Prostate cancer is the second most common cause of cancer death, after lung and bronchus cancers, in men in the United States. According to the American Cancer Society, 192 280 men were diagnosed with prostate cancer and 27 360 men died of the disease in 2009.1 In the past 2 years, prostate cancer has been diagnosed earlier in the course of the disease because of widespread use of the serum prostate specific antigen.2 Until recently, imaging had a minor role in the early detection and localization of prostate cancer; however, ultrasound and magnetic resonance (MR) imaging use is increasing.
Because of its superior contrast resolution, MR is invaluable for diagnosing, staging and monitoring prostate cancer.2 Anatomical T2-weighted MR in 3 planes can depict the location of the tumor and determine whether it is confined to the prostate gland or if there is an extracapsular extension or metastasis of the tumor to adjacent tissues.3 More specialized MR sequences such as MR spectroscopic imaging, dynamic contrast-enhanced (DCE)-MR imaging and diffusion-weighted imaging sequences can provide functional information (eg, biochemical changes, vascularity, permeability and free water path length, respectively). At the National Cancer Institute’s Molecular Imaging Clinic, we use a multiparametric MR approach to prostate cancer imaging including T2-weighted, diffusion-weighted imaging, MR spectroscopy and DCE-MR imaging.
We will review our technique in this article to share our experience with others who are interested in performing this study.
Preparation
Upon scheduling, all of our patients receive an informational sheet to explain the steps of the procedure. When patients arrive for their imaging appointment, a radiologist explains the procedure and answers any questions. We make every effort to ensure patients understand the procedure, which increases patient cooperation and results in scans that are more successful.
We do not require our patients to undergo bowel cleansing before scanning and have not found this to interfere with the quality of the study. We also do not routinely use medications to suppress bowel motion. Patients who recently had a prostate biopsy receive a Tl-weighted scan to check for prostate hemorrhage before we insert the endorectal coil. Tl-weighted images show biopsy-related hemorrhage as hyperintense regions.4 Because hemorrhages invalidate the sequences and render the study meaningless, if a significant one is seen, the patient is rescheduled 3 to 4 weeks later to allow the residual hemorrhage to resolve.
We use a 3-Tesla MR scanner with combined endorectal coil and anterior half of a 32-channel cardiac coil. A radiologist or trained technologist performs a digital rectal exam to check the rectal vault for obstruction, localize the prostate gland and ensure there are no abnormalities (eg, internal hemorrhoids) that might contraindicate the use of the endorectal coil. The balloon of the endorectal coil is lubricated with 1% lidocaine gel to minimize the discomfort. The patient is asked to lie in the left decubitis position with knees bent and the uninflated endorectal coil is then gently placed in the rectum. The coil should be inserted slowly with steady pressure, with the coil antenna facing toward the prostate gland. The patient may experience some discomfort or pressure, but there should not be pain. If the patient reports pain, the coil should be repositioned and gently reinserted. It is useful to follow the course of the rectum as determined by the digital rectal exam for the insertion. Despite the unpleasant aspects of endorectal coil MR, we have had very few problems.
After insertion, the balloon is inflated with approximately 50 cc to 60 cc of perfluorocarbon liquid. Perfluorocarbon is used to avoid air-tissue interface susceptibility artifacts that can render nondiagnostic images if the balloon is inflated with air. After endorectal coil placement with perfluorocarbon instillation, the patient is asked to turn onto his back, and the upper portion of the 32-channel coil is placed on his pelvis, centering on the symphysis pubis. Before starting the procedure, good intravenous access is obtained with an 18- to 20-gauge angiocatheter needle preferably in the antecubital fossa.
Examination
MR acquisition starts with an initial 3-plane localizer of the full pelvis and a smaller field of view localizer around the prostate, followed by appropriate MR sequences (see Table).
Table.
| Sequence | TR/TE | FOV (mm) | Pixel Size | Matrix | Flip Angles | Slice Thickness (mm) | Scan Time (min) |
|---|---|---|---|---|---|---|---|
| T2W sagittal | 2925/120 | 140 | 0.27 × 0.27 | 304 × 234 | 90/100 | 3 | 1:51 |
| T2W axial | 8869/120 | 140 | 0.27 × 0.27 | 304 × 234 | 90/180 | 3 | 5:37 |
| T2W coronal | 2632/120 | 140 | 0.27 × 0.27 | 304 × 234 | 90/100 | 3 | 1:40 |
| DWI | 3709/52 | 140 | 1.02 × 1.02 | 112 × 108 | 90/180 | 2.73/0.27 | 4:46 |
| 3-D MRSI | 980/100 | 72 | 6 × 6 | 10 × 10 | 90/180 | 6 | 12:51 |
| T1 axial GRE | 3.7/2.2 | 262 | 1.02 × 1.02 | 256 × 186 | 2 | 6 | 0:22 |
| T1 axial DCE | 3.7/2.2 | 262 | 1.02 × 1.02 | 256 × 186 | 8.5 | 6 | 5:12 |
| Axial THRIVE | 5.3/2.6 | 440 | 1.5 × 1.5 | 280 × 199 | 10 | 5 | 0:57 |
T2-Weighted Imaging
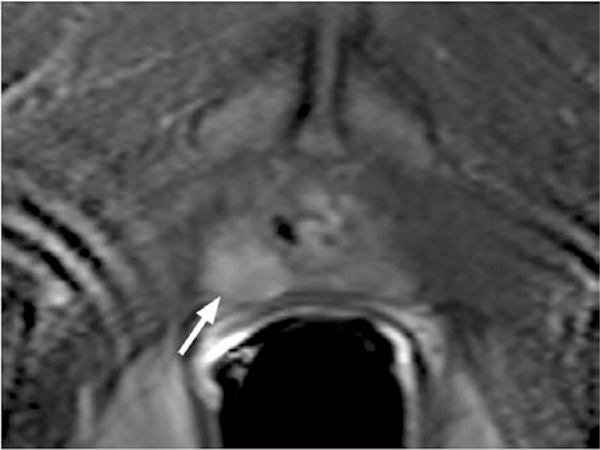
T2-weighted turbo spin echo MR images are obtained in axial, sagittal and coronal planes. The T2-weighted MR sequence is the main sequence for anatomical imaging because of its high spatial resolution. These images help identify prostate gland lesions and assess whether a tumor is within the gland or extending outside the prostate (see Figures 1 and 2). Treatment decisions often are based on the information obtained from this sequence regarding the capsule. T2-weighted images depict prostate cancer lesions as well-circumscribed, low-signal intensity foci that are often multiple.2
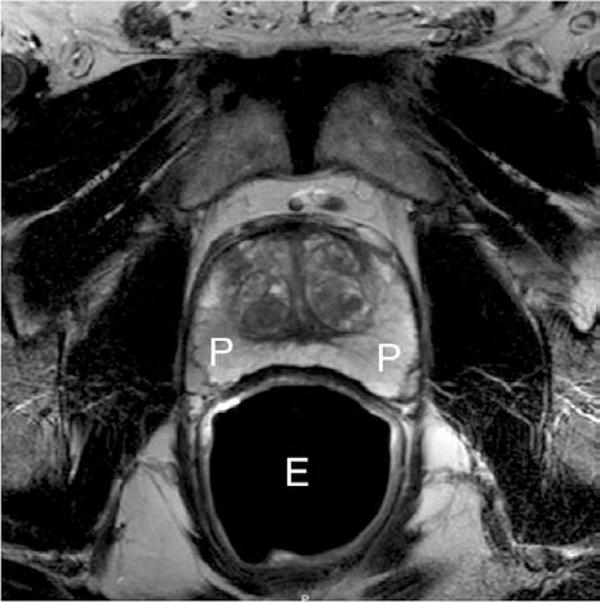
Figure. 1.
Normal axial T2-weighted MR image of prostate peripheral zone (P) with endorectal coil (E).
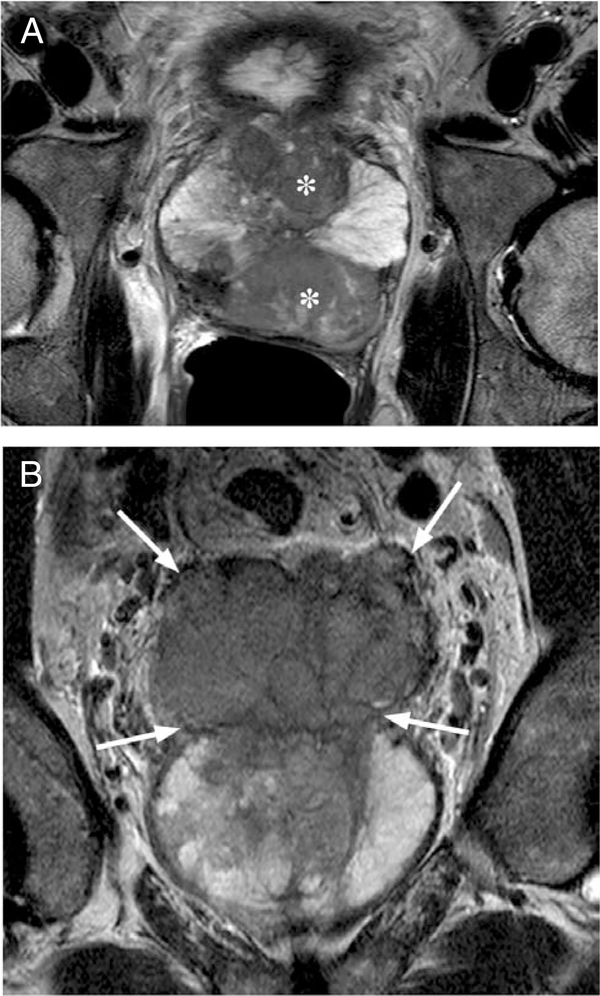
Figure 2.
T2-weighted axial (A) and coronal (B) MR image of a large prostate tumor (asterisks) with seminal vesicle invasion (arrows).
Diffusion-Weighted Imaging
In normal tissue, water molecules may move freely in the space between cells. In tumor tissue, however, water movement is restricted by the increased cellularity and fibrosis associated with tumors. Diffusion-weighted imaging is an important sequence in prostate MR imaging because it exploits this property of tissue to create diagnostic images. Despite its low signal-to-noise ratio and its susceptibility artifacts, diffusion-weighted imaging provides a sensitive method of detecting prostate cancer with higher contrast than T2-weighted scans, albeit with lower spatial resolution. Diffusion-weighted imaging depicts any area in the gland where there is restricted water diffusion (see Figure 3). Our diffusion-weighted imaging sequence is a single-shot echo planar imaging sequence that includes 5 evenly spaced b values starting from 0 s/mm2 to a maximum b value of 750 s/mm2. The images at different b values are used to calculate apparent diffusion coefficient maps. Restricted water appears as a low apparent diffusion coefficient value, which is seen as a hypotense focus on the maps. Prostate tumors appear hyperintense on diffusion-weighted imaging and hypointense on apparent diffusion coefficient maps.2
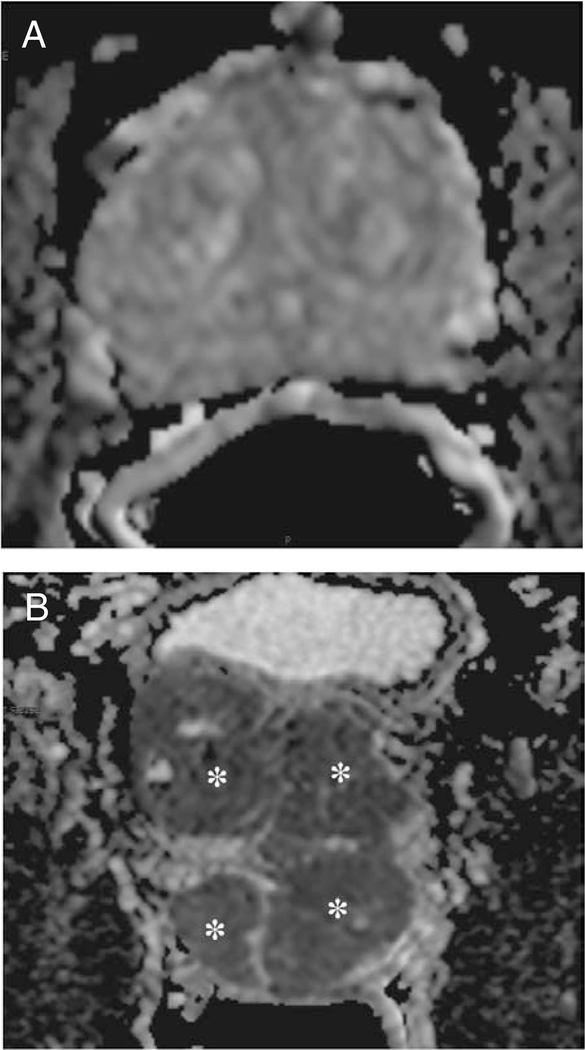
Figure 3.
Axial apparent diffusion coefficient maps of diffusion- weighted images of normal prostate gland (A) and prostate gland with large multiple tumors (B). Asterisks indicate the tumors.
MR Spectroscopic Imaging
MR spectroscopic imaging depicts the “chemical signature” of tissue. As such, it is the most technically challenging of all the sequences described here. Prostate MR spectroscopic imaging evaluates the relative signals arising from citrate, normally produced by the prostate,and choline, associated with cell membrane synthesis.5 For example, elevated choline and diminished citrate peaks increase the suspicion of cancer. We currently use a multivoxel 3-D point-resolved spatially localized spectroscopy sequence with fat suppression to cover the entire prostate gland. It is not always possible to cover the entire gland if the prostate is very large. The spectral dimension covers 2 kHz in 1024 points. Second order shimming is used to improve B0 field homogeneity. This sequence requires almost 15 minutes of scan time, including setup and acquisition. It therefore represents a substantial fraction (about 25%) of the total imaging time required for prostate imaging. To analyze the spectroscopy data, we use a research software tool provided by the manufacturer. Prostate tumors contain high levels of choline and lower levels of citrate when compared to normal background prostate5 and can be visualized in choline/citrate ratio maps (see Figure 4).
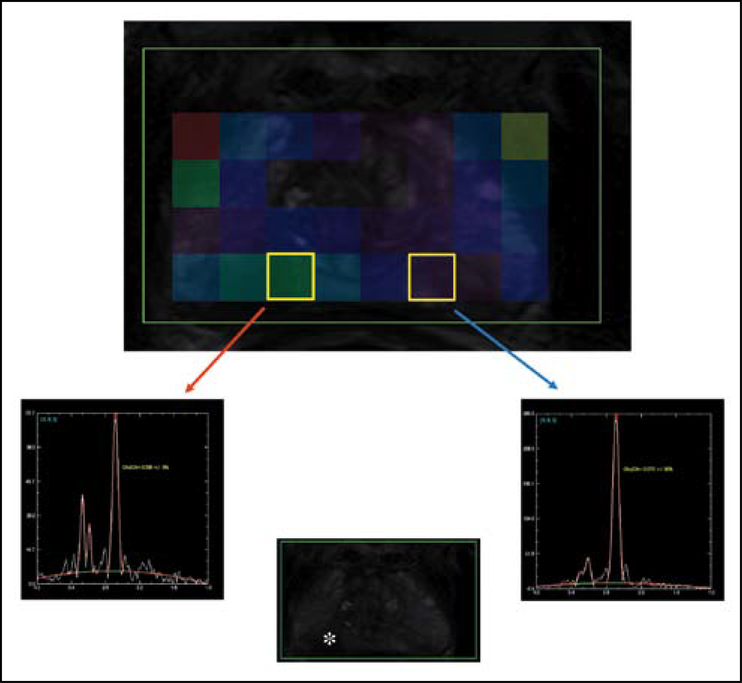
Figure 4.
A chemical shift spectra superimposed on T2 images depicting both normal spectra peaks (blue arrow) and abnormal spectra (red arrow) of area in the prostate gland (asterisk).
Dynamic Contrast-Enhanced MR
DCE-MR is an important functional sequence in prostate imaging. It shows the enhancement patterns of the prostate (see Figure 5). Many but not all tumors are more vascular than the surrounding tis- sue.6 However, benign conditions such as prostatitis and hyperplasia can be positive on DCE-MR imaging. The key strategy of DCE-MR is to obtain rapid 3-D images of the entire prostate gland in multiple passes after the contrast is administered. The signal-to-noise ratio and spatial resolution are intentionally sacrificed for rapid temporal resolution. We currently use ultra-fast T1-weighted spoiled gradient echo sequence with a scan time of 5.6 seconds for each set of images for 53 passes over 5 minutes without a pause. DCE-MR imaging, displayed as a cine movie, depicts contrast uptake kinetics. For instance, if a lesion enhances early and/or de-enhances rapidly, malignancy is suspected.3,6,7 DCE-MR images can be processed with special software to obtain color-coded enhancement patterns illustrating the rate of contrast wash-in and wash-out. Both raw DCE-MR images viewed in cine mode and postprocessed color maps are used to interpret DCE-MR.
Figure 5.
Dynamic contrast-enhanced MR image of a rapidly enhancing lesion (arrow) in the right peripheral zone.
Postcontrast 3-D T1-Weighted Imaging
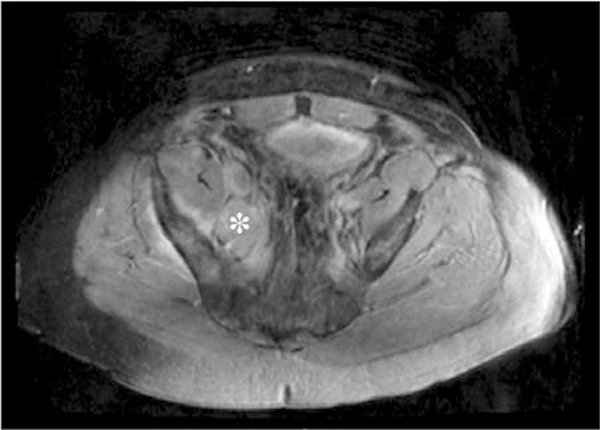
The final sequence is a multistack abdomen and pelvis postcontrast, fat-suppressed axial T1-weighted high-resolution isotropic volume excitation (THRIVE). We use the THRIVE sequence with 3 stacks covering the pelvis and middle and upper abdomen (TR/TE, 5.3/2.6; matrix, 280 × 199; voxel size, 1.5 × 1.5; flip angle, 10°; slice thickness, 5 mm; and FOV, 440). This is used to check for enlarged nodes and early bone metastases (see Figure 6). It also is useful in screening for additional incidental pathology. For instance, this sequence has enabled us to detect pancreatic cancers, kidney cancers and retroperitoneal masses that reprioritize the treatment of prostate cancer.
Figure 6.
Axial THRIVE image of metastatic right iliac lymph nodes (asterisk) in a patient with prostate cancer.
Interpretation of Prostate MR
Although technologists do not interpret studies, it is important to understand how these sequences fit into prostate cancer diagnosis. As mentioned previously, T2-weighted images provide anatomic imaging. Apparent diffusion coefficient maps, MR spectroscopic imaging and DCE-MR imaging provide additional supporting data. If only the T2-weighted sequence is positive then there is a low likelihood that a lesion is a cancer, whereas if all sequences are positive then it is highly likely that the lesion is cancerous. Thus, these parameters work in concert to determine the probability of cancer.
Note that MR imaging does not directly diagnose prostate cancer; the diagnosis remains in the realm of biopsy and histological analysis. However, MR imaging allows clinicians to understand the location, size and extent of a cancer in a way that is not possible with prostate-specific antigen values or digital rectal exams.
Successful MR of the Prostate
Prostate MR imaging with endorectal coil is a challenging examination for both patients and technologists. The following tips can help technologists obtain better MR images while helping patients cope with the procedure.
Sit with patients outside the scanner and explain each detail of the procedure thoroughly. If possible, send a procedure information sheet to the patient when the exam is scheduled.
Make sure the patient understands that he must stay still during the exam. Inform the patient that motion will compromise the diagnostic quality of his image and therefore could affect his treatment.
Ask the patient to empty his bladder before the procedure to avoid interruptions during the scan.
Use perfluorocarbon liquid instead of air to inflate the endorectal coil if your protocol includes diffusion-weighted imaging and MR spectroscopic imaging at 3 T. (However, the balloon can be inflated with air or barium at 1.5 T.)
Use semianesthetic gel on the balloon of the endorectal coil prior to placement to minimize discomfort.
Consider administering antispasm medication to minimize rectal spasm and motion in sensitive patients.
Closely monitor MR images as they are processed and check for motion artifact caused by rectal spasm and patient motion. Repeat the sequence if sufficient motion is seen.
In patients who cannot tolerate the coil, the study can be done without a coil. However, the quality of the examination may diminish and the quality of the information derived may be affected.
Conclusion
MR imaging of the prostate is a complex examination that requires skill and attention. It is highly rewarding to localize a tumor that has evaded biopsy or provide information that aids a patient’s treatment decision. The quality of MR imaging of the prostate continues to improve and ideally, it will become even more specific for cancer. In the meantime, endorectal coil MR continues to be an important tool in the fight against prostate cancer. ◆
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Contributor Information
Dagane Daar, Clinical Research Directorate/CMRP, SAIC-Frederick Inc, National Cancer Institute (NCI)-Frederick, in Frederick, Maryland..
Marcelino Bernardo, Clinical Research Directorate/CMRP, SAIC-Frederick Inc, National Cancer Institute (NCI)-Frederick, in Frederick, Maryland..
Peter L Choyke, NCI, National Institutes of Health (NIH), in Bethesda, Maryland..
Yolanda McKinney, MIP, NCI, NIH..
Baris Turkbey, MIP, NCI, NIH..
References
- 1.What are the key statistics about prostate cancer? American Cancer Society website. www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_prostate_cancer_36.asp?sitearea=. Updated April 29, 2010 Accessed May 12, 2010.
- 2.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection — histopathologic correlation. Radiology. 2010;255(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkbey B, Pinto P, Choyke PL. Imaging techniques for prostate cancer: implications for focal therapy. Nat Rev Urol. 2009;6(4):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turkbey B, Albert PS, Kurdziel K, Choyke PL. Imaging localized prostate cancer: current approaches and new developments. AJRAm J Roentgenol. 2009;192(6):1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma S, Rajesh A, Fütterer JJ, Turkbey B, et al. Prostate MRI and 3D MR spectroscopy: how we do it. AJR Am J Roentgenol. 2010;194(6):1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocak I, Bernardo M, Metzger G, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol. 2007;189(4):849. [DOI] [PubMed] [Google Scholar]
- 7.Kim CK, Park BK, Lee HM, Kim SS, Kim E. MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR Am J Roentgenol. 2008;190(5):1180–1186. [DOI] [PubMed] [Google Scholar]