Pulmonary artery catheter (PAC) (i.e. Swan–Ganz catheter) is a diagnostic tool for quantitative hemodynamic measurements. The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial in 2005 discouraged the use of PAC. This is because PAC use demonstrates an unanticipated increase in the adverse events and resource utilizations in the ESCAPE trial.1 In addition, recent studies have demonstrated PAC to increase short-term mortality and resource utilization associated with critically ill patients.2, 3 However, recent studies have demonstrated an increase in the utilization of PAC for heart failure (HF) admissions.4
Currently, the American College of Cardiology/American Heart Association guidelines recommend using PAC for HF with cardiogenic shock (CS) or those on a mechanical circulatory support (class I, level of evidence, C).5 However, PAC use is discouraged for routine management of HF.5 The role of PAC for hospitalizations with CS is not well described and remains somewhat enigmatic in its use. We sought to investigate the use of PAC for the management of CS hospitalizations and in-hospital mortality associated with it.
This study retrospectively analyzed the National Inpatient Sample (NIS) from 2005 to 2014.6 The NIS is a subset of the Healthcare Cost and Utilisation Project sponsored by the Agency for Healthcare Research and Quality. The details about the NIS database have been described earlier.7 This study used the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic code 785.51 to identify hospitalizations with CS (N = 855,252).8 Hospitalizations of patients younger than 18 years of age were excluded from this study (N = 12,884). Utilization of PAC was identified using ICD-9-CM procedures codes 89.63, 89.64, 89.66, 89.67, 89.68 which have been validated previously (N = 71,452).9 To represent national estimates, discharge weights were utilized. The primary end point of this study was to observe the utilization of PAC for CS hospitalizations. Trends in the hospitalizations were calculated using the Jonckheere–Terpstra test. This study further stratifies PAC use into clinically important subgroups by the presence of acute coronary syndrome (ACS). Rates of PAC use was calculated using the number of PAC procedures per 1000 hospitalization with CS. Continuous variables were analyzed using Student's t-test and were represented as the mean ± standard deviation. Categorical variables were analyzed using the chi-squared test or Fisher's exact test and were represented as frequencies and percentages. All p-values were two sided, and a value of less than 0.05 was considered statistically significant. A hierarchical, mixed-effect, multivariate logistic regression was performed to calculate adjusted in-hospital mortality, and data on age, gender, race, hospital location and Charlson's comorbidity index10 were included.
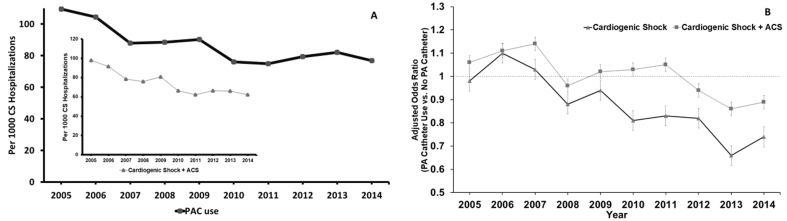
Hospitalizations with CS increased from 55,074 in 2005 to 123,590 in 2014 (Ptrend<0.001) during the study period. PAC utilization was 8.5% for overall hospitalizations with CS (Table 1). PAC utilization decreased from 109.5 in 2005 to 76.9 in 2014 per 1000 CS hospitalizations (Ptrend<0.001) during the study period. ACS occurred in 45.5% of hospitalizations with PAC use and 53.2% of hospitalizations without PAC use. PAC use in hospitalizations with ACS decreased from 97.9 in 2005 to 62.3 in 2014 per 1000 CS hospitalizations (Fig. 1, panel A). The majority of PAC utilization was at urban teaching hospitals (73.9%). Vasopressor was utilized in 62,398 hospitalizations (8.1%) without PAC use and 7207 (10.1%) hospitalizations with PAC use. An intraaortic balloon pump was utilized in 36.5% of hospitalizations with PAC use and in 23.4% of hospitalizations without PAC use (P=<0.001). Percutaneous ventricular assist device was utilized in 2.4% of hospitalizations with PAC use and in 1.1% of hospitalizations without PAC use. Overall in-hospital mortality was lower when using PAC as compared to those without (33.9% vs. 38.8%, P < 0.001). This remains significantly low even after performing adjusted analysis (odds ratio: 0.90; confidence interval: 87–0.93, P < 0.001) (Table 1). In-hospital mortality decreased consistently during the study period (46% in 2005 to 37.4% in 2014, Ptrend<0.001). Furthermore, in-hospital mortality was lower when using PAC (44.5% in 2005 to 29.4% in 2014, Ptrend<0.001) throughout the study period as compared in those without (46.2% in 2005 to 38% in 2014, Ptrend=<0.001). Adjusted mortality significantly decreased in overall CS and CS plus ACS (Fig. 1, panel B). However, the significant benefits of PAC in the subset of CS + ACS were observed after the year 2012, and the significant benefits of PAC in the subset of CS only were observed after the year 2008.
Table 1.
Baseline characteristics and in-hospital mortality with and without PAC use in cardiogenic shock hospitalizations.
| Variables | PAC use (N = 71,452) | No PAC use (N = 770,917) | P value |
|---|---|---|---|
| Age, years (median with interquartile range) | 65 (55–75) | 69 (59–79) | <0.001 |
| Femalesa | 25,491 (35.7) | 312,109 (40.5) | <0.001 |
| Caucasians | 43,391 (60.7) | 486,544 (63.1) | <0.001 |
| African-Americans | 7725 (10.8) | 81,116 (10.5) | |
| Othersb | 20,330 (28.5) | 203,248 (26.4) | |
| Charlson's comorbidity index | |||
| 0 | 3114 (4.4) | 44,685 (5.8) | <0.001 |
| 1 | 15,619 (21.9) | 163,825 (21.2) | |
| 2 | 17,662 (24.7) | 188,509 (24.5) | |
| ≥3 | 35,057 (49.1) | 373,898 (48.5) | |
| Elective admissionc | 9,968 (14.0) | 86,870 (11.3) | <0.001 |
| Emergent/urgent admission | 61,373 (86.0) | 682,506 (88.7) | |
| Hospital location | |||
| Rural | 2250 (3.2) | 47,817 (6.2) | <0.001 |
| Urban, nonteaching | 16,339 (22.9) | 272,301 (35.5) | |
| Urban, teaching | 52,650 (73.9) | 447,492 (58.3) | |
| Elixhauser comorbidities | |||
| Chronic lung disease | 16,235 (22.7) | 188,344 (24.4) | <0.001 |
| Diabetes | 18,209 (25.5) | 194,589 (25.2) | 0.15 |
| Hypertension | 35,187 (49.3) | 408,630 (53.0) | <0.001 |
| Liver disease | 2708 (3.8) | 25,791 (3.4) | <0.001 |
| Peripheral vascular disease | 8307 (11.6) | 96,461 (12.5) | <0.001 |
| Renal failure | 20,366 (28.5) | 209,143 (27.1) | <0.001 |
| Valvular disease | 4585 (6.4) | 48,706 (6.3) | 0.30 |
| In-hospital outcome | |||
| In-hospital mortalityd | 24,216 (33.9) | 298,660 (38.8) | <0.001 |
| Adjusted in-hospital mortalitye | Odds ratio: 0.90 Confidence interval: 0.87–0.93 |
<0.001 | |
CS, cardiogenic shock; PAC, pulmonary artery catheter.
52 missing.
14 missing.
1652 missing.
529 missing.
Adjusted using age, sex, race, hospital location and Charlson's comorbidity index.
Fig. 1.
Panel A: utilization of PAC for hospitalizations with CS. The inset demonstrates PAC use per 1000 hospitalizations with CS + ACS. Trend value for all is P < 0.001 by Jonckheere–Terpstra trend test. Panel B: adjusted in-hospital mortality in the overall CS hospitalizations and CS + ACS hospitalizations. Bar represents a 95% confidence interval, and dot represents the odds ratio. The dotted line represents the odds ratio of 1 below which is a beneficial effect of PAC. ACS, acute coronary syndrome; CS, cardiogenic shock; PAC, pulmonary artery catheter.
PAC use is declining even though the observed mortality is lower when using it. Several reasons have been postulated for the decline in PAC use, including the lack of guidelines driving the use of PAC in CS or higher mortality observed when using PAC in a previous study.9 Also, the evolution of bedside echocardiography provides detailed information regarding the mechanisms of CS which enables more cause-oriented treatment and may be the reason for the decline in PAC use. Possible reasons for the reduction in mortality may include an increase in the use of newer mechanical circulatory support (MCS) devices for the management of CS or the earlier use of percutaneous support devices which are much easier and ubiquitous.11, 12 From our data set, we cannot claim that this reduction in mortality is truly associated with PAC and MCS use. However, we postulate that the appropriate use of PAC when using MCS may help in reducing mortality associated with CS. Finally, previous studies utilized selection criteria which excluded patients in whom PAC use was deemed useful by the practitioner, whereas we utilized a national all-comers database. There remains a valid concern for the reduction in the utilization of PAC, especially when the use of MCS has been increasing. The management of these MCS devices is hemodynamically driven, and the use of a PAC remains the only reliable direct measurement of real-time hemodynamics available to us today. As important as the PAC itself, the physicians who are interpreting and the trainees learning to interpret need to have adequately mastered PAC insertion and interpretation for the device to demonstrate a truly beneficial effect.
This study has several limitations as with any retrospective analysis. There may remain differences in baseline mortality risk between the PAC and non-PAC groups that could be driving the observed differences in mortality rates, rather than the use of PAC. Even after analyzing adjusted in-hospital mortality, several unmeasured confounders still remain as this study did not account for those more serious hospitalizations in which patients died before the use of PAC.
In summary, the mortality of CS associated with PAC use is decreasing, but the use of PAC has declined and is much lower than expected. This may suggest a deleterious effect from the results of previous observational studies. However, given the decreased mortality among patients with CS in whom PAC was used, it behooved us as a community of physicians to use PAC in selected CS cases, especially in whom MCS is planned or being utilized.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Binanay C., Califf R.M., Hasselblad V., Investigators E and Coordinators ES Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 2.Rajaram S.S., Desai N.K., Kalra A. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD003408.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doshi R., Patel H., Shah P. Pulmonary artery catheterization use and mortality in hospitalizations with HFrEF and HFpEF: a nationally representative trend analysis from 2005 to 2014. Int J Cardiol. 2018;269:289–291. doi: 10.1016/j.ijcard.2018.07.069. [DOI] [PubMed] [Google Scholar]
- 4.Pandey A., Khera R., Kumar N., Golwala H., Girotra S., Fonarow G.C. Use of pulmonary artery catheterization in US patients with heart failure, 2001–2012. JAMA Intern Med. 2016;176:129–132. doi: 10.1001/jamainternmed.2015.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 6.Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. NIS database documentation archive. Rockville, MD. June 2016. www.hcup-us.ahrq.gov/db/nation/nis/nisarchive.jsp [Google Scholar]
- 7.Patel N., Kalra R., Doshi R. Hospitalization rates, prevalence of cardiovascular manifestations, and outcomes associated with sarcoidosis in the United States. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolte D., Khera S., Aronow W.S. Trends in incidence Bar represents a 95% confidence interval and dot represents the odds ratio. The dotted line, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiener R.S., Welch H.G. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–429. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 10.Austin S.R., Wong Y.N., Uzzo R.G., Beck J.R., Egleston B.L. Why summary comorbidity measures such as the Charlson comorbidity index and elixhauser score work. Med Care. 2015;53:e65–e72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khera R., Cram P., Lu X. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941–950. doi: 10.1001/jamainternmed.2014.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meraj P.M., Doshi R., Schreiber T., Maini B., O'Neill W.W. Impella 2.5 initiated prior to unprotected left main PCI in acute myocardial infarction complicated by cardiogenic shock improves early survival. J Interv Cardiol. 2017;30:256–263. doi: 10.1111/joic.12377. [DOI] [PubMed] [Google Scholar]