Abstract
Aim
The aim of the study was to compare the immediate and late clinical outcomes of balloon mitral valvotomy (BMV), based on the immediate post-BMV valve area and percentage gain in mitral valve area (MVA).
Methods
Clinical data of 818 consecutive patients who underwent BMV in our institute from 2000 to 2008 were analyzed retrospectively. They were categorized into three groups based on the postprocedural MVA and percentage gain in valve area—(1) 50% gain with final MVA <1.5 cm2, group 1 (fair result); (2) final MVA of ≥1.5 cm2, group 2 (good result); and (3) <50% gain with final MVA <1.5 cm2, group 3 (suboptimal result).
Results
The baseline characteristics of the three patient groups were clearly distinct. Those who had <50% gain with final MVA <1.5 cm2 were older and had higher incidence of atrial fibrillation (17 [22.4%]), heart failure (32 [42.1%]), pulmonary artery hypertension (45 [59.2%]), and significantly deformed valves (39 [51.3%]) at baseline. At a mean follow-up period of 5.64 ± 3.84 years, incidence of redo BMV (23 [4.6%]) and mitral valve replacement (17 [3.4%]) was higher in them than those with immediate MVA ≥1.5 cm2. Among those with MVA <1.5 cm2, events on follow-up were similar irrespective of the percentage gain in MVA.
Conclusions
Immediate postprocedural MVA of ≥1.5 cm2, and not percentage gain, predicts better long-term clinical outcomes after BMV. Patients who had less than 50% gain with final MVA <1.5 cm2 represent high-risk population with advanced mitral valve disease and comorbidities.
Keywords: Balloon mitral valvotomy, Mitral valve area, Percentage gain, Late outcome, Procedural success
Balloon mitral valvotomy (BMV) has emerged as the treatment of choice for hemodynamically significant mitral stenosis (MS).1, 2 Immediate procedural success, defined as an increase in the mitral valve area (MVA) of at least 1.5 cm2, in the absence of more than grade 2 mitral regurgitation (MR) of more than 90%, has been reported in various studies.1, 2, 3 It has been shown that the most important predictor of postprocedural MVA is the anatomy of the valve.4, 5 Even though a post-BMV MVA of 1.5 cm2 is often targeted, it cannot be attained in all patient groups. We hypothesized that patients who had at least 50% gain in MVA after BMV (even though the absolute valve area is <1.5 cm2) should have better clinical outcomes than those who failed to have a 50% gain. Moreover, literature on modified natural history of post-BMV patients who were categorized based on the percentage gain in MVA is scarce.
The study was planned with the aim to determine and compare the immediate and late clinical outcomes of post-BMV patients who were categorized based on the absolute postprocedural MVA and percentage gain in valve area.
1. Patients and methods
1.1. Study population
Clinical, echocardiographic, and hemodynamic data of 818 consecutive patients who underwent BMV in a tertiary care institute from 2000 to 2008 were analyzed retrospectively after getting the ethical clearance from the institute ethics committee. Patients were categorized into three groups based on the postprocedural MVA and percentage gain in the valve area (Table 1). Those who had postprocedural gain in MVA of at least 50% but final MVA <1.5 cm2 were categorized as group 1 (fair result group). Patients with postprocedural MVA ≥1.5 cm2 were categorized as group 2 (good result group). Those who had postprocedural gain in MVA of less than 50% with postprocedural MVA <1.5 cm2 were placed in group 3 (suboptimal result group). Baseline demographic data; pre- and post-BMV echocardiographic and hemodynamic data; clinical and echocardiographic data at the first year and the last follow-up after BMV; and data on events on follow-up including death, cerebrovascular accidents (CVAs), redo BMV, and mitral valve replacement (MVR) were collected and analyzed retrospectively. Events on follow-up were analyzed primarily as a composite of mortality, CVA, redo BMV, or MVR and secondarily as an individual event.
Table 1.
Subgroup definition.
| Group 1, fair result | Group 2, good result | Group 3, suboptimal result |
|---|---|---|
| Immediate postprocedural MVA of <1.5 cm2 and at least 50% gain in MVA. | Immediate postprocedural MVA of at least 1.5 cm2. | Immediate postprocedural MVA of <1.5 cm2 and less than 50% gain in MVA. |
MVA, mitral valve area.
2. Methods
Detailed clinical and echocardiographic (two-dimensional echocardiography [2D Echo] and Doppler color flow imaging) evaluation was carried out in all patients to assess the severity of MS, valve morphology, and MR. The Wilkins echocardiographic scoring system6 was used to assess the severity of mitral valve thickness, leaflet mobility, valvular calcification, and subvalvular disease, each being graded from 1 to 4 to a maximum score of 16.
The MVA was determined by 2D Echo planimetry in the parasternal short-axis view and by continuous-wave Doppler scanning using the pressure half-time method.7 Transesophageal echocardiography (TEE) was also routinely performed. Transthoracic echocardiogram was performed during BMV, 24 h after the procedure, and at follow-up visits. The contraindications to the procedure were MR of Seller's grade8 more than 2, left atrial (LA) thrombus on TEE performed before BMV, and extensive commissural calcification.
In all patients, the entry site was the right femoral vein. Surgical standby was available for all procedures. Antibiotic cover was given for all patients, and all were heparinized after septal dilatation. Septal puncture was performed by the Brockenbrough technique.9 The left and right heart pressures and the transmitral pressure gradients (TMGs) were measured immediately before and after the BMV. Left ventricular angiogram in the 30° right anterior oblique view was performed before the procedure in all patients suspected to have more than mild MR. MR was graded 1–4 as described.8 BMV was performed using the antegrade transseptal technique as already described.10 Final valve area of at least 1.5 cm2 in the absence of more than grade 2 MR1 was defined as procedural success. Loss of more than 50% of the initial gain in MVA in patients who had at least 50% gain in the MVA or postprocedural MVA ≥ 1.5 cm2 was considered as restenosis.1, 2
2.1. Statistical analysis
Statistical analysis was performed using the software SPSS, version 21.0. Z test of proportion was used to compare preintervention qualitative variables and in-hospital events after intervention. One-way analysis of variance test and post hoc test were used to compare preintervention quantitative variables. Analysis of covariance test and post hoc test were used to compare postintervention quantitative variables, quantitative variables at the first year of follow-up, and quantitative variables at the last follow-up. Average-adjusted posttest value for initial difference was compared. Kaplan–Meier analysis and Cox regression analysis were used to compare the event rate. A p value < 0.05 was considered as significant.
3. Results
3.1. Demographic data
Demographic data are listed in Table 2. It was observed that among the patient population, patients who were in the good result group were younger ([29.97 ± 10.33 years vs. 35.21 ± 9.99 years, good result group vs. suboptimal result group; p < 0.001] and [30.62 ± 11.31 years vs. 35.21 ± 9.99 years, fair result group vs. suboptimal result group; p = 0.006]). Patients in the suboptimal result group had the smallest balloon (mm) ([25.77 ± 1.08 vs. 25.43 ± 1.03, good result group vs. suboptimal result group; p = 0.115] and [25.5 ± 1.09 vs. 25.43 ± 1.03, fair result group vs. suboptimal result group; p = 0.901]) chosen and had the lowest balloon size on maximal dilatation (mm) ([24.76 ± 1.07 vs. 24.32 ± 1.09, good result group vs. suboptimal result group; p = 0.015] and [24.38 ± 1.1 vs. 24.32 ± 1.09, fair result group vs. suboptimal result group; p = 0.902) performed. Patients who had good results after BMV had the lowest incidence of atrial fibrillation (AF) ([47 {8.6%} vs. 17 {22.4%},good result group vs. suboptimal result group; p < 0.001] and [31 {15.7%} vs. 17 {22.4%}, fair result group vs. suboptimal result group; p = 0.192]), heart failure ([176 {32.4%} vs. 32 {42.1%}, good result group vs. suboptimal result group; p = 0.092] and [81 {40.9%} vs. 32 {42.1%}, fair result group vs. suboptimal result group; p = 0.857), and pulmonary artery hypertension (PAH) ([262 {48.2%} vs. 45 {59.2%}, good result group vs. suboptimal result group; p = 0.071] and [110 {55.6%} vs. 45 {59.2%}, fair result group vs. suboptimal result group; p = 0.585]). They also had the lowest incidence of deformed valves ([114 {21%} vs. 39 {51.3%}, good result group vs. suboptimal result group; p < 0.001] and [72 {36.4%} vs. 39 {51.3%}, fair result group vs. suboptimal result group; p = 0.024), and Wilkins echocardiographic score ([7.38 ± 1.32 vs. 8.45 ± 1.47, good result group vs. suboptimal result group; p < 0.001] and [8.04 ± 1.23 vs. 8.45 ± 1.47, fair result group vs. suboptimal result group; p = 0.071]) was lower in them than in the other patient groups. The incidence of prior interventions including prior surgical commissurotomy ([58 {10.7%} vs. 19 {25%}, good result group vs. suboptimal result group; p < 0.001] and [31 {15.7%} vs. 19 {25%}, fair result group vs. suboptimal result group; p = 0.290) and prior BMV ([21 {3.9%} vs. 8 {10.5%}, good result group vs. suboptimal result group; p = 0.010] and [12 {6.1%} vs. 8 {10.5%}, fair result group vs. suboptimal result group; p = 0.204]) was more in the patients who belonged to the suboptimal result group. The patients, who had suboptimal results, were relatively sicker and older and had more advanced disease with more deformed valves than the patients who had good results.
Table 2.
Preprocedural clinical and morphologic variables.
| Preprocedural clinical and morphologic variables | Group 1, fair result | Group 2, good result | Group 3, suboptimal result | p value 1 vs. 2 | p value 1 vs. 3 | p value 2 vs. 3 |
|---|---|---|---|---|---|---|
| Number (N) | 198 | 544 | 76 | |||
| Males, N (%) | 35 (17.7) | 130 (23.9) | 14 (18.4) | 0.072 | 0.886 | 0.290 |
| Age (years) | 30.62 ± 11.31 | 29.97 ± 10.33 | 35.21 ± 9.99 | 0.762 | 0.006 | <0.001 |
| Body surface area | 1.37 ± 0.15 | 1.42 ± 0.15 | 1.44 ± 0.18 | <0.001 | 0.006 | 0.694 |
| Prior surgical commissurotomy, N (%) | 38 (19.2) | 58 (10.7) | 19 (25) | 0.002 | 0.290 | <0.001 |
| Prior balloon mitral valvotomy, N (%) | 12 (6.1) | 21 (3.9) | 8 (10.5) | 0.199 | 0.204 | 0.010 |
| Size of balloon at rest (mm) | 25.5 ± 1.09 | 25.77 ± 1.08 | 25.43 ± 1.03 | 0.001 | 0.901 | 0.115 |
| Size of balloon on maximal dilatation (mm) | 24.38 ± 1.1 | 24.76 ± 1.07 | 24.32 ± 1.09 | <0.001 | 0.902 | 0.015 |
| Heart failure, N (%) | 81 (40.9) | 176 (32.4) | 32 (42.1) | 0.030 | 0.857 | 0.092 |
| Atrial fibrillation, N (%) | 31 (15.7) | 47 (8.6) | 17 (22.4) | 0.006 | 0.192 | <0.001 |
| Wilkins echocardiographic score of >8, N (%) | 72 (36.4) | 114 (21) | 39 (51.3) | <0.001 | 0.024 | <0.001 |
| Wilkins echocardiographic score (mean) | 8.04 ± 1.23 | 7.38 ± 1.32 | 8.45 ± 1.47 | <0.001 | 0.071 | <0.001 |
| Pulmonary artery hypertension, N (%) | 110 (55.6) | 262 (48.2) | 45 (59.2) | 0.075 | 0.585 | 0.071 |
3.2. Preprocedural echocardiographic and hemodynamic data
Preprocedural data are given in Table 3. The preprocedural MVA (in cm2) ([0.93 ± 0.17 vs 0.75 ± 0.12, good result group vs. suboptimal result group; p < 0.001] and [0.84 ± 0.14 vs. 0.75 ± 0.12, fair result group vs. suboptimal result group; p < 0.001]) assessed by echocardiography was lowest among the patients who belonged to the suboptimal result group. They also had the highest grade of MR ([0.86 ± 0.8 vs. 1.12 ± 0.82, good result group vs. suboptimal result group; p = 0.032] and [1.01 ± 0.82 vs. 1.12 ± 0.82, fair result group vs. suboptimal result group; p = 0.609]) and mitral valve gradient (mean – mmHg) ([14.14 ± 5.35 vs 17.06 ± 6.62, good result group vs. suboptimal result group; p = 0.007] and [16.03 ± 7.1 vs. 17.06 ± 6.62, fair result group vs. suboptimal result group; p = 0.196]) as assessed by echocardiography before BMV.
Table 3.
Preprocedural echocardiographic and hemodynamic data.
| Preprocedural echocardiographic and hemodynamic data | Group 1, fair result | Group 2, good result | Group 3, suboptimal result | p value 1 vs. 2 | p value 1 vs. 3 | p value 2 vs. 3 |
|---|---|---|---|---|---|---|
| Mitral valve area, cm2 (by echo) | 0.84 ± 0.14 | 0.93 ± 0.17 | 0.75 ± 0.12 | <0.001 | <0.001 | <0.001 |
| Mitral valve gradient (mean), mm Hg (by echo) | 16.03 ± 7.1 | 14.14 ± 5.35 | 17.06 ± 6.62 | 0.079 | 0.196 | 0.007 |
| Grade of mitral regurgitation (by echo) | 1.01 ± 0.82 | 0.86 ± 0.8 | 1.12 ± 0.82 | 0.077 | 0.609 | 0.032 |
| Left atrial pressure (mean), mmHg (by catheterization) | 23.35 ± 8.09 | 22.84 ± 6.98 | 24.98 ± 8.51 | 0.875 | 0.149 | 0.055 |
| Pulmonary artery pressure (mean), mmHg (by catheterization) | 35.51 ± 13.5 | 35.28 ± 13.9 | 40.59 ± 17.58 | 0.892 | 0.024 | <0.001 |
| Transmitral gradient, mmHg (by catheterization) | 15.46 ± 6.73 | 15.7 ± 6.83 | 17.54 ± 6.64 | 0.769 | 0.021 | <0.001 |
Patients of the suboptimal result group had the highest mean LA pressure (mmHg) ([22.84 ± 6.98 vs 24.98 ± 8.51, good result group vs. suboptimal result group; p = 0.055] and [23.35 ± 8.09 vs. 24.98 ± 8.51, fair result group vs. suboptimal result group; p = 0.149]), highest mean pulmonary artery (PA) pressure (mmHg) ([35.28 ± 13.9 vs 40.59 ± 17.58, good result group vs. suboptimal result group; p < 0.001] and [35.51 ± 13.5 vs. 40.59 ± 17.58, fair result group vs. suboptimal result group; p = 0.024]), and highest transmitral gradient [TMG] (mmHg) ([15.7 ± 6.83 vs 17.54 ± 6.64, good result group vs. suboptimal result group; p < 0.001] and [15.46 ± 6.73 vs. 17.54 ± 6.64, fair result group vs. suboptimal result group; p = 0.021]) assessed by catheterization.
3.3. Postprocedural echocardiographic and hemodynamic data
Postprocedural data are given in Table 4. The mean MVAs in group 1, group 2, and group 3 are 1.36 ± 0.09 cm2, 1.74 ± 0.2 cm2, 1.25 ± 0.23 cm2, respectively. The postprocedural MVA (cm2) ([1.74 ± 0.2 vs. 1.25 ± 0.23, good result group vs. suboptimal result group; p < 0.001] and [1.36 ± 0.09 vs. 1.25 ± 0.23, fair result group vs. suboptimal result group; p < 0.001]) assessed by echocardiography was lowest among the patients who had suboptimal result after the procedure. They also had the highest grade of MR ([1.6 ± 0.8 vs. 1.79 ± 1, good result group vs. suboptimal result group; p = 0.065] and [1.68 ± 0.98 vs. 1.79 ± 1, fair result group vs. suboptimal result group; p = 0.418]) and mitral valve gradient (mean – mm Hg) ([5.56 ± 2.72 vs. 7.89 ± 4.18, good result group vs. suboptimal result group; p < 0.001] and [7.51 ± 4.9 vs. 7.89 ± 4.18, fair result group vs. suboptimal result group; p = 0.552]) assessed by echocardiography after the procedure.
Table 4.
Postprocedural echocardiographic and hemodynamic data.
| Post-procedural echocardiographic and hemodynamic data | Group 1, fair result | Group 2, good result | Group 3, suboptimal result |
p value 1 vs. 2 |
p value 1 vs. 3 |
p value 2 vs. 3 |
|---|---|---|---|---|---|---|
| Mitral valve area, cm2 (by echo) | 1.36 ± 0.09 | 1.74 ± 0.2 | 1.25 ± 0.23 | <0.001 | <0.001 | <0.001 |
| Mitral valve gradient (mean), mmHg (by echo) | 7.51 ± 4.9 | 5.56 ± 2.72 | 7.89 ± 4.18 | <0.001 | 0.552 | <0.001 |
| Grade of mitral regurgitation (by echo) | 1.68 ± 0.98 | 1.6 ± 0.8 | 1.79 ± 1 | 0.263 | 0.418 | 0.065 |
| Left atrial pressure (mean), mmHg (by catheterization) | 15.58 ± 6.74 | 13.7 ± 5.04 | 16.57 ± 7.11 | <0.001 | 0.287 | <0.001 |
| Pulmonary artery pressure (mean), mmHg (by catheterization) | 28.67 ± 13.29 | 25.38 ± 10.71 | 29.96 ± 12.71 | 0.015 | 0.457 | <0.001 |
| Transmitral gradient, mmHg (by catheterization) | 7.09 ± 3.7 | 5.61 ± 2.79 | 7.76 ± 4.1 | <0.001 | 0.198 | <0.001 |
Patients of the suboptimal result group had the highest mean LA pressure (mmHg) ([13.7 ± 5.04 vs. 16.57 ± 7.11, good result group vs. suboptimal result group; p < 0.001] and [15.58 ± 6.74 vs. 16.57 ± 7.11, fair result group vs. suboptimal result group; p = 0.287]), highest mean PA pressure (mmHg) ([25.38 ± 10.71 vs. 29.96 ± 12.71, good result group vs. suboptimal result group; p < 0.001] and [28.67 ± 13.29 vs. 29.96 ± 12.71, fair result group vs. suboptimal result group; p = 0.457]), and highest TMG (mm Hg) ([5.61 ± 2.79 vs. 7.76 ± 4.1, good result group vs. suboptimal result group; p < 0.001] and [7.09 ± 3.7 vs. 7.76 ± 4.1, fair result group vs. suboptimal result group; p = 0.198]) assessed by catheterization (mm Hg) immediately after BMV.
3.4. In-hospital events
In-hospital events are listed in Table 5. The incidence of in-hospital events including thromboembolism, pericardial tamponade, major bleeding, stroke, MR of grade 3 or more, and mortality was comparable among the study cohorts. Patients of the good result group have the lowest incidence of emergent MVR ([3 {0.6%} vs. 3 {3.9%}, good result group vs. suboptimal result group; p = 0.005] and [6 {3%} vs. 3 {3.9%}, fair result group vs. suboptimal result group; p = 0.703]) after the procedure.
Table 5.
In-hospital events.
| In-hospital events | Group 1, fair result | Group 2, good result | Group 3, suboptimal result | p value 1 vs. 2 | p value 1 vs. 3 | p value 2 vs. 3 |
|---|---|---|---|---|---|---|
| Thromboembolism, N (%) | 4 (2) | 6 (1.1) | 3 (3.9) | 0.338 | 0.366 | 0.052 |
| Pericardial tamponade, N (%) | 0 (0) | 2 (0.4) | 1 (1.3) | 0.393 | 0.107 | 0.265 |
| Major bleeding, N (%) | 0 (0) | 1 (0.2) | 0 (0) | 0.546 | 1.000 | 0.709 |
| Stroke, N (%) | 3 (1.5) | 4 (0.7) | 0 (0) | 0.331 | 0.281 | 0.454 |
| Emergent mitral valve replacement, N (%) | 6 (3) | 3 (0.6) | 3 (3.9) | 0.006 | 0.703 | 0.005 |
| Mortality, N (%) | 1 (0.5) | 0 (0) | 0 (0) | 0.097 | 0.536 | 1.000 |
| Grade 3 or more mitral regurgitation, N (%) | 23 (11.6) | 41 (7.5) | 11 (14.5) | 0.080 | 0.799 | 0.366 |
3.5. Clinical follow-up
3.5.1. At one year
Data of 784 patients were available 1 year after the procedure (Table 6). Patients with good results had the greatest MVA (cm2) ([1.69 ± 0.01 vs. 1.28 ± 0.02, good result group vs. suboptimal result group; p < 0.001] and [1.4 ± 0.01 vs. 1.28 ± 0.02, fair result group vs. suboptimal result group; p < 0.001]) and the lowest mitral valve gradient mean (mm Hg) ([5.38 ± 0.15 vs. 8.08 ± 0.42, good result group vs. suboptimal result group; p < 0.001] and [6.85 ± 0.25 vs. 8.08 ± 0.42, fair result group vs. suboptimal result group; p = 0.012]) among the three groups at 1 year. The grades of MR and PA systolic pressure (mmHg) were comparable among the groups.
Table 6.
Follow-up at the first year.
| Follow-up at the first year | Group 1, fair result | Group 2, good result | Group 3, suboptimal result |
p value 1 vs. 2 |
p value 1 vs. 3 |
p value 2 vs. 3 |
|---|---|---|---|---|---|---|
| Mitral valve area (cm2) | 1.4 ± 0.01 | 1.69 ± 0.01 | 1.28 ± 0.02 | <0.001 | <0.001 | <0.001 |
| Mitral valve gradient (mean), mmHg | 6.85 ± 0.25 | 5.38 ± 0.15 | 8.08 ± 0.42 | <0.001 | 0.012 | <0.001 |
| Grade of mitral regurgitation | 1.49 ± 0.06 | 1.46 ± 0.03 | 1.41 ± 0.09 | 0.663 | 0.460 | 0.601 |
| Pulmonary artery systolic pressure, mmHg | 41 ± 0.85 | 40.35 ± 0.51 | 39.5 ± 1.41 | 0.512 | 0.360 | 0.570 |
3.5.2. At 5 years
Data of 732 patients were available at 5 years after the procedure (Table 7). The mean follow-up period was 5.64 ± 3.84 years. Patients with good results after procedure were seen to have the greatest MVA (1.54 ± 0.32 cm2), lowest mitral valve gradient (6.5 ± 0.19 mm Hg), lowest grade of MR (1.57 ± 0.04), and lowest PA systolic pressure (39.41 ± 0.52 mmHg) at 5 years of follow-up. Patients who belonged to the suboptimal result group were observed to have a lowest MVA (cm2) (1.27 ± 0.29 vs. 1.18 ± 0.3, fair result group vs. suboptimal result group; p = 0.046), greatest mitral valve gradient mean (mm Hg) (8.7 ± 0.33 vs. 9.83 ± 0.54, fair result group vs suboptimal result group; p = 0.072), and highest PA systolic pressure (mmHg) (41.86 ± 0.9 vs. 45.44 ± 1.47, fair result group vs. suboptimal result group; p = 0.038) on follow-up at 5 years.
Table 7.
Last follow-up.
| Last follow-up | Group 1, fair result | Group 2, good result | Group 3, suboptimal result |
p value 1 vs. 2 |
p value 1 vs. 3 |
p value 2 vs. 3 |
|---|---|---|---|---|---|---|
| Mitral valve area (cm2) | 1.27 ± 0.29 | 1.54 ± 0.32 | 1.18 ± 0.3 | <0.001 | 0.046 | <0.001 |
| Mitral valve gradient (mean), mmHg | 8.7 ± 0.33 | 6.5 ± 0.19 | 9.83 ± 0.54 | <0.001 | 0.072 | <0.001 |
| Grade of mitral regurgitation | 1.59 ± 0.07 | 1.57 ± 0.04 | 1.59 ± 0.11 | 0.848 | 0.999 | 0.900 |
| Pulmonary artery systolic pressure, mmHg | 41.86 ± 0.9 | 39.41 ± 0.52 | 45.44 ± 1.47 | 0.019 | 0.038 | <0.001 |
3.6. Events on follow-up
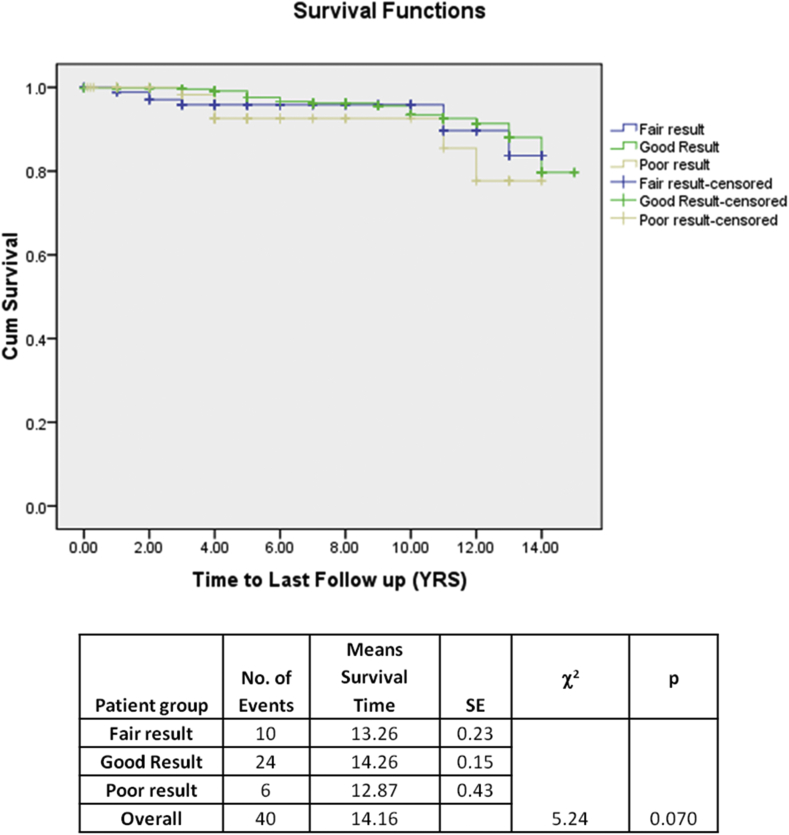
The event rate of the patient groups is provided in Table 7. Occurrence of composite events was significantly higher in patients with post-BMV MVA <1.5 cm2 than in those with post-BMV MVA ≥1.5 cm2 (Fig. 1). The increase was primarily due to the increased occurrence of redo BMV and MVR in those patient populations (redo BMV 23 [4.6%] and MVR 17 [3.4%]) (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Table 8), whereas occurrence mortality and CVA remained nonsignificant. Among patients with post-BMV MVA <1.5 cm2 (fair result group vs. suboptimal result group), no significant difference was observed in the occurrence of composite events or individual event. Cox regression analysis has shown that postprocedural MVA of 1.5 cm2 is associated with lesser redo interventions irrespective of the baseline characteristics. The occurrence of events was similar with those groups who had immediate postprocedural MVA of less than 1.5 cm2, irrespective of the percentage gain in MVA (Table 9).
Fig. 1.
Kaplan–Meier curve showing any event on follow-up—Mortality, cerebrovascular accident (CVA), redo balloon mitral valvotomy (BMV), or mitral valve replacement (MVR).
Fig. 2.
Kaplan–Meier curve showing the event on follow-up—Mortality.
Fig. 3.
Kaplan–Meier curve showing the event on follow-up—CVA. CVA, cerebrovascular accident.
Fig. 4.
Kaplan–Meier curve showing the event on follow-up—Redo BMV. BMV, balloon mitral valvotomy.
Fig. 5.
Kaplan–Meier curve showing the event on follow-up—MVR. MVR, mitral valve replacement.
Table 8.
Events on follow-up.
| Events on follow-up | Group 1, fair result | Group 2, good result | Group 3, suboptimal result |
|---|---|---|---|
| Mortality | 3 (1.8) | 3 (0.6) | 1 (1.6) |
| Cerebrovascular accident | 10 (5.8) | 24 (4.8) | 6 (9.4) |
| Redo balloon mitral valvotomy | 12 (7.1) | 23 (4.6) | 7 (11.3) |
| Mitral valve replacement | 24 (12.1) | 17 (3.1) | 9 (11.8) |
Table 9.
Events on follow-up (Cox regression analysis).
| Event | Group comparison | B | S.E. | p |
|---|---|---|---|---|
| Redo balloon mitral valvotomy | Good result vs. fair result | −0.709 | 0.381 | 0.063 |
| Poor result vs. fair result | 0.527 | 0.590 | 0.371 | |
| Good result vs. poor result | −1.237 | 0.546 | 0.023 | |
| Mitral valve replacement | Good result vs. fair result | −0.843 | 0.479 | 0.078 |
| Poor result vs. fair result | −0.429 | 0.491 | 0.382 | |
| Good result vs. poor result | −1.272 | 0.353 | <0.001 |
4. Discussion
Excellent acute hemodynamic results have been reported in numerous clinical studies involving a large number of patients undergoing BMV.1, 2, 3, 11, 16 The definition of procedural success adopted in most studies is a postprocedural MVA of at least 1.5 cm2 without significant MR.1, 2, 12 Literature on clinical follow-up of post-BMV patients based on the percentage gain in postprocedural MVA is scarce.
We have analyzed the modified natural history of post-BMV patients by categorizing them into three groups, depending on the postprocedural MVA and the percentage gain in MVA. Not only the in-hospital events but also the events on long-term clinical follow-up have been found to be significantly more in the group that had postprocedural MVA of at least 1.5 cm2.
4.1. Demographic factors and immediate procedural success
The patients who had postprocedural MVA of <1.5 cm2, especially those who did not have 50% gain in MVA (suboptimal result group), were relatively sicker and had more advanced disease than the patients who had postprocedural MVA of at least 1.5 cm2, as suggested by the higher incidence of AF (17 [22.4%]), heart failure (32 [42.1%]), and PAH (45 [59.2%]). They were relatively older (35.21 ± 9.99 years), had deformed mitral valve with higher Wilkin's echocardiographic score (8.45 ± 1.47), and had more prior surgical (19 [25%]) and balloon (8 [10.5%]) interventions. Our findings were consistent with those of a previous study that showed a significant favorable impact on immediate post-BMV in patients with <8 Wilkins score.17 They also had higher TMG and LA and PA pressures, both before and after the procedure.
It has been shown that older age, smaller valve area, unfavorable valve anatomy, previous commissurotomy, and baseline MR are potential predictors for poor immediate outcome with a similar predictive strength as valve calcification.18 In our study, advanced age, more deformed valve at the time of the procedure, and relatively lower preprocedural MVA might have contributed to a lesser postprocedural MVA in those patients. Apart from the patient-related factors, the balloon size and the extent of maximal balloon dilatation during the procedure was lowest (24.32 ± 1.09 mm) (as assessed by the balloon size on maximal dilatation) with the suboptimal result group. The patient-related factors might have led the operators to choose a smaller sized balloon and go for a lesser dilatation. Hence, it may be presumed that patient-related factors including advanced age and a more deformed mitral valve have precluded them in attaining an optimal postprocedural MVA.
4.2. In-hospital events
The incidence of in-hospital events including thromboembolism, tamponade, major bleeding, stroke, MR of grade 3 or more, and mortality was comparable among the study groups. Seventy-five (9.2%) patients demonstrated a postprocedural MR of at least moderate grade, and urgent MVR was needed in 12 (1.46%) of them. The incidence of at least moderate MR after procedure and the incidence of significant MR requiring urgent MVR were lowest in those with fair results. The reason for a lower incidence of at least moderate MR after procedure in them may be due to the fact that they had a relatively less deformed valve than the others.4 A more deformed valve may result in undesirable transmission of balloon pressure force, resulting in valve disruption instead of the expected commissural cleavage.13
4.3. Follow-up
The best results of BMV are seen in young patients who have MS with favorable anatomic characteristics (i.e., pliable noncalcified valves and moderate impairment of the subvalvular apparatus). Fawzy et al19 reported an event-free survival rate of 79% at 10 years and 43% at 15 years in relatively younger patients (mean age 31 ± 11 years), and the rates were significantly higher for patients with optimal valve anatomy (88% at 10 years and 66% at 15 years).
In our study, patients who had less than 50% gain in MVA along with absolute postprocedural MVA of <1.5 cm2 after BMV were older with unfavorable valve anatomy and had the lowest absolute MVA, highest mitral valve gradient, highest grade of MR, and highest PA pressure at 1 year and 5 years of follow-up, whereas those with post-BMV MVA of ≥1.5 cm2 were relatively young and had pliable valves, and the number of events including redo BMV and MVR was lowest among them. Suboptimal immediate results led to relatively early intervention, which explains the presence of an early drop of event-free survival after the procedure. Other events (mortality and CVA) remained statistically nonsignificant when compared with patients having post-BMV MVA < 1.5 cm2. This is in accordance with other series14, 15 that have shown similar outcomes. It has also been shown that postprocedural MVA of 1.5 cm2 is associated with lesser redo interventions irrespective of the baseline characteristics.
In patients with post-BMV MVA of <1.5 cm2, the incidence of mortality, CVA, MVR, and redo BMV on follow-up showed no statistical difference, irrespective of percentage gain in MVA, even though they had more redo interventions than those with post-BMV MVA of 1.5 cm2. Patients with post-BMV MVA of <1.5 cm2 represent a high-risk patient population with advanced mitral valve disease and significant comorbidity. Irrespective of the percentage gain in post-BMV MVA, events on follow-up did not differ significantly between them.
4.4. Limitations of the study
Of the 818 patients, 86 patients were lost to follow-up. Because it is likely that patients may have not received follow-up because of an adverse event, this may have affected the results of our study.
5. Conclusions
Patients who had less than 50% gain with final MVA <1.5 cm2 immediately after BMV represent high-risk population with advanced mitral valve disease and comorbidities including AF, heart failure, and PAH, and events on follow-up did not vary significantly, irrespective of the percentage gain in MVA. Immediate postprocedural MVA of ≥1.5 cm2 predicts better long-term clinical outcomes after BMV.
Conflicts of interest
All authors have none to declare.
Disclosures
The authors have no competing interests, funding, or financial relationships to disclose.
References
- 1.Nobuyoshi M., Arita T., Shirai S.I. Percutaneous balloon mitral valvuloplasty a review. Circulation. 2009;119(8):e211–e219. doi: 10.1161/CIRCULATIONAHA.108.792952. [DOI] [PubMed] [Google Scholar]
- 2.Nair K.K., Pillai H.S., Thajudeen A. Comparative study on safety, efficacy, and midterm results of balloon mitral valvotomy performed with triple lumen and double lumen mitral valvotomy catheters. Cathet Cardiovasc Interv. 2012;80(6):978–986. doi: 10.1002/ccd.24284. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Farhat M., Betbout F., Gamra H. Predictors of long-term event-free survival and of freedom from restenosis after percutaneous balloon mitral commissurotomy. Am Heart J. 2001;142(6):1072–1079. doi: 10.1067/mhj.2001.118470. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez R., Banuelos C., Alfonso F. Long-term clinical and echocardiographic follow-up after percutaneous mitral valvuloplasty with the Inoue balloon. Circulation. 1999;99(12):1580–1586. doi: 10.1161/01.cir.99.12.1580. [DOI] [PubMed] [Google Scholar]
- 5.Cavalcante J.L., Rodriguez L.L., Kapadia S., Tuzcu E.M., Stewart W.J. Role of echocardiography in percutaneous mitral valve interventions. JACC Cardiovasc Imaging. 2012;5(7):733–746. doi: 10.1016/j.jcmg.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Wilkins G.T., Weyman A.E., Abascal V.M., Block P.C., Palacios I.F. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60(4):299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Authors/Task Force Members. Vahanian A., Alfieri O., Andreotti F. Guidelines on the management of valvular heart disease (version 2012) the Joint Task Force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European association for Cardio-thoracic Surgery (EACTS) Eur J Cardio Thorac Surg. 2012;42(4):S1–S44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 8.Sellers R.D., Levy M.J., Amplatz K., Lillehei C.W. Left retrograde cardioangiography in acquired cardiac disease: technic, indications and interpretations in 700 cases∗. Am J Cardiol. 1964;14(4):437–447. doi: 10.1016/0002-9149(64)90027-x. [DOI] [PubMed] [Google Scholar]
- 9.Earley M.J. How to perform a transseptal puncture. Heart. 2009;95(1):85–92. doi: 10.1136/hrt.2007.135939. [DOI] [PubMed] [Google Scholar]
- 10.Arora R., Nair M., Kalra G.S., Nigam M., Khalilullah M. Immediate and long-term results of balloon and surgical closed mitral valvotomy: a randomized comparative study. Am Heart J. 1993;125:1091–1094. doi: 10.1016/0002-8703(93)90118-s. [DOI] [PubMed] [Google Scholar]
- 11.Carabello B.A. Modern management of mitral stenosis. Circulation. 2005;112(3):432–437. doi: 10.1161/CIRCULATIONAHA.104.532498. [DOI] [PubMed] [Google Scholar]
- 12.Saeki F., Ishizaka Y., Tamura T. Long-term clinical and echocardiographic outcome in patients with mitral stenosis treated with percutaneous transvenous mitral commissurotomy. Jpn Circ J. 1999;63(8):597–604. doi: 10.1253/jcj.63.597. [DOI] [PubMed] [Google Scholar]
- 13.Arora R., Kalra G.S., Singh S. Percutaneous transvenous mitral commissurotomy: immediate and long-term follow-up results. Cathet Cardiovasc Interv. 2002;55(4):450–456. doi: 10.1002/ccd.10109. [DOI] [PubMed] [Google Scholar]
- 14.Nair M., Kumar K., Pillai H.S. Immediate and long-term results following balloon mitral valvotomy in patients with atrial fibrillation. Clin Cardiol. 2012;35(12):E35–E39. doi: 10.1002/clc.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathan A.Z., Mahdi N.A., Leon M.N. Is redo percutaneous mitral balloon valvuloplasty (PMV) indicated in patients with post-PMV mitral restenosis? J Am Coll Cardiol. 1999;34(1):49–54. doi: 10.1016/s0735-1097(99)00176-x. [DOI] [PubMed] [Google Scholar]
- 16.Sarmiento R.A., Blanco R., Gigena G. Initial results and long-term follow-up of percutaneous mitral valvuloplasty in patients with pulmonary hypertension. Heart Lung Circ. 2017;26(1):58–63. doi: 10.1016/j.hlc.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Palacios I.F., Sanchez P.L., Harrell L.C., Weyman A.E., Block P.C. Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplasty variables that predict long-term outcome. Circulation. 2002;105(12):1465–1471. doi: 10.1161/01.cir.0000012143.27196.f4. [DOI] [PubMed] [Google Scholar]
- 18.Iung B., Cormier B., Ducimetiere P. Immediate results of percutaneous mitral commissurotomy: a predictive model on a series of 1514 patients. Circulation. 1996;94:2124–2130. doi: 10.1161/01.cir.94.9.2124. [DOI] [PubMed] [Google Scholar]
- 19.Fawzy M.E., Shoukri M., Al Buraiki J. Seventeen years' clinical and echocardiographic follow up of mitral balloon valvuloplasty in 520 patients, and predictors of long-term outcome. J Heart Valve Dis. 2007;16:454–460. [PubMed] [Google Scholar]