses on multi-drug resistant organisms [28] and remains controversial [29]. Translating the identification of colonized patients into a subsequent reduction in risk for infection will only be possible if effective interventions are available [30] and if appropriately high-risk patients can be selected prior to implementing any intervention. This study’s results suggest that Enterococcus burden may complement VRE status as a useful surrogate target for studies testing probiotics or conventional infection control-based strategies. Future studies may also wish to examine whether a tipping point can be identified for Enterococcus domination, and whether specific interventions can affect the relative abundance of Enterococcus in ICU patients.
In these data, the risk associated with gastrointestinal colonization depended on the organism. The most dramatic results were seen with Enterococcus whereas C. difficile, Pseudomonas, and Klebsiella were each individually associated with specific infections but not with overall infection. Enterococci clones such as E.faecium ST796 exhibit broad antibiotic resistance and starvation tolerance, characteristics that help them to thrive under unique ecological conditions including the selective pressure of vancomycin [31]. Selective killing of Gram-negative bacteria by antibiotics reduces RegIII-γ levels and allows expansion of Gram-positives including Enterococcus [32]. As a result, when the right conditions exist, Enterococcus exhibits a densely dominating phenotype [33]. When other conditions exist, such as high levels of nitrate and colonic inflammation, a more diverse set of pathogens may appear including multiple Gammaproteobacteria species [34]. This may explain why Enterococcus associated with all–cause infection whereas the other organisms did not: the ecological features of Enterococcus mean that it uniquely reflects loss of gastrointestinal colonization resistance.
This study’s results regarding fecal microbial diversity contrast with the results of Taur et al., who found that fecal microbial diversity prior to BMT was an important predictor of death and infection [12]. Compared to healthy volunteers, ICU patients have low fecal microbial diversity [35, 36], which further declines with prolonged admission [37], and are likely to show Enterococcus domination [38]. BMT patients also have low diversity compared to healthy subjects, but have profound immunological changes compared to ICU patients. In BMT patients, lack of transplant engraftment mediates the development of infection and high gastrointestinal biodiversity may reflect a Th1 and Th17 phenotype driven by obligate anaerobes that defends against infection [39]. In contrast for ICU patients, who do not have to completely reconstitute their immunity, diversity may be less important as a marker of loss of colonization resistance. Acuity of illness also may be a factor. ICU patients are heterogeneous and include patients who lack sufficient time to lose diversity between the onset of illness and ICU admission.
There are several strengths to this study. It was prospective, relatively large, incorporated both culture and sequencing data, and involved a standard medical ICU population that facilitates generalization of the results. The primary outcome—death or culture-proven infection—was important and rigorously adjudicated. The study also has limitations. There is no single gold standard for the ascertainment of infection in the ICU; the definition used here was designed to maximize specificity rather than sensitivity. Sequencing results equate OTUs with specific bacteria but rarely distinguish bacteria at the species level (e.g., E.faecium vs E.faecalis) and may not correlate with culture-based tests. This study used rarefied data to focus on relatively abundant gut bacteria, and alternative methods would be necessary to examine very scarce taxa. Last, although the types of bacterial infections observed here parallel results from other ICUs in the developed world, results from this study may not apply to all institutions or ICUs [40].
In sum, the specific pathogens responsible for most medical ICU infections could be identified at the time of ICU admission. Colonization with VRE and domination by Enterococcus were important risk factors for all-cause infection and mortality, with domination a risk factor even in the absence of VRE. The presence of VRE or Enterococcus domination may be useful surrogate outcomes for studies seeking to intervene on the gastrointestinal microbiome to prevent infections in critically ill patients or similarly at-risk patient populations.
Supplementary Material
Acknowledgments
Funding: This research was supported in part by the National Institutes of Health (DK111847) and the American Gastroenterological Association Research Scholar Award (to D.E.F.).
Footnotes
Conflicts of interest
None of the authors have conflicts of interest.
REFERENCES
- 1.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, Investigators EIGo (2009). International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–9 [DOI] [PubMed] [Google Scholar]
- 2.Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar JR, Soufir L, Lazard T, Jamali S, Mourvillier B, Cohen Y, De Lassence A, Azoulay E, Cheval C, Descorps-Declere A, Adrie C, Costa de Beauregard MA, Carlet J, Group OS (2006). Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: a reappraisal. Clin Infect Dis 42:1118–26 [DOI] [PubMed] [Google Scholar]
- 3.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA (2016). Estimating National Trends in Inpatient Antibiotic Use Among US Hospitals From 2006 to 2012. JAMA Intern Med 176:1639–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burillo A, Bouza E (2014). Use of rapid diagnostic techniques in ICU patients with infections. BMC Infect Dis 14:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskey CJ (2006). Antibiotic regimens and intestinal colonization with antibiotic-resistant gram-negative bacilli. Clin Infect Dis 43 Suppl 2:S62–9 [DOI] [PubMed] [Google Scholar]
- 6.Johanson WG, Pierce AK, Sanford JP (1969). Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram-negative bacilli. N Engl J Med 281:1137–40 [DOI] [PubMed] [Google Scholar]
- 7.Hentges DJ, Freter R (1962). In vivo and in vitro antagonism of intestinal bacteria against Shigella flexneri. I. Correlation between various tests. J Infect Dis 110:30–7 [DOI] [PubMed] [Google Scholar]
- 8.Doki N, Suyama M, Sasajima S, et al. (2017). Clinical impact of pre-transplant gut microbial diversity on outcomes of allogeneic hematopoietic stem cell transplantation. Ann Hematol 96:1517–23 [DOI] [PubMed] [Google Scholar]
- 9.Harris B, Morjaria SM, Littmann ER, Geyer AI, Stover DE, Barker JN, Giralt SA, Taur Y, Pamer EG (2016). Gut Microbiota Predict Pulmonary Infiltrates after Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med 194:450–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, Ponce DM, Barker JN, Giralt S, van den Brink M, Pamer EG (2014). The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124:1174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu K, Ogura H, Hamasaki T, Goto M, Tasaki O, Asahara T, Nomoto K, Morotomi M, Matsushima A, Kuwagata Y, Sugimoto H (2011). Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci 56:1171–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, Perales MA, Jenq RR, van den Brink MR, Pamer EG (2012). Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 55:905–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziakas PD, Thapa R, Rice LB, Mylonakis E (2013). Trends and significance of VRE colonization in the ICU: a meta-analysis of published studies. PLoS One 8:e75658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung E, Byun S, Lee H, Moon SY, Lee H (2014). Vancomycin-resistant Enterococcus colonization in the intensive care unit: clinical outcomes and attributable costs of hospitalization. Am J Infect Control 42:1062–6 [DOI] [PubMed] [Google Scholar]
- 15.Budding AE, Grasman ME, Eck A, Bogaards JA, Vandenbroucke-Grauls CM, van Bodegraven AA, Savelkoul PH (2014). Rectal swabs for analysis of the intestinal microbiota. PLoS One 9:e101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glockner FO (2013). Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Kuczynski J, Stombaugh J, et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurdie PJ, Holmes S (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011). Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC (2016). Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315:762–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR, Investigators S (2005). SAPS 3--From evaluation of the patient to evaluation of the intensive care unit. Part 1: Objectives, methods and cohort description. Intensive Care Med 31:1336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez de Villota E, Mosquera JM, Rubio JJ, Galdos P, Diez Balda V, de la Serna JL, Tomas MI (1980). Association of a low serum albumin with infection and increased mortality in critically ill patients. Intensive Care Med 7:19–22 [DOI] [PubMed] [Google Scholar]
- 23.Garrouste-Orgeas M, Timsit JF, Kallel H, Ben Ali A, Dumay MF, Paoli B, Misset B, Carlet J (2001). Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 22:687–92 [DOI] [PubMed] [Google Scholar]
- 24.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A (2011). Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 365:1693–703 [DOI] [PubMed] [Google Scholar]
- 25.Frencken JF, Wittekamp BHJ, Plantinga NL, Spitoni C, van de Groep K, Cremer OL, Bonten MJM (2017). Associations Between Enteral Colonization With Gram-Negative Bacteria and Intensive Care Unit-Acquired Infections and Colonization of the Respiratory Tract. Clin Infect Dis [DOI] [PubMed] [Google Scholar]
- 26.Ruiz M, Torres A, Ewig S, Marcos MA, Alcon A, Lledo R, Asenjo MA, Maldonaldo A (2000). Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med 162:119–25 [DOI] [PubMed] [Google Scholar]
- 27.Raven KE, Reuter S, Gouliouris T, Reynolds R, Russell JE, Brown NM, Torok ME, Parkhill J, Peacock SJ (2016). Genome-based characterization of hospital-adapted Enterococcus faecalis lineages. Nat Microbiol 1:15033. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention, Methicillin-resistant Staphylococcus aureus infections: ELC Prevention Collaboratives, accessed on-line at https://www.cdc.gov/HAI/pdfs/toolkits/MRSA_toolkit_white_020910_v2.pdf on December 22, 2017.
- 29.Peterson LR, Diekema DJ (2010). To screen or not to screen for methicillin-resistant Staphylococcus aureus. J Clin Microbiol 48:683–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pena C, Pujol M, Ardanuy C, Ricart A, Pallares R, Linares J, Ariza J, Gudiol F (1998). Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 42:53–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao W, Howden BP, Stinear TP (2017). Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr Opin Microbiol 41:76–82 [DOI] [PubMed] [Google Scholar]
- 32.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG (2008). Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 455:804–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arias CA, Murray BE (2012). The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Baumler AJ (2017). Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357:570–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, Lemieux M, Derenski K, King J, Vis-Kampen C, Knight R, Wischmeyer PE (2016). Extreme Dysbiosis of the Microbiome in Critical Illness. mSphere 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, Minami Y, Sugano M, Kubota N, Uegaki S, Kamoshida H, Sawamura A, Nomoto K, Gando S (2011). Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci 56:2361–5 [DOI] [PubMed] [Google Scholar]
- 37.Zaborin A, Smith D, Garfield K, Quensen J, Shakhsheer B, Kade M, Tirrell M, Tiedje J, Gilbert JA, Zaborina O, Alverdy JC (2014). Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio 5:e01361–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iapichino G, Callegari ML, Marzorati S, Cigada M, Corbella D, Ferrari S, Morelli L (2008). Impact of antibiotics on the gut microbiota of critically ill patients. J Med Microbiol 57:1007–14 [DOI] [PubMed] [Google Scholar]
- 39.Kamada N, Seo SU, Chen GY, Nunez G (2013). Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13:321–35 [DOI] [PubMed] [Google Scholar]
- 40.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated I, Antimicrobial Use Prevalence Survey T (2014). Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


