Abstract
The S100 protein family is involved in epithelial cell maturation and inflammation. Some S100 members are dysregulated during carcinogenesis and have been established as tumour markers. Psoriasin (S100A7) and koebnerisin (S100A15) form a highly homologous S100-subfamily that is regulated throughout tumor progression in epithelial cancers. Despite their homology, both S100 proteins are functionally distinct but synergize as antimicrobial peptides (AMP), chemoattractants for immune cells and proinflammatory ‘alarmins’. High cytoplasmic psoriasin expression prevents further tumour progression but nuclear translocation or secretion of psoriasin is associated with tumour growth and poor prognosis. The present review outlines the opposing effects of psoriasin and koebnerisin in multifunctional pathways and in mechanisms that are known to affecttumour cells (‘seeds’), tumour environment (‘soil’) and tumour cell migration (‘seeding’) thereby influencing epithelial carcinogenesis.
Keywords: S100A7, S100A15, psoriasin, koebnerisin, antimicrobial peptides, alarmins, cancer progression
Introduction:
In Paget’s theory on breast cancer metastasis cancer cells are regarded as “seed” that grow at distinct sites of the body, which he termed “soil” [1]. Tumour cells rarely invent new pathways, they rather exploit existing signalling cascades to survive and even to progress. Similar to immune cells that are attracted by chemokine gradients to the site of inflammation, cancer cells can metastasize to their “soil”, sites where they are able to re-attach and to grow.
Multiple pathways are involved in epithelial carcinogenesis depending on the type of tissue, lesion and stage during tumour progression [2]. Genes of the S100 protein family are among the pioneers that have been found to be dysregulated during carcinogenesis and today, some of the S100 members are considered as markers for tumour progression [3–5].
The S100 protein family constitutes a multigenic family of small (9–13kDa) calcium-binding EF-hand proteins with highly diverse but mostly elusive functions [6]. They are important regulators of a variety of intracellular functions, such as cell maturation and survival. As part of the innate immune response, some S100 proteins are released into the extracellular space in order to function as natural antimicrobials or as chemoattractants for inflammatory cells [7–9]. Most S100 protein family members are encoded on chromosome 1 [8,9], which is frequently associated with epithelial cancers and has been regarded as tumour susceptibility locus [10,11].
S100 gene duplications throughout vertebrate evolution led to an increase in number and diversity within the S100 family. Two evolutionary new and highly homologous genes on chromosome 1, psoriasin (S100A7) and koebnerisin (S100A15), were cloned because of their high expression in hyperplastic inflammatory psoriatic skin. Despite their high homology, psoriasin and koebnerisin are distinct in expression, function and mechanism of action, and therefore exemplary for the diversity within the S100 family. The corresponding single ortholog in mice, mS100a7a15, shares expressional and functional characteristics of both of the human proteins [12,13].
Psoriasin has been intensively studied in various cancers tissues, such as of breast, skin, head and neck, bladder and lung. [14–18]. In epithelial breast cancer, psoriasin is associated with poor prognosis and considered a marker for tumour progression [16,18]. Because of the high homology between psoriasin and koebnerisin, both S100 proteins are difficult to distinguish when co-expressed. Recent work shows that psoriasin and koebnerisin are co-regulated throughout different types of epithelial breast cancer [17]. Here, we review how these highly homologous S100 proteins modulate tumour cells (‘seeds’), tumour environment (‘soil’) and tumour cell migration (‘seeding’) thereby influencing epithelial carcinogenesis.
Tumour ‘Seed’
Psoriasin (S100A7) and koebnerisin (S100A15) are co-expressed in mature epithelial cells of breast lobules and ducts and differentiated epithelial skin layers [13,17]. Their expression is induced in calcium-differentiated keratinocytes along with late differentiation markers [19–21]. In terminally differentiated keratinocytes, psoriaisn and koebnerisin distribute to the cell periphery, where they are secreted and act as innate antimicrobial peptides (AMP) against pathogens [17,22,23]. Further, both S100 are potential substrates of the membrane bound transglutaminase to participate in the cornified envelope [19,24,25]. The cornified envelope forms a cellular barrier that is rich of lipids and transglutaminase-crosslinked high-molecular weight proteins that protects the skin from water loss, mechanical and chemical attacks [25].
In epithelial cancers, psoriasin and likely koebnerisin are usually elevated within early tumour stages, such as in pre-invasive carcinomas [15,26,27]. Within the tumour tissue, they are expressed in well differentiated tumour cells and show an expression pattern similar to that observed in normal mature epithelial cells [28].
For instance, psoriasin is expressed in the cytoplasm of differentiated epithelial cells, where it interferes with cellular β-catenin levels [29]. As a component of the Wnt signalling pathway, cytoplasmic β-catenin can translocate to the nucleus where it activates c-Myc, a prominent tumour oncogene that is overexpressed in several epithelial cancers [30,31]. When cytoplasmic, psoriasin degrades β-catenin via glycogen synthase kinase 3 (GSK3)-β or e-cadherin dependent mechanisms, and thus prevents nuclear β-catenin tranlocation and subsequent c-Myc activation [29,32]. Therefore, cytoplasmic psoriasin serves as a negative regulator of β-catenin-mediated oncogenic c-Myc activity thereby inhibiting tumour growth.
Psoriasin is usually downregulated in adjacent invasive cancer tissue, but when the expression persists, the nuclear translocation of psoriasin is associated with a poor clinical prognosis [16,33]. In the nucleus, psoriasin is thought to bind and to activate c-jun activation domain binding protein 1 (Jab1) [33]. Jab1 is a transcription co-factor that stabilizes c-Jun and drives activator protein (AP)-1 mediated gene transcription [34]. Jab-1 activated AP-1 transcription mediates cell survival through Akt by inhibition of pro-apoptotic Bad, a member of the Bcl-2 family. Akt further activates NF-κB mediated transcription of prosurvival genes, such as B-cell lymphomas 2 (Bcl-2) and survivin [35,36]. Jab1 further stabilizes hypoxia-inducible factor-1 (HIF-1) that upregulates HIF-1 dependent gene transcription, such as vascular endothelial growth factor (VEGF) and erythropoietin [37].
Jab1 is also a component of the enzymatic COP9 signalosome protein complex. In the nucleus Jab1 as part of the COP9 signalosome downregulates the cell cycle inhibitor p27Kip1 resulting in proliferation [38–40]. Thus, nuclear translocation of psoriasin and Jab1-dependent effects results in enhanced cell survival and proliferation. [17]. In invasive breast carcinomas, psoriasin and koebnerisin are co-regulated and associated with estrogen/ progesterone receptor negative and more aggressive tumours [17]. Koebnerisin nuclear translocation has not been studied, however due to mutations in the Jab1 binding site, koebnerisin might not be able to bind to Jab1 compared to psoriasin [17]. (IS THIS AN APPROPRIATE REFERENCE FOR THIS STATEMENT?)
Besides nuclear translocation, oncogenic effects could be mediated by S100 protein release into the extracellular space where it interacts with cell surface receptors such as RAGE, that is known to sustain inflammation and to promote carcinogenesis (Ref. Gebhardt et al., 2008, JEM). Elevated serum levels of psoriasin have been considered as a potential marker for epithelial cancer progression [18]. Extracellular psoriasin can bind to RAGE and thereby activates NF-κB that controls the transactivation of several genes involved in immune responses (IL-8, TNF-α) as well as cell proliferation (cyclin D1) and apoptosis (cIAP-1) [41]. In inflammation-associated carcinogenesis, NF-κB is a key player that assists pre-neoplastic and malignant cells to escape tumour-surveillance mechanisms by activating anti-apoptotic genes such as survivin, BCL-2, and cellular inhibitor of apoptosis protein 1 and 2 (cIAP-1, cIAP-2) [42]. Koebnerisin is not able to induce RAGE signalling but interacts with a yet unknown Gi-protein coupled receptor [17]. In tumour cells, psoriasin translocation to the nucleus and the extracellular release mediates oncogenic effects, which are further potentiated by subsequently low cytoplasmic levels that might unblock preventive anti-oncogenic characteristics.
Like the human psoriasin (S100A7) and koebnerisin (S100A15) orthologs, mS100a7a15 is expressed in mature epithelial cells of breast lobules and ducts and differentiated epithelial skin layers [12,43]. mS100a7a15 marks calcium and protein kinase C (PKC) dependent cell differentiation, is a transglutaminase substrate in keratinocytes, and proposes a function as antimicrobial peptide (AMP) that is upregulated by E.coli dependent TLR4 activation [12,22]. Well differentiated tumour cells may take advantage of the functions of mS100a7a15 as AMP and component of the protective cornified envelope similar to corresponding human psoriasin and koebnerisin.
Bi-transgenic mice with elevated mS100a7a15 expression in the mammary gland reveal ductal hyperplasia. In the ductal tissue, mS100a7a15 enhances proliferation as indicated by elevated CyclinD1 and Ki67 expression [44]. CyclinD1 is positive cell cycle regulator and can be activated by NF-κB and c-Jun/AP-1 that are both downstream of psoriasin in human [45]. Similar to psoriasin, mS100a7a15 reveals a putative Jab1- binding motif for nuclear AP-1 activation. When secreted into the extracellular space, mS100a7a15 can activate RAGE on epithelial cell like psoriasin, however other downstream signalling that could be important for tumour cell survival remain to be investigated [43]. However, secreted proteins of the S100A7/S100A15 subfamily are not just autocrine factors for the tumour ‘seed’, they may also influence the tumour environment, ‘soil’.
Tumour ‘Soil’
Multiple mechanisms steer the preparation of the microenvironment in order to allow tumor cell seeding. The surrounding matrix needs to be prepared to provide space for tumour growth and invasion. Moreover, a tumour supplying vasculature is needed for nutrition, oxygen and for tumour spreading.
Matrix metalloproteinases (MMPs) are zinc-dependent endo-peptidases that are capable of degrading extracellular matrix proteins [46]. Some MMPs are regulated by AP-1 transcription factors, which can be activated by psoriasin (S100A7) [47,48]. In prostate cancer cells, psoriasin is able to induce matrix metalloproteinases MMP-1, −3, and −9, which promote the degradation of the tumour surrounding tissue [49]. VEGF, a major angiogenic factor for tumour vascularisation and progression is upregulated by hypoxia and dependent on HIF-1, a Jab1 stabilized transcription factor that can be activated by psoriasin [50,51]. Furthermore, extracellular psoriasin induces reactive oxygen species (ROS) generation in epithelial cells that may also induce VEGF expression, probably mediated through RAGE signalling as shown in endothelial cells [52]. In a breast cancer model, the mouse S100a7a15 ortholog shows similar oncogenic effects by inducing matrix metalloproteinases (MMP-2) and angiogenic factors, like VEGF to enhance tumour malignancy [44].
Tumour-surveillance is thought to be dependent on certain leukocyte subtypes, e.g. CD8+ cytotoxic T lymphoytes (CTLs), CD4+ Th1 cells and natural killer cells. Cancer cells can evade immunologic surveillance by attenuating the immune defence, e.g. through TGF-β. They are thought to “re-program” certain leukocytes to become friendly and supportive, e.g. tumour-associated macrophages (TAM-M2) produce growth factors, matrix metalloproteases and angiogenic factors to support tumour growth and invasion [53]. In a breast cancer model, mS100a7a15 recruits leukocytes and tumour-associated macrophages (TAM) via RAGE/Stat3 signalling and thus promotes tumour progression and metastasis [44]. As mentioned before, the human orthologs psoriasin and koebnerisin are chemoattractants and able to recruit myeloid cells, like monocytes [41]. Wether they could assist in the evasion of tumour-surveillance by attracting tumour-associated macrophages (TAM) has not been investigated yet. Compared to psoriasin, koebnerisin is produced additionally by tumour surrounding non-epithelial cells such as dendritic cells, epithelial-derived myoepithelial cells around acini, and by surrounding blood vessels [17,21].
As secreted factors, psoriasin and koebnerisin can further mediate immune responses via extracellular receptors, like RAGE to enhance TNF-α, IL-1, IL-6, and IL-8 production as ‘alarmins’[54]. In immune cells, these proinflammatory cytokines lead to NF-κB dependent secretion of growth factors that enhance proliferation and survival of malignant cells [55]. Furthermore, also attracted macrophages, mast cells and neutrophils can also upregulate non-specific immune responses that may lead to enhanced tumour development [42]. Similar to psoriasin and koebnerisin, mS100a7a15 induces NF-κB dependent pro- inflammatory molecules like CXCL1, CXCL8, IL-1α, IL-11 and CSF2, which might exaggerate immune responses and promotes tumour development, including tumour spread ‘seeding’ [2,44,56].
Tumour ‘Seeding’
Metastasis requires tumour cells to detach from the primary tumour to traffic and to re-attach, to “seed”, in the suitable ‘soil’. Tumour cells utilize mechanisms similar to leukocytes to migrate through solid tissues and vessels, and to extravasate into tissues [57]. Like leukocytes, tumour cells seem to “sense” their “soil” by chemokine gradients, e.g. through the leukokine CXCL12 that is expressed in organs that are frequently affected by breast cancer metastases [58,59]. CXCL12 recruits lymphocytes and monocytes that express the CXCL12 receptor CXCR4 [60]. Breast cancer cells express functionally active CXCR4 and are therefore recruited to CXCL12 expressing tissues, like lung, lymph nodes, bone marrow and liver. Secreted epithelial-derived psoriasin (S100A7) and koebnerisin (S100A15) are able to attract leukocytes into the skin through different extracellular receptors, such as RAGE. Furthermore, psoriasin is overexpressed in brain metastases derived from the lung squamous cell carcinoma [28]. Recent work shows that psoriasin promotes migration of epithelial tumour cell that is dependent on RAGE [61,62] and NF-κB signalling [63–65].
The next step of successful migration and invasion of new tissues includes the three major steps of extravasation: rolling, adhesion and diapedesis. The first two steps are dependent on various integrins, cadherins and selectins. In this context, psoriasin is able to bind to the integrin β6 subunit. This interaction between psoriasin and β6 is required for αvβ6-dependent invasion of carcinoma cells [66].
Conclusion:
In concordance to Paget’s ‘seed’ and ‘soil’ theory, psoriasin (S100A7) and koebnerisin (S100A15) are two proteins that are exploited by the tumour i) to adapt cellular signalling pathways that regulate the survival of the tumour ‘seeds’ ii) to modify the surrounding microenvironment (‘soil’) to escape tumour-surveillance, iii) and to promote the migration of the cancer cells (‘seeding’). However, little is known about the recently discovered koebnerisin and furthermore, several functions that are referred to psoriasin might actually be due to koebnerisin signalling. Their different properties are compelling reasons to discriminate psoriasin (S100A7) and koebnerisin (S100A15) in epithelial homeostasis, inflammation and epithelial carcinogenesis.
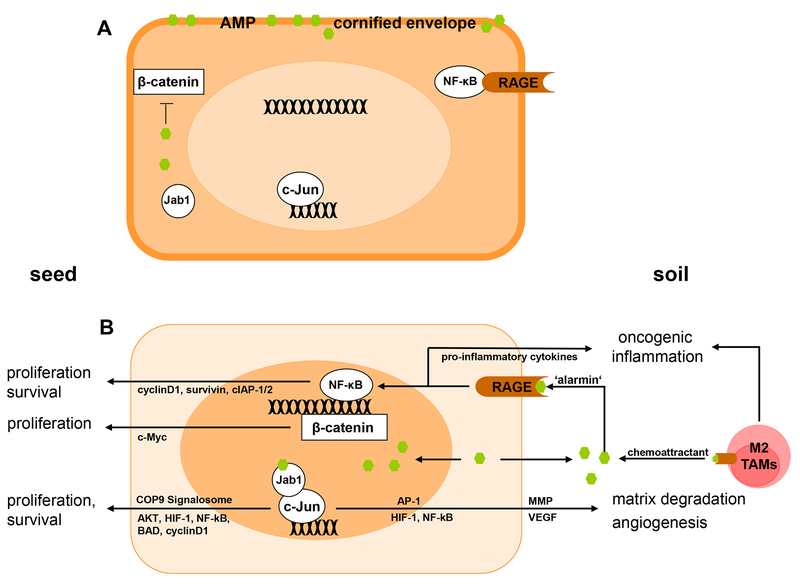
Figure 1. Multifunctional psoriasin (S100A7) and koebnerisin (S100A15) control the tumour ‘seed’ and ‘soil’.
Cellular distribution and release of psoriasin (S100A7) and koebnerisin (S100A15) into the extracellular space effect tumour survival and progression A) Cytoplasmic psoriasin and koebenrisin may protect the tumour cell (‘seed’) from physical (cornified envelope) and biological (AMP) damage, and psoriasin prevents β-catenin translocation to the nucleus and its downstream oncogenic activity. B) In invasive carcinomas, a psoriasin - Jab1 is thought to translocate into the nucleus, to activate AP-1 target genes as well as COP9 signalosome signalling. Psoriasin and likely koebnerisin are secreted into the extracellular space (‘soil’) and act as chemoattractants for tumour-associated inflammatory cells, such as macrophages (TAMs). Their secreted function as ‘alarmins’ by binding to extracellular receptors, such as RAGE, thereby mediating tumor-promoting inflammation. Furthermore, extracellular psoriasin is able to induce tumour cell migration via RAGE. Additionally, extracellular and nuclear distribution of psoriasin leads to a lack of cytoplasmic peptide levels and unlocks the β-catenin-mediated oncogenic signalling.
Acknowledgements
The authors are very grateful to Christoffer Gebhardt, German Cancer Research Center (DKFZ) and Department of Dermatology, Venerology and Allergology University Hospital Mannheim,Germany, for his expertise and valuable suggestions.
References
- 1.Paget S: The distribution of secondary growths in cancer of the breast. 1889 Cancer Metastasis Rev 1989, 8:98–101. [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA: The hallmarks of cancer. Cell 2000, 100:57–70. [DOI] [PubMed] [Google Scholar]
- 3.Salama I, Malone PS, Mihaimeed F, Jones JL: A review of the S100 proteins in cancer. Eur J Surg Oncol 2008, 34:357–364. [DOI] [PubMed] [Google Scholar]
- 4.Lukanidin E, Sleeman JP: Building the niche: the role of the S100 proteins in metastatic growth. Semin Cancer Biol 2012, 22:216–225. [DOI] [PubMed] [Google Scholar]
- 5.Gebhardt C, Nemeth J, Angel P, Hess J: S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 2006, 72:1622–1631. [DOI] [PubMed] [Google Scholar]
- 6.Donato R: Intracellular and extracellular roles of S100 proteins. Microsc Res Tech 2003, 60:540–551. [DOI] [PubMed] [Google Scholar]
- 7.Donato R: Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1999, 1450:191–231. [DOI] [PubMed] [Google Scholar]
- 8.Donato R: S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001, 33:637–668. [DOI] [PubMed] [Google Scholar]
- 9.Marenholz I, Heizmann CW, Fritz G: S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun 2004, 322:1111–1122. [DOI] [PubMed] [Google Scholar]
- 10.Tirkkonen M, Tanner M, Karhu R, Kallioniemi A, Isola J, Kallioniemi OP: Molecular cytogenetics of primary breast cancer by CGH. Genes Chromosomes Cancer 1998, 21:177–184. [PubMed] [Google Scholar]
- 11.Moinzadeh P, Breuhahn K, Stutzer H, Schirmacher P: Chromosome alterations in human hepatocellular carcinomas correlate with aetiology and histological grade--results of an explorative CGH meta-analysis. Br J Cancer 2005, 92:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf R, Voscopoulos CJ, FitzGerald PC, Goldsmith P, Cataisson C, Gunsior M, Walz M, Ruzicka T, Yuspa SH: The mouse S100A15 ortholog parallels genomic organization, structure, gene expression, and protein-processing pattern of the human S100A7/A15 subfamily during epidermal maturation. J Invest Dermatol 2006, 126:1600–1608. [DOI] [PubMed] [Google Scholar]
- 13.Wolf R, Ruzicka T, Yuspa SH: Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids 2011, 41:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celis JE, Rasmussen HH, Vorum H, Madsen P, Honore B, Wolf H, Orntoft TF: Bladder squamous cell carcinomas express psoriasin and externalize it to the urine. J Urol 1996, 155:2105–2112. [PubMed] [Google Scholar]
- 15.Moubayed N, Weichenthal M, Harder J, Wandel E, Sticherling M, Glaser R: Psoriasin (S100A7) is significantly up-regulated in human epithelial skin tumours. J Cancer Res Clin Oncol 2007, 133:253–261. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi SC, Matta A, Kaur J, Grigull J, Chauhan SS, Thakar A, Shukla NK, Duggal R, DattaGupta S, Ralhan R, et al. : Nuclear S100A7 is associated with poor prognosis in head and neck cancer. PLoS One 2010, 5:e11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf R, Voscopoulos C, Winston J, Dharamsi A, Goldsmith P, Gunsior M, Vonderhaar BK, Olson M, Watson PH, Yuspa SH: Highly homologous hS100A15 and hS100A7 proteins are distinctly expressed in normal breast tissue and breast cancer. Cancer Lett 2009, 277:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Zhao Q, Chen Y, Wang Y, Gao S, Mao Y, Li M, Peng A, He D, Xiao X: Selective expression of S100A7 in lung squamous cell carcinomas and large cell carcinomas but not in adenocarcinomas and small cell carcinomas. Thorax 2008, 63:352–359. [DOI] [PubMed] [Google Scholar]
- 19.Broome AM, Ryan D, Eckert RL: S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem 2003, 51:675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinsson H, Yhr M, Enerback C: Expression patterns of S100A7 (psoriasin) and S100A9 (calgranulin-B) in keratinocyte differentiation. Exp Dermatol 2005, 14:161–168. [DOI] [PubMed] [Google Scholar]
- 21.Wolf R, Lewerenz V, Buchau AS, Walz M, Ruzicka T: Human S100A15 splice variants are differentially expressed in inflammatory skin diseases and regulated through Th1 cytokines and calcium. Exp Dermatol 2007, 16:685–691. [DOI] [PubMed] [Google Scholar]
- 22.Buchau AS, Hassan M, Kukova G, Lewerenz V, Kellermann S, Wurthner JU, Wolf R, Walz M, Gallo RL, Ruzicka T: S100A15, an antimicrobial protein of the skin: regulation by E. coli through Toll-like receptor 4. J Invest Dermatol 2007, 127:2596–2604. [DOI] [PubMed] [Google Scholar]
- 23.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM: Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 2005, 6:57–64. [DOI] [PubMed] [Google Scholar]
- 24.Eckert RL, Lee KC: S100A7 (Psoriasin): a story of mice and men. J Invest Dermatol 2006, 126:1442–1444. [DOI] [PubMed] [Google Scholar]
- 25.Ruse M, Lambert A, Robinson N, Ryan D, Shon KJ, Eckert RL: S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochemistry 2001, 40:3167–3173. [DOI] [PubMed] [Google Scholar]
- 26.Leygue E, Snell L, Hiller T, Dotzlaw H, Hole K, Murphy LC, Watson PH: Differential expression of psoriasin messenger RNA between in situ and invasive human breast carcinoma. Cancer Res 1996, 56:4606–4609. [PubMed] [Google Scholar]
- 27.Alowami S, Qing G, Emberley E, Snell L, Watson PH: Psoriasin (S100A7) expression is altered during skin tumorigenesis. BMC Dermatol 2003, 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Wang Y, Chen Y, Sun S, Li N, Lv D, Liu C, Huang L, He D, Xiao X: Identification and validation of S100A7 associated with lung squamous cell carcinoma metastasis to brain. Lung Cancer 2007, 57:37–45. [DOI] [PubMed] [Google Scholar]
- 29.Deol YS, Nasser MW, Yu L, Zou X, Ganju RK: Tumor-suppressive effects of psoriasin (S100A7) are mediated through the beta-catenin/T cell factor 4 protein pathway in estrogen receptor-positive breast cancer cells. J Biol Chem 2011, 286:44845–44854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amundadottir LT, Johnson MD, Merlino G, Smith GH, Dickson RB: Synergistic interaction of transforming growth factor alpha and c-myc in mouse mammary and salivary gland tumorigenesis. Cell Growth Differ 1995, 6:737–748. [PubMed] [Google Scholar]
- 31.Chen CH, Shen J, Lee WJ, Chow SN: Overexpression of cyclin D1 and c-Myc gene products in human primary epithelial ovarian cancer. Int J Gynecol Cancer 2005, 15:878–883. [DOI] [PubMed] [Google Scholar]
- 32.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R: E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci 1999, 112 (Pt 8):1237–1245. [DOI] [PubMed] [Google Scholar]
- 33.Emberley ED, Alowami S, Snell L, Murphy LC, Watson PH: S100A7 (psoriasin) expression is associated with aggressive features and alteration of Jab1 in ductal carcinoma in situ of the breast. Breast Cancer Res 2004, 6:R308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emberley ED, Niu Y, Curtis L, Troup S, Mandal SK, Myers JN, Gibson SB, Murphy LC, Watson PH: The S100A7-c-Jun activation domain binding protein 1 pathway enhances prosurvival pathways in breast cancer. Cancer Res 2005, 65:5696–5702. [DOI] [PubMed] [Google Scholar]
- 35.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS: Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev 2008, 22:1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME: Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91:231–241. [DOI] [PubMed] [Google Scholar]
- 37.Bae MK, Ahn MY, Jeong JW, Bae MH, Lee YM, Bae SK, Park JW, Kim KR, Kim KW: Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem 2002, 277:9–12. [DOI] [PubMed] [Google Scholar]
- 38.Chamovitz DA, Segal D: JAB1/CSN5 and the COP9 signalosome. A complex situation. EMBO Rep 2001, 2:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emberley ED, Niu Y, Leygue E, Tomes L, Gietz RD, Murphy LC, Watson PH: Psoriasin interacts with Jab1 and influences breast cancer progression. Cancer Res 2003, 63:1954–1961. [PubMed] [Google Scholar]
- 40.Tomoda K, Kubota Y, Arata Y, Mori S, Maeda M, Tanaka T, Yoshida M, Yoneda-Kato N, Kato JY: The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem 2002, 277:2302–2310. [DOI] [PubMed] [Google Scholar]
- 41.Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, et al. : Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol 2008, 181:1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eiro N, Vizoso FJ: Inflammation and cancer. World J Gastrointest Surg 2012, 4:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webb M, Emberley ED, Lizardo M, Alowami S, Qing G, Alfia’ar A, Snell-Curtis LJ, Niu Y, Civetta A, Myal Y, et al. : Expression analysis of the mouse S100A7/psoriasin gene in skin inflammation and mammary tumorigenesis. BMC Cancer 2005, 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasser MW, Qamri Z, Deol YS, Ravi J, Powell CA, Trikha P, Schwendener RA, Bai XF, Shilo K, Zou X, et al. : S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res 2012, 72:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaulian E, Karin M: AP-1 as a regulator of cell life and death. Nat Cell Biol 2002, 4:E131–136. [DOI] [PubMed] [Google Scholar]
- 46.Woessner JF Jr.: Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991, 5:2145–2154. [PubMed] [Google Scholar]
- 47.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B: IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 2007, 293:H3356–3365. [DOI] [PubMed] [Google Scholar]
- 48.Kim HS, Kim MH, Jeong M, Hwang YS, Lim SH, Shin BA, Ahn BW, Jung YD: EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res 2004, 24:747–753. [PubMed] [Google Scholar]
- 49.Ye L, Sun PH, Martin TA, Sanders AJ, Mason MD, Jiang WG: Psoriasin (S100A7) is a positive regulator of survival and invasion of prostate cancer cells. Urol Oncol 2012. [DOI] [PubMed] [Google Scholar]
- 50.Shweiki D, Itin A, Soffer D, Keshet E: Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359:843–845. [DOI] [PubMed] [Google Scholar]
- 51.McMahon G: VEGF receptor signaling in tumor angiogenesis. Oncologist 2000, 5 Suppl 1:3–10. [DOI] [PubMed] [Google Scholar]
- 52.Shubbar E, Vegfors J, Carlstrom M, Petersson S, Enerback C: Psoriasin (S100A7) increases the expression of ROS and VEGF and acts through RAGE to promote endothelial cell proliferation. Breast Cancer Res Treat 2012, 134:71–80. [DOI] [PubMed] [Google Scholar]
- 53.Sica A, Schioppa T, Mantovani A, Allavena P: Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006, 42:717–727. [DOI] [PubMed] [Google Scholar]
- 54.Hegyi Z, Zwicker S, Bureik D, Peric M, Koglin S, Batycka-Baran A, Prinz JC, Ruzicka T, Schauber J, Wolf R: Vitamin D analog calcipotriol suppresses the Th17 cytokine-induced proinflammatory S100 “alarmins” psoriasin (S100A7) and koebnerisin (S100A15) in psoriasis. J Invest Dermatol 2012, 132:1416–1424. [DOI] [PubMed] [Google Scholar]
- 55.Karin M: Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441:431–436. [DOI] [PubMed] [Google Scholar]
- 56.Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011, 144:646–674. [DOI] [PubMed] [Google Scholar]
- 57.Friedl P, Wolf K: Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 2003, 3:362–374. [DOI] [PubMed] [Google Scholar]
- 58.Balkwill F, Mantovani A: Inflammation and cancer: back to Virchow? Lancet 2001, 357:539–545. [DOI] [PubMed] [Google Scholar]
- 59.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. : Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410:50–56. [DOI] [PubMed] [Google Scholar]
- 60.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA: A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 1996, 184:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kataoka K, Ono T, Murata H, Morishita M, Yamamoto KI, Sakaguchi M, Huh NH: S100A7 promotes the migration and invasion of osteosarcoma cells via the receptor for advanced glycation end products. Oncol Lett 2012, 3:1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winston J, Wolf R: Psoriasin (S100A7) promotes migration of a squamous carcinoma cell line. J Dermatol Sci 2012, 67:205–207. [DOI] [PubMed] [Google Scholar]
- 63.Helbig G, Christopherson KW 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H: NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 2003, 278:21631–21638. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Lau G, Chen L, Yuan YF, Huang J, Luk JM, Xie D, Guan XY: Interleukin 23 promotes hepatocellular carcinoma metastasis via NF-kappa B induced matrix metalloproteinase 9 expression. PLoS One 2012, 7:e46264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al. : NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 2007, 13:62–69. [DOI] [PubMed] [Google Scholar]
- 66.Morgan MR, Jazayeri M, Ramsay AG, Thomas GJ, Boulanger MJ, Hart IR, Marshall JF: Psoriasin (S100A7) associates with integrin beta6 subunit and is required for alphavbeta6-dependent carcinoma cell invasion. Oncogene 2011, 30:1422–1435. [DOI] [PubMed] [Google Scholar]