Abstract
Objectives
The role of physical inactivity in relation to cardiovascular disease (CVD) among postmenopausal women is understudied. The main objective of this study was to measure the physical activity levels (PALs) and evaluate its relation to other CVD risk factors among postmenopausal rural women of Bangladesh.
Methods
A cross-sectional study was conducted among 265 postmenopausal women aged 40–70 years who visited the outpatient department of a primary health-care center situated in the village Karamtola of Gazipur district. A pretested modified questionnaire of STEP-wise approach to Surveillance (STEPS) of noncommunicable disease risk factors was used to collect data on sociodemographic and lifestyle factors. PAL was determined by the Estimated Energy Requirement (EER) equation of the Dietary Reference Intakes (DRIs) Committee, and association with CVD risk factors was examined by Spearman's rank correlation.
Results
More than half (58.1%) of the postmenopausal women were identified as sedentary with high prevalence of central obesity (73.2%) among them. CVD risk factors including age (r = −0.228, p < 0.01), age at menopause (r = −0.129, p < 0.05), duration of menopause (r = −0.183, p < 0.05), 2-h plasma glucose (r = −0.148, p < 0.05), total cholesterol (r = −0.138, p < 0.05), low-density lipoprotein cholesterol (r = −0.122, p < 0.05), and triglyceride (r = −0.168, p < 0.01) showed a significant as well as inverse association with Metabolic Equivalent of Task (MET) of physical activity.
Conclusion
Low PAL and significant inverse correlation with various CVD risk factors demand interventions to maintain higher PAL among postmenopausal women of Bangladesh.
Keywords: Cardiovascular risk factors, Central obesity, Lipid profile, Physical activity, Postmenopausal women, Bangladesh
1. Introduction
Physical activity is globally considered as an important modifiable risk behavior that contributes to prevent major noncommunicable diseases (NCDs).1 In terms of cardiovascular benefits, physical activity improves different parameters of heart including myocardial contractility, cardiac output, endothelial function, and fibrinolytic activity.2 Although physical activity has various cardiovascular benefits, worldwide the proportion of physically inactive adult population is 31%, and among them, women form a predominant section.3 Again, it has been reported that the surge of cardiovascular disease (CVD) in postmenopausal women may be partially explained by their lifestyle factors, especially low physical activity level (PAL).4 Current evidence reveals that there is, in fact, a beneficial relationship that exists between PAL and CVD among postmenopausal women.5 However, the results of research on the relation between PAL and the risk of CVD in postmenopausal women are inconsistent.6 Hence, further studies are required to provide new data to clarify the role of physical activity in the prevention of CVD development in postmenopausal women.6
Bangladesh is bearing a huge burden of CVD resulting from ischemic heart disease and stroke.7 A recent rural study demonstrated a dramatic increase in CVD mortality among Bangladeshi women (47 folds) compared with men (30 folds).8 This finding is similar to other studies conducted in different parts of the world where an increased prevalence of coronary artery disease (CAD) in postmenopausal women was observed.9, 10 It has been reported that women experience the first event of CVD usually after menopause, and hence this period is a landmark for impending CVD in this group.11 Again, although previous studies reported inverse relationship between PAL and CVD, most of them were conducted in men.12, 13 The relationship between PAL and risk of CVD in menopausal women however remains understudied, although the benefits as found in men are also anticipated in them.14 In Bangladesh, as per our knowledge, only one representative study has been conducted in the year 2010 on the PAL of adult population, which was based on STEP-wise approach to Surveillance (STEPS) of NCD risk factors.15 The study determined PAL among general population and provided only baseline data. Thus, evidence-based information about PAL of Bangladeshi postmenopausal women is absolutely lacking. In these circumstances, the main objective of this study was to measure the PAL and evaluate its association with other CVD risk factors among the study population. In addition to this, we assessed the distribution of CVD risk factors at various PALs among these women.
2. Materials and methods
2.1. Study population
A total 265 postmenopausal women in the age range of 40–70 years of age who visited the outpatient department of a primary health-care center situated in the village Karamtola of Gazipur district were enrolled in this cross-sectional study from February to December 2016. Menopausal status of them was defined as no menstrual bleeding for a period of at least 12 months and no other clinical condition causing amenorrhea.16 The selection was carried out through convenient sampling technique, and individuals were categorized as having ‘no CVDs’ based on their self-reported statement, clinical history, and medical records review. Postmenopausal women with acute illness or unwilling to participate were excluded from the study. A standardized pretested questionnaire was used to collect data through face-to-face interview, and informed written consent was obtained accordingly. A modified STEPS questionnaire of the World Health Organization (WHO) was used to gather sociodemographic and behavioral information of the participants.17 Their PAL was measured as described in the following.
2.2. Measurement of PALs
To determine the PAL, a Microsoft Excel spreadsheet template was used which was based on the Estimated Energy Requirement (EER) equation of the Dietary Reference Intakes (DRIs) Committee, proposed by Gerrior et al.18 The basic components of the spreadsheet were age, sex, weight, height, basal energy expenditure (BEE), list of activities with number of days and duration, Metabolic Equivalent of Tasks (METs), total caloric cost of the activity (Δ PAL), PAL, physical activity coefficient (PA), and total energy expenditure (TEE).
In the Excel spreadsheet, five predefined formulas were used to determine BEE, Δ PAL, PAL, PA, and TEE (not used in this study). At first, values of age, sex, weight, height, and last 24 hours' activities with duration and MET against each activity were entered into the required data field of the Excel spreadsheet. As the values were entered in the required data fields, BEE was automatically calculated based on the following equation:
The next step was the automatic calculation of the impact of each reported PA on Δ PAL. This formula, set in the template, was as follows:
Then, the PAL was determined based on the basal activity impact on energy expenditure (a factor of 1.1) and the sum of all activities (sum of Δ PAL). This factor counted for thermic effect of food (TEF) and postexercise increased in energy expenditure. The PAL was automatically calculated according to following predefined formula:
PAL = 1.1 + sum of Δ PALi, where Δ PALi is the list of each reported activity impact on energy expenditure.
The value of PAL was used to generate PA by the Microsoft Excel logic function, and following criteria were used to determine the PAL of an individual:
Sedentary: PA = 1.0, when 1.0 ≤ PAL < 1.4.
Low active: PA = 1.14, when 1.4 ≤ PAL < 1.6.
Active: PA = 1.27, when 1.6 ≤ PAL < 1.9.
Very active: PA = 1.45, when 1.9 ≤ PAL < 2.5.
The formula to determine the physical activity for women using the Microsoft Excel logic function was
IF (PAL≥1.9, “1.45”, IF (PAL≥1.6,“1.27”, IF (PAL≥1.4,“1.14”, IF (PAL≥1,“1”, “”))))
A previous relevant study in Bangladesh used the Global Physical Activity Questionnaire (GPAQ) to measure the level of PA.15 In the GPAQ, increases in heart rate and breathing are used to address the PALs. But, it was reported that heart rate–based measurement of physical activity may not provide accurate data other than a general picture of physical activity pattern.18 Hence, in the present study, we decided to apply a new method of physical activity measurement based on the EER equation of the DRIs Committee. Pretesting was done to validate the method as it was not applied for Bangladeshi population before. However, this method used doubly labeled water data of both predicted and observed levels of physical activity to assess physical activity patterns. This doubly labeled water (DLW or free-living indirect calorimetry) is a gold standard method commonly used to increase the precision and accuracy of physical activity measurement worldwide.18, 19 Further details about the EER equation, its components, and the calculation of PA have been described elsewhere.18
2.3. Cardiovascular risk factors assessed
In this study, we assessed distribution of CVD risk factors at different PALs and examined the relationship between them. We analyzed the following CVD risk factors:
Nonmodifiable risk factors: age, age at menopause, duration of menopause, and family history of NCD.
Behavioral risk factors: use of oral contraceptive pill (OCP), tobacco use (smoking and smokeless), extra salt intake (>5 g/day), and physical activity.
Cardiometabolic risk factors: generalized obesity, central obesity, hypertension, diabetes mellitus (DM) (fasting plasma glucose and 2-h plasma glucose), hypercholesterolemia, low level of high-density lipoprotein (HDL), and high level of triglyceride (TG) and low-density lipoprotein (LDL).
2.4. Physical measurement
Anthropometric parameters (height, weight, waist circumference, and hip circumference) and blood pressure (BP) were measured following the methods described in the STEPS survey of Bangladesh.20 Body mass index (BMI) was used to determine generalized obesity and classified according to the international guideline.21 Again, to determine central obesity, we followed the WHO guideline.22 Hypertension was diagnosed based on the “Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)” when systolic BP was ≥140 mmHg and/diastolic BP was ≥90 mmHg or a person was a known hypertensive or on antihypertensive drug.23
2.5. Biochemical parameters
In this study, diabetes population comprised newly diagnosed DM using WHO criteria (fasting plasma glucose ≥7.0 mmol/l or 2-h plasma glucose ≥11.1 mmol/l) and self-statement of a person as known diabetic or on antidiabetic medication.24 Oral glucose tolerance test (OGTT) was carried out for those who did not know their glycemic status. To define lipid abnormality, the Adult Treatment Panel (ATP) III cutoff value was considered as ≥240 mg/dl for hypercholesterolemia, <40 mg/dl for low level of HDL, >199 mg/dl for high level of TG, and >159 mg/dl for high level of LDL.25 For biochemical tests, 5 cc of venous blood was collected with aseptic precaution in fasting state, and again 3 cc was collected after 2 h of 75 g of glucose intake.
Ethical approval to conduct the study was obtained from the Ethical Review Committee of Bangladesh University of Heath Sciences.
3. Data processing and analysis
Data were analyzed using the Statistical Package for Social Science (SPSS), version 20.0. The CVD risk factors were categorized as ‘Yes’ or ‘No’, and the ‘Yes’ category was showed and analyzed in the frequency table. The prevalence of CVD risk factors at various levels of PA were presented as proportions with 95% confidence intervals (CIs). Pie diagram was used to represent the prevalence of various categories of physical activity as proportions. Again, age was categorized into four groups (40–49 years, 50–59 years, 60–69 years, and >69 years) to show the distribution of various levels of PA among the age groups. This distribution was presented as percentage using multiple bar graphs. The spearman's correlation was run between physical activities, MET value, and other CVD risk factors to show the association between them. The r value was considered statistically significant at a threshold of p < 0.05.
4. Results
4.1. Physical activity levels
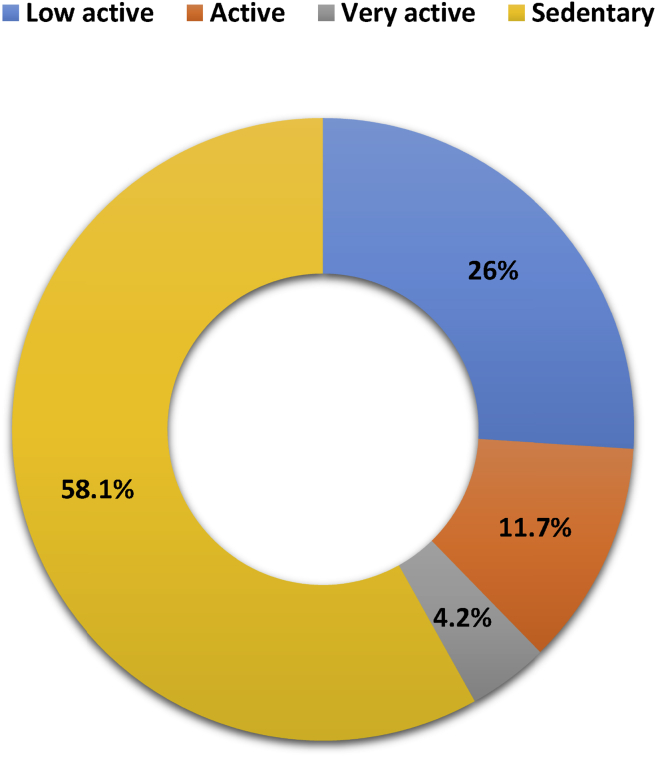
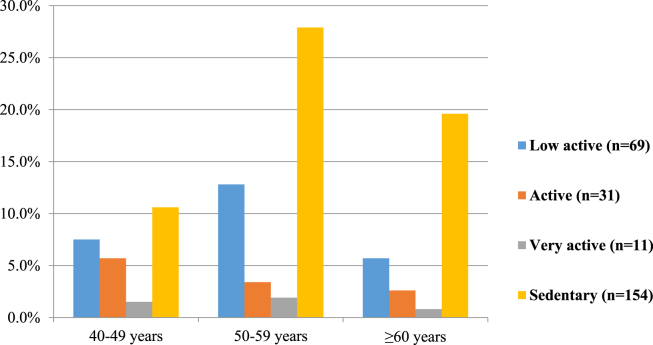
Among 265 postmenopausal women, more than half (58.1%) were habituated to lead a sedentary lifestyle, one fourth (26%) were detected as low active, and rest of the participants' physical activity varied from active to very active (Fig. 1). In each age group, sedentary participants were comparatively predominant, and their distribution was highest in the age category of 50–59 years (Fig. 2).
Fig. 1.
Physical activity levels among postmenopausal women, n = 265.
Fig. 2.
Distribution of physical activity levels at different age groups of postmenopausal women, n = 265.
4.2. Burden of CVD risk factors at different PALs
All the CVD risk factors were distributed with high proportion among the sedentary participants compared with others. Overall, one third of the participants had a family history of major NCDs, and more than half of them were found sedentary in their lifestyle. Same findings were also observed in OCP users. Nearly half of the total participants had the bad habit of extra salt intake in meal and use of smokeless tobacco. Regarding cardiometabolic risk factors, prevalence of central obesity was highest (73.2%) and really at alarming level. Next to central obesity, 37.7% of the postmenopausal women were hypertensive, and prevalence of hypercholesterolemia was more than that of DM (25.7% vs 20.8%). Among the components of lipid profile, low level of cardiac-friendly HDL cholesterol was distributed in higher proportion than others, and proportion of each component of lipid profile was almost same (more than half of overall percentage) among the sedentary respondents (Table 1).
Table 1.
Distribution of cardiovascular disease risk factors according to physical activity levels, n = 265.
| Variables | Low active (n = 69) |
Active (n-31) |
Very active (n = 11) |
Sedentary (n = 154) |
Overall (n = 265) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Family history of NCD | 8.7 | 2.0–15.4 | 2.3 | −3 to 7.6 | 1.1 | −5.1 to 7.3 | 18.1 | 12.0–24.2 | 30.2 | 24.7–35.7 |
| Oral contraceptives use | 9.8 | 2.8–16.8 | 4.9 | −2.7 to 12.5 | 1.9 | −6.2 to 10.0 | 17.7 | 11.7–23.7 | 34.3 | 28.6–40.0 |
| Overweight | 6.8 | 0.9–12.7 | 2.3 | 3.0–7.6 | 1.9 | −6.2 to 10.0 | 12.8 | 7.5–18.1 | 23.8 | 18.7–18.9 |
| Generalized obesity | 0.8 | −1.3 to 2.9 | 0.8 | −2.3 to 3.9 | 0.8 | −4.5 to 6.1 | 5.3 | 1.8–8.8 | 7.5 | 4.3–10.7 |
| Central obesity | 17.4 | 8.5–26.3 | 9.4 | −0.9 to 19.7 | 2.6 | −6.8 to 12.0 | 43.8 | 36–51.6 | 73.2 | 67.9–78.5 |
| Smoking | 0.8 | −1.3 to 2.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | −0.6 to 2.2 | 1.5 | 0.0–3.0 |
| Smokeless tobacco use | 12.1 | 4.4–19.8 | 5.3 | −2.6 to 13.2 | 2.6 | −6.8 to 12.0 | 32.5 | 25.1–39.9 | 47.5 | 41.5–53.5 |
| Extra salt intakea | 9.8 | 2.8–16.8 | 4.5 | −2.8 to 11.8 | 1.5 | −5.7 to 8.7 | 29.1 | 21.9–36.3 | 44.9 | 38.9–50.9 |
| Diabetes mellitus | 4.2 | −0.53 to 8.9 | 1.5 | −2.8 to 5.8 | 1.9 | −6.2 to 10.0 | 13.2 | 7.9–18.5 | 20.8 | 15.9–25.7 |
| Hypertension | 9.1 | 2.3–15.9 | 4.9 | −2.7 to 12.5 | 1.5 | −5.7 to 8.7 | 22.3 | 15.7–28.9 | 37.7 | 31.9–43.5 |
| Hypercholesterolemia | 5.7 | 0.23–11.1 | 2.3 | −3.0 to 7.6 | 0.0 | 0.0 | 17.7 | 11.7–23.7 | 25.7 | 20.4–31.0 |
| Low HDLb | 7.2 | 1.1–13.3 | 2.6 | −3.0 to 8.2 | 1.1 | −5.1 to 7.3 | 15.1 | 9.4–20.8 | 26.0 | 20.7–31.3 |
| High triglyceridec | 5.7 | 0.23–11.2 | 1.9 | −2.9 to 6.7 | 1.1 | −5.1 to 7.3 | 15.5 | 9.8–21.2 | 24.2 | 19.0–29.4 |
| High LDLd | 5.7 | 0.23–11.2 | 1.1 | −2.6 to 4.8 | 0.0 | 0.0 | 14.0 | 8.5–19.5 | 20.8 | 15.9–25.7 |
NCD, noncommunicable disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Salt intake >5 g per day.
HDL <40 mg/dl.
Triglyceride >199 mg/dl.
LDL >159 mg/dl.
4.3. Association between PA and CVD risk factors
A negative and statistically significant correlation was found between MET value and age (r = −0.228, p < 0.01), age at menopause (r = −0.129, p < 0.05), duration of menopause (r = −0.183, p < 0.05), 2-h plasma glucose (r = −0.148, p < 0.05), total cholesterol (r = −0.138, p < 0.05), LDL cholesterol (r = −0.122, p < 0.05), and TG (r = −0.168, p < 0.01) (Table 2).
Table 2.
Spearman's correlation between MET of physical activity and CVD risk factors among postmenopausal women, (n = 265).
| Variables | r | p-value |
|---|---|---|
| Age | −0.228 | 0.000* |
| Age at menopause | −0.129 | 0.035* |
| Duration of menopause | −0.183 | 0.003* |
| Fasting blood glucose | −0.092 | 0.135 |
| 2-h plasma glucose | −0.148 | 0.028* |
| Total cholesterol | −0.138 | 0.024* |
| HDL cholesterol | 0.085 | 0.166 |
| LDL cholesterol | −0.122 | 0.048* |
| Triglyceride | −0.168 | 0.006* |
| Systolic blood pressure | −0.062 | 0.316 |
| Diastolic blood pressure | −0.073 | 0.235 |
| Body mass index | −0.013 | 0.831 |
| Waist hip ratio | −0.047 | 0.445 |
MET, Metabolic Equivalent of Tasks; CVD, cardiovascular diseases; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*Spearman's correlation coefficient r was significant at a threshold of p < 0.05.
5. Discussion
Postmenopausal women of Bangladesh are an apparently high-risk group of CVD, but without including them in the nationwide CVD prevention program, the NCD-related global target will remain unachievable. In this perspective, the present study detected more than half of the postmenopausal women as sedentary in their lifestyle, residing in a rural area of Bangladesh. This result is nearly double of the study by Moniruzzaman et al which reported that 31.6% of the Bangladeshi adult women (25 years or older) residing in a rural area are inactive.15 Again, countrywide prevalence of insufficient physical activity among adult women reported by Moniruzzaman et al was also lower (53.6%) than that in the present study. This comparative finding also remained consistent in accordance with other studies conducted among postmenopausal women of low- and middle-income countries such as India and Cameroon where the proportion of physically inactive participants was 55% and 51.9%, respectively.26, 27
Physical inactivity is a behavioral risk factor that gives rise to develop certain cardiometabolic risk factors such as obesity, hypertension, DM, and abnormal lipid profile. In our study, all these risk factors were highly prevalent and showed agreement with the previous studies that reported high proportion of central obesity,26, 28, 29 hypertension,28, 29 diabetes,28, 29 hypercholesterolemia,28, 29 and hypertriglyceridemia28, 30, 31 among postmenopausal women. The high prevalence of low HDL cholesterol level in the present study is supported by another study conducted among postmenopausal women of Iran.32 Other than physical inactivity, some other underlying factors may be contributed for emergence of these cardiometabolic risk factors among Bangladeshi postmenopausal women. One possible reason is ovarian failure with estrogen deficiency. The estrogen deficiency during postmenopausal period is thought to be responsible for a significant proportion of increased cardiometabolic risk factors among postmenopausal women.33 High central adiposity trait in the present study is not surprising as high level of adiponectin present in women's body favors centralization of body fat during menopausal transition.34 In our study, parameters of blood pressure showed no significant correlation with the physical activity. So possible explanation for hypertension in the present study could be high salt intake behavior of the respondents.
Our study found significant association between MET value and CVD risk factors. Among the modifiable risk factors, significant inverse relationship between components of lipid profile (total cholesterol, LDL cholesterol, and TG) and MET is notable. Evidence suggested that lipid profile in postmenopausal women is a controversial issue and that the associated physical inactivity makes it more complex.35 Moreover, relationship between physical activity and each component of lipid profile showed inconsistent results. The inverse relationship of our study between physical activity and components of lipid profile was consistent with the findings of other studies for total cholesterol,35, 36, 37 LDL cholesterol,35, 37 and TG.35, 36 However, there are some other studies that found no relationship for total cholesterol,38, 39 LDL cholesterol,38, 39 and TG.37, 39 The possible reasons of this variation may be due to (1) the method of assessment of physical activity, (2) consideration of hormone replacement therapy (HRT), and (3) assessment of physical fitness in terms of physical activity. It has been documented that some studies used VO2 to assess physical fitness35, 36, 37, 38, 39 rather than physical activities, whereas other studies considered HRT35 and other excluded,40 even some studies adjusted current HRT35, 38 during analysis and others considered past HRT37 to establish relation between lipids and PAL among postmenopausal women. In Bangladesh, among rural postmenopausal women with inadequate access to essential drugs, use of HRT is very rare, and hence, we did not include HRT in our study. Regarding methodological concern, subjective and objective measurement of physical activity is an important issue which may impact on variation in the results of the study. It has documented in both cross-sectional40 and longitudinal study41 that physical activity as measured objectively was not related to lipid levels. So the relation between physical activity and lipids demands further studies to reach a valid conclusion considering the aforementioned factors.
DM is considered as a prognostic factor of CVD, and the present study identified a significant inverse relationship between blood glucose (2-h plasma glucose) and MET value. This indicates that physically inactive postmenopausal women are at high risk of developing DM, and the same findings are also described in another study conducted among postmenopausal women.42 The 2-h plasma glucose is an independent and important metabolic parameter to detect progression of subclinical atherosclerosis as it increases carotid intima-media thickness and subsequently the risk of CAD.43 According to the report of a WHO/International Diabetic Federation consultation,24 increasing 2-h plasma glucose is associated with increased risk of fatal and nonfatal CVD across the diabetic to nondiabetic range. An Australian study also demonstrated an independent and strong dose–response relationship between physical activity and 2-h plasma glucose44
Several factors might impact on the results of this study. The most important one is the subjective measurement of physical activity which is associated with the probability of recall bias. Moreover, rural population usually is not conscious about its duration of activities in minutes and hours. Besides this, the small sample size limits to generalize the data.
Other than these limitations, the study is important from the view of public health as it addresses an important health issue of a high-risk population that remains neglected most often by the policy makers of developing countries. This study is unique as the first study of Bangladesh which assessed PALs and their association with CVD risk factors in a vulnerable population of rural setting. Again, this study generated baseline data for PALs of postmenopausal women which may provide direction to conduct a large-scale cohort study in future.
6. Conclusion
The high proportion of physically inactive postmenopausal women in a rural area of Bangladesh alarms us about the future explosion of CVD among them. Again, inverse relation of CVD risk factors with MET value demands further lifestyle interventional studies among this high-risk population of Bangladesh.
Key messages
What is already known?
Beneficial relationship exists between physical activity and CVD among postmenopausal women.
What this study adds?
First time, PALs and their association with CVD risk factors are assessed among postmenopausal women of Bangladesh.
Role of contributors
L.B. conceptualized the research idea and prepared the manuscript; P.C.B. provided support to L.B. in designing the study and acquisition and analysis of data; M.F. interpreted the findings and helped in data presentation; L.A. critically revised the draft for final version and gave the intellectual input as a supervisor to submit the manuscript.
Conflict of interest
All authors have none to declare.
Funding
None declared.
Acknowledgments
The authors would like to thank the Director, Dr. Heun Guyn Jung, for his kind permission to conduct the study in his primary health-care centre and provide full facilities to do relevant laboratory tests with staff support.
Contributor Information
Lingkan Barua, Email: lingkanbarua@gmail.com.
Mithila Faruque, Email: mithilafaruque@gmail.com.
Palash Chandra Banik, Email: palashcbanik@gmail.com.
Liaquat Ali, Email: vc@buhs.ac.bd.
References
- 1.NCDs| Physical Inactivity: A Global Public Health Problem. World Health Organization; September 4, 2018. http://www.who.int/ncds/prevention/physical-activity/inactivity-global-health-problem/en/ Accessed 5 October 2018. [Google Scholar]
- 2.Press V., Freestone I., George C. Physical activity: the evidence of benefit in the prevention of coronary heart disease. QJM: Int J Med. 2003;96(4):245–251. doi: 10.1093/qjmed/hcg041. [DOI] [PubMed] [Google Scholar]
- 3.Physical Inactivity: A Global Public Health Problem. World Health Organization; October 6, 2014. http://www.who.int/dietphysicalactivity/factsheet_inactivity/en/ Accessed 5 October 2018. [Google Scholar]
- 4.Gudmundsdottir S.L., Flanders W.D., Augestad L.B. Physical activity and cardiovascular risk factors at menopause: the Nord-TrØndelag health study. Climacteric. 2013;16(4):438–446. doi: 10.3109/13697137.2013.768231. [DOI] [PubMed] [Google Scholar]
- 5.Owens J.F., Matthews K.A., Räikkönen K., Kuller L.H. It is never too late: change in physical activity fosters change in cardiovascular risk factors in middle-aged women. Prev Cardiol. 2003;6(1):22–28. doi: 10.1111/j.1520-037x.2003.00972.x. [DOI] [PubMed] [Google Scholar]
- 6.Mazurek K., Żmijewski P., Kozdroń E. Cardiovascular risk reduction in sedentary postmenopausal women during organised physical activity. Kardiol Pol. 2017;75(5):476–485. doi: 10.5603/KP.a2017.0035. [DOI] [PubMed] [Google Scholar]
- 7.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1980–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karar Z.A., Alam N., Streatfield P.K. Epidemiological transition in rural Bangladesh, 1986–2006. Glob Health Action. 2009;2(1):1904. doi: 10.3402/gha.v2i0.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto A., Horibe H., Mabuchi H. Analysis of serum lipid levels in Japanese men and women according to body mass index. Increase in risk of atherosclerosis in postmenopausal women. Atherosclerosis. 1999;143(1):55. doi: 10.1016/s0021-9150(98)00275-5. [DOI] [PubMed] [Google Scholar]
- 10.Trémollières F.A., Pouilles J.-M., Cauneille C., Ribot C. Coronary heart disease risk factors and menopause: a study in 1684 French women. Atherosclerosis. 1999;142(2):415–423. doi: 10.1016/s0021-9150(98)00252-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonow R.O., Braunwald E. Elsevier Saunders; Philadelphia, PA: 2012. Braunwalds Heart Disease: A Textbook of Cardiovascular Medicine. [Google Scholar]
- 12.Bouchard C., Després J.-P. Physical activity and health: atherosclerotic, metabolic, and hypertensive diseases. Res Q Exerc Sport. 1995;66(4):268–275. doi: 10.1080/02701367.1995.10607911. [DOI] [PubMed] [Google Scholar]
- 13.Berlin J.A., Colditz G.A. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132(4):612–628. doi: 10.1093/oxfordjournals.aje.a115704. [DOI] [PubMed] [Google Scholar]
- 14.Sesso H.D., Paffenbarger R.S., Ha T., Lee I.-M. Physical activity and cardiovascular disease risk in middle-aged and older women. Am J Epidemiol. 1999;150(4):408–416. doi: 10.1093/oxfordjournals.aje.a010020. [DOI] [PubMed] [Google Scholar]
- 15.Moniruzzaman M., Zaman M.M., Islalm M., Ahasan H., Kabir H., Yasmin R. Physical activity levels in Bangladeshi adults: results from STEPS survey 2010. Publ Health. 2016;137:131–138. doi: 10.1016/j.puhe.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soules M.R., Sherman S., Parrott E. Executive summary: stages of reproductive aging workshop (STRAW) Climacteric. 2001;4(4):267–272. [PubMed] [Google Scholar]
- 17.Bonita R. World Health Organization; 2001. Surveillance of Risk Factors for Noncommunicable Diseases: The WHO STEPwise Approach: Summary. Geneva: Noncommunicable Disease and Mental Health.http://www.who.int/ncd_surveillance/media/en/269.pdf [Google Scholar]
- 18.Gerrior S., Juan W., Basiotis P. An easy approach to calculating estimated energy requirements. Prev Chronic Dis. 2006;3:A129. http://www.cdc.gov/pcd/issues/2006/oct/06_0034.htm [PMC free article] [PubMed] [Google Scholar]
- 19.Clow A., Edmunds S. Human Kinetics; Champaign, IL: 2014. Physical Activity and Mental Health. [Google Scholar]
- 20.Non-communicable Disease Risk Factors Survey Bangladesh 2010. World Health Organization; July 2011. http://www.searo.who.int/bangladesh/publications/ncd_risk_factor_2010/en/ Accessed 5 October 2018. [Google Scholar]
- 21.Body Mass Index – BMI. World Health Organization. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed October 5, 2018.
- 22.Waist Circumference and Waist-hip Ratio. World Health Organization; Geneva: 2011. http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf [Google Scholar]
- 23.Chobanian A.V. The seventh report of the Joint national committee on prevention, detection, evaluation, and treatment of high blood pressure the JNC 7 report. JAMA. 2003;289(19):2560. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 24.Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia Report of a WHO/IDF Consultation. World Health Organization; 2006. http://apps.who.int/iris/bitstream/10665/43588/1/9241594934_eng.pdf [Google Scholar]
- 25.Expert Panel On Detection Evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Tandon V., Mahajan A., Sharma S., Sharma A. Prevalence of cardiovascular risk factors in postmenopausal women: a rural study. J Mid Life Health. 2010;1(1):26. doi: 10.4103/0976-7800.66993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moor V.J.A., Nansseu J.R.N., Nouaga M.E.D. Assessment of the 10-year risk of cardiovascular events among a group of Sub-Saharan African post-menopausal women. Cardiol J. 2016;23(2):123–131. doi: 10.5603/CJ.a2015.0056. [DOI] [PubMed] [Google Scholar]
- 28.Jesmin S., Islam A.S., Akter S. Metabolic syndrome among pre- and post-menopausal rural women in Bangladesh: result from a population-based study. BMC Res Notes. 2013;6(1):157. doi: 10.1186/1756-0500-6-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahan M.S., Billah S.M.B. Metabolic syndrome in Bangladeshi menopausal women. J Bangladesh Coll Phys Surg. 2016;34(1):15. [Google Scholar]
- 30.Ebrahimpour P., Fakhrzadeh H., Heshmat R., Ghodsi M., Bandarian F., Larijani B. Metabolic syndrome and menopause: a population-based study. Diabetes Metab Syndrome Clin Res Rev. 2010;4(1):5–9. [Google Scholar]
- 31.Cameron A. Metabolic Syndrome Measurement and Worldwide Prevalence. Nutrit Interv Metab Syndr. 2015:3–16. [Google Scholar]
- 32.Marjani A., Moghasemi S. The metabolic syndrome among postmenopausal women in Gorgan. Int J Endocrinol. 2012;2012:1–6. doi: 10.1155/2012/953627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H.M., Park J., Ryu S.Y., Kim J. The effect of menopause on the metabolic syndrome among Korean women: the Korean national health and nutrition examination survey, 2001. Diabetes Care. 2007;30(3):701–706. doi: 10.2337/dc06-1400. [DOI] [PubMed] [Google Scholar]
- 34.Rathmann W., Haastert B., Herder C. Differential association of adiponectin with cardiovascular risk markers in men and women? The KORA survey 2000. Int J Obes (Lond). 2007;31(5):770–776. doi: 10.1038/sj.ijo.0803471. [DOI] [PubMed] [Google Scholar]
- 35.Hagberg J.M., Mccole S.D., Ferrell R.E. Physical activity, hormone replacement therapy and plasma lipoprotein-lipid levels in postmenopausal women. Int J Sports Med. 2003;24(1):22–29. doi: 10.1055/s-2003-37198. [DOI] [PubMed] [Google Scholar]
- 36.Lindheim S., Notelovitz M., Feldman E., Larsen S., Khan F. The independent effects of exercise and estrogen on lipids and lipoproteins in postmenopausal women. Int J Gynecol Obstet. 1994;47(1):88–89. [PubMed] [Google Scholar]
- 37.Binder E.F., Birge S.J., Kohrt W.M. Effects of endurance exercise and hormone replacement therapy on serum lipids in older women. J Am Geriatr Soc. 1996;44(3):231–236. doi: 10.1111/j.1532-5415.1996.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson E.T., Davy K.P., Seals D.R. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physically active and less active postmenopausal women. Arterioscler Thromb Vasc Biol. 1995;15(5):669–677. doi: 10.1161/01.atv.15.5.669. [DOI] [PubMed] [Google Scholar]
- 39.Klebanoff R., Miller V.T., Fernhall B. Effects of exercise and estrogen therapy on lipid profiles of postmenopausal women. Med Sci Sports Exerc. 1998;30(7):1028–1034. doi: 10.1097/00005768-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Cauley J.A., Porte R.E.L., Sandler R.B., Orchard T.J., Slemenda C.W., Petrini A.M. The relationship of physical activity to high density lipoprotein cholesterol in postmenopausal women. J Chron Dis. 1986;39(9):687–697. doi: 10.1016/0021-9681(86)90152-9. [DOI] [PubMed] [Google Scholar]
- 41.Cauley J.A., Kriska A.M., Laporte R.E., Sandler R.B., Pambianco G. A two year randomized exercise trial in older women: effects on HDL-cholesterol. Atherosclerosis. 1987;66(3):247–258. doi: 10.1016/0021-9150(87)90068-2. [DOI] [PubMed] [Google Scholar]
- 42.Hsia J., Wu L.L., Allen C., Oberman A., Lawson W.E. Physical activity and diabetes risk in postmenopausal women. Am J Prev Med. 2005;28(1):19–25. doi: 10.1016/j.amepre.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Kim H.-J., Ahn C.-W., Kang E.-S. The level of 2-h post-challenge glucose is an independent risk factor of carotid intima-media thickness progression in Korean type 2 diabetic patients. J Diabet Complicat. 2007;21(1):7–12. doi: 10.1016/j.jdiacomp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Healy G.N., Dunstan D.W., Shaw J.E., Zimmet P.Z., Owen N. Beneficial associations of physical activity with 2-h but not fasting blood glucose in Australian adults: the AusDiab study. Diabetes Care. 2006;29(12):2598–2604. doi: 10.2337/dc06-0313. [DOI] [PubMed] [Google Scholar]