Abstract
Purpose of review
Prostate cancer is the most common solid organ cancer type among American men. Screening and imaging aim to detect early-stage disease that is biologically aggressive. The focus of this study is to review multiparametric MRI in the detection and risk stratification of prostate cancer.
Recent findings
MP-MRI has been shown to be the most accurate noninvasive technique to localize prostate cancer. Recent studies reported that using MRI for guidance during prostate biopsies increases the yield of prostate biopsies. Moreover, multiparametric and particular MRI sequences such as apparent diffusion coefficient values of diffusion-weighted MRI have been found to correlate negatively with tumor Gleason scores.
Summary
Among the existing imaging modalities, multiplanar magnetic resonance is the best at detecting prostate cancers. Some risk stratification is possible based on size, extent and apparent diffusion coefficient values. However, prostate MRI remains nonspecific and biopsies must be performed to confirm whether an abnormality is benign or malignant and to assign Gleason scores.
Keywords: multiparametric MRI, prostate cancer, tumor aggressiveness
INTRODUCTION
Prostate cancer is a significant healthcare issue in the US. An estimated 241740 men will be diagnosed withprostatecancerin2012.Prostate cancer will kill an estimated 28000 men, making it the second leading cause of cancer-related death in American men [1]. Early detection of prostate cancer results in high cure rates since successful treatment is still possible if the disease is organ-confined; on the contrary, diagnosis and treatment at later stages of the disease result in relatively poor out comes despite increased morbidity, mortality and cost. The introduction of prostate specific antigen (PSA) screening has resulted in dramatically increased rates of prostate cancer diagnoses. Many of these cancers are indolent and do not need to be treated; however, some are biologically aggressive. Recent studies of the impact of PSA on health outcomes have been inconclusive, in part, because the PSA test is so ubiquitous that itis hard to find control populations that have never been tested. However, it is incontrovertible that the disease-specific death rate of prostate cancer is declining starting in early 2000. This is likely a result of screening with PSA and improved treatments for advanced disease.
The current screening approach, which includes a random transrectal ultrasound (TRUS)-guided biopsy, however, has a considerable false-negative rate [2]. Whole regions of the prostate are not even sampled and tumors may be missed even if they are within the biopsy ‘template’. This leads to missed diagnosis and underestimation of the extent of disease. Imaging could play a major role in screening by limiting biopsies and enabling more targeted biopsies. Accurate localization of prostate cancers could also potentially assist in decision-making regarding the use of active treatment or active surveillance in selected patients. Herein, we will review multiparametric MRI (MP-MRI) for localized prostate cancer, emphasizing its potential role in risk stratification and improving outcomes.
MULTIPARAMETRIC MRI
MP-MRI provides the best anatomic imaging of the prostate gland due to its superior spatial and contrast resolution compared with other imaging techniques. The introduction of high field strength magnets (3 Tesla) and dedicated coil designs (endorectal coil) have resulted in higher signal-to-noise ratios which can be used to increase resolution, contrast and/or speed, and this has led MRI to surpass other existing techniques in the delineation of intra-prostatic tumor foci. Currently, the most optimal technique for MRI of the prostate involves a multiparametric approach which includes anatomic and functional sets of sequences.
Anatomic sequences
Anatomic sequences of multiparametric prostate MRI are T1 and T2-weighted (W) MRI. T1W-MRI is of limited value as one cannot delineate the zonal anatomy or tumor foci. T1 weighting is useful for detecting biopsy-related hemorrhage, which interferes with the diagnostic capability of other prostate MRI sequences. The presence of significant hemorrhage in a prostate gland precludes an accurate study and patients should be rescheduled at a later time. T2W-MRI is universally accepted as the ‘fulcrum’ of MP-MRI since it provides the highest soft tissue resolution for visualization of tumors, the zonal anatomy, capsule, neurovascular bundles, anterior fibrous stroma, and seminal vesicles. On T2W-MRI, peripheral-zone cancers are usually round or ill-defined, low signal intensity foci; however, this pattern is nonspecific and can be seen in prostatitis, hemorrhage, atrophy, benign hyperplasia, biopsy-related scars, and post-treatment (hormone, ablation, etc.) changes [3]. Cancers of the central gland are more challenging to detect due to the significant overlap between signal characteristics of tumors and benign hyperplastic nodules. Typical T2WMRI features of a central gland tumor are defined as homogenous, low-signal-intensity lesion with ill-defined margins, without a distinct capsule, and invasion of the pseudocapsule, with lenticular, urethral or anterior fibromuscular invasion [4]. Many lesions are positive on T2 yet are not cancer upon biopsy. Nevertheless, a major role of MRI is in detecting localized focal lesions for subsequent biopsy. This enables patients whose tumors evade detection on routine random biopsy to be correctly diagnosed often because the tumor is located outside the normal template of the TRUS biopsy. Detecting extracapsular extension (ECE) or seminal vesicle invasion is of importance to risk stratification. Such findings can influence how the treatment is modified. On T2W images, ECE usually appears as a direct extension of the tumor into the periprostatic fat tissue; however, overt ECE may not be seen in all situations, and secondary findings such as asymmetry of the neurovascular bundle, envelopment of the neurovascular bundle, contour angulation and bulging, irregular gland margin, capsular obscuration or retraction, and obliteration of the recto-prostatic angle should be assessed [5]. Seminal vesicle invasion (SVI) can be directly visualized as an extension of tumor from the base of the prostate into the seminal vesicles and/or the presence of focal low-signal-intensity filling defects within the hyper-intense seminal vesicles (which subsequently enhance after contrast media) [6]. However, especially in older men, the seminal vesicles can be affected by atrophy and hyperplasia, making it more difficult to detect tumor.
T2W-MRI by itself is reported to have a wide range of sensitivity and specificity for cancer detection with results varying from 27 to 100% and 32 to 99%, respectively. Sensitivity and specificity for local staging and predicting the presence of extracapsular extension also demonstrate a wide range from 14.4 to 100% and 67 to 100%, respectively. These wide ranges of sensitivities and specificities are due to the significant variability in the patient populations, magnet strengths, coil designs, gold standard correlation methodologies (biopsy vs. surgery), and level of expertise used in different studies [7–13,14∎]. Higher-risk lesions are those that are large (>1cm), very low in signal intensity and exhibit ECE or SVI. In this mannerT2Wimaging can be used to stratify prostate cancers.
Functional sequences
Functional MRI sequences include diffusion-weighted MRI (DW-MRI), magnetic resonance spectroscopy imaging (MRSI) and dynamic contrast-enhanced MRI (DCE-MRI) (Figs 1 and 2). DW-MRI evaluates the Brownian motion of free water within tissues and this technique was initially developed and used in the early detection of acute cerebrovascular stroke. Its use in oncology imaging is growing since densely cellular tissues (such as tumors) have more restricted water diffusion since the mean water path length is interrupted by cell membranes. The resultant reduction in the diffusion of water can be depicted by DW-MRI. DW-MRI is quantified by calculating the apparent diffusion coefficient (ADC) and is displayed as a parametric map reflecting the relative ADC. Prostate cancers demonstrate hyperintense signal characteristics when compared to healthy peripheral zone on raw high ‘b’ field DW-MRI, whereas on ADC maps they show decreased signal intensity relative to healthy peripheral zone, reflecting lower water diffusion [15]. DW-MRI demonstrates a wide range of sensitivity and specificity (57–93.3% and 57–100%, respectively) for tumor detection in various studies, depending on field strength, imaging parameters, patient selection, and validation [14∎,15–19]. Recently, there is a growing interest in predicting the aggressiveness of prostate cancer via quantitative ADC values extracted from DW-MRI which have been demonstrated to vary with Gleason scores, thus helping to stratify low-risk from higher-risk prostate cancers [20–23].
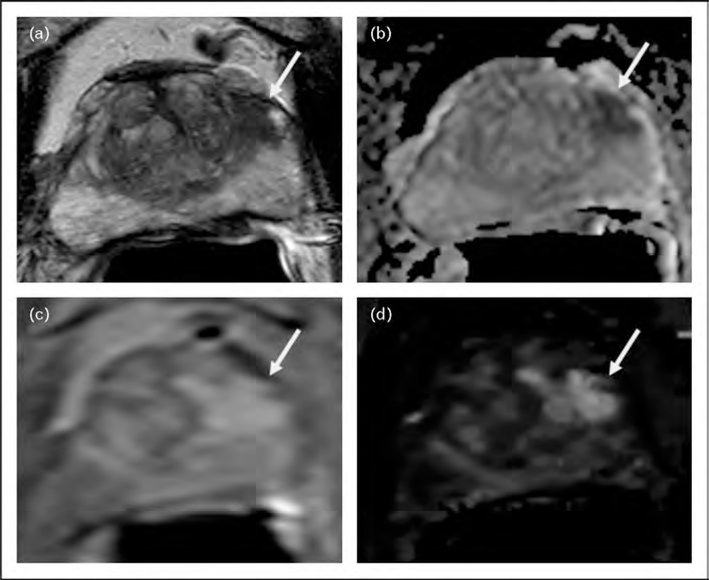
FIGURE 1.
A 72-year-old man with a serum PSA of 14.7 ng/dl with two prior TRUS-guided biopsies which revealed no tumor. Axial T2W-MRI (a), ADC map of DW-MRI (b), raw DCE-MRI (c), and Ktrans map derived from DCE-MRI (d) demonstrate a lesion in the left mid-anterior horn peripheral zone (arrows). Subsequent TRUS/MRI fusion-guided biopsy revealed Gleason 3 + 4 (40%) tumor within that left-sided anterior peripheral zone lesion. ADC, apparent diffusion coefficient; DCE-MRI, dynamic contrast-enhanced MRI; DW-MRI, diffusion-weighted MRI; TRUS, transrectal ultrasound.
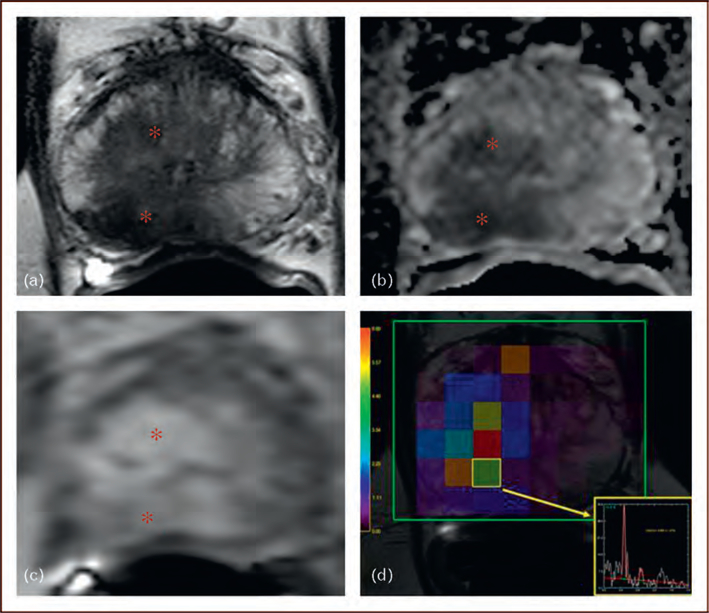
FIGURE 2.
A 64-year-old man with a serum PSA of 19.5 ng/dl with no prior biopsy. Axial T2W-MRI (a), ADC map of DWMRI (b), raw DCE-MRI (c), and magnetic resonance spectroscopy (d) demonstrate a lesion in the right mid-peripheral zone and central gland (asterix). Subsequent TRUS/MRI fusion-guided biopsy revealed Gleason 5 þ 5 (100%) tumor within that right-sided lesion. ADC, apparent diffusion coefficient; DCE-MRI, dynamic contrast-enhanced MRI; DW-MRI, diffusion-weighted MRI; TRUS, transrectal ultrasound.
MRSI provides a display of the chemical composition of the prostate gland through specific metabolites, such as citrate, choline, and creatine, which have characteristic resonance frequencies that can be measured with MRSI. The normal prostate gland can be considered a ‘citrate’ factory and thus contains low levels of choline and high levels of citrate, whereas prostate cancers demonstrate increased levels of choline and diminished levels of citrate. Hence, the ratio of choline to citrate can be used as an index of malignancy. Integration of MRSI into multiparametric prostate MRI improves specificity, tumor detection rates, and helps to estimate tumor volume [13,14∎,24,25,26]. Despite its benefits, MRSI has a few disadvantages such as requiring expertise, long acquisition times, and specialized equipment. Moreover, there is no consensus on how to analyze MRSI data and to interpret results. Nonetheless, very high choline-to-citrate ratios are considered to indicate a higher risk for aggressive disease.
Dynamic contrast-enhanced MRI is another functional sequence used in the MP-MRI approach. It aims to depict vascularity and permeability changes within the prostate gland since tumor foci tend to include neoangiogenic vessels, which can be anarchic, inefficient, and highly permeable. DCEMRI is performed by obtaining fast T1W gradient echo images before, during, and after the rapid bolus administration of a low-molecular-weight gadolinium chelate. On raw DCE-MRI, tumors typically demonstrate early and intense enhancement and de-enhancement compared to the background tissue. Raw DCE-MRI data can be used to generate time vs. enhancement curves. The enhancement curves can be converted to gadolinium concentration values [Gd]. These [Gd]–time curves can be mathematically fit to a two-compartment pharmacokinetic model to obtain several quantitative permeability parameters, such as Ktrans (transendothelial transport of contrast medium from vascular compartment to the tumor interstitium), kep (reverse transport parameter of contrast medium from the extracellular space back to the plasma space), fpV (fraction of plasma volume compared to whole tissue volume), and Ve (extravascular, extracellular volume fraction of the tumor (the fraction of tumor volume occupied by extravascular space) [27]. Tumors tend to have higher quantitative permeability values; however, there may be an overlap between those values of tumor and benign hyperplasia, especially in the central gland. Moreover, the reproducibility and comparability of those parameters remain a challenge and need further research. Similar to other MRI sequences, DCE-MRI has a wide range of sensitivity (46–96%) and specificity (74–96%) for tumor detection depending on the details of the study [13,14∎,28–30]. Additionally, DCE-MRI appears to be a promising MRI sequence to detect residual and recurrent tumors in patients who undergo treatment for prostate cancer [31–33]. Focal, rapidly, and intensely enhancing lesions with rapid washout are indicative of higher-grade tumors. As fewer of these features are noted the risk of aggressive tumors is also reduced.
Currently, based on the collective experience in the literature, it is evident that no single MRI sequence is sufficient to accurately detect prostate cancer. Each sequence has its own advantages and limitations; however, a multiparametric approach results in higher detection rates in the prostate [14∎], and further research is needed to figure out the best combination of MP-MRI for a high yield lesion detection. Moreover, because of the complexity of this method and lack of familiarity with it, new methods of providing imagers with automated diagnostic maps, combining the features of all the relevant parameters, are being explored. Such decision support systems may assist imagers in selecting lesions for image-guided biopsy.
RISK STRATIFICATION VIA MULTIPARAMETRIC MRI
Determining biological aggressiveness and risk stratification has implications for treatment. The decision to undergo active surveillance or radical treatment is based on several factors, including serum PSA, PSA density, Gleason score at biopsy, and percentage of the involvement in the core. MP-MRI has been reported to predict more aggressive disease. Rastinehad et al. [34∎∎] reported a positive correlation of MP-MRI findings with D’Amico risk scores in a cohort of 101 patients. Moreover, individual MRI sequences such as ADC maps derived from DW-MRI and quantitative parameters of DCE-MRI have been reported to predict tumor aggressiveness in a noninvasive manner. Turkbey et al. [21] reported a negative correlation between ADC values and tumor Gleason scores as well as with D’Amico risk scores. Vargas et al. [35] demonstrated a similar negative correlation between ADC values and tumor Gleason score in 51 patients. Oto et al. [36∎] reported a moderate correlation between ADC values and Gleason scores and between Kep and microvessel density of prostate cancer, which may facilitate noninvasive assessment of prostate cancer aggressiveness and angiogenesis. Hambrock et al. [37] reported an inverse relationship between ADC values and tumor Gleason grades and achieved a high discriminatory performance in the differentiation of low, intermediate, and high-grade tumors in 51 patients. It is obvious that MP-MRI or any particular sequence of MP-MRI cannot replace a biopsy; however, information from MP-MRI can provide data about the risk of aggressiveness of prostate cancer and this can potentially be used in conjunction with existing clinical variables for risk stratification and decision-making. MRI currently does not exist in standard-of-care decision-making algorithms or in nomograms. However, a few groups are conducting research in implementing MRI into such algorithms and nomograms. Recently, Shukla-Dave et al. [38] incorporated T2W-MRI and magnetic resonance spectroscopy into Kattan’s nomogram and reported an improved prediction of insignificant prostate cancers. These results need validation in multiple centers and in larger patient populations.
CONCLUSION
The role of imaging in prostate cancer is rapidly evolving. Whereas previously, imaging played a minor role in prostate cancer management, now it is beginning to become much more important. The rise of PSA screening, combined with the recognition that such screening can lead to both overtreatment and under-treatment, has prompted a re-evaluation of the role of imaging. Clearly, the blind prostate biopsy misses many tumors outside of the normal prostate biopsy template, while also underestimating the grade of the cancer. Men who might otherwise choose active surveillance are dissuaded by the requirement for recurrent biopsies and the fear of missing curable disease. Imaging can help direct biopsies to the appropriate site and monitor existing tumors for growth, in some cases obviating the need for repeated biopsies. Localization of prostate cancer has become more important that local staging in justifying the added expense of imaging.
Among the existing imaging modalities, multiplanar magnetic resonance is the best at detecting prostate cancers. However, prostate MRI is nonspecific and biopsies must be performed to confirm whether an abnormality is benign or malignant. Current research focuses on improving the diagnostic capabilities of MRI including developing methods of computer-assisted diagnosis. This will bring the power of prostate MRI to nonmagnetic resonance experts.
Prostate cancer is the most common solid organ cancer type among American men. Screening and imaging aim to detect early-stage disease that is biologically aggressive. The development of imaging modalities will further improve tumor detection, help stratify patients according to risk and improve the quality of patient care.
KEY POINTS.
Multiparametric MRI (MP-MRI) is currently the best imaging modality to localize prostate cancer.
MP-MRI can be used to direct biopsies, which can increase the yield of prostate biopsies.
Information from T2-weighted images, ADC values derived from diffusion-weighted MRI (DW-MRI) and dynamic contrast-enhanced MRI (DCE-MRI) are promising for prediction of aggressiveness of prostate cancer.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
∎ of special interest
∎∎ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 342–343).
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29. [DOI] [PubMed] [Google Scholar]
- 2.Levine MA, Ittman M, Melamed J, Lepor H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol 1998; 159:471–475. [DOI] [PubMed] [Google Scholar]
- 3.Turkbey B, Bernardo M, Merino MJ, et al. MRI of localized prostate cancer: coming of age in the PSA era. Diagn Interv Radiol 2012; 18:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akin O, Sala E, Moskowitz CS, et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology 2006; 239:784–792. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Mullerad M, Chen HN, et al. Prostate cancer: incremental value of endorectal MR imaging findings for prediction of extracapsular extension. Radiology 2004; 232:133–139. [DOI] [PubMed] [Google Scholar]
- 6.Sala E, Akin O, Moskowitz CS, et al. Endorectal MR imaging in the evaluation of seminal vesicle invasion: diagnostic accuracy and multivariate feature analysis. Radiology 2006; 238:929–937. [DOI] [PubMed] [Google Scholar]
- 7.Wefer AE, Hricak H, Vigneron DB, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol 2000; 164:400–404. [PubMed] [Google Scholar]
- 8.Nakashima J, Tanimoto A, Imai Y, et al. Endorectal MRI for prediction of tumor site, tumor size, and local extension of prostate cancer. Urology 2004; 64:101–105. [DOI] [PubMed] [Google Scholar]
- 9.Sala E, Eberhardt SC, Akin O, et al. Endorectal MR imaging before salvage prostatectomy: tumor localization and staging. Radiology 2006; 238:176–183. [DOI] [PubMed] [Google Scholar]
- 10.Fütterer JJ, Engelbrecht MR, Huisman HJ, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology 2005; 237:541–549. [DOI] [PubMed] [Google Scholar]
- 11.Bloch BN, Furman-Haran E, Helbich TH, et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging: initial results. Radiology 2007; 245:176–185. [DOI] [PubMed] [Google Scholar]
- 12.Weinreb JC, Blume JD, Coakley FV, et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy: results of ACRIN prospective multiinstitutional clinicopathologic study. Radiology 2009; 251:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology 2010; 255:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ∎.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol 2011; 186:1818–1824. Evaluates four MRI sequences and uses a novel correlation method as a gold standard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbs P, Pickles MD, Turnbull LW. Diffusion imaging of the prostate at 3.0 tesla. Invest Radiol 2006; 41:185–188. [DOI] [PubMed] [Google Scholar]
- 16.Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest Radiol 2007; 42:842–847. [DOI] [PubMed] [Google Scholar]
- 17.Haider MA, van der Kwast TH, Tanguay J, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. Am J Roentgenol 2007; 189:323–328. [DOI] [PubMed] [Google Scholar]
- 18.Reinsberg SA, Payne GS, Riches SF, et al. Combined use of diffusion-weighted MRI and 1H MR spectroscopy to increase accuracy in prostate cancer detection. Am J Roentgenol 2007; 188:91–98. [DOI] [PubMed] [Google Scholar]
- 19.Mazaheri Y, Shukla-Dave A, Hricak H, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging: correlation with pathologic findings. Radiology 2008; 246:480–488. [DOI] [PubMed] [Google Scholar]
- 20.Woodfield CA, Tung GA, Grand DJ, et al. Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. Am J Roentgenol 2010; 194:316–322. [DOI] [PubMed] [Google Scholar]
- 21.Turkbey B, Shah V, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images. Radiology 2010; 258:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bittencourt LK, Barentsz JO, de Miranda LC, Gasparetto EL. Prostate MRI: diffusion-weighted imaging at 1.5T correlates better with prostatectomy Gleason Grades than TRUS-guided biopsies in peripheral zone tumours. Eur Radiol 2012; 22:468–475. [DOI] [PubMed] [Google Scholar]
- 23.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Wetter A, Engl TA, Nadjmabadi D, et al. Combined MRI and MR spectroscopy of the prostate before radical prostatectomy. Am J Roentgenol 2006; 187:724–730. [DOI] [PubMed] [Google Scholar]
- 25.Weis J, Ahlstro¨m H, Hlavcak P, et al. Two-dimensional spectroscopic imaging for pretreatment evaluation of prostate cancer: comparison with the stepsection histology after radical prostatectomy. Magn Reson Imaging 2009; 27:87–93. [DOI] [PubMed] [Google Scholar]
- 26.Coakley FV, Kurhanewicz J, Lu Y, et al. Prostate cancer tumor volume: measurement with endorectal MR and MR spectroscopic imaging. Radiology 2002; 223:91–97. [DOI] [PubMed] [Google Scholar]
- 27.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 1999; 10:223–232. [DOI] [PubMed] [Google Scholar]
- 28.Futterer JJ, Heijmink SW, Scheenen TW, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 2006; 241:449–458. [DOI] [PubMed] [Google Scholar]
- 29.Ocak I, Bernardo M, Metzger G, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. Am J Roentgenol 2007; 189:849. [DOI] [PubMed] [Google Scholar]
- 30.Villers A, Puech P, Mouton D, et al. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol 2006; 176:2432–2437. [DOI] [PubMed] [Google Scholar]
- 31.Panebianco V, Sciarra A, Lisi D, et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP). Eur J Radiol 2011. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32.Akin O, Gultekin DH, Vargas HA, et al. Incremental value of diffusion weighted and dynamic contrast enhanced MRI in the detection of locally recurrent prostate cancer after radiation treatment: preliminary results. Eur Radiol 2011; 21:1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouviere O, Valette O, Grivolat S, et al. Recurrent prostate cancer after external beam radiotherapy: value of contrast-enhanced dynamic MRI in localizing intraprostatic tumor: correlation with biopsy findings. Urology 2004; 63:922–927. [DOI] [PubMed] [Google Scholar]
- 34. ∎∎.Rastinehad AR, Baccala AA Jr, Chung PH, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol 2011; 185:815–820. Correlates MP-MRI findings with D’Amico risk score system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 2011; 259:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. ∎.Oto A, Yang C, Kayhan A, et al. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am J Roentgenol 2011; 197:1382–1390. Evaluates both utility of quantitative DW-MRI and DCE-MRI for predicting aggressive prostate cancers. [DOI] [PubMed] [Google Scholar]
- 37.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol 2012; 61:177–1784. [DOI] [PubMed] [Google Scholar]
- 38.Shukla-Dave A, Hricak H, Akin O, et al. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int 2011. doi: 10.1111/j.1464-410X.2011.10612.x. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]