Abstract
Background & Aims:
In patients with acute severe ulcerative colitis (ASUC), standard infliximab induction therapy has modest efficacy. There are limited data on the short-term or long-term efficacy of accelerated infliximab induction therapy for these patients.
Methods:
In a retrospective study, we collected data from 213 patients with steroid refractory ASUC who received infliximab rescue therapy at 3 centers, from 2005 through 2017. Patients were classified that received standard therapy (5 mg/kg infliximab at weeks 0, 2, and 6) or accelerated therapy (>5 mg/kg infliximab at shorter intervals). The primary outcome was colectomy in-hospital and at 3, 6, 12, and 24 months. Multivariable regression models were adjusted for relevant confounders. We also performed a meta-analysis of published effects of standard vs accelerated infliximab treatment of ASUC.
Results:
In the retrospective analysis, 81 patients received accelerated infliximab therapy and 132 received standard infliximab therapy. There were no differences in characteristics between the groups, including levels of C-reactive protein or albumin. Similar proportions of patients in each group underwent in-hospital colectomy (9% receiving accelerated therapy vs 8% receiving standard therapy; adjusted odds ratio, 1.35; 95% CI, 0.38–4.82). There was no significant difference between groups in proportions that underwent colectomy at 3, 6, 12, or 24 months (P>.20 for all comparisons). Among those in the accelerated group, an initial dose of 10 mg/kg was associated with a lower rate of colectomy compared to patients who initially received 5 mg/kg followed by subsequent doses of 5mg/kg or higher. Our systematic review identified 7 studies (181 patients receiving accelerated infliximab and 436 receiving standard infliximab) and found no significant differences in short- or long-term outcomes.
Conclusion:
In a retrospective study and meta-analysis, we found no association between accelerated infliximab induction therapy and lower rates of colectomy in patients with ASUC, compared to standard induction therapy. However, confounding by disease severity cannot be excluded. Randomized trials are warranted to compare these treatment strategies.
Keywords: Hospitalization, colectomy, infliximab, acute severe ulcerative colitis, TNF antagonist, surgery, IBD treatment, multicenter, patient management
INTRODUCTION
Acute severe ulcerative colitis (ASUC) affects nearly a quarter of patients with ulcerative colitis (UC), often within the first two years after diagnosis1–5. One-third of patients are refractory to intravenous corticosteroids6, the cornerstone of management of ASUC, and are offered medical rescue therapy or undergo colectomy1–5. A small but important trial by Jarnerot et al. established the efficacy of infliximab (IFX) in rescuing steroid-refractory ASUC, reducing the number of patients who needed colectomy during the hospitalization by half7. However, one-quarter of patients who received a single intravenous infusion of IFX required colectomy by 3 months. Two prospective clinical trials comparing infliximab and cyclosporine demonstrated comparable clinical efficacy of both agents, but nevertheless a significant proportion of patients initiating therapy with standard dose IFX failed to respond and required colectomy8, 9.
An elegant study by Brandse et al. demonstrated that significant fecal losses of IFX occur in the setting of ASUC10, suggesting that greater therapeutic benefit may be achieved by attempting to overcome such losses by repeating IFX infusions at a shorter interval than the standard 5mg/kg at weeks 0, 2, and 6 induction dosing or at a higher upfront dose. A small but growing body of literature has examined whether an ‘accelerated induction’ protocol, administering IFX at higher doses or shorter intervals, results in superior outcomes11–15. However, the results of these studies have been mixed and from small patient cohorts. An initial study by Gibson et al. demonstrated a benefit with this accelerated induction protocol in the short term13, however other studies failed to demonstrate an advantage to accelerated induction12, 14, 15. Limiting interpretation of this data is that each study had small numbers of patients and inadequate statistical power. Survey-based studies have highlighted the considerable variation in infliximab dosing in the hospitalized setting16, further highlighting the importance of a robust-evidence base to guide clinical practice.
We performed this retrospective multi-center study with the following aims: (1) to compare the outcomes of accelerated compared to standard IFX induction in patients with ASUC; and (2) to perform a systematic review and meta-analysis of the literature to determine if accelerated IFX induction is associated with short- or long-term benefits in ASUC.
METHODS
Study Population
This retrospective study included patients hospitalized with ASUC at Massachusetts General Hospital (MGH) (Boston, MA), Indiana University Hospital (IUH) (Indianapolis, IN), or Johns Hopkins Hospital (JHH) (Baltimore, MD) who received IFX rescue therapy for steroid-refractory ASUC. Eligible patients received treatment with intravenous corticosteroids (methylprednisolone 60mg daily or equivalent) and had inadequate clinical and laboratory response after 3–5 days of treatment, and initiated IFX therapy for medical rescue. Patients who had previously received IFX, had Crohn’s disease or IBD-unclassified, or with previous IBD-related surgery were excluded. All patients were tested Clostridium difficile and cytomegalovirus colitis and were excluded if positive.
Patients were divided into two groups based on IFX administration schedule. Those who received IFX at 5mg/kg at weeks 0, 2, and 6 were considered to have received “standard induction”. Patients who either had upfront administration of IFX at 10mg/kg and/or had received their IFX infusions at shorter intervals were grouped in the “accelerated induction” group. The choice of regimen varied by provider. At MGH and JHH, patients perceived to have more severe disease clinically or based on endoscopic severity often received upfront 10mg/kg, while those with partial response to the initial 5mg/kg dose received either another infusion at the same dose or 10mg/kg in 3–5 days. At IUH, patients with a CRP/albumin ratio > 1 received up front 10mg/kg infliximab; responders received a subsequent infusion at 10mg/kg in 2 weeks while partial or non-responders received a second dose at 10mg/kg in 3–5 days.
Covariates and Outcomes
Information on relevant covariates was obtained by review of the medical record by study investigators and included age, sex, duration of disease, age at diagnosis, disease extent per the Montreal classification, prior treatment history for UC, and concomitant immunomodulator use (azathioprine, 6-mercaptopurine, methotrexate). Relevant admission laboratory parameters included hemoglobin, serum albumin, CRP, platelet count, and erythrocyte sedimentation rate (ESR). Where flexible sigmoidoscopy was performed, disease was classified as endoscopically ‘severe’ in the presence of ulcerations and spontaneous bleeding.
Our primary study outcome was colectomy during the index hospitalization. Secondary study outcomes included colectomy at 3, 6, 12, and 24 months after the index hospitalization and length of hospital stay.
Statistical Analysis
The study was approved by the Institutional Review Board at each of the three study sites. Statistical analysis was performed using Stata 13.0 (StataCorp, College Station, TX). Continuous variables were summarized using means and standard deviations and compared using the t-test while categorical variables were expressed as proportions and compared using the chi-square test. First, we performed univariate logistic regression with in-hospital colectomy as our outcome to identify potential clinical and laboratory predictors including IFX dosing regimen. Multivariable logistic regression was performed including those with a p-value < 0.15 on univariate analysis or those that had been noted to be influential previously. A two-sided p-value < 0.05 indicated independent statistical significance. Similar analyses were repeated for each of the study outcomes and using linear regression models for our continuous outcome of length of stay.
As selection of IFX dosing was non-random, we conducted a propensity score adjusted analysis17, 18 assigning each patient a score indicating likelihood of receiving accelerated IFX induction. This included a model incorporating endoscopic severity, number of bowel movements over a 24-hour period, hemoglobin, serum albumin and CRP levels. This propensity score was then included in the multivariable model. Pre-planned subgroup analysis included stratifying by whether patients received an upfront 10mg/kg infusion as their first dose or whether it was with a step-up protocol of 5mg/kg infusions administered within a few days of each other (‘chaser’ regimen).
Systematic review and meta-analysis
We performed a systematic review of the published literature by searching PubMed/Medline for relevant manuscripts using combination of the MeSH headings “infliximab” “ulcerative colitis” and “hospitalization” between 1998 and December 2017. In addition to full text manuscripts identified, manual review of abstracts from the major gastroenterology conferences (Digestive Diseases Week, Crohn’s and Colitis Foundation Annual Conference, United European Gastroenterology Week, and Annual Meeting of the European Crohn’s and Colitis Organization) was performed. Two study investigators (DA, ANA) independently reviewed the identified studies and confirmed eligibility for inclusion in the meta-analysis based on the following criteria: (a) published as an abstract or full text; (b) include patients with steroid-refractory ASUC initiating IFX; (c) present outcomes of both standard and accelerated IFX induction; and (d) provide sufficient information to calculate OR and 95% CI. From each eligible study, we extracted the number of patients included in the accelerated and standard induction arms, and number of events in each arm. The study outcomes extracted included in-hospital colectomy (or colectomy at 1 month), and surgery at 3, 6, 12, and 24 months after the index hospitalization where available. A DerSimonian and Laird random effects meta-analysis was performed to quantitatively pool the results of the included studies19. Meta-regression was performed to identify relevant influential predictors. Publication bias was assessed by visual inspection of the funnel plot and Begg’s and Egger tests.
RESULTS
Study Population
Our study cohort included 213 patients with ASUC receiving infliximab rescue therapy (107 MGH, 50 IUH, 56 JHH) between 2005–17. The mean age of the included patients was 31 years, and 40% were women. Table 1 compares the characteristics of patients who received accelerated IFX induction (n=81) to those who received standard dosing (n=132). Among the 81 patients in the accelerated IFX group, 21 received 5mg/kg followed by chaser doses of 5mg/kg or 10mg/kg while 60 received an upfront dose of 10mg/kg as the first dose. Among those in the standard induction, 5mg/kg chaser arm, and up front 10mg/kg arm, there were 97 (73%), 10 (50%), and 53 (88%) patients who received at least 3 doses of IFX. Both groups were similar in age, gender, disease duration and extent of UC. There was no statistically significant difference in the baseline CRP (35.5 vs. 29.6 mg/dL, p=0.49), albumin (3.1 vs. 3.2 g/dL, p=0.49), ESR (45mm/hr vs 38 mm/hr, p=0.07), or hemoglobin (11.1g/dL vs. 11.5g/dL, p=0.15) between the two groups. Among the 177 patients who underwent endoscopic evaluation, there was a similar proportion of those classified as having severe inflammation in the accelerated (83%) compared to the standard induction groups (73%, p=0.10). There was a larger proportion of accelerated IFX induction at IUH (52%) compared to JHH (38%) or MGH (32%) but there was no difference in patient characteristics between the three centers.
Table 1:
Characteristics of the Study Population
| Characteristic | Standard infliximab induction (n=132) | Accelerated infliximab induction (n=81) | p-value |
|---|---|---|---|
| Female (%) |
45% | 32% | 0.05 |
| Caucasian (%) | 89% | 84% | 0.38 |
| Mean Age at diagnosis, (SD) (in years) | 33.0 (16.6) | 31.0 (14.6) | 0.36 |
| Mean Disease duration, (SD) (in years) | 4.2 (3.1) | 4.6 (2.8) | 0.67 |
| UC extent pancolitis (%) | 63% | 76% | 0.06 |
| Current smoking (%) | 3% | 1% | 0.39 |
| Hospital site (%) | 0.05 | ||
| Massachusetts General Hospital | 55% | 42% | |
| Indiana University Hospital | 18% | 32% | |
| Johns Hopkins Hospital | 27% | 26% | |
| Concomitant medications (%) | |||
| Aminosalicylates | 58% | 43% | 0.03 |
| Immunomodulators | 24% | 17% | 0.23 |
| Mean Laboratory results at hospital admission, (SD) | |||
| C-reactive protein (mg/dL) | 29.6 (51.9) | 35.5 (54.9) | 0.50 |
| ESR (mm/hr) | 38 (24.9) | 46 (26.2) | 0.07 |
| Albumin (g/dL) | 3.2 (0.7) | 3.1 (0.7) | 0.49 |
| Hemoglobin (g/dL) | 11.5 (2.4) | 11.0 (2.3) | 0.20 |
| Severe endoscopic inflammation (%)+ | 73% | 84% | 0.10 |
ESR – erythrocyte sedimentation rate
Endoscopic evaluation (sigmoidoscopy/colonoscopy) performed in 177 patients
In-hospital and long-term outcomes with accelerated IFX induction.
The proportion of patients who required in-hospital colectomy was similar between the accelerated (9%) and standard IFX groups (8%, p=0.80) (OR 1.14, 95% CI 0.42 – 3.13). We also identified no difference in the length of index hospitalization between the two groups (11.3 vs 12.3 days, p=0.48). Examining longer term outcomes at 3, 6, 12, and 24 months also revealed no differences in the rates of colectomy between the two groups (Figure 1). At 2 years after the index hospitalization, 31% of patients in the accelerated IFX group had undergone colectomy compared to 33% of those who received standard IFX induction (p=0.88) (OR 0.95, 95% CI 0.50 – 1.82). There was no difference in the association between accelerated induction and colectomy outcomes at each of the three centers when analyzed separately (Supplemental Table 1).
Figure 1:
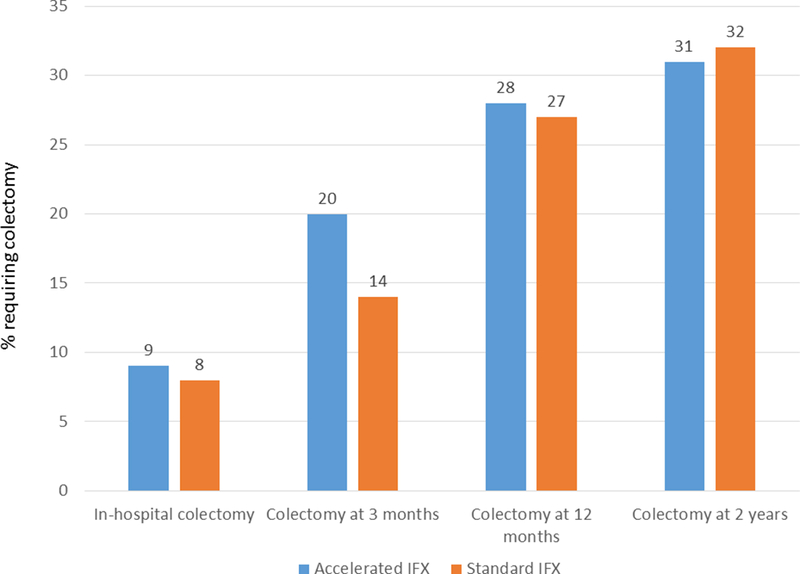
Rates of colectomy in-hospital, at 3, 12, and 24 months following hospitalization for acute severe ulcerative colitis and infliximab rescue therapy
On univariate analysis, age at hospitalization (p=0.017), serum albumin (p < 0.001), and pancolitis (p=0.06) met our a priori statistical threshold for inclusion in the multivariable model. Endoscopic severity also predicted need for colectomy. Two patients (5%) in the non-severe colonoscopy group underwent surgery compared to fourteen (11%) of those with severe inflammation (Fisher’s p=0.26). In a multivariable model, additionally adjusting for institution, there was no difference between accelerated and standard IFX in need for colectomy in-hospital (OR 1.35, 95% CI 0.38 – 4.83), or at 3, 6, 12, or 24 months (Table 2). The only variable that was consistently an independent predictor of colectomy was low serum albumin. An increase in serum albumin by 1g/dL was associated with lower likelihood of colectomy (OR 0.10, 95% CI 0.03 – 0.33). This association remained significant till almost 2 years after hospitalization (OR 0.61, 95% CI 0.36 – 1.04, p=0.067).
Table 2:
Multivariable analysis of risk of colectomy with accelerated compared to standard infliximab induction schedule for acute severe ulcerative colitis
| Time point | OR* | 95% CI | p-value |
|---|---|---|---|
| In-hospital | 1.35 | 0.38 – 4.82 | 0.64 |
| 3 months | 1.64 | 0.70 – 3.87 | 0.26 |
| 6 months | 1.19 | 0.54 – 2.61 | 0.66 |
| 1 year | 0.93 | 0.45 – 1.95 | 0.86 |
| 2 years | 0.86 | 0.42 – 1.75 | 0.67 |
additionally adjusted for pancolitis, albumin, age and hospital site
CI – confidence interval; OR – odds ratio
Among those who received accelerated induction, we then compared those who received 10mg/kg as their first IFX dose to those who received 5mg/kg and subsequently received either 5 or 10mg/kg as ‘chaser’ doses before week 2. On multivariable analysis, compared to those in the latter group, those who had IFX 10mg/kg up front had a numerically lower risk of colectomy in-hospital (OR 0.21, 95% CI 0.04 – 1.04) and 3 months (OR 0.33, 95% CI 0.10 – 1.05). There remained a lower risk of colectomy at 12 months (p=0.03), and 24 months (OR 0.23, 95% CI 0.07 – 0.73, p=0.012) in the upfront 10mg/kg groups. Supplemental Table 2 compares the outcomes of the upfront 10mg/kg IFX group to standard induction. While there was no statistically significant difference, there were numerically lower rates of in-hospital and long-term colectomy in the 10mg/kg group with a trend towards statistical significance at 2 years (OR 0.44, 95% CI 0.18 – 1.12, p=0.08).
Sensitivity and Subgroup analyses
We performed several sensitivity and subgroup analysis. We repeated the analysis in the subset of patients with available CRP levels and found no statistically significant difference between the two IFX induction protocols. Compared to standard IFX induction, accelerated induction was not associated with a significant reduction in risk of colectomy in-hospital (OR 0.63, 95% CI 0.13 – 3.10) or at any other time point when adjusting for admission CRP levels. Subgroup analysis of those with severe endoscopic inflammation (n=136) also revealed no significant difference between the two groups in in-hospital (p=0.48) or other outcomes. Restricting the analysis to patients who completed 2 or 3 doses of infliximab also did not reveal a reduction or short or long-term colectomy risk with accelerated IFX induction
Propensity scores were calculated to determine likelihood of receiving accelerated IFX induction. On adjustment for the propensity score in the multivariable model, there remained no difference between the accelerated and standard induction groups for in-hospital (OR 0.70, 95% CI 0.16 – 3.01), 6 month (p = 0.75), 12 month (p = 0.76) or 24 month (p=0.62) colectomy. We then stratified patients by whether their CRP / albumin ratio was above or below the median value for the entire cohort (0.29). Accelerated IFX induction as not associated with lower rates of in-hospital colectomy in either of the two groups (p-interaction=0.25).
Systematic review and meta-analysis
The systematic review identified 7 eligible studies (3 full text and 4 abstract) comparing accelerated and standard infliximab infusions11–15, 20 (Table 3). This included 181 patients receiving accelerated IFX infusion and 436 on standard IFX infusion. Four studies were from North America14, 15, 20, 1 from Europe13, and 2 from Australia11, 12. Four studies presented the rates of colectomy in-hospital (3 studies) or 1 month (1 study) that were pooled together using a random-effects model. There was no heterogeneity between the studies (i2=17%). Using a DerSimonian and Laird random effects model, there was no difference in the rate of colectomy with accelerated compared to standard infliximab infusions (OR 0.76, 95% CI 0.36 – 1.61, p=0.47) (Figure 2). While there was a trend towards a higher frequency of colectomy at 3 months in the accelerated induction group (OR 1.93, 95% CI 0.80 – 4.66, p=0.14), there was no difference at 12 months (OR 0.96, 95% CI 0.57 – 1.60) or 2 years (OR 1.79, 95% CI 0.50 – 6.38) (Table 4). Only two included studies defined accelerated infliximab induction as being an upfront dose of 10mg/kg20, 21. We could not quantitative pool together the two studies as one provided information only up to 1 year21 while the other provided only 2 year data20. However, individually, neither study demonstrated 10mg/kg infliximab up front to be superior to standard induction. Analysis excluding these two studies in the meta-analysis revealed no difference between an accelerated 5mg/kg dose induction regimen or standard infliximab induction for in-hospital as well as long-term rates of colectomy.
Table 3:
Characteristics of studies included in the systematic review and meta-analysis
| Number of patients | |||||
|---|---|---|---|---|---|
| First Author | Year | Location | Publication type | Standard Infliximab Induction | Accelerated infliximab induction |
| Gibson | 2015 | Europe | Full text | 35 | 15 |
| Govani | 2016 | North America | Abstract | 40 | 17 |
| Choy | 2016 | Australia | Abstract | 26 | 9 |
| An | 2017 | Australia | Abstract | 16 | 28 |
| Shah | 2017 | North America | Full text | 120 | 26 |
| Al Khoury | 2017 | North America | Abstract | 67 | 5 |
| Nalagatla (present study) | 2018 | North America | Full text | 132 | 81 |
Figure 2:
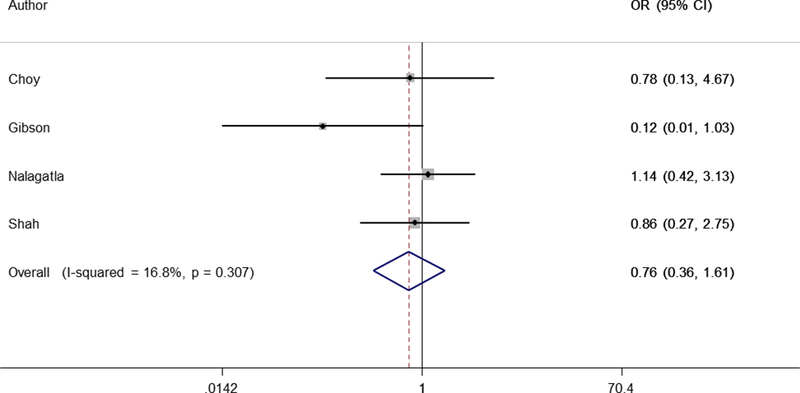
Forest Plot of random effects meta-analysis of rate of in-hospital colectomy with standard and accelerated infliximab induction for acute severe ulcerative colitis
Table 4:
Results of random effects meta-analysis of pooled rates of colectomy with accelerated compared to standard infliximab induction schedule for acute severe ulcerative colitis
| Colectomy time point | Pooled OR | 95% CI | Number of studies | I2 (%) |
|---|---|---|---|---|
| In-hospital/1 month | 0.76 | 0.36 – 1.61 | 4 | 16.8 |
| 3 months | 1.93 | 0.80 – 4.66 | 3 | 56.4 |
| 1 year | 0.96 | 0.57 – 1.60 | 3 | 0.0 |
| 2 years | 1.79 | 0.50 – 6.38 | 3 | 62.1 |
CI – confidence interval; OR – odds ratio
DISCUSSION
One-third of patients with ASUC fail to respond to intravenous steroid therapy and require either medical rescue therapy or colectomy2, 3, 5, 6, 22, 23. Despite IFX being efficacious in this setting, a significant proportion of patients with ASUC do not respond adequately to standard induction dosing7, 24. In this multi-center retrospective cohort study, we did not find that an accelerated IFX induction was associated with lower rates of in-hospital, short-term, or long-term colectomy when compared to standard induction dosing. However, among those receiving accelerated induction, upfront administration of 10mg/kg reduced risk of colectomy in-hospital and at 1 and 2 years when compared to just reducing the interval between 5mg/kg doses. Our main results were confirmed in a quantitative meta-analysis of published literature.
An elegant study by Brandse et al. examined serum and fecal IFX levels in 30 patients with active UC initiating therapy10. Nearly two-thirds of the fecal samples demonstrated detectable levels, and patients who were non-responders at week 2 had greater fecal loses than those who responded. This finding and rate of non-response to IFX in ASUC kindled interest in an accelerated induction protocol with either higher doses or more frequent infusions to optimize serum and tissue concentrations and increase rates of response. One of the earliest reports on this was by Gibson et al. who reviewed their experience with 50 hospitalized patients, among whom 15 received an accelerated induction regimen, completing three doses at 5mg/kg within 24 days13. While the rate of colectomy during induction was lower in the accelerated IFX cohort, there was no difference in colectomy rates at 2 years. Shah et al. found no difference in the rate of colectomy by 30 days or 1 year between 26 patients receiving high dose 10mg/kg and 120 patients receiving standard dose IFX15. Similarly, Choy et al. also reported no difference in rate of colectomy between accelerated and standard IFX induction12 while Govani et al. noted lower rates of colectomy at 3 months with accelerated IFX induction14.
There are several reasons for conflicting results in the literature, and the potential lack of benefit of accelerated IFX regimen in the meta-analysis and our own pooled analysis. First, it may be that there truly is no benefit of an accelerated regimen and need for more than the standard IFX dose represents a marker of severity that predicts need for colectomy irrespective of the therapeutic intervention. Despite greater fecal losses, there was no difference in the serum IFX level between responders and non-responders in the study by Brandse et al.10 suggesting that beyond a threshold, there may be little incremental benefit. However, the findings could also reflect limitations of the literature. Most individual studies had few patients, limiting statistical power. Second, the studies differed in their definition of accelerated induction with some using it to exclusively refer to those receiving 10mg/kg up front15 while others used the term to comprise any dosing more frequent than the standard induction13. Interestingly, we observed in our study that those who received 10mg/kg upfront as their first dose had lower rates of colectomy than those who got more frequent ‘chaser’ infusions. Thus, an early, aggressive approach aimed at overcoming fecal losses and achieving optimal serum and tissue IFX levels may be critical to optimizing outcomes in severe colitis. Whether doses even higher than 10mg/kg may be beneficial in some remains to established. Finally, one can hypothesize that there may be specific subgroups of patients most likely to benefit from an accelerated induction regimen. Some have proposed using CRP/albumin ratio25 or absolute CRP values to determine who receives more aggressive induction therapy. While we did not note an effect modification by this in our analyses, future prospective studies are important to answer this robustly.
There are many next steps to accurately defining the role of accelerated IFX induction in ASUC. While we did not find a significant benefit of accelerated IFX induction in ASUC, studies were limited by small sample sizes and inability to reliably subgroup patients into smaller strata who may benefit. Larger prospective cohorts are needed to more robustly define if accelerated IFX infusion has an effect. Further effort is also essential to determine if there are pre-specified criteria for a priori identifying those who may be most likely to benefit such as has been proposed with using a CRP/albumin ratio. In addition, comparative clinical trials of different strategies are much needed and results of ongoing studies are awaited (NCT02770040, NCT03209232). Third, IFX dose beyond a specific threshold may not yield clinical benefit and there is a need for further translational research into mechanisms for lack of response to IFX. It is plausible that non-response to IFX in the setting of ASUC is not pharmacokinetically mediated but rather represents distinct inflammatory pathways in the tissue26. Further exploration of such mechanisms can yield novel therapeutic targets.
We readily acknowledge several limitations to our study. First, while it was a multicenter study and among the largest to examine this question thus far, the number of patients was still relatively small particularly for subgroup analysis and there was heterogeneity in dosing and timing of administration of IFX without a fixed protocol. Owing to the retrospective design, laboratory parameters were not homogenously available at specific time points (such as after the induction) and we did not have information on serum or fecal infliximab levels as this was not systematically available. However, we believe this is useful initial data to inform design of prospective clinical trials going forward.
In conclusion, we did not find accelerated infliximab induction to be superior to standard induction in all patients with ASUC. However, confounding by disease severity cannot be excluded as the common practice at each center was to favor accelerated induction for those felt to have more severe disease. There is a need for prospective randomized clinical trials and larger multi-center prospective cohorts to better identify the optimal medical rescue strategy in patients with ASUC to reduce morbidity and improve patient outcomes.
Supplementary Material
Acknowledgments
Source of funding: Ananthakrishnan is supported in part by grants from the National Institutes of Health (R03 DK112909) and the Crohn’s and Colitis Foundation.
Footnotes
Conflicts of Interest: Ananthakrishnan has served on scientific advisory boards for Abbvie, Takeda, Gilead, and Merck and has received research support from Pfizer. The other authors have no conflicts to declare.
REFERENCES
- 1.Bernstein CN, Ng SC, Lakatos PL, et al. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis 2013;19:2001–10. [DOI] [PubMed] [Google Scholar]
- 2.Caprilli R, Viscido A, Latella G. Current management of severe ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2007;4:92–101. [DOI] [PubMed] [Google Scholar]
- 3.Cesarini M, Collins GS, Ronnblom A, et al. Predicting the Individual Risk of Acute Severe Colitis at Diagnosis. J Crohns Colitis 2017;11:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pola S, Patel D, Ramamoorthy S, et al. Strategies for the care of adults hospitalized for active ulcerative colitis. Clin Gastroenterol Hepatol 2012;10:1315–1325 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther 2016;43:482–513. [DOI] [PubMed] [Google Scholar]
- 6.Turner D, Walsh CM, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 2007;5:103–10. [DOI] [PubMed] [Google Scholar]
- 7.Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805–11. [DOI] [PubMed] [Google Scholar]
- 8.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet 2012;380:1909–15. [DOI] [PubMed] [Google Scholar]
- 9.Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol 2016;1:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of Infliximab Into Feces Is Associated With Lack of Response to Therapy in Patients With Severe Ulcerative Colitis. Gastroenterology 2015;149:350–5 e2. [DOI] [PubMed] [Google Scholar]
- 11.An YK, Chen CY, White LS, et al. Accelerated dosing of infliximab induction and endoscopic mucosal healing in patients with acute severe ulcerative colitis. J Gastroenterol Hepatol 2017;32:121. [Google Scholar]
- 12.Choy MC, Seah D, Gorelik A, et al. Comparison of Accelerated Infliximab Induction vs Standard Induction Treatment in Acute Severe Ulcerative Colitis. Gastroenterology 2016;150:S803. [Google Scholar]
- 13.Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:330–335 e1. [DOI] [PubMed] [Google Scholar]
- 14.Govani SM, Waljee AK, Stidham RW, et al. Accelerated Dosing of Infliximab Prevents Colectomy Within 90 Days in Only Half of Patients With Severe Ulcerative Colitis. Gastroenterology 2016;150:S106. [Google Scholar]
- 15.Shah SCN S, Panchal HJ, Sands BE, et al. Accelerated infliximab dosing increases 30-day coletomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis 2017;(in press). [DOI] [PubMed] [Google Scholar]
- 16.Shah SC, Naymagon S, Cohen BL, et al. There is Significant Practice Pattern Variability in the Management of the Hospitalized Ulcerative Colitis Patient at a Tertiary Care and IBD Referral Center. J Clin Gastroenterol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health 2000;21:121–45. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 20.Al Khoury A, Chao C-Y, Bessissow T, et al. Intensified Infliximab Rescue Therapy for Acute Severe Ulcerative Colitis does not Improve Long Term Colectomy-Free Survival. Gastroenterology 2017;152:S399. [Google Scholar]
- 21.Shah SC, Naymagon S, Panchal HJ, et al. Accelerated infliximab dosing increases 30-day coletomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis 2017;(in press). [DOI] [PubMed] [Google Scholar]
- 22.Bojic D, Radojicic Z, Nedeljkovic-Protic M, et al. Long-term outcome after admission for acute severe ulcerative colitis in Oxford: the 1992–1993 cohort. Inflamm Bowel Dis 2009;15:823–8. [DOI] [PubMed] [Google Scholar]
- 23.Van Assche G, Vermeire S, Rutgeerts P. Management of acute severe ulcerative colitis. Gut 2011;60:130–3. [DOI] [PubMed] [Google Scholar]
- 24.Halpin SJ, Hamlin PJ, Greer DP, et al. Efficacy of infliximab in acute severe ulcerative colitis: a single-centre experience. World J Gastroenterol 2013;19:1091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson DJ, Hartery K, Doherty J, et al. CRP/Albumin Ratio: An Early Predictor of Steroid Responsiveness in Acute Severe Ulcerative Colitis. J Clin Gastroenterol 2017. [DOI] [PubMed] [Google Scholar]
- 26.Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut 2009;58:1612–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


