Abstract
Introduction:
Spread through air spaces (STAS) is a form of invasion wherein tumor cells extend beyond the tumor edge within the lung parenchyma. In lung adenocarcinoma (ADC), we investigated the (a) association between STAS and procedure-specific outcomes (sublobar resection and lobectomy), (b) effect of surgical margin/tumor diameter ratio in STAS-positive patients, and (c) potential utility of frozen section (FS) for detecting STAS intraoperatively.
Methods:
We investigated 1497 patients who underwent lobectomy (n=970) or sublobar resection (n=527) for T1N0M0 lung ADC, following propensity-score matching. Outcomes were analyzed using a competing-risks approach. The effect of margin/tumor ratio on recurrence pattern (locoregional and distant) was investigated in sublobar patients. Five pathologists evaluated the feasibility of intraoperatively identifying STAS using FS (sensitivity, specificity, interrater reliability).
Results:
On multivariable analysis following propensity-score matching (349 pairs/procedure), sublobar resection was significantly associated with recurrence (subhazard ratio, 2.84; P<0.001) and lung cancer–specific death (subhazard ratio, 2.63; P=0.021) in patients with STAS but not in those without STAS. Patients with STAS who underwent sublobar resection had a higher risk of locoregional recurrence regardless of margin/tumor ratio (margin/tumor ratio ≥1 vs. <1: 5-year cumulative incidence of recurrence [CIR], 16% and 25%); among patients without STAS, locoregional recurrences occurred in patients with margin/tumor ratio <1 (5-year CIR, 7%). Sensitivity and specificity for detecting STAS by FS were 71% and 92%, with substantial interrater reliability (Gwet’s AC1, 0.67).
Conclusions:
In T1 lung ADC patients with STAS, lobectomy was associated with better outcomes than sublobar resection. Pathologists can recognize STAS on FS.
Keywords: Lung adenocarcinoma, Wedge resection, Recurrence, Lobectomy
INTRODUCTION
Anatomical surgical resection by lobectomy is the standard of care for the management of early-stage lung adenocarcinoma (ADC), the most common histologic subtype of non-small cell lung cancer (NSCLC).1 This follows the Lung Cancer Study Group 821 randomized trial,2 which showed that sublobar resection was associated with a higher risk of recurrence than lobectomy for patients with T1N0M0 NSCLC.3 Despite ongoing concerns about the adequacy of sublobar resection for cure,2,4–6 its use is increasing.7 Randomized trials assessing the outcomes of sublobar versus lobar resection for small (≤2 cm) tumors are ongoing (Japan Clinical Oncology Group 0804 and Cancer and Leukemia Group B 140503).
An analysis of patients with stage I NSCLC from the Surveillance, Epidemiology, and End Results database (1998–2009) showed that both the incidence of small (≤2 cm) NSCLC tumors (the majority of which are lung ADCs) and the use of sublobar resection are increasing.7 Numerous retrospective and ongoing prospective studies have established that tumor size alone is often used to decide the type of resection to perform.8,9 We and others have suggested that the presence and predominance of aggressive histological subtypes determines the outcome independent of the size of the tumor.10–13 More importantly, we identified that the presence of micropapillary (MIP) histologic subtype predispose patients undergoing sublobar resection for small lung ADC to a higher risk of locoregional recurrence,10 despite negative surgical margin. This observation led us to investigate the resected lung beyond the edge of the tumor. We thereby identified a previously unrecognized pattern of invasion: tumor spread through air spaces (STAS), which is defined as tumor cells existing within air spaces in the lung parenchyma beyond the tumor edge. STAS is present in 38% of T1a lung ADCs.14 We were the first to report that STAS is significantly associated with a higher risk of locoregional recurrence following sublobar resection.14 The prognostic importance of STAS has been validated in cohorts from multiple institutional databases15–20 and for other NSCLC histologic subtypes.21–24
Achieving a surgical margin greater than the diameter of the tumor has been recommended as a strategy to decrease the incidence of recurrence following sublobar resection.3,25 A recent study reported that, among 31 STAS-positive tumors, the distance between the farthest STAS lesion and the tumor edge did not exceed the tumor diameter.17 We hypothesized that, in STAS-positive T1N0M0 lung ADCs, achieving a surgical margin greater than the tumor diameter may reduce the incidence of recurrence following sublobar resection. The aim of this study was to investigate the effect of STAS and surgical margin on procedure-specific outcomes (recurrence and lung cancer–specific death) in patients with early-stage lung ADC. Propensity-score matching between patients who underwent lobectomy and sublobar resection was performed using clinical and pathologic factors to address selection bias and differential outcomes between patients who undergo lobectomy versus sublobar resection.
Although histologic subtype can affect outcomes in a procedure-specific manner (lobectomy versus sublobar resection), preoperative imaging and frozen section (FS) is unable to accurately identify the predominant or presence of histologic subtype, which would aid in determining the most appropriate resection to perform.26 In this study, we assessed the potential utility of FS analysis for detecting STAS intraoperatively by investigating the sensitivity, specificity, and interrater reliability of identifying STAS on FS across five pathologists.
METHODS
Study Cohort
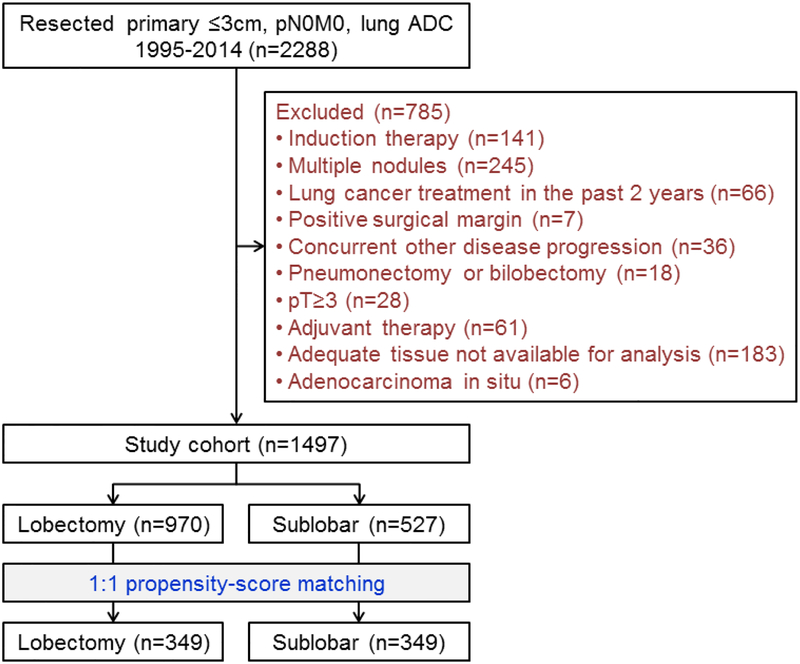
This retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK; WA0269–08). The MSK Thoracic Service’s prospectively maintained lung cancer database was reviewed to identify consecutive patients who had been surgically treated for ≤3 cm pathologic stage I lung ADC between January 1, 1995, and December 31, 2014. Pathologic stage was based on the eighth edition of the American Joint Committee on Cancer Staging Manual.27 Exclusion criteria are shown in Figure 1. In total, 1497 patients met the inclusion criteria. Additional information on pathologic lymph node evaluation and data collection is available in Supplementary Method S1, Method S2, and Table S1.
Figure 1.
CONSORT diagram. ADC, adenocarcinoma.
Recurrence and Lung Cancer–Specific Death as Endpoints
The study endpoints were recurrence and lung cancer–specific death. All recurrences were confirmed by clinical, radiologic, and pathologic assessment and were classified as locoregional or distant.28 Lung cancer–specific death was defined as death due to recurrent disease associated with resected lung cancer (Supplementary Method S3).29
Histologic Evaluation
Tumor slides were reviewed by two experienced thoracic pathologists (S.L. and W.D.T.; Supplementary Methods S4) who were blinded to patient clinical outcomes. Tumor STAS was defined as tumor cells either in clusters, solid nests or aggregates of single cells within air spaces beyond the edge of the main tumor.14 Artifacts were excluded based on previously described criteria.14
Assessment of Surgical Margin Distance and Effect of Margin/Tumor Diameter on Recurrence Pattern
Surgical margin distance was defined as the distance between the surgical staple margin and the nearest tumor edge, which was assessed by gross measurement using a ruler placed along the tumor and surrounding lung parenchyma in the gross specimen after cross-section of the tumor.10 The relationship between surgical margin distance, tumor diameter, and recurrence patterns was evaluated by use of the ratio of surgical margin distance to tumor diameter (margin/tumor ratio).25 Cumulative incidence of each type of recurrence at 5-years (only locoregional recurrence, or distant recurrence including both locoregional and distant) was summarized separately in patients with or without STAS and compared between margin/tumor ratio ≥1 (surgical margin ≥ tumor diameter) and <1 (surgical margin < tumor diameter) using Gray’s test.
Propensity-Score Matching
To reduce potential selection bias related to using a nonrandomized cohort to generate two groups (lobectomy and sublobar resection) with comparable characteristics, we performed propensity score–matched analyses. Year of surgery, age at surgery, sex, smoking status, chronic obstructive pulmonary disease (COPD), cardiovascular disease (CVD), diabetes mellitus (DM), prior lung cancer, prior other malignancies, body mass index, forced expiratory volume in one second (FEV1, predicted), diffusion capacity of the lungs for carbon monoxide (DLCO, predicted), p-Stage, pathologic tumor size, invasive tumor size, lymphovascular invasion (LVI), visceral pleural invasion (VPI), necrosis, and STAS were used to achieve balance in covariates between the two groups. Balance of covariates between the groups was assessed by the absolute standardized mean difference (ASMD) before and after the matching procedure. ASMD≤0.1 indicates balance in the covariate between the two groups.30 Additional information is available in Supplementary Method S5.
Prognostic Analyses
The outcomes of interest were recurrence and lung cancer–specific death, both analyzed in the competing-risk framework. For recurrence, death from any cause without recurrence was considered a competing event. For lung cancer–specific death, death from causes other than lung cancer or from unknown causes was considered a competing event. Cumulative incidence of recurrence (CIR) and lung cancer–specific cumulative incidence of death (LC-CID) were used to estimate the probability of recurrence or lung cancer–specific death following surgical resection with curative intent.31 Patients who did not experience recurrence or die during the study period were censored at the time of the last available follow-up. Differences in CIR or LC-CID between groups were tested using the Gray method.32
Associations between variables and CIR or LC-CID were estimated using Gray and Fine models.33 Multivariable models were constructed in a backwards-selection approach starting with variables with P<0.1 from the univariable analyses. Statistical analyses were performed using R 3.1.1; the “survival” and “cmprsk” software packages were used in the analyses. All P values were two-sided; significance was set at 5%.
FS Analysis for Detection of STAS
To evaluate the feasibility of FS for intraoperative detection of STAS, we assessed the performance of FS slide reporting. FS slides were selected to identify cases that had substantial non-neoplastic lung parenchyma to allow for evaluation of STAS. Performance was quantified by sensitivity, specificity, and interrater reliability (agreement). FS analysis was performed on 48 lung ADC tumors for which complete FS slides, FS control (FSC) slides, and permanent tumor slides were available with adequate adjacent lung parenchyma. The FS slides were independently reviewed for STAS status by five pathologists (S.L., J.C.C., J.M., N.R., W.D.T.) who were blinded to patient clinicopathologic data. This FS slide review to identify STAS was performed specifically for the purposes of this study, after reporting of the final pathologic results. Gwet’s AC1 statistic34 was applied to evaluate interrater reliability, which is an alternative to the Kappa statistic35 when there is potential extreme distribution across categories. The degree of agreement was interpreted as follows: slight agreement, AC1=0.00 to 0.20; fair agreement, AC1=0.21 to 0.40; moderate agreement, AC1=0.41 to 0.60; substantial agreement, AC1=0.61 to 0.80; and almost perfect agreement, AC1≥0.81. Additional information is available in Supplementary Method S6.
RESULTS
Patient Characteristics–Lobectomy vs. Sublobar Resection Before and After Propensity-Score Matching
Table 1 lists patient clinicopathologic characteristics and the differences between the lobectomy and sublobar cohorts before and after propensity-score matching. Before matching, 18 of 25 covariates were unbalanced (ASMD≥0.1) between lobectomy and sublobar resection. The 1:1 matching for lobectomy versus sublobar resection resulted in 349 matched pairs (n=698) with balanced covariates (ASMD≤0.1) except for prior lung cancer (ASMD=0.105).
Table 1. Patient demographic characteristics and difference between lobar and sublobar resection before and after propensity-score matching.
| Before matching (n=1497) | After matching (n=698) | ||||||
|---|---|---|---|---|---|---|---|
| Lobectomy N=970 |
Sublobar N=527 |
Lobectomy N=349 |
Sublobar N=349 |
||||
| Clinicopathologic variables | ASMD* | ASMD* | |||||
| Period of surgery | 1995–1999 | 68 (7) | 22 (4) | 0.435 | 15 (4) | 15 (4) | 0.030 |
| 2000–2004 | 244 (25) | 63 (12) | 45 (13) | 46 (13) | |||
| 2005–2009 | 341 (35) | 179 (34) | 117 (34) | 121 (35) | |||
| 2010–2014 | 317 (33) | 263 (50) | 172 (49) | 167 (48) | |||
| Age at surgery (year) | 69 (61–75) | 70 (64–76) | 0.203 | 69 (64–75) | 70 (63–76) | 0.045 | |
| Sex | Female | 601 (62) | 337 (64) | 0.041 | 222 (64) | 222 (64) | <0.001 |
| Male | 369 (38) | 190 (36) | 127 (36) | 127 (36) | |||
| Smoking | Never | 181 (19) | 87 (17) | 0.066 | 64 (18) | 59 (17) | 0.085 |
| Former | 669 (69) | 379 (72) | 236 (68) | 249 (71) | |||
| Current | 120 (12) | 61 (12) | 49 (14) | 41 (12) | |||
| COPD positive | 157 (16) | 135 (26) | 0.234 | 73 (21) | 78 (22) | 0.035 | |
| CVD positive | 175 (18) | 141 (27) | 0.210 | 84 (24) | 84 (24) | <0.001 | |
| DM positive | 90 (9) | 75 (14) | 0.154 | 43 (12) | 54 (15) | 0.091 | |
| Prior LC positive | 24 (2) | 105 (20) | 0.576 | 18 (5) | 27 (8) | 0.105 | |
| Prior malignancy positive | 240 (25) | 199 (38) | 0.280 | 120 (34) | 129 (37) | 0.054 | |
| BMI (n=1495) | 26 (23–30) | 27 (24–30) | 0.098 | 27 (23–30) | 27 (24–30) | 0.054 | |
| FEV1 (%) (n=1435) | 92 (80–105) | 86 (69–101) | 0.323 | 89 (78–101) | 90 (71–104) | 0.071 | |
| DLCO (%) (n=1377) | 84 (70–97) | 79 (64–92) | 0.328 | 82 (67–95) | 82 (66–94) | 0.097 | |
| Serum creatinine (mg/dL) (n=1450) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.044 | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.038 | |
| SUVmax (n=1226) | 2.5 (1.4–4.7) | 2.1 (1.0–3.6) | 0.259 | 2.1 (1.1–4.0) | 2.2 (1.2–3.9) | 0.062 | |
| Pathologic tumor size (cm) | 1.8 (1.3–2.3) | 1.4 (1.0–1.8) | 0.670 | 1.5 (1.1–1.9) | 1.5 (1.1–1.9) | 0.037 | |
| Invasive tumor size (cm) | 1.4 (1.0–2.0) | 1.0 (0.6–1.5) | 0.678 | 1.1 (0.7–1.5) | 1.1 (0.7–1.5) | 0.017 | |
| p-Stage | IA1 | 144 (15) | 172 (33) | 0.573 | 96 (28) | 96 (28) | 0.019 |
| IA2 | 453 (47) | 223 (42) | 158 (45) | 160 (46) | |||
| IA3 | 252 (26) | 49 (9) | 43 (12) | 41 (12) | |||
| IB | 121 (12) | 83 (16) | 52 (15) | 52 (15) | |||
| LVI positive | 384 (40) | 165 (31) | 0.174 | 108 (31) | 111 (32) | 0.019 | |
| VPI positive | 120 (12) | 83 (16) | 0.097 | 52 (15) | 52 (15) | <0.001 | |
| Necrosis (n=1478) positive | 121 (13) | 35 (7) | 0.201 | 34 (10) | 28 (8) | 0.060 | |
| Histologic grade | Low | 132 (14) | 129 (24) | 0.281 | 73 (21) | 78 (22) | 0.066 |
| Intermediate | 604 (62) | 292 (55) | 197 (56) | 201 (58) | |||
| High | 234 (24) | 106 (20) | 79 (23) | 70 (20) | |||
| Presence (≥5%) of LEP pattern | 616 (64) | 366 (69) | 0.126 | 242 (69) | 241 (69) | 0.006 | |
| Presence (≥5%) of MIP pattern | 473 (49) | 231 (44) | 0.099 | 160 (46) | 143 (41) | 0.098 | |
| Presence (≥5%) of SOL pattern | 386 (40) | 194 (37) | 0.061 | 122 (35) | 128 (37) | 0.036 | |
| STAS positive | 389 (40) | 218 (41) | 0.026 | 141 (40) | 135 (39) | 0.035 | |
| Mutation status (n=1306) | Wild type | 440 (51) | 230 (52) | 0.117 | 161 (50) | 151 (52) | 0.057 |
| EGFR | 181 (21) | 75 (17) | 61 (19) | 49 (17) | |||
| KRAS | 240 (28) | 140 (31) | 99 (31) | 91 (31) | |||
| Outcomes | P | ||||||
| Number of any recurrence | 99 | 102 | 30 | 63 | |||
| Only locoregional recurrence | 24 | 57 | 6 | 34 | |||
| Distant recurrence | 75 | 45 | 24 | 29 | |||
| Number of any death | 212 | 155 | 68 | 96 | |||
| Lung cancer-specific death | 57 | 58 | 16 | 34 | |||
| Other cause / unknown death | 155 | 97 | 52 | 62 | |||
| 5-year CIR (%) | 12 (10–14) | 21 (18–26) | 10 (7–14) | 20 (16–25) | <0.001 | ||
| 5-year LC-CID (%) | 6 (4–7) | 11 (8–15) | 6 (4–10) | 9 (6–13) | 0.013 | ||
| 5-year Overall survival (%) | 84 (82–87) | 74 (69–78) | 82 (77–87) | 78 (74–83) | 0.015 | ||
Data are number (%), median (25, and 75 percentiles), or 5-year cumulative incidence or survival rate (95% confidence interval [lower-upper]).
ASMD (absolute standardized mean difference) ≤0.1 indicates balance in the covariate between the two groups.
Abbreviation: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DLCO, diffusion capacity of the lung for carbon monoxide; DM, diabetes mellitus; FEV1, forced expiratory volume in one second; LC, lung cancer; LEP, lepidic; MIP, micropapillary; p-Stage, pathologic stage; SOL, solid; STAS, spread through air spaces; SUVmax, maximum standardized uptake value; VPI, visceral pleural invasion.
CIR and LC-CID Analysis After Matching: Sublobar Resection vs. Lobectom
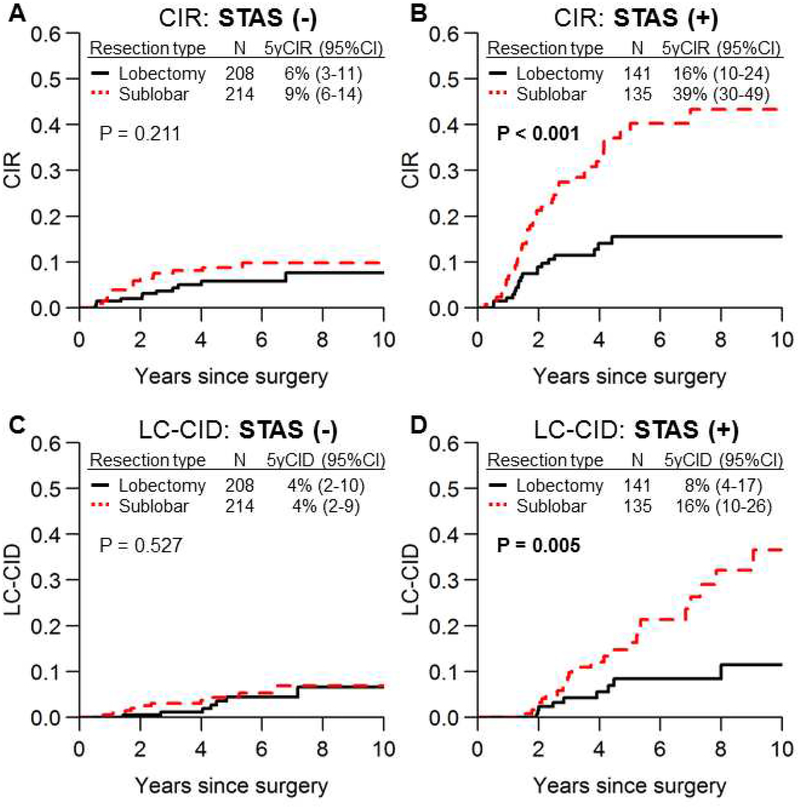
There was no significant difference in CIR between lobectomy and sublobar resection in patients without STAS (Figure 2A); however, in patients with STAS, sublobar resection was associated with a significantly higher risk of recurrence than lobectomy (Figure 2B; 5-year CIR, 39% vs. 16%; P<0.001). Similar results were observed in LC-CID analyses (Figure 2C and 2D). Further analyses to validate the significance of STAS demonstrated that (1) there were no unbalanced clinicopathologic covariates between lobectomy and sublobar resection in both the STAS-positive and STAS-negative cohorts (Supplementary Table S2), and (2) the magnitude of difference in survival outcomes between lobectomy and sublobar resection by STAS status was not influenced by p-Stage, LVI, necrosis, or MIP and solid (SOL) patterns (Supplementary Figure S1 and S2). For example, sublobar resection had a worse prognosis than lobectomy regardless of necrosis status (Supplementary Figure S2B).
Figure 2.
Cumulative incidence of recurrence (CIR) and lung cancer–specific cumulative incidence of death (LC-CID) curves for lobectomy and sublobar resection. CIR (A, B) and LC-CID (C, D) curves comparing lobectomy versus sublobar resection in patients with spread through air spaces (STAS)–negative tumors and patients with STAS-positive tumors are shown. In patients with STAS-negative tumors, risk of recurrence (A) and lung cancer–specific death (C) did not significantly differ between lobectomy and sublobar resection; however, in patients with STAS-positive tumors, sublobar resection was associated with a significantly higher risk of recurrence (B) and lung cancer–specific death (D) (sublobar resection vs. lobectomy: 5-year CIR, 39% vs. 16% [P<0.001]; 5-year CID, 16% vs. 8% [P=0.005]). CI, confidence interval.
In the present study, owing to the small number of patients undergoing segmentectomy, segmentectomy and wedge resection were treated as one group. However, we include a CIR and LC-CID analysis that compares three procedures (lobectomy, segmentectomy, and wedge resection) in Supplementary Figure S3. In patients with STAS, both segmentectomy and wedge resection had higher CIR and LC-CID than lobectomy.
Univariable and Multivariable Competing-Risk Regression Analysis After Matching
Table 2 shows the results of univariable analyses for both survival endpoints of interest. Factors significantly associated with a higher risk of recurrence were sublobar resection, COPD, prior lung cancer, lower FEV1, lower DLCO, higher creatinine level, higher maximum standardized uptake value, larger tumor size, larger invasive tumor size, higher stages, LVI, VPI, necrosis, higher histologic grade, absence of lepidic pattern, presence of MIP, presence of SOL, and absence of EGFR mutation. Factors significantly associated with a higher risk of lung cancer–specific death were sublobar resection, smoking, COPD, lower FEV1, lower DLCO, larger invasive tumor size, higher stages, LVI, VPI, necrosis, higher histologic grade, presence of MIP, presence of SOL, and absence of EGFR mutation.
Table 2. Univariable competing risk regression for recurrence and lung cancer–specific death after propensity-score matching.
| Recurrence | Lung cancer—specific death | ||||||
|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P | SHR | 95% CI | P | ||
| Sublobar resection (vs. lobectomy) | 2.19 | (1.42 – 3.37) | <0.001 | 2.10 | (1.16 – 3.80) | 0.014 | |
| Age at surgery (per 1 year increase) | 1.01 | (0.99 – 1.03) | 0.4 | 1.02 | (0.99 – 1.05) | 0.2 | |
| Male sex (vs. female) | 1.38 | (0.92 – 2.08) | 0.12 | 1.60 | (0.92 – 2.77) | 0.094 | |
| Smoking (vs. never) | Former | 1.29 | (0.71 – 2.34) | 0.4 | 9.42 | (1.29 – 68.85) | 0.027 |
| Current | 1.25 | (0.57 – 2.77) | 0.6 | 11.34 | (1.42 – 90.56) | 0.022 | |
| COPD history (vs. no COPD) | 1.75 | (1.12 – 2.71) | 0.013 | 2.25 | (1.27 – 3.98) | 0.005 | |
| CVD history (vs. no CVD) | 0.86 | (0.52 – 1.41) | 0.5 | 1.02 | (0.53 – 1.94) | 1 | |
| DM history (vs. no DM) | 0.78 | (0.40 – 1.51) | 0.5 | 0.80 | (0.32 – 2.01) | 0.6 | |
| Prior LC (vs. no prior LC) | 2.96 | (1.67 – 5.23) | <0.001 | 1.67 | (0.66 – 4.23) | 0.3 | |
| Prior malignancy (vs. no prior malignancy) | 0.80 | (0.52 – 1.24) | 0.3 | 0.69 | (0.38 – 1.25) | 0.2 | |
| BMI (per 1 index) | 0.99 | (0.95 – 1.02) | 0.5 | 0.96 | (0.92 – 1.00) | 0.076 | |
| FEV1 (per 1%) | 0.99 | (0.98 – 1.00) | 0.009 | 0.99 | (0.97 – 1.00) | 0.039 | |
| DLCO (per 1%) | 0.99 | (0.98 – 1.00) | 0.014 | 0.99 | (0.97 – 1.00) | 0.021 | |
| Serum creatinine (per 1 mg/dL increase) | 1.93 | (1.15 – 3.24) | 0.013 | 2.84 | (1.37 – 5.89) | 0.005 | |
| SUVmax (per 1 SUV increase) | 1.07 | (1.03 – 1.12) | 0.001 | 1.07 | (1.01 – 1.14) | 0.025 | |
| Pathologic tumor size (per 1 cm increase) | 1.44 | (1.00 – 2.06) | 0.048 | 1.24 | (0.73 – 2.12) | 0.4 | |
| Invasive tumor size (per 1 cm increase) | 2.27 | (1.71 – 3.01) | <0.001 | 1.95 | (1.33 – 2.87) | <0.001 | |
| p-Stage (vs. IA1) | IA2 | 2.83 | (1.43 – 5.57) | 0.003 | 1.98 | (0.80 – 4.88) | 0.14 |
| IA3 | 2.37 | (0.96 – 5.85) | 0.062 | 1.90 | (0.53 – 6.78) | 0.3 | |
| IB | 6.15 | (2.98 – 12.67) | <0.001 | 6.12 | (2.47 – 15.43) | <0.001 | |
| LVI (vs. no LVI) | 4.10 | (2.70 – 6.24) | <0.001 | 4.68 | (2.59 – 8.46) | <0.001 | |
| VPI (vs. no VPI) | 2.84 | (1.82 – 4.45) | <0.001 | 3.73 | (2.11 – 6.58) | <0.001 | |
| Necrosis (vs. no necrosis) | 3.35 | (2.02 – 5.57) | <0.001 | 3.94 | (2.05 – 7.55) | <0.001 | |
| Histologic grade (vs. low) | Intermediate | 8.76 | (2.77 – 27.71) | <0.001 | 11.71 | (1.61 – 85.13) | 0.015 |
| High | 9.12 | (2.76 – 30.18) | <0.001 | 16.37 | (2.19 – 122.59) | 0.007 | |
| Presence of LEP pattern (vs. no LEP pattern) | 0.59 | (0.39 – 0.89) | 0.012 | 0.59 | (0.34 – 1.02) | 0.060 | |
| Presence of MIP pattern (vs. no MIP pattern) | 3.14 | (2.01 – 4.89) | <0.001 | 2.11 | (1.19 – 3.74) | 0.010 | |
| Presence of SOL pattern (vs. no SOL pattern) | 3.05 | (2.02 – 4.62) | <0.001 | 4.47 | (2.45 – 8.15) | <0.001 | |
| STAS (vs. no STAS) | 3.80 | (2.45 – 5.90) | <0.001 | 3.31 | (1.85 – 5.95) | <0.001 | |
Abbreviation: BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DLCO, diffusion capacity of the lung for carbon monoxide; DM, diabetes mellitus; FEV1, forced expiratory volume in one second; LC, lung cancer; LEP, lepidic; LVI, lymphovascular invasion; MIP, micropapillary; p-Stage, pathologic stage; SHR, subhazard ratio; SOL, solid; STAS, spread through air spaces; SUVmax, maximum standardized uptake value; VPI, visceral pleural invasion.
Table 3 shows the results of the final multivariable competing risk regression model. In all patients after matching (n=698), sublobar resection, resection type, prior lung cancer, pathologic stage IB (versus IA1) disease, LVI, necrosis, presence of MIP, presence of SOL, and STAS were independent risk factors for recurrence. Sublobar resection, LVI, presence of SOL, and STAS were independent risk factors for lung cancer–specific death (Table 3, top).
Table 3. Final multivariable competing risk regression model for recurrence and lung cancer-specific death after propensity-score matching in all patients (top), patients with STAS (middle), and patients without STAS (bottom).
| Recurrence | Lung cancer-specific death | ||||||
|---|---|---|---|---|---|---|---|
| SHR | 95% CI | P | SHR | 95% CI | P | ||
| All patients after matching (n=698) | |||||||
| Sublobar resection (vs. lobectomy) | 2.33 | (1.46 – 3.70) | <0.001 | 1.95 | (1.07 – 3.58) | 0.030 | |
| Prior LC (vs. no prior LC) | 2.96 | (1.67 – 5.26) | <0.001 | ||||
| p-Stage (vs. IA1) | IA2 | 1.60 | (0.79 – 3.25) | 0.19 | 1.14 | (0.43 – 3.00) | 0.8 |
| IA3 | 1.74 | (0.70 – 4.34) | 0.2 | 1.58 | (0.47 – 5.34) | 0.5 | |
| IB | 2.30 | (1.02 – 5.19) | 0.044 | 2.46 | (0.89 – 6.80) | 0.082 | |
| LVI (vs. no LVI) | 2.00 | (1.18 – 3.29) | 0.009 | 2.16 | (1.03 – 4.18) | 0.042 | |
| Necrosis (vs. no necrosis) | 2.17 | (1.20 – 3.91) | 0.010 | 1.70 | (0.84 – 3.41) | 0.14 | |
| Presence of MIP pattern (vs. no MIP pattern) | 1.86 | (1.10 – 3.15) | 0.021 | ||||
| Presence of SOL pattern (vs. no SOL pattern) | 1.51 | (0.93 – 2.45) | 0.098 | 2.07 | (1.03 – 4.18) | 0.042 | |
| STAS (vs. no STAS) | 1.88 | (1.07 – 3.30) | 0.028 | 2.03 | (1.05 – 3.94) | 0.035 | |
| Patients with STAS after matching (n=276) | |||||||
| Sublobar resection (vs. lobectomy) | 2.84 | (1.59 – 5.08) | <0.001 | 2.63 | (1.16 – 5.95) | 0.021 | |
| Prior LC (vs. no prior LC) | 2.64 | (1.30 – 5.33) | 0.007 | ||||
| p-Stage (vs. IA1) | IA2 | 1.34 | (0.58 – 3.06) | 0.5 | 0.90 | (0.29 – 2.84) | 0.9 |
| IA3 | 1.86 | (0.65 – 5.35) | 0.2 | 1.29 | (0.25 – 6.56) | 0.8 | |
| IB | 1.62 | (0.63 – 4.18) | 0.3 | 2.15 | (0.66 – 7.08) | 0.2 | |
| LVI (vs. no LVI) | 1.48 | (0.85 – 2.59) | 0.17 | 1.54 | (0.68 – 3.50) | 0.3 | |
| Necrosis (vs. no necrosis) | 2.22 | (1.11 – 4.43) | 0.023 | 1.52 | (0.68 – 3.44) | 0.3 | |
| Presence of MIP pattern (vs. no MIP pattern) | 1.34 | (0.73 – 2.47) | 0.3 | ||||
| Presence of SOL pattern (vs. no SOL pattern) | 1.08 | (0.60 – 1.92) | 0.8 | 1.38 | (0.62 – 3.08) | 0.4 | |
| Patients without STAS after matching (n=422) | |||||||
| Sublobar resection (vs. lobectomy) | 1.93 | (0.88 – 4.21) | 0.10 | 1.56 | (0.56 – 4.30) | 0.4 | |
| Prior LC (vs. no prior LC) | 4.60 | (1.65 – 12.8) | 0.004 | ||||
| p-Stage (vs. IA1) | IA2 | 1.97 | (0.59 – 6.56) | 0.3 | 1.30 | (0.22 – 7.84) | 0.8 |
| IA3 | 1.53 | (0.23 – 9.96) | 0.7 | 2.20 | (0.27 – 18.0) | 0.5 | |
| IB | 4.19 | (1.06 – 16.46) | 0.040 | 2.27 | (0.35 – 14.9) | 0.4 | |
| LVI (vs. no LVI) | 4.23 | (1.60 – 11.2) | 0.004 | 3.32 | (0.70 – 15.76) | 0.13 | |
| Necrosis (vs. no necrosis) | 1.74 | (0.55 – 5.54) | 0.3 | 2.12 | (0.48 – 9.28) | 0.3 | |
| Presence of MIP pattern (vs. no MIP pattern) | 2.29 | (1.04 – 5.03) | 0.040 | ||||
| Presence of SOL pattern (vs. no SOL pattern) | 2.06 | (0.76 – 5.54) | 0.15 | 3.79 | (0.88 – 16.2) | 0.073 | |
Abbreviation: CI, confidence interval; LC, lung cancer; LVI, lymphovascular invasion; MIP, micropapillary; p-Stage, pathologic stage; SHR, subhazard ratio; SOL, solid; STAS, spread through air spaces.
Given that the impact of lobectomy and sublobar resection on recurrence and lung cancer–specific death significantly varied by STAS status (Figure 2), we conducted further multivariable analyses using the same variables in two separate cohorts that were stratified by STAS status. In this analysis, sublobar resection was independently associated with both recurrence and lung cancer–specific death in patients with STAS (Table 3, middle; subhazard ratio for recurrence, 2.84, P<0.001; and subhazard ratio for lung cancer-specific death, 2.63, P=0.021) but not in patients without STAS (Table 3, bottom).
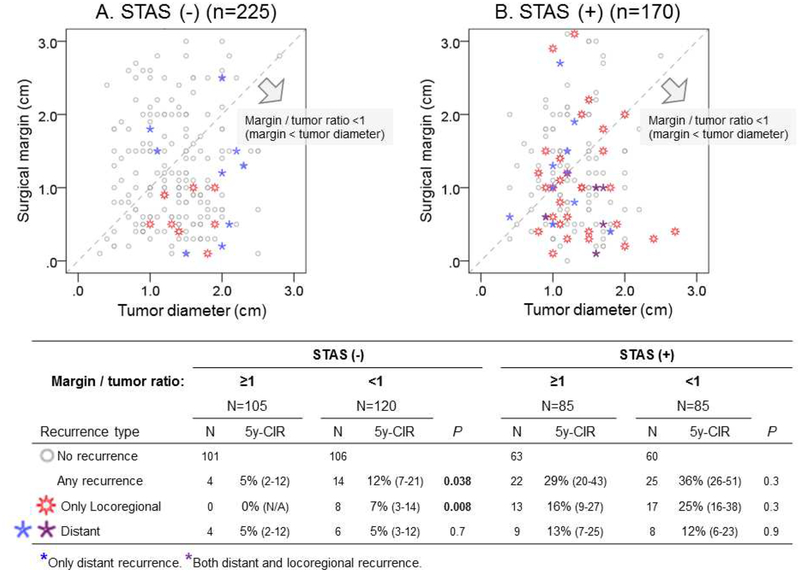
Impact of Margin/Tumor Ratio on Recurrence Following Sublobar Resection in STAS-Positive Tumors
The relationship between margin/tumor ratio and recurrence patterns is shown in Figure 3. Among patients with STAS-negative tumors, if margin/tumor ratio ≥1 (surgical margin ≥ tumor diameter), recurrence was rare and no locoregional recurrence was observed. In STAS-negative tumors, margin/tumor ratio ≥1 was associated with significantly lower risk of recurrence, particularly locoregional recurrence compared with margin/tumor ratio <1 (margin/tumor ratio ≥1 vs. <1: 5-year CIR for any recurrence, 5% vs. 12%, P=0.038; only locoregional recurrence, 0% vs. 7%, P=0.008; and distant recurrence, 5% vs. 5%, P=0.7).
Figure 3.
Relationship between margin/tumor ratio and recurrence pattern after sublobar resection by tumor spread through air spaces (STAS) status. Margin/tumor ratio was defined as the ratio of surgical margin distance to tumor diameter. Patients who underwent sublobar resection with available surgical margin assessment were divided into two groups on the basis of STAS status: (A) STAS negative and (B) STAS positive. Each dot represents a patient and is plotted on the basis of tumor size (x-axis) and surgical margin (y-axis). Each patient (dot) is categorized into one of four groups on the basis of recurrence pattern: gray dot, no recurrence; red dot, locoregional recurrence; blue dot, distant recurrence; and purple dot, both locoregional and distant recurrence. A dot located in the area under the dotted diagonal line represents a patient whose surgical margin was smaller than their tumor diameter. The number of cases and 5-year cumulative incidence of recurrence (CIR) for each recurrence type are shown in the bottom table. Recurrence was rare (n=4, no locoregional recurrence) in patients with STAS-negative tumors (A) with margin/tumor ratio ≥1 (surgical margin ≥ tumor size, above the dotted diagonal line); in contrast, 14 patients with STAS-negative tumors with margin/tumor ratio <1 (surgical margin < tumor size, under the dotted diagonal line) had recurrence, of which 8 were locoregional. Among patients with STAS (B), >25% had recurrence at 5 years after surgery, regardless of margin/tumor ratio (margin/tumor ratio ≥1 vs. <1: 5-year CIR for any recurrence, 29% vs. 36%, P=0.3; only locoregional recurrence, 16% vs. 25%, P=0.3).
Among patients with STAS-positive tumors, the risk of recurrence was high, regardless of margin/tumor ratio (margin/tumor ratio ≥1 vs. <1: 5-year CIR for any recurrence, 29% vs. 36%, P=0.3; only locoregional recurrence, 16% vs. 25%, P=0.3; and distant recurrence, 13% vs. 12%, P=0.3).
FS Analysis
Tumor cells were observed in the lung parenchyma beyond the edge of the main tumor in both FS and FSC slides (Supplementary Figure S4; demographic characteristics of the 48 patients are shown in Supplementary Table S3). Across five pathologists, sensitivity ranged from 59% to 86% and specificity from 74% to 100% (Supplementary Table S4). The overall sensitivity and specificity across the five pathologists, derived from the generalized estimating equation logistic regression model, were 71% (95% confidence interval [CI], 63% to 78%) and 92% (95% CI, 84% to 96%). The observed percent agreement was 75.4%. Interrater reliability across the five pathologists was substantial on the basis of Gwet’s AC1 (coefficient, 0.67 [95% CI, 0.57 to 0.77]).
DISCUSSION
Our study demonstrates that, in lung ADC the presence of STAS was associated with higher CIR and LC-CID in patients with sublobar resection (both segmentectomy and wedge) than those undergoing lobectomy and that the benefit of a surgical margin wider than the tumor diameter in sublobar resections in protecting against recurrence, especially locoregional recurrence, was found in patients without STAS, but not in those with STAS. On multivariable analysis following propensity-score matching, sublobar resection was an independent risk factor for recurrence and lung cancer–specific death only in patients with STAS. The majority of recurrences in patients with STAS who underwent sublobar resection were locoregional, suggesting that a wider resection margin per se may not provide protection against recurrence in these patients.
Our study provides important insight and identifies a factor—STAS—that should be investigated in prospective studies comparing the impact of lobectomy and sublobar resection, in addition to tumor size, on recurrence and survival. Based on our literature review comparing survival outcomes between sublobar resection and lobectomy (Supplementary Table S5),6,9,36–48 no study demonstrated factors that significantly change the magnitude of difference in survival outcomes between sublobar resection and lobectomy, such as STAS in the present study. In addition, no study investigated histologic subtypes of lung ADC and STAS. While our study confirms that lobectomy should remain the standard of care for early-stage lung ADC, especially for STAS-positive patients, it also raises awareness for investigations or considerations of alternate therapies, such as stereotactic body radiation therapy (SBRT) or other ablative therapies. Our group, in collaboration with radiation oncologists and intervention radiologists, has already published that MIP and SOL histologic subtypes in core biopsy specimens are associated with a high risk of locoregional failure and metastasis following SBRT49 or ablation.50 The present study highlights the significance of STAS in the normal lung surrounding the tumor; without adequate tissue to analyze STAS, alternate therapies such as SBRT or ablation remain a suboptimal alternative to lobectomy.
Recent studies have suggested that STAS might be the observation of an ex vivo artifact caused by mechanical spread of “dissociated” tumor cells by the knife surface during slide preparation.51 However, our findings confirm that STAS is not an ex vivo artifact but is a clinically significant biologic phenomenon, on the basis of the multiple independent studies which have shown this to be an important prognostic factor in all major histologic types of lung cancer14–24 as well as the following observations in this study: (1) STAS was an independent risk factor for both recurrence and lung cancer–specific death in multivariable analysis that included high-grade histologic subtypes and p-Stages, and (2) the difference in survival outcomes between sublobar resection and lobectomy differed significantly by STAS status but not by other factors, such as p-Stage and presence of high-grade histologic subtype (MIP or SOL).
Since the impact of STAS on recurrence and lung cancer-specific death appears to be significantly reduced by lobectomy compared to sublobar resection, we investigated whether pathologists can reliably recognize STAS on intraoperative frozen section to guide thoracic surgeons in cases where there is an option for limited resection vs lobectomy. Walts et al reported a study evaluating frozen section from resected lung ADC and found a low sensitivity of 50%, but a high specificity of 100% and 100% positive predictive value.52 This confirms our finding of high specificity for STAS in frozen sections. In Walts et al study, it is possible their lower sensitivity may be due to more limited sampling of adjacent lung parenchyma in their cases while in our study, we specifically selected cases where sufficient nonneoplastic lung parenchyma was present to optimize evaluation for STAS. In clinical practice, it may be important to sample the non-neoplastic lung surrounding the main tumor to evaluate for STAS.
In our previous study, FS analysis had high specificity (94%) but low sensitivity (37%) for detecting the presence of MIP pattern.26 In the present study, we found that FS analysis has relatively better sensitivity (71%) and similar specificity (92%) for detecting STAS. One of the reasons for higher sensitivity for detection of STAS could be that the tumor cells are readily distinguished from benign inflammatory cells such as macrophages within alveolar spaces, while recognizing the MIP pattern within the main tumor is more difficult due to the challenge in distinguishing it from the other lung ADC histological subtypes. The finding that STAS can be detected by FS analysis with good sensitivity/specificity and substantial interpathologist agreement is promising and provides rationale to investigate FS analysis in a prospective study. It is important both in prospective studies and in clinical practice to include appropriate/adequate normal lung parenchyma surrounding the tumor and, furthermore, to avoid various forms of frozen section–related artifacts (e.g., floaters, tangential sections, and rugged or folded tissues).
One of the limitations of this study is its retrospective nature. Although we performed propensity-score matching, preoperative selection bias between lobectomy and sublobar resection remains—for example, in tumors that were located close to the hilum or in an intersegmental plane. Another limitation of our study is that our cohort included patients who did not undergo pathologic lymph node evaluation (see Study Cohort in Methods and Supplementary Table S1). The inclusion of these patients may have affected our outcomes. Nevertheless, that the incidence of locoregional recurrence in patients with T1 lung ADC remains high despite negative resection margins is an important issue that requires attention.
In conclusion, our propensity score–matched analysis demonstrates that, compared with lobectomy, sublobar resection is associated with a significantly higher risk of recurrence and subsequent lung cancer–specific death in patients with STAS. Our data confirm that lobectomy should remain the standard treatment option for patients with early-stage lung ADC, especially those with STAS-positive tumors. FS analysis may be useful to intraoperatively detect STAS and aiding intraoperative decisions regarding the most appropriate resection type for patients with early-stage lung ADC.
Supplementary Material
ACKNOWLEDGMENTS
We thank David B. Sewell of the Memorial Sloan Kettering Thoracic Surgery Service for editorial assistance.
Funding: The author’s laboratory work is supported by grants from the National Institutes of Health (R01 CA236615, R01 217169 and P30 CA008748), the U.S. Department of Defense (LC160212, CA170630, and BC132124), the Joanne and John DallePezze Foundation, the Derfner Foundation, and the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
*The funding sources played no role in any aspect of the study or the manuscript.
Glossary
- ADC
adenocarcinoma
- ASMD
absolute standardized mean difference
- CIR
cumulative incidence of recurrence
- COPD
chronic obstructive pulmonary disease
- CVD
cardiovascular disease
- DLCO
diffusion capacity of the lungs for carbon monoxide
- DM
diabetes mellitus
- FEV1
forced expiratory volume in one second
- FS
frozen section
- FSC
frozen section control
- LC-CID
lung cancer–specific cumulative incidence of death
- LVI
lymphovascular invasion
- MIP
micropapillary
- MSK
Memorial Sloan Kettering Cancer Center
- NSCLC
non-small cell lung cancer
- SBRT
stereotactic body radiation therapy
- SOL
solid
- STAS
spread through air spaces
- VPI
visceral pleural invasion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.The health consequences of smoking – 50 years of progress: a report of the Surgeon General. In: U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, ed.Atlanta, GA.: 2014. [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–622; discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Centers: NCCN clinical practice guidelines in oncology (NCCN Guidelines): Non-small cell lung cancer v5. 2017. Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. [DOI] [PubMed]
- 4.Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg. 2003;125:924–928. [DOI] [PubMed] [Google Scholar]
- 5.Wolf AS, Richards WG, Jaklitsch MT, et al. Lobectomy versus sublobar resection for small (2 cm or less) non-small cell lung cancers. Ann Thorac Surg. 2011;92:1819–1823; discussion 1824–1815. [DOI] [PubMed] [Google Scholar]
- 6.Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol. 2015;33:3447–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varlotto JM, Medford-Davis LN, Recht A, et al. Identification of stage I non-small cell lung cancer patients at high risk for local recurrence following sublobar resection. Chest. 2013;143:1365–1377. [DOI] [PubMed] [Google Scholar]
- 8.Dai C, Shen J, Ren Y, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer </= 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol. 2016;34:3175–3182. [DOI] [PubMed] [Google Scholar]
- 9.Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest. 2011;139:491–496. [DOI] [PubMed] [Google Scholar]
- 10.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol. 2015;33:2877–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–1446. [DOI] [PubMed] [Google Scholar]
- 13.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. [DOI] [PubMed] [Google Scholar]
- 14.Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol. 2015;10:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol. 2015;39:793–801. [DOI] [PubMed] [Google Scholar]
- 16.Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol. 2017;12:1788–1797. [DOI] [PubMed] [Google Scholar]
- 17.Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2016;152:64–72 e61. [DOI] [PubMed] [Google Scholar]
- 18.Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol. 2017;12:1052–1060. [DOI] [PubMed] [Google Scholar]
- 19.Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2016;23:567–572. [DOI] [PubMed] [Google Scholar]
- 20.Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol. 2013;37:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol. 2017;12:223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol. 2017;41:1077–1086. [DOI] [PubMed] [Google Scholar]
- 23.Aly RG, Eguchi T, Kadota K, et al. Spread through air spaces (STAS) correlates with prognosis in lung neuroendocrine tumors (LNET). Mod Pathol. 2018. (suppl; abstr);31:724. [Google Scholar]
- 24.Toyokawa G, Yamada Y, Tagawa T, et al. High Frequency of Spread Through Air Spaces in Resected Small Cell Lung Cancer. Anticancer Res. 2018;38:1821–1825. [DOI] [PubMed] [Google Scholar]
- 25.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–932; discussion 932–923. [DOI] [PubMed] [Google Scholar]
- 26.Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of </= 3 cm: accuracy and interobserver agreement. Histopathology. 2015;66:922–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin MB, Cancer. AJCo AJCC Cancer Staging manual. New York: Springer; 2016. [Google Scholar]
- 28.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142:1620–1635. [DOI] [PubMed] [Google Scholar]
- 29.Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol. 2017;35:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 33.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 34.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61:29–48. [DOI] [PubMed] [Google Scholar]
- 35.Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378–382. [Google Scholar]
- 36.Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today. 2016;46:1370–1382. [DOI] [PubMed] [Google Scholar]
- 37.Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg. 2016;102:1702–1710. [DOI] [PubMed] [Google Scholar]
- 38.Koike T, Kitahara A, Sato S, et al. Lobectomy Versus Segmentectomy in Radiologically Pure Solid Small-Sized Non-Small Cell Lung Cancer. Ann Thorac Surg. 2016;101:1354–1360. [DOI] [PubMed] [Google Scholar]
- 39.Kodama K, Higashiyama M, Okami J, et al. Oncologic Outcomes of Segmentectomy Versus Lobectomy for Clinical T1a N0 M0 Non-Small Cell Lung Cancer. Ann Thorac Surg. 2016;101:504–511. [DOI] [PubMed] [Google Scholar]
- 40.Altorki N, Kohman LJ, Veit LJ, et al. Limited resection as a cure for early lung cancer: time to challenge the gold standard? Bull Am Coll Surg. 2015;100:57–58. [PubMed] [Google Scholar]
- 41.Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol. 2014;32:2449–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg. 2013;146:358–364. [DOI] [PubMed] [Google Scholar]
- 43.Zhao ZR, Situ DR, Lau RWH, et al. Comparison of Segmentectomy and Lobectomy in Stage IA Adenocarcinomas. J Thorac Oncol. 2017;12:890–896. [DOI] [PubMed] [Google Scholar]
- 44.Qu X, Wang K, Zhang T, et al. Long-term outcomes of stage I NSCLC (</=3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: a PSM based analysis. J Thorac Dis. 2017;9:4561–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Yuan C, Zhang Y, et al. Survival following segmentectomy or lobectomy in elderly patients with early-stage lung cancer. Oncotarget. 2016;7:19081–19086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khullar OV, Liu Y, Gillespie T, et al. Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol. 2015;10:1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg. 2010;251:550–554. [DOI] [PubMed] [Google Scholar]
- 49.Leeman JE, Rimner A, Montecalvo J, et al. Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;97:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao S, Stein S, Petre EN, et al. Erratum to: Micropapillary and/or Solid Histologic Subtype Based on Pre-Treatment Biopsy Predicts Local Recurrence After Thermal Ablation of Lung Adenocarcinoma. Cardiovasc Intervent Radiol. 2017;40:1658. [DOI] [PubMed] [Google Scholar]
- 51.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walts AE, Marchevsky AM. Current Evidence Does Not Warrant Frozen Section Evaluation for the Presence of Tumor Spread Through Alveolar Spaces. Arch Pathol Lab Med. 2018;142:59–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.