Abstract
Background
Despite the increasing popularity of transcatheter aortic valve replacement (TAVR), only about 10,000 TAVR cases have been performed in Asia to date. The procedure is still in a nascent stage in India with very few centers offering this state-of-art technique. Here, we present the early results of TAVR experience at our center.
Methods
Forty-nine patients with severe symptomatic aortic stenosis (AS) were referred to our center for TAVR from November 2015 to February 2018. Twenty-five patients underwent TAVR at our conventional cardiac catheterization laboratory under local or general anesthesia, with standby surgical team support.
Results
The mean age of the patients was 72.0 ± 8.1 years. The mean Society of Thoracic Surgeons score was 13.8 ± 10.2. Baseline mean ejection fraction was 50.3 ± 14.8%. Baseline mean aortic valve gradient was 55.8 ± 24.7 mmHg. There was one procedural-related death. Two of the patients required urgent surgery: one for contained annular rupture and one underwent vascular repair for femoral artery occlusion. Mild and moderate paravalvular leak was seen in 11 and 3 patients, respectively. Four patients (16%) required permanent pacemaker. Eighty percent were in New York Heart Association class I-II at discharge. One-year all-cause mortality was 8%, with no hospitalizations or major adverse cardiac event during the 1-year follow-up.
Conclusion
Our early data clearly shows that in our country, TAVR is a good alternative for symptomatic severe AS for high surgical risk cases. Large-scale multicenter studies are required to study the real impact of TAVR in the Indian scenario. During initial years of implementation of a nationwide TAVR program, it may be prudent to focus on creating TAVR Centers of Excellence by developing an ideal hub and spokes model.
Keywords: Transcatheter aortic valve replacement (TAVR), Aortic stenosis, Indian experience
1. Introduction
The most common cause of isolated acquired aortic stenosis (AS) in both developed and developing countries is age-related degeneration of the aortic valve. Prevalence of isolated AS was found to be as high as 7.3% in an Indian hospital-based echocardiographic survey, with most of the patients being aged more than 60 years.1 Studies from Western data have revealed that in patients aged over 75 years overall prevalence of AS is 12.4% and that of severe symptomatic AS is 3.4%.2 These patients have a survival rate as low as 15–50% at 5 years without aortic valve replacement.3 By extrapolating Western prevalence data to the Indian population, it is estimated that nearly 300,000 patients with AS are likely to be eligible for aortic valve replacement. Until recent times, the standard of care therapy was surgical aortic valve replacement (SAVR) for symptomatic severe AS. It has been observed that SAVR has good outcomes across broad populations with low complication rates. However, it has been reported that up to 30% of patients with severe symptomatic AS do not undergo SAVR, because of reasons such as advanced age, associated comorbid conditions, previous surgery, inoperability or high risk, and unwillingness to undergo open heart surgery. Transcatheter aortic valve replacement (TAVR), a percutaneous procedure, has transformed care of high risk and inoperable AS. Since the introduction of TAVR, there has been continuous innovation in the technology and improvement in patient selection and implantation techniques. SAVR is now challenged by TAVR for severe AS today, with >3,00,000 procedures being performed in more than 1000 global centers and a substantial amount of emerging data. TAVR was initially shown to be efficacious and safe in inoperable patients, also known to be at extreme risk for surgery.4 In these patients, TAVR reduced overall mortality as compared with medical therapy or balloon valvuloplasty. Also, both balloon-expandable and self-expandable TAVR have demonstrated noninferiority to surgery across high-risk patient populations.5 Owing to proven advantage of TAVR, the US Food and Drug Administration subsequently granted marketing approval, and now, it has become the standard of care in high-risk patients. As per the Placement of AoRTic TraNscathetER Valve Trial - IIA (PARTNER-IIA) trial6 and Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients - (SURTAVI) trial,7 TAVR was noninferior to SAVR in intermediate-risk groups with respect to primary clinical outcomes, expanding the indication to intermediate-risk patients. Despite the increasing popularity of TAVR, only about 10,000 TAVR cases have been performed in Asia to date. The procedure is still in its nascent stage in India with very few centers offering this state-of-art technique. Here, we present the early results of our TAVR experience in our center
2. Materials and methods
2.1. Patient inclusion and preprocedure evaluation
A total of 49 patients with severe symptomatic AS were evaluated and found to be at high risk for SAVR and were referred to our center for TAVR, during the period from November 2015 to February 2018. Baseline data of all the patients were recorded. Parameters such as demographic details, history of present illness, Society of Thoracic Surgeons (STS) score, and European System for Cardiac Operative Risk Evaluation (EURO) scores were recorded. Baseline echocardiography was performed. Patients were selected for TAVR based on the risk assessment and after a detailed discussion by the heart team that included a cardiologist, cardiothoracic surgeon, and cardiac anesthetist. The risk assessment not only involves STS/EURO score but also other high risks features such as frailty, chest deformities, and oxygen-dependent respiratory failure. Computed tomography (CT) was performed in all the patients, and information about annulus size, optimal angiographic projection for device implantation, and other information such as sinus width, leaflet size, and calcification were collected. Echo parameters were repeated postoperatively, at the time of discharge, after 1 month of the procedure, and 6 and 12 months after the procedure along with assessment of comorbidities. If the CT Coronaries coronary angiography was normal or showed insignificant disease, regular catheter coronary angiography was avoided. Patients who had significant left main disease, proximal left anterior descending artery (LAD) stenosis, or acute coronary syndrome were considered for percutaneous coronary intervention (PCI) before TAVR. However, patients having non-LAD single disease were left with medical management.
2.2. TAVR procedure protocol
At our center, TAVR is performed in a regular catheterization laboratory which has adequate space to accommodate device preparation, echocardiographic equipment, anesthesia equipment, and other surgical instruments in a sterile environment. The preferred access site for the procedure was through the transfemoral route whenever feasible. The procedure was performed under conscious sedation or general anesthesia, depending on compromised hemodynamics or patient preference. Conscious sedation offers advantage of better hemodynamic status, shorter procedural duration, and shorter length of intensive care unit stay. Transesophageal echocardiogram (TEE) was planned for selected cases. Intraprocedural TEE was not performed routinely as it usually requires general anesthesia. Also, the probe used for TEE may partially obstruct the fluoroscopic view, necessitating multiple retractions and advancements during the procedure. However, it was considered in patients having severe native valve calcifications and in patients who had previous mitral prosthesis. Before the procedure, a temporary pacing catheter (a balloon-tipped lead) was positioned in the right ventricle via the right jugular or femoral venous route. Both the groins were used for vascular access: on one side, a 6F or 7F access for a pigtail catheter and on the other side, preinsertion of two proglide and an initial 9F sheath, which was later upgraded to the larger TAVR introducer sheath. Aortography was performed, and native aortic valve was crossed by a straight guidewire followed by pressure gradient measurement. An undersized balloon was used for balloon dilatation, and simultaneous aortogram was performed during a brief period of rapid ventricular pacing. During balloon valvuloplasty, the risk of coronary occlusion was assessed. Balloon-expandable valves were deployed under rapid ventricular pacing, whereas the self-expanding valve was slowly released usually without pacing as per protocol. Subsequently, aortic valve gradients were again measured along with angiographic and echocardiographic assessments for paravalvular leaks (PVLs). In case of significant PVL, postdilatation was performed. After the completion of the procedure, catheters and guidewires were withdrawn, and hemostasis was achieved.
3. Results
3.1. Baseline data
Of the 49 patients with severe symptomatic AS who were evaluated by us, 24 patients did not undergo the procedure (Table 1). Seventeen patients did not undergo due to monetary constraints, 2 patients had sudden cardiac death during the evaluation period, 1 patient had pseudosevere AS, 3 patients with severe coronary artery disease required cardiac bypass graft (CABG), and one was post-SAVR patient having severe concentric hypertrophy with severe left ventricular outflow tract gradient. Of the 25 patients who underwent TAVR, 17 (68%) were males and 8 (32%) were females. The mean age of the patients was 72.0 ± 8.1 years. Diabetes mellitus was found in 16 of 25 (64%), hypertension in 16 of 25 (64%), chronic kidney disease in 10 of 25 (40%), moderate-to-severe chronic obstructive pulmonary disease in 10 of 25 (40%) patients. At baseline, most of them (88%) had New York Heart Association (NYHA) class III or IV dyspnea. Four patients had a history of syncope while none had a history of angina. All patients underwent CT coronary angiogram. Conventional coronary angiogram was performed in 13 patients, who had coronary artery disease revealed by CT. One patient had left main disease, and 8 patients had LAD disease. Four patients underwent PCI of LAD before TAVR. Three were post-CABG patients, and 2 were post-mitral valve replacement patients. Two patients had significant peripheral vascular disease of right iliac and femoral arteries, which required the use of the left femoral route for valve introduction. Three had chronic liver disease. Baseline mean STS score was 13.8 ± 10.2, while the mean baseline EURO score was 17.54 ± 4.6. Baseline ejection fraction (EF) was found to be 50.3 ± 14.8, with 7 patients having severe left ventricular dysfunction. Baseline mean aortic valve (AV) gradient was 55.8 ± 24.7 mmHg. Five patients had low-flow, low-gradient AS. Dobutamine stress echo diagnosed 4 patients with severe AS and 1 with pseudosevere AS, who was excluded.
Table 1.
Baseline data.
| Gender distribution | Males: 17/25 (68%) Females: 8/25 (32%) |
| Mean age | 72 ± 8.1 years |
| Baseline angina | Nil |
| Baseline dyspnea | Grade II: 2 (8%) |
| Grade III: 8 (32%) | |
| Grade IV: 15 (60%) | |
| Comorbid conditions | Diabetes mellitus: 16/25 (64%) |
| Hypertension: 16/25 (64%) | |
| Chronic kidney disease: 10/25 (40%) | |
| Coronary artery disease: 13/25 (52%) | |
| COPD: 10/25 (40%) | |
| PVD: 2/25 (8%) | |
| CLD: 3/25 (12%) | |
| STS score | 13.8 ± 10.2 |
| EURO score | 17.54 ± 4.6 |
| PAH | Nil: 12 |
| Mild: 4 | |
| Moderate: 5 | |
| Severe: 4 | |
| Aortic regurgitation | Nil: 4 |
| Mild: 12 | |
| Moderate: 9 | |
| Severe: Nil | |
| Ejection fraction | <35%: 7 |
| 35–55%: 8 | |
| >55%: 10 | |
| Mean EF | 50.3 ± 14.8 |
| Mean aortic valve gradient | 55.8 ± 24.7 mmHg |
| Mean annulus perimeter | 72.6 ± 12.4 mm |
| Mean annulus area (perimeter derived) | 410.1 ± 121.8 mm |
| Mean annulus diameter (perimeter derived) | 25.4 ± 8.2 mm |
| Mean annulus diameter (area based) | 22.1 ± 5.2 mm |
| Previous history | CABG: 3 |
| MVR: 2 | |
| CVA: Nil |
Data are presented as mean ± SD or as number and percentage.
CABG, cardiac bypass graft; COPD, chronic obstructive pulmonary disease; CLD, chronic liver disease; CVA, cerebrovascular accident; EF, ejection fraction; EURO, European System for Cardiac Operative Risk Evaluation; MVR, mitral valve replacement; PAH, pulmonary arterial hypertension; PVD, peripheral vascular disease; STS, Society of Thoracic Surgeons.
3.2. Hospital stay and discharge
Procedural details are given in Table 2. There was one procedure-related death, where the patient with baseline severe left ventricular dysfunction developed asystole post-valve deployment with rapid pacing, he was revived and placed on extracorporeal membrane oxygenation support, but died 2 days later. One patient required urgent surgery for contained annular rupture, and one patient underwent vascular repair for femoral artery occlusion. Two patients required peripheral stent placement, and one required balloon dilatation for femoral artery occlusion. Four patients developed complete heart block (two in self-expandable valve and two in balloon expandable valve) requiring permanent pacemaker implantation. Two patients had acute kidney injury not requiring dialysis.
Table 2.
Procedural details.
| Anesthesia | General: 15 (60%) Conscious sedation: 10(40%) |
| Access route | Femoral: 25 (100%) |
| Use of transesophageal | 16/25 (64%) |
| Echocardiogram | |
| LV wire | Amplatz super/extrastiff wire: 17/25 (64%), |
| Confida wire: 6/25 (24%), | |
| Lunderquist wire: 2/25 (8%) | |
| Preprocedure vascular cutdown | Nil |
| Preprocedure proglide | 25/25 (100%) |
| Predilatation | Evolut R/Core valve: 5 |
| Sapien 3: 5 | |
| Valve type | Evolut R: 15 (60%) |
| SAPIEN 3: 7 (28%) | |
| Core valve: 2 (8%) | |
| Balloon-expandable study valve: 1 (4%) | |
| Valve size | 23 mm: 6/25 patients (24%) |
| 26 mm: 11/25 patients (44%) | |
| 29 mm: 8/25 patients (32%) | |
| Paravalvular leak | Severe PVL: Nil |
| Moderate PVL: 3 (12%) | |
| Mild PVL: 11 (44%) | |
| No PVL: 11 (44%) | |
| Postdilatation required | 3 (12%) |
| Immediate postprocedure mean LV ejection fraction | 55.4 ± 9.4% |
| Immediate postprocedure mean aortic valve gradient | 8.5 ± 4.9 mmHg |
| Procedural success | 25/25 (100%) |
Data are presented as mean ± SD or as number and percentage.
LV, left ventricle; PVL, paravalvular leak.
At discharge, majority of the patients were in NYHA class I to II status (80%), while five patients had class III dyspnea (Fig. 1). None of the patients had angina at discharge. Mean left ventricular ejection fraction (LVEF) at discharge was 55.4 ± 9.4%, while mean AV gradient was 8.5 ± 4.9 mmHg. Mild PVL was seen in 11 patients and moderate PVL in 3 patients. None had severe PVL. All patients were on dual antiplatelet therapy at discharge, except 3 who were on oral anticoagulation for atrial fibrillation.
Fig. 1.
Dyspnea grade during follow-up period as compared with the baseline. Postprocedure period there was significant improvement in dyspnea, which maintained on 1-year follow-up.
3.3. Follow-up
1 month: One-month follow-up was completed in all 24 patients (Table 3). None of the patients had any major adverse cardiac events (MACEs). All patients were in NYHA class I to II status. Mean LVEF was 55.4 ± 9.6%, while mean AV gradient was 8.8 ± 3.6 mmHg. Mild PVL was seen in 7 patients.
6 months: Six-month follow-up was completed in 19 patients. There were no MACEs during the 6-month follow-up. All the patients were in class I to II status. All except one patient showed significant improvement in EF with mean LVEF of 56 ± 8.9%, while the mean AV gradient was 8.3 ± 2.8 mmHg. Mild PVL was seen in 3 patients.
12 months: One-year follow-up was completed in 12 (50%) patients. The overall all-cause mortality during 1-year follow-up was 8%. There was 1 unrelated noncardiac cause of death due to dengue shock syndrome. All the patients were in NYHA class I to II status. Mean LVEF was 55 ± 9%, while the mean gradient AV was 8 ± 3.5 mmHg. None of the patients had PVL.
24 months: Two-year follow-up was completed in 3 patients. All 3 patients are in NYHA class I-II status with no morbidity or mortality. All had normal LV function with no significant increase in the mean gradient.
Table 3.
In-hospital, 1-month, 6-month, 1-year, and 2-year follow-up results.
| In-hospital complications and mortality | |
| Coronary occlusion | Nil |
| Annular rupture | 1/25 (4%) |
| Minor vascular complications | 4/25 (16%) |
| Femoral occlusion | 4/25 (16%) |
| Femoral artery stenting | 2/25 (8%) |
| Femoral balloon dilatation | 1/25 (4%) |
| Groin hematoma | 1/25 (4%) |
| Complication requiring surgical intervention | 2/25 (8%) |
| Conduction abnormality | 4/25 (16%) |
| Pacemaker implantation | 4/25 (16%) |
| Acute kidney injury (recovered) | 2/25 |
| Acute kidney injury requiring dialysis | Nil |
| Stroke or transient ischemic attack | Nil |
| Atrial fibrillation | 3/25 (12%) |
| Mortality | 1/25 (4%) |
| Median length of stay (LoS) | 6 days |
| Prolonged hospital stay more than 10 days | 2/25 (8%) |
| 30-day MACCE | Nil (n = 24) |
| 6-month MACCE | Nil (n = 19) |
| 1-year MACCE | Nil (n = 12) |
| 2-year MACCE | Nil (n = 3) |
| All-cause 1-year mortality (including cardiac and noncardiac causes) | 2/25 (8%) |
MACCEs, major adverse cardiac and cerebrovascular events.
4. Discussion
TAVR is a novel innovation and is a dawn of a new era in the field of minimal invasive procedure in the treatment of severe symptomatic AS. The experience in India about the procedure is limited with a very few centers which are equipped with expertise and personnel to carry out the TAVR procedure. The present study included 25 patients suffering from AS who underwent TAVR. Baseline characteristics showed that majority of patients were males with most having age more than 70 years and multiple comorbid conditions. Procedural success was (100%) with no major intraoperative and postoperative complications in most of the patients. The vascular complications were slightly higher in our series (16%); advancing a cross-over wire from the contralateral femoral artery and using a routine balloon inflation at the time of Perclose suture deployment have shown to significantly reduce the vascular complications to <3–4% in the transcatheter valve therapy registry. At baseline, 92% patients had severe dyspnea which showed significant improvement at discharge with 80% in class I to II status and remained so in subsequent follow ups (Fig. 1). LVEF and mean flow gradient showed significant improvement, and these improvements were maintained till the end of 1-year follow-up (Fig. 3, Fig. 4). Mild PVL was seen in 44% and moderate PVL in 12% of the patients at discharge. All PVL disappeared at the end of first year (Fig. 2). Study results varied based on the type of valve implanted with the self-expanding valve showing no effect of PVL on mortality, with nearly 80% of severe PVL reducing to mild or disappearing by 1 year.8 However, studies with balloon-expandable valves showing a significant increase in mortality on follow-up if the paravalvular regurgitation is more than mild.6 Post-TAVR conduction abnormalities resulting in permanent pacemaker implantation (PPI) vary from 4 to 6.0% for the Edwards SAPIEN4, 5, 6 to about 19–22% for the Medtronic Core Valve.6, 7 In our series, overall 16% of the patients required permanent pacemaker. However, the mortality rates at 2-year follow-up are not different between patients requiring PPI and patients not requiring PPI.7 Pacemaker requirement and residual PVLs after the procedure are higher than the reported in the current era and may be related to the initial experience of the performing team. In our study group, in-hospital and 1-month mortality was 4%, and one-year all-cause mortality was 8%. The initial studies with balloon-expandable valves conducted on high-risk patients showed a 1-month mortality of 3–5% at 1 month and 24–30% at 1 year.4, 5 Study using self-expanding valves on high-risk patients had shown 1-year mortality of 14%.8 In the intermediate-risk group, the 1-year mortality was 14% with balloon-expandable valves and 8.8% with the self-expanding valve.6, 7 The risk of stroke was about 2–4% in trials using both balloon- and self-expanding valves.4, 5, 6, 7 Also, data from the Western countries have limited information on the outcomes of TAVR performed on bicuspid aortic valves. In our series, nearly half (48%) of our TAVR cases had bicuspid aortic valves; however, because the overall numbers were less, there was no comparison. Our average length of stay had been 6 days, and two of our cases with SAPIEN 3 valve replacement were discharged the next day. TAVR is a less invasive option, provides early ambulation, has fewer complications, improves the quality of life, and results in durable outcomes. So far, the long-term data with TAVR are encouraging for patients completing the 5-year follow-up and beyond, with the risk of death similar to SAVR and no need for re-replacement of the valve.9 In India, in the current scenario, TAVR can be safely considered as the first-line therapy for patients with severe AS who are at high risk for surgery.
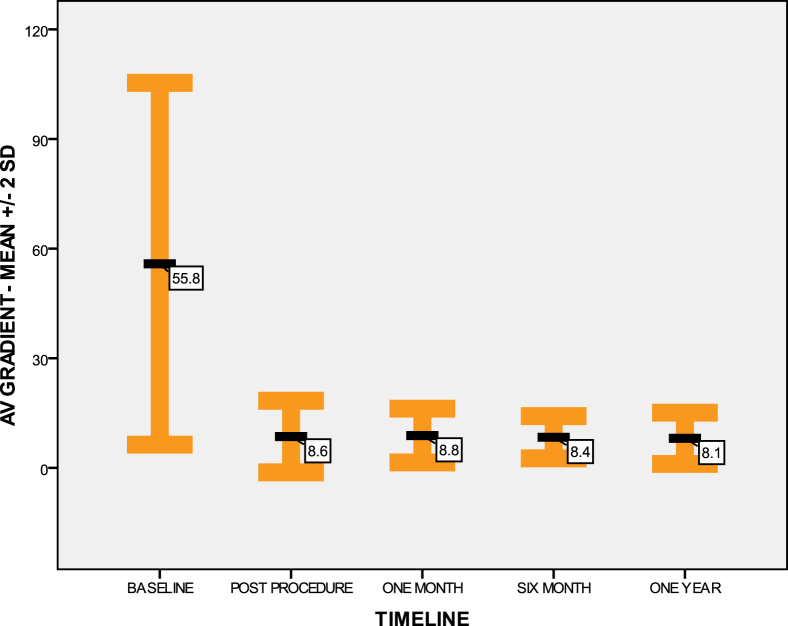
Fig. 3.
Aortic valve (AV) mean gradient ± 2 SD, at the baseline and during 1-year follow-up. Baseline mean gradient was 55.8 ± 24.7 mmHg. Immediate postprocedure mean gradient was 8.5 ± 4.9 mmHg. One-month mean gradient was 8.8 ± 3.6 mmHg. Six-month mean gradient was 8.3 ± 2.8 mmHg. One-year mean gradient was 8 ± 3.5 mmHg. SD, standard deviation.
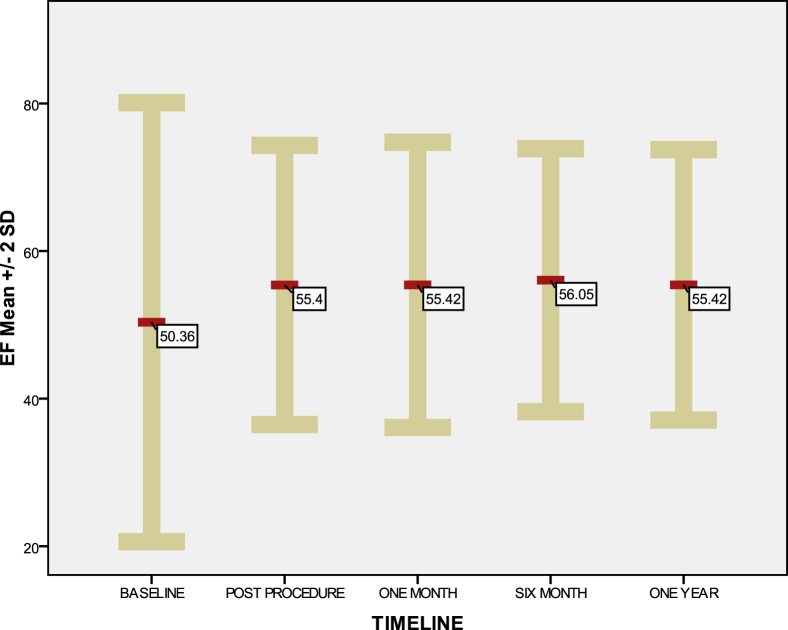
Fig. 4.
Mean ejection fraction (EF) ± 2 SD, at the baseline and during 1-year follow-up. Baseline mean EF was 50.3 ± 14.8%. Immediate postprocedure mean EF was 55.4 ± 9.6%. The 1-month, 6-month, and 1-year mean EF are almost equal. SD, standard deviation.
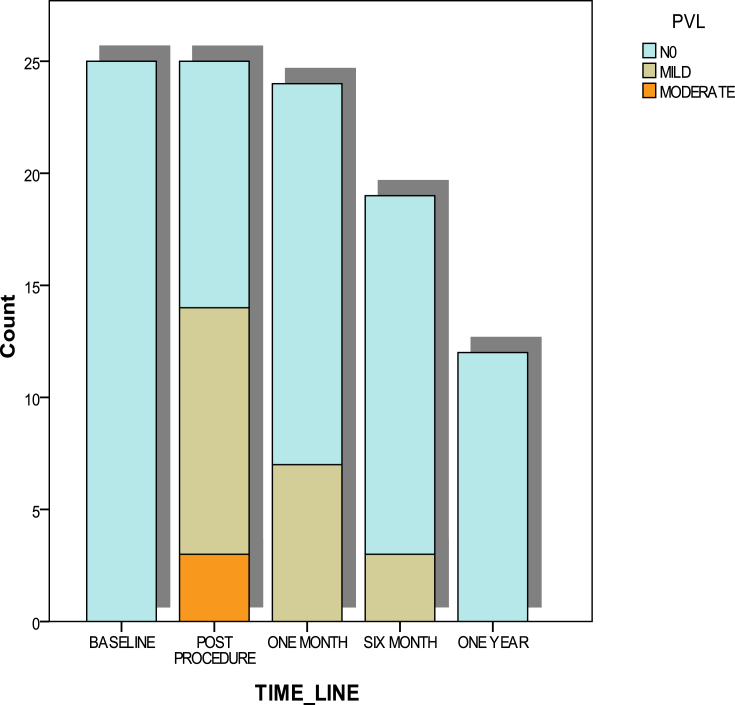
Fig. 2.
Paravalvular leak (PVL) grade during follow-up period as compared with the baseline. Immediate postprocedure moderate PVL was seen in 3 patients, and 11 patients had mild PVL. All PVL disappeared by the end of 1 year.
4.1. Economic challenges in India
In 2016, mean procedure costs in the United States were USD 69,592 for TAVR and USD 58,332 for SAVR.10 When compared with the cost in the United States, the cost of TAVR in India is almost half (USD 34,900), still TAVR costs roughly 6 times that of conventional SAVR, of which two-third of the costs are contributed by valve costs alone. In our series, 40% of patients did not undergo TAVR because of cost constrains. At current costs, it is certainly a mammoth task for our government to support such an expensive program for the entire country. This sets an uphill task for the clinicians to get the financing models for the patients to undergo TAVR as most of the insurance agencies in the country reimburse for the conventional procedures, and the balance pie has to be the out-of-pocket expenditure. An important concern has been the elderly subsets of patients wherein the TAVR costs and managing the comorbidities are high, which has been a matter of due diligence and a critical decision-making from the patient families to buy-in for TAVR. Technological advancements and procedural simplification, availability of locally manufactured devices with an advent of indigenous native Indian valves, and increase in volume of cases performed may ultimately reduce the overall cost of a TAVR procedure.
4.2. Regulatory challenges for TAVR program
Regulatory body approval was one of the major hurdles in India till early 2016, wherein the valves had to be imported under patient individual license. Ever growing data from TAVR trials on the benefits and effectiveness of the procedure resulted in the fast tracked approvals of the two major TAVR valves by the Indian regulatory authority by 2016.
4.3. Organizing heart team and training challenges for TAVR program
A successful procedure relies on multiple factors such as appropriate patient selection involving a multidisciplinary “heart team,” meticulous planning, and maintaining high standards of care during and after the procedure. All patients were assessed and thoroughly investigated by blood tests, echocardiography, and CT imaging before discussion in the heart team conference. Our heart team includes interventional cardiologists, imaging specialists, heart surgeons, and cardiac anesthesiologists. Neurologists are involved in the initial heart team discussion, if the patient had a history of previous stroke or any neurological disorder requiring assessment. Asian/Indian patients have several unique anatomical characteristics. Iliofemoral vessel diameter is likely to be smaller.11 Body size and aortic valve annulus tends to be smaller, predisposing to higher risk of annular rupture, residual gradients, and vascular complications.12, 13 Third, we have seen more bicuspid valves in India, and they are associated with unique challenges. The companies involved in the manufacture of prostheses have an organized training and proctoring program for TAVI teams, which is usually provided at one of the high-volume centers around the world. After a certain adequate number of proctored cases, we started performing TAVI independently without supervision.
4.4. Limitations of the study
The main limitation was the small sample size and also 1-year follow-up was completed only in 50% of the patients.
5. Conclusion
TAVR is a good alternative for symptomatic severe AS for high and intermediate surgical risk cases. TAVR in India has just commenced, and quaternary care hospitals with their strong heart team and TAVR program have embarked on these newer procedures. As data from large-scale studies become available, TAVR may soon replace the conventional SAVR for high-risk AVR cases in the next few years once costs are scaled down from 50% to 70% of the current scenario. During initial years of implementation of a nationwide TAVI program, it may be prudent to collate the outcomes of large volume centers performing TAVR and focus on creating TAVR centers of excellence. With the burgeoning growth of TAVR in India, scientific collaborations with different advocacy groups should be created for developing a successful hub and spoke model. However, financial implications, nonavailability of expertise, and lack of awareness remain the major challenges in India.
Conflicts of interest
All authors have none to declare.
References
- 1.Manjunath C.N., Srinivas P., Ravindranath K.S., Dhanalakshmi C. Incidence and patterns of valvular heart disease in a tertiary care high-volume cardiac centre: a single center experience. IHJ. 2014;66(3):320–326. doi: 10.1016/j.ihj.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osnabrugge R.L.J., Mylotte D., Head S.J. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modelling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Vahanian A., Alfieri O., Andreotti F. Guidelines on the management of valvular heart disease (version 2012): the joint task force on the management of valvular heart disease of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS) Eur J Cardio Thorac Surg. 2012;42:S1–S44. doi: 10.1093/ejcts/ezs455. [DOI] [PubMed] [Google Scholar]
- 4.Leon M.B., Smith C.R., Mack M., Miller D.C., Moses J.W. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 5.Smith C.R., Leon Martin B., Mack Michael J., Craig Miller D., Moses Jeffrey W. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 6.Leon M.B., Smith C.R., Mack M.J. Transcatheter or surgical aortic valve replacement in intermediate- risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 7.Reardon M.J., Van Mieghem N.M., Popma J.J. Surgical or transcatheter aortic valve placement in intermediate risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 8.Adams D.H., Popma J.J., Reardon M.J. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 9.Mack M.J., Leon M.B., Smith C.R. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (partner 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi: 10.1016/S0140-6736(15)60308-7. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds M.R., Lei Y., Wang K. Cost-effectiveness of transcatheter aortic valve replacement with a self-expanding prosthesis versus surgical aortic valve replacement. J Am Coll Cardiol. 2016;67(1):29–38. doi: 10.1016/j.jacc.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiam P.T., Koh A.S., Ewe S.H. Iliofemoral anatomy among Asians: implications for transcatheter aortic valve implantation. Int J Cardiol. 2013;167(4):1373–1379. doi: 10.1016/j.ijcard.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe Y., Hayashida K., Takayama M. First direct comparison of clinical outcomes between European and Asian cohorts in transcatheter aortic valve implantation: the Massy study group vs. the prevail Japan trial. J Cardiol. 2015;65:112–116. doi: 10.1016/j.jjcc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran H.S.R., Seshayyan S., Victor A., Rajapandian G. Aortic valve annular dimension in indian population. J Clin Diagn Res. 2013;7(9):1842–1845. doi: 10.7860/JCDR/2013/5776.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]