Genetic evidence and experimental studies have underlined the importance of IL-17-mediated immunity in the protection against mucosal fungal infections. Specifically, the role of IL-17 in antifungal immunity has been extensively studied in the mouse model of oropharyngeal candidiasis (OPC). CD4+ T helper 17 (Th17) cells represent the most abundant source of IL-17 upon activation of adaptive antifungal immune responses. However, innate sources such as γδ T cells and the newly identified innate TCRαβ+ cells have been shown to rapidly produce IL-17 at the early stages of oral Candida albicans infection (1). It was previously demonstrated that this rapid innate Type-17 response is essential for fungal clearance (1). However, the triggers that activate innate immunity in response to C. albicans invasion of the oral mucosa are not fully understood. Transition from the yeast to the hyphal form has been shown to be essential for C. albicans invasion of the oral epithelium (OEC) and leads to barrier injury (2). It was recently discovered that Candidalysin, a pore-forming peptide toxin secreted by C. albicans hyphae, is a crucial molecular determinant of epithelial cell damage (3). In this issue of Science Immunology, Verma et. al (4) have identified Candidalysin as the missing link between C. albicans invasion and the expansion of innate TCRαβ+ cells in the oral mucosa.
Given that C. albicans is a human commensal, but is absent in rodents, mice are immunologically naïve to this fungus (1), thus providing a neat experimental system to study the rapid activation of innate immunity in response to Candida. Here, Verma et al. have studied the sequence of events that lead to activation of CD4+CD44hiCCR6+ innate TCRαβ+ cells in response to primary Candida infection in the mouse OPC model. Despite high levels of CCR6 expression, Verma et al. now show that innate TCRαβ+ cells expansion during C. albicans infection is not due to CCR6-CCL20 mediated recruitment to the oral mucosa, but rather to local proliferation or recruitment through other mechanisms (4). Upon secondary C. albicans infection, activation of phagocytic cells through the Dectin-1/Syk/CARD9 and/or TLR2 pathways synergizes with Candida-specific memory Th17 cells to contain the infection (5–7). Here, by studying the activation of innate innate TCRαβ+ cells to primary C. albicans infection, Verma et al. demonstrate that proliferation of TCRαβ+ cells does not rely on the activation of TCR signaling or on signaling through Dectin-1 or TLR2. This suggests that antigen specificity or cell activation through these pattern recognition receptors is dispensable for innate TCRαβ+ cells response in the OPC model.
How do innate TCRαβ+ cells sense C. albicans invasion? Hyphal formation is essential for C. albicans mucosal invasion and epithelial cell damage, and the authors demonstrate that yeast locked C. albicans mutant was not able to activate IL-17 production in the oral mucosa. The hyphae-associated virulence factor Candidalysin, has been previously shown to be secreted exclusively by invasive hyphae (3). Thus, Verma et al. explored whether Candidalysin might be the trigger of the innate Type-17 response by TCRαβ+ cells. Indeed, when mice were infected with a C. albicans ece1Δ/Δ, a strain lacking the Candidalysin-encoding extent of cell elongation 1 gene (ECE1), both the expansion of innate TCRαβ+ cells and the expression of Il17 were strongly reduced. In contrast to earlier observation by Moyes et al. (3), the authors now report a comparable fungal load between the wild type and the C. albicans ece1Δ/Δ, suggesting that the reduced innate TCRαβ+ cells proliferation was not due to reduced fungal exposure.
To explain the observed phenotype, the authors next focused on understanding the mechanisms behind the Candidalysin-mediated initiation of the innate IL-17 response in the oral mucosa. The induction of rapid innate responses to Candida infection has been previously linked to epithelial damage (8). Candidalysin is essential to trigger such damage through the activation of a MAPK phosphatase MKP1/c-Fos dependent danger response, inducing pro-inflammatory IL-1β and IL-6 production (3). Pro-IL-1α stored in oral keratinocytes allows for the rapid release of active IL-1α during C. albicans infection (8); a mechanism conserved in response to other pathogens causing EC damage (9). Verma et al. found that Il1β expression was induced in a Candidalysin-dependent manner while Il1α was only partially dependent on Candidalysin. Consistently, the authors detected decreased numbers of innate TCRαβ+ cells in IL-1R deficient mice. Verma at al. set to identify whether IL-1 signaling is essential for innate TCRαβ+ cell activation during oral C. albicans infection. The authors irradiated congenically marked wild type and Il1r1−/− mice and reconstituted them with the same or reciprocal bone marrow cells. However, this effort failed to identify a clear role for IL-1 signaling in hematopoietic or non-hematopoietic cell compartments, suggesting that either the effect of IL-1 on innate TCRαβ+ cells expansion was indirect or the involvement of radio-resistant cells mediating this effect.
Although assessed only in vitro, IL-17 production appears to further act in a positive feedback loop in synergy with Candidalysin to amplify the upregulation of several cytokines and damage-associated molecular patterns (DAMPs), including IL-1α/β by epithelial cells, which are crucial to drive proliferation of IL-17-producing innate oral TCRαβ+ cell (Fig. 1). Overall, the work of Verma et al. suggests that Candidalysin acts as a danger signal during the invasion of hyphae through the epithelium and promotes OEC damage that triggers protective innate IL-17-mediated antifungal immunity.
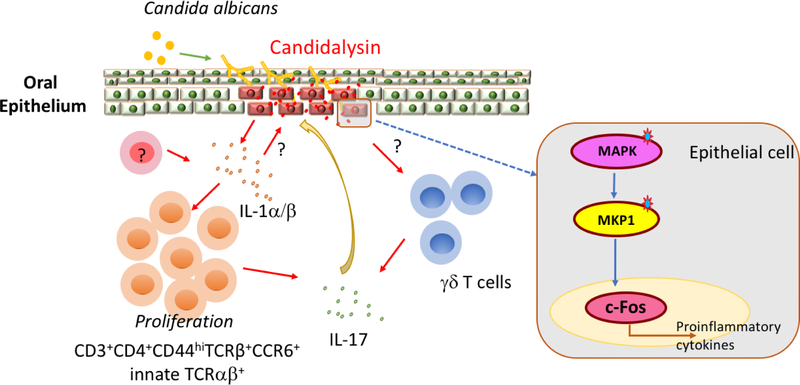
Figure 1. Candidalysin drives innate Type-17 response to oral Candidiasis.
C. albicans switches from yeast to hyphal form upon adhesion to host cells in the oral mucosa. At the invasive hyphal stage, Candidalysin is secreted by invasive hyphae and triggers epithelial cell damage through the activation of a MAPK phosphatase MKP1/c-Fos dependent danger response. Candidalysin-induced oral epithelial damage leads to the production of IL-1α/β from epithelial cells and/or other unidentified cells types. IL-1α/β further drives innate TCRαβ+ cells expansion and IL-17 production, possibly by both innate TCRαβ+ and γδ T. IL-17 and Candidalysin synergistically amplify the danger responses, inducing IL-1α/β and other inflammatory mediators. This positive feedback-loop, linking pro-inflammatory cytokines with fungal virulence factor, is essential for the establishment of a protective innate response to oral candidiasis.
Despite establishing a role for candidalysin in alerting the innate T cell response, some important questions remain. What is the mechanism behind Candidalysin triggered initial release of cytokines and DAMPs by the epithelium? Inflammasomes are possible candidates since NLRP3 (7) and NLRC4 (10) responses are important for the control of Candida infection. Are innate TCRαβ+ cells able to directly sense EC damage caused by Candidalysin or do they rely on help by other innate cells, such as resident phagocytes or innate lymphoid cells (ILCs)? In the present study, the authors have assessed the effect of Candidalysin using a single strain of C. albicans: SC5314. Yet, a recent study performed with several C. albicans isolates reports that the expression of Candidalysin does not always correlate with in vivo pathogenicity (8). Additional factors are thus likely to further contribute to the capacity of C. albicans to cause epithelial damage. In this regard, further studies on C. albicans produced toxins might shed light on how this opportunistic fungus modulates innate and adaptive immunity to influence the balance between pathogenicity and commensalism. As humans are constantly exposed to C. albicans and likely harbor adaptive memory to this fungus, the role of Candidalysin during a secondary oral infection remains to be explored.
Acknowledgements:
The Iliev laboratory is supported by grants from the US National Institutes of Health (DK113136, DK098310 and AI123819) and the Kenneth Rainin Foundation. Irina Leonardi is supported by the Swiss National Science Foundation (Fellowship P2ZHP3_164850).
REFERENCES
- 1.Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, Osborne LC, Bishu S, Ghilardi N, Siebenlist U, Watkins SC, Artis D, McGeachy MJ, Gaffen SL, Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med 211, 2075–2084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wachtler B, Citiulo F, Jablonowski N, Forster S, Dalle F, Schaller M, Wilson D, Hube B, Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One 7, e36952 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Forster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Hader A, Kurzai O, Luo T, Kruger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR, Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532, 64–+ (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma AH, Richardson JP, Zhou CS, Coleman BM, Moyes DL, Ho J, Huppler AR, Ramani K, McGeachy MJ, Mufazalov IA, Waisman A, Kane LP, Biswas PS, Hube B, Naglik JR, Gaffen SL, Oral epithelial cells orchestrate innate Type 17 responses to Candida albicans through the virulence factor Candidalysin. Sci. Immunol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Sousa CRE, Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8, 630–638 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD, Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8, 31–38 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA, An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schonherr FA, Sparber F, Kirchner FR, Guiducci E, Trautwein-Weidner K, Gladiator A, Sertour N, Hetzel U, Le GT, Pavelka N, d’Enfert C, Bougnoux ME, Corti CF, LeibundGut-Landmann S, The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol, (2017). [DOI] [PubMed] [Google Scholar]
- 9.Sorenson BS, Khammanivong A, Guenther BD, Ross KF, Herzberg MC, IL-1 receptor regulates S100A8/A9-dependent keratinocyte resistance to bacterial invasion. Mucosal Immunol 5, 66–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG, A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. Plos Pathog 7, e1002379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]