Abstract
The present research investigated the response of silver maple (Acer saccharinum L.) to salt treatment. The short- and long-term effects of NaCl and CaCl2 treatments on plant fitness characteristics (growth parameters, leaf chlorophyll content) and biochemical stress-coping mechanisms (proline accumulation as well as enzymatic activities) were examined. We found that the silver maple response to salt stress strictly depended on salt type and dose—calcium chloride was less toxic than sodium chloride, but high concentrations of both salts negatively influenced plant growth. The accumulation of proline, slight changes in the activity of superoxide dismutase and marked changes in catalase and peroxidase activities in the roots and leaves indicated complexity of the plant response. It was also shown that after one year, enzymatic parameters were restabilized, which indicates plant recovery, but the reduced mass of seedlings suggests that one year is not enough to cope with the prolonged cyclic salt stress, both resulting from NaCl and CaCl2 application. Therefore, seedlings of silver maple should be considered as moderately susceptible to salinity. Hence, it is recommended to use silver maple on non-de-iced urban areas, while planting on often de-iced roads should be avoided.
Keywords: Acer saccharinum L., Sodium chloride, Calcium chloride, Salt stress, Plant growth, Proline, Antioxidant enzymes
Introduction
In urban areas, each winter, various chemical and abrasive materials are used on roads and sidewalks to prevent ice formation. The two most commonly used de-icing salts worldwide are sodium (Na+) chloride (NaCl) and calcium (Ca2+) chloride (CaCl2). Although CaCl2 is better for melting ice (Nixon, 2008) and less damaging to plants (Trajkova, Papadantonakis & Savvas, 2006), NaCl is used most extensively (c.a. 9–10 million tons per year compared to 0.3 million tons of calcium chloride—Ramakrishna & Viraraghavan, 2005) because it is less expensive and easier to handle (Nixon, 2008). The commonly used salts are dispensed directly or mixed with sand before they are applied to the road (Simini & Leone, 1986).
Salinity of soils located near de-iced roads changes with the distance from road margin. For example, it was indicated that Na+ concentration within five meters from road margin remains at constant concentration (101–154 mg kg−1) and then drastically decreased within next five meters (Bryson & Barker, 2002). The maximal salt accumulation zone is located about one meter from road margin where trees are often planted in urban environment (Cunningham et al., 2008). It is also worth noticing that salt accumulation depends on abiotic factors (e.g., soil properties, landscape, weather) as well as biotic factors (e.g., local vegetation) (Cunningham et al., 2008) and may show strong variation (Bryson & Barker, 2002; Cunningham et al., 2008). Therefore, in many cases, accurate estimation of salt concentration within the nearest area from de-iced roads is hard, but severe accumulation of salts in non-permeable soils is highly probable due to winter maintenance of roads. Although the application of salts is necessary for traffic safety, it can cause damage to adjacent roadside trees and shrubs. Several de-icing investigations have attributed roadside plant damage to the combination of aerial spray of road salts, direct foliar contact with salt ions and high soil salt concentrations (Davison, 1971; Dirr, 1976; Hofstra, Hall & Lumis, 1979; Czerniawska-Kusza, Kusza & Duzyński, 2004; Gałuszka et al., 2011; Douglas, 2011). It is established that at least along major highways, salt spray causes more damage to trees and shrubs than salt absorbed from the soil (Dirr, 1976; Hofstra, Hall & Lumis, 1979; Sucoff, Hong & Wood, 1976; Langille, 1976). Salt spray injury is more commonly observed in evergreen coniferous tree species, while soil uptake injury is more common in deciduous trees (Hofstra, Hall & Lumis, 1979; Mekdaschi et al., 1988; Kozłowski, 1997; Bryson & Barker, 2002). Salts affect plant growth in several ways. De-icing salts cause damage through direct contact of the salt solution with plant foliage (referred to as “spray zone” injury) and through chemical and physical modification of the soil as a result of salt accumulation (Douglas, 2011). Dissolved salt ions originating from chemical de-icers (e.g., Na+, Cl−) can cause osmotic stress in plants (Paul, Rocher & Impens, 1987). High concentrations of inorganic chloride salts in the soil make cations (such as potassium, calcium, and magnesium) unavailable to plant roots (White & Broadley, 2001; Tester & Davenport, 2003). Furthermore, the accumulation of specific ions can cause toxicity within plant cells (Levitt, 1980; White & Broadley, 2001; Raveh & Levy, 2005) and reduce both frost hardiness (Sucoff, Hong & Wood, 1976) and drought tolerance (Maas, 1985).
Salinity is a stress factor enhancing the production of reactive oxygen species (ROS) which can lead to oxidative damage in plant cells. Therefore, robust metabolism of ROS is believed to protect plant tissues from injuries under salt conditions as well as during other abiotic stresses (e.g., drought and light stress) (Miller et al., 2010). On the other hand, tuned ROS balance play role in signal transduction pathways and are among factors activating plant responses to environmental stimuli (Miller et al., 2010). This can be modulated and fine-tuned by enzymatic antioxidants, such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD). Increasing SOD activity was shown to be involved in salt stress tolerance in herbaceous (reviewed by Gill & Tuteja, 2010) and woody plant species (Wang et al., 2010). A similar conclusion could be drawn with regard to studies on the involvement of CAT and POD in the response of Populus cathayana Rehder to salt stress (Yang et al., 2009). The accumulation of osmolytes is also one of the salt-induced stress coping mechanisms. During NaCl stress, Na+ ions are stored in vacuoles, while potassium ions (K+) and compatible chemicals (such as proline, sucrose, glycine, betaine, mannitol) are accumulated in the cytosol and other organelles to balance the osmotic pressure of ions in the vacuoles (Wang et al., 2005; Lee et al., 2007; Székely et al., 2008). Similar changes can be observed during cold (Naidu et al., 1991), drought (Choudhary, Sairam & Tyagi, 2005), oxidative (Yang, Lan & Gong, 2009) and heavy metal (Siripornadulsil et al., 2002) stress. Proline is probably the most widely distributed osmolyte, and it occurs not only in plants but also in many other organisms (Delauney & Verma, 1993). In addition to regulating osmotic pressure, proline is implicated in plant tissue defense against osmotic stress and in the protection of plasma membrane integrity (Mansour, 1998) or as a source of carbon and nitrogen (Peng, Lu & Verma, 1997; Soshinkova et al., 2013).
Silver maple (Acer saccharinum L.) is a common, floodplain deciduous tree originating from North America that is adapted to saturated soils and flooding (Saeki et al., 2011). It is also common and widely cultivated in the northern temperate zone (Day, Seiler & Persaud, 2000). In many countries, including Poland (where silver maple is not protected species), this tree species is planted in street settings, parks, and residential and commercial landscapes. Its widespread popularity results from its rapid growth and attractive appearance—leaves are gently-lobed, deeply dissected and glaucous silvery-white on abaxial side (Saeki et al., 2011). From a practical point of view, appropriate tree selection should be based on information about the relationship between tree growth and stress-coping mechanisms that pertain to the urban environment, such as salt stress, soil compaction and heat island effect. Although some studies have suggested that silver maple is a plant sensitive to salinity (Buschbom, 1968; Dmuchowski, Baczewska & Bra̧goszewska, 2011; Dmuchowski, Brogowski & Baczewska, 2011), the available information about the salinity tolerance level of silver maple at different stages of growth is scarce, e.g., the salinity tolerance of silver maple seedlings is not well known. Furthermore, studies on the role of antioxidant enzymes during salt stress and on the plant growth parameters accompanying it allow us to estimate range and amplitude of response to salinity in this woody species. Therefore, the aim of this investigation was to determine the effects of de-icing salts on the silver maple at four time-steps (14 d, 28 d, 180 d and 360 d). We also assayed how different salinity levels altered the biochemical stress-coping mechanism and changed the fitness of this widely used plant.
Material and Methods
Seed material
Mature seeds were collected from 10 randomly selected silver maple (Acer saccharinum L.) trees (c.a. 10% of total seed pool were gathered from single mother tree) in Lodz, central Poland (19°20′N/19°38′E), on 30 June 2013. The local climate is temperate, and the seasons are clearly differentiated. Meteorological data (Lodz Meteorological Station) based on a 10-year period (2000–2010) indicated that the mean annual temperature was 8.8 °C. The average low temperature during winter was ≤2.5 °C, and the average high temperature during summer was 22.4 °C. Annual total precipitation (rain and snow) was 587.2 mm. The frost-free period averages 271 d (days).
Seed germination
The silver maple seeds were mixed before tests in order to fulfill the randomization requirement. The seeds were surface sterilized with 70% (v/v) ethanol for 1 min and with 10% (v/v) sodium hydrochloride for 20 min. Then, the sterilized seeds were soaked for 4 h (hours) in sterile distilled water. The seeds were germinated on trays (100 seeds per tray) containing wet perlite (previously tested not to release salt) at 25 °C. All used seeds were viable and the final germination rate was above 75%.
Growth conditions
After a week, seedlings of similar size were individually transplanted into 500-cm3 pots filled with perlite. The pots were watered to saturation twice daily with Hoagland’s solution (half strength) (Hoagland & Arnon, 1950). The plants were grown in a growth chamber with a 16:8 h photoperiod and a light intensity of 900–1,200 µmol m−2 s−1. Relative humidity was maintained between 60% and 70% and thermoperiod of 25 °C/18 °C (day/night). The pots of replicate treatments (see below) were rotated periodically within the chamber rooms to reduce the effects of possible temperature and/or light variation.
Plant treatments
After 2 months, the seedlings were divided into 12 groups. Each group was treated with one solution of NaCl (0, 10, 30, 60, 100 and 120 mM) or CaCl2 (0, 6.7, 20, 40, 66 and 80 mM). The control groups (0 NaCl and a 0 CaCl2) were not treated with any salt solution. The plants were treated three times at two-week intervals with 25 cm3 of the appropriate salt solutions. To prevent the accumulation of salts in perlite, distilled water was applied every three days. The concentrations of NaCl and CaCl2 were chosen to achieve the same concentration of dissociated ions in the corresponding solutions (10 mM NaCl and the corresponding 6.7 mM CaCl2, 120 mM NaCl and 80 mM corresponding CaCl2). NaCl and CaCl2 are used in Poland both is solid (10–30 g m−2 of NaCl) and dissolved (40–160 cm3 m−2 of 25% NaCl or 15/30% CaCl2 solution) form (Czarna, 2013). The concentrations of salt solutions used in this study were chosen to simulate distribution of road salt residues which depends on distance from salted surfaces (the maximal accumulation of salt can be observed in area located nearest to the road margin).
Then, 14 and 28 d after treatment (three doses of salt solution), leaves and roots were collected for the analysis of enzymatic and nonenzymatic parameters. These parameters were analyzed again 360 d after treatment. In addition, seedling growth was measured 180 and 360 d after treatment. For each treatment in each time point, four plants were subjected to biochemical analysis and another four plants were subjected to analysis of growth parameters.
Growth parameters
Fresh (FW) and dry (DW) weights of the plant shoots and roots were measured at 180 and 360 d. To determine dry mass, the material was dried for 48 h in a forced-draft oven at 60 °C. To determine the relative growth rate (RGR), plants were harvested immediately prior to the beginning of the salt treatments. Thereafter, successive harvests were taken at 180 and 360 d. The relative growth rate (RGR) was calculated using the following formula: RGR = (ln mass2 * ln mass1/time) (Khan, Unagr & Showater, 2000). The dry mass of each harvest was used for calculations. RGRs were expressed in g g−1 FW d−1.
Biochemical analysis
Chlorophyll a and b contents in the fresh leaf samples were measured using the method of Arnon (1949) and Ashraf et al. (1994). Pigment concentrations were calculated from the absorbance of extract at 663 nm (A663) and 645 nm (A656) using the following formula:
Chlorophyll a (mg g−1 FW) = [12.7 * (A663) − 2.69 * (A645)] * 0.5
Chlorophyll b (mg g−1 FW) = [22.9 * (A645) − 4.69 * (A663)] * 0.5
The content of photosynthetic pigments was expressed in mg g−1 FW.
Free proline accumulation was determined using the method of Bates, Waldren & Teare (1973). The content of free proline was expressed in mg g−1 FW. For enzymatic analysis, the leaf fragments were homogenized (1:10 ratio) in 0.05 phosphate buffer (pH = 7.0) containing 1mM EDTA and 1% soluble PVP (polyvinylpyrrolidone). Then, the homogenates were centrifuged at 15,000 rpm for 15 min at 4 °C. The resulting supernatants were immediately used to analyse protein content and enzymatic activity. Superoxide dismutase (SOD, EC 1.15.1.1) activity was determined spectrophotometrically using the assay of Beauchamp & Fridovich (1971). The absorbance was measured 10 min after starting at a wavelength of 560 nm. The total reaction mixture of 2.7 cm3 contained 50 mM potassium phosphate buffer pH 7.8, 13 mM methionine, 75 µM NBT, 2 µM riboflavin, 0.1 mM EDTA and the enzyme extract. The reaction was started by turning on the UV lamp. The enzyme activity was expressed in units, each representing the amount of the enzyme required to inhibit 50% of the photochemical reduction of NBT, min−1 mg−1 protein. Catalase (CAT, EC 1.11.1.6) activity was determined spectrophotometrically using the assay of Dhindsa, Plumb-Dhindsa & Thorpe (1981). Absorbance was measured at a wavelength of 240 nm (ε = 36.1 mM−1 cm−1). The total reaction mixture of 2 cm3 contained 50 mM potassium phosphate buffer pH 7.0, 15 mM H2O2 and the enzyme extract. The enzyme activity was expressed in units, each representing 1 mM H2O2 decomposed, min−1 mg−1 protein. Peroxidase (POD, EC 1.11.1.7) activity was determined spectrophotometrically using the assay of Maehly & Chance (1954). Absorbance was measured at a wavelength of 470 nm (ε = 26.6 mM−1 cm−1). The total reaction mixture of 2 cm3 contained 25 mM acetate buffer pH 5.6, 5 mM guaiacol, 15 mM H2O2 and the enzyme extract. The enzyme activity was expressed in units, each representing 1 µmol tetraguaiacol formed, min−1 mg−1 protein. Protein content was assayed by the Bradford method (1976) using bovine serum albumin as a standard.
Statistical analysis
Measurements at each time point were obtained from four independent replicates. The data for all statistical tests were log10 transformed to meet the assumptions of normality and homogeneity of variances implicit in parametric statistical procedures. The data were analyzed by one-, two- or three-way ANOVA. When significant differences were found among means, Tukey’s multiple comparison post hoc test (HSD-test) after one-way ANOVA was carried out to determine if significant (P < 0.05) differences occurred between individual treatments. Statistical analysis was carried out using Statistica 10 PL.
Ethics statement
The plant material (seeds of Acer saccharinum L.) was collected in Lodz. The area of Lodz city reported in this paper is controlled by the government of the Poland and is not privately owned, nor protected. The species studied here is not yet a protected species in Poland and permit for collection of seeds was not required (Poland Ministry of the Environment, 2013; Poland Ministry of the Environment, 2014). Furthermore, neither local population size nor population fitness was affected.
Results
Growth Parameters
In general, both salt types caused a reduction in the fresh weight of the seedlings; however, a greater reduction in the shoot and root fresh weight occurred after NaCl treatments (all corresponding NaCl vs CaCl2 comparisons in each time point were significant at P < 0.001). The total dry weight accumulation was not significantly inhibited at low salinities (<30 mM)—this parameter was strongly inhibited at concentrations >60 mM of NaCl and >40 mM of CaCl2 (Table 1). On the other hand, weight of seedlings showed substantial promotion at low salinity caused by CaCl2 (6.7 and 20 mM for both fresh and weight dry weight) after the first harvest (Table 1). Further increasing salinity caused a progressive decline in weight. Analysis of variance of the salt-treated plants indicated a greater reduction of the fresh weight of roots than of shoots. In the contrary, dry weight of shoots were much more affected by increasing salt concentration than dry weight of roots. (Table 1). Only dry weight of roots were not significantly affected by interaction of all studied factors, while other weight parameters were strongly affected by the salt type, dose and time as well as their interaction (Table S2).
Table 1. The fresh and dry weight of the roots and shoots from the silver maple seedlings exposed to different salt type (NaCl or CaCl2) and dose after 180 and 360 d.
| Salinity (mM) | Fresh weight (mg plant−1) | Dry weight (mg plant−1) | ||||
|---|---|---|---|---|---|---|
| root | shoot | total | root | shoot | total | |
| After 180 d | ||||||
| 0 NaCl | 480.6 ± 12.91 | 310.2 ± 10.91 | 790.8 ± 12.01 | 62.7 ± 1.81 | 214.5 ± 8.91 | 277.2 ± 8.41 |
| 10 NaCl | 467.0 ± 8.91 | 305.7 ± 5.31 | 772.7 ± 21.61 | 60.3 ± 5.41 | 227.0 ± 11.31 | 287.3 ± 5.71 |
| 30 NaCl | 322.4 ± 5.52 | 258.4 ± 9.82 | 580.8 ± 16.62 | 56.1 ± 4.712 | 188.9 ± 6.82 | 245.0 ± 8.12 |
| 60 NaCl | 309.1 ± 7.62 | 242.7 ± 7.623 | 551.8 ± 12.82 | 55.9 ± 8.912 | 155.0 ± 4.63 | 210.9 ± 4.23 |
| 100 NaCl | 244.5 ± 12.23 | 225.7 ± 12.13 | 470.2 ± 8.93 | 51.5 ± 2.812 | 123.2 ± 5.24 | 174.7 ± 3.34 |
| 120 NaCl | 217.0 ± 15.04 | 197.0 ± 5.84 | 414.0 ± 9.84 | 46.0 ± 6.32 | 100.7 ± 6.15 | 146.7 ± 4.15 |
| 0 CaCl2 | 536.5 ± 4.83 | 414.1 ± 13.52 | 950.8 ± 12.82 | 87.2 ± 5.22 | 272.4 ± 6.31 | 359.6 ± 5.72 |
| 6.7 CaCl2 | 565.9 ± 6.52 | 416.5 ± 6.52 | 982.4 ± 21.82 | 103.1 ± 5.71 | 280.5 ± 7.51 | 383.6 ± 6.31 |
| 20 CaCl2 | 596.3 ± 7.41 | 447.2 ± 7.91 | 1043.5 ± 23.21 | 108.9 ± 2.81 | 236.6 ± 9.52 | 345.5 ± 8.72 |
| 40 CaCl2 | 499.0 ± 12.24 | 385.3 ± 11.23 | 884.3 ± 14.23 | 81.6 ± 7.523 | 197.0 ± 6.83 | 278.6 ± 7.63 |
| 66 CaCl2 | 457.7 ± 8.65 | 358.6 ± 8.14 | 816.3 ± 15.94 | 72.5 ± 4.63 | 152.7 ± 6.34 | 225.2 ± 3.54 |
| 80 CaCl2 | 412.5 ± 8.66 | 336.7 ± 6.85 | 749.2 ± 11.65 | 59.6 ± 4.64 | 134.8 ± 5.75 | 194.4 ± 4.25 |
| After 360 d | ||||||
| 0 NaCl | 553.8 ± 12.91 | 670.9 ± 10.42 | 1224.7 ± 21.62 | 86.4 ± 4.31 | 407.1 ± 6.52 | 493.5 ± 9.52 |
| 10 NaCl | 573.2 ± 8.91 | 771.4 ± 27.31 | 1344.6 ± 25.21 | 81.5 ± 5.612 | 448.0 ± 6.31 | 529.5 ± 6.81 |
| 30 NaCl | 412.6 ± 5.52 | 607.5 ± 18.23 | 1020.1 ± 19.73 | 70.8 ± 7.12 | 344.5 ± 11.83 | 415.3 ± 5.63 |
| 60 NaCl | 379.5 ± 7.63 | 463.1 ± 14.94 | 842.6 ± 12.54 | 69.0 ± 5.423 | 229.1 ± 5.94 | 298.1 ± 4.34 |
| 100 NaCl | 276.3 ± 12.24 | 398.3 ± 17.55 | 674.6 ± 10.65 | 58.0 ± 6.634 | 145.5 ± 8.05 | 203.5 ± 5.25 |
| 120 NaCl | 243.2 ± 15.05 | 394.6 ± 13.25 | 637.8 ± 9.86 | 49.0 ± 4.84 | 122.1 ± 8.96 | 171.1 ± 3.46 |
| 0 CaCl2 | 536.7 ± 4.84 | 634.0 ± 45.83 | 1170.7 ± 27.13 | 124.7 ± 5.93 | 580.8 ± 17.91 | 705.5 ± 8.92 |
| 6.7 CaCl2 | 865.9 ± 6.51 | 1383.4 ± 23.21 | 2249.3 ± 21.51 | 140.9 ± 6.22 | 615.5 ± 15.91 | 756.4 ± 9.61 |
| 20 CaCl2 | 652.2 ± 7.42 | 897.0 ± 15.32 | 1549.2 ± 17.42 | 172.9 ± 7.11 | 530.0 ± 17.52 | 702.9 ± 7.62 |
| 40 CaCl2 | 588.0 ± 12.23 | 596.5 ± 14.93 | 1184.5 ± 12.63 | 109.4 ± 6.44 | 346.0 ± 12.03 | 455.4 ± 6.43 |
| 66 CaCl2 | 477.2 ± 8.65 | 525.5 ± 21.14 | 1002.7 ± 12.54 | 92.4 ± 4.35 | 240.1 ± 3.94 | 332.5 ± 5.34 |
| 80 CaCl2 | 465.0 ± 14.45 | 473.2 ± 10.45 | 938.2 ± 8.95 | 73.5 ± 3.86 | 213.5 ± 5.95 | 287.0 ± 3.85 |
Notes.
Values are mean ± SD (n = 4). Values in each column with the same number are not significantly different at P < 0.05, Tukey’s multiple test.
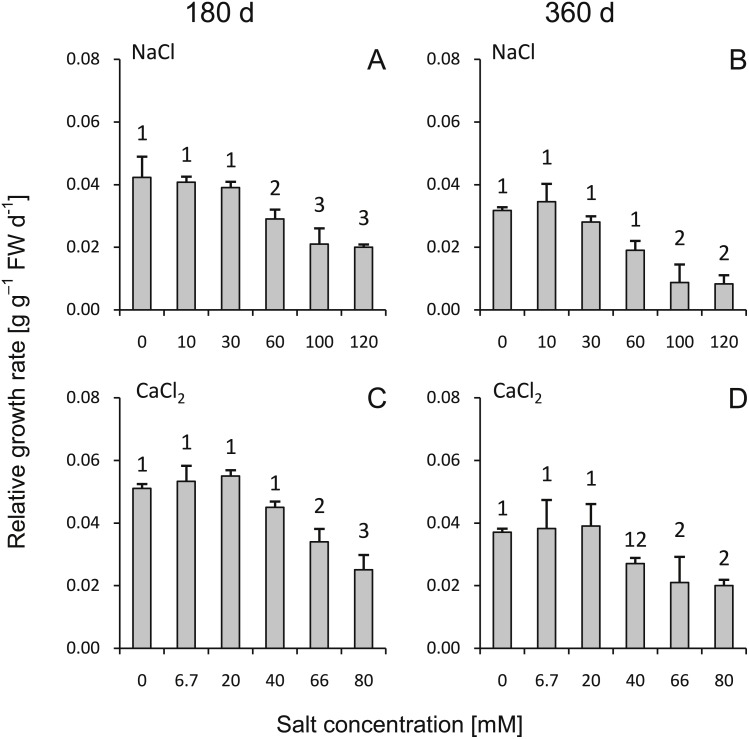
During the 180- to 360-d periods, the highest RGR was observed in the groups treated with low salinity (both NaCl and CaCl2, <30 mM and <20 mM, respectively) as well as with medium salt dose (60 mM NaCl and 40 mM CaCl2) (Fig. 1). The highest salinity substantially inhibited seedlings growth. Higher growth rates were usually observed during the 0- to 180-d period. Non saline controls and the groups subjected to the low salinity treatments were not significantly different from each other. Leaves of the plants from both NaCl and CaCl2 treatments (except control groups—0 mM NaCl and 0 mM CaCl2) developed a reddish color and became more brittle and dry; however, this was more prevalent for the NaCl-treated plants.
Figure 1. Effects of saline stress on the relative growth rate (RGR) of the silver maple seedlings exposed to different salinity levels (NaCl or CaCl2) after 180 (A, C) and 360 (B, D) days (g g−1 FW d−1).
Values are mean ± SD (n = 4). Different numbers above each bar indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
Photosynthetic pigments
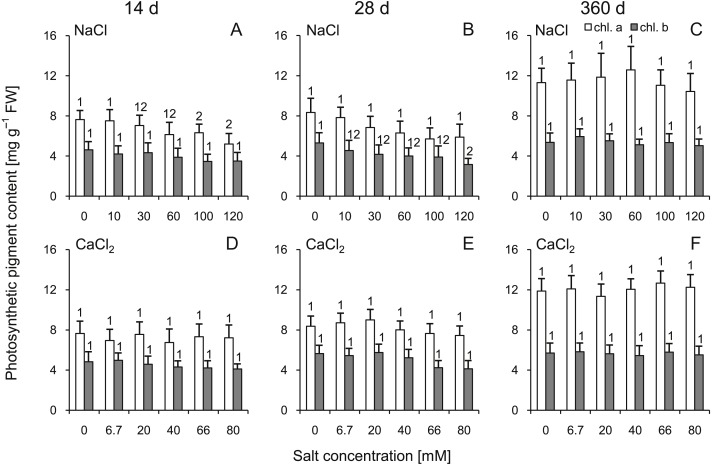
Comparing to control groups, reduced concentrations of chlorophyll a were observed under the influence of solutions >100 mM NaCl at 14 d (Fig. 2). Significant reduction of chlorophyll b concentration was observed only at 28 d in group treated with 120 mM NaCl. CaCl2 solutions did not trigger any significant changes of chlorophyll a and b concentration in any group. At 360 d, the chlorophyll a and b contents were similar in all groups (no significant differences were observed for all corresponding NaCl vs CaCl2 comparisons; ANOVA with Tukey’s post-hoc test). Interestingly, this parameter were affected by all individual factors, while their interaction were not significant (Table S3).
Figure 2. Effects of saline stress on the content of photosynthetic pigments (chlorophyll a and b) in the leaves from the silver maple seedlings exposed to different salinity levels (NaCl or CaCl2) after 14 (A, D), 28 (B, E) and 360 (C, F) days.
Values are mean ± SD (n = 4). Different numbers above each bar indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
Proline content
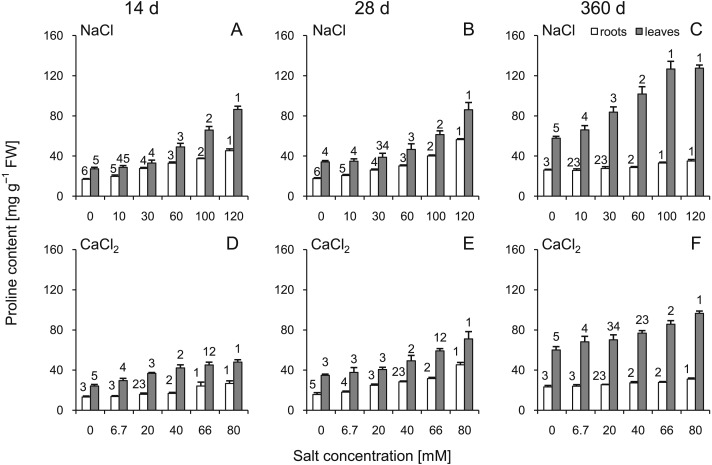
The proline content was significantly greater in the roots and leaves under all stress conditions (Fig. 3). The highest levels of proline compared to the control plants were observed at 360 d. In addition, greater changes were observed after treatment with high concentrations of NaCl than with CaCl2 (significant changes >60 mM NaCl in leaves and 100 mM in roots; ANOVA with Tukey’s post-hoc test). Both leaf and root proline amount were dependent of all tested factors and all their interactions (Tables S3 and S4).
Figure 3. Effects of saline stress on the content of proline in the roots and leaves from the silver maple seedlings exposed to different salinity levels (NaCl or CaCl2) after 14 (A, D), 28 (B, E) and 360 (C, F) days.
Values are mean ± SD (n = 4). Different numbers above each bar indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
Superoxide dismutase activity
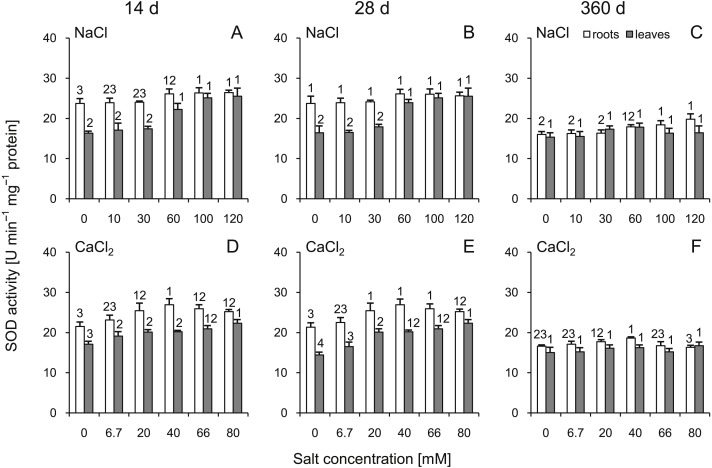
A statistically significant increase in SOD activity was demonstrated in the roots and leaves at 14 d after treatment with NaCl as well as at 14 and 28 d after treatment with CaCl2 (Fig. 4). It is noteworthy that after 360 d of treatment, SOD activity in most cases did not differ from the control plants. The slight increase was recorded only in the roots of plants treated with 100 and 120 mM NaCl. Comparing corresponding salt treatments, some differences were observed in leaves (100 mM NaCl vs 66 mM CaCl2 at 14 d; 60 mM NaCl vs 40 mM CaCl2 and 100 mM NaCl vs 66 mM CaCl2 at 28 d) as well as in roots (only 120 mM NaCl vs 80 mM CaCl2 at 360). A three-way ANOVA showed significant influence of all factors, except interaction of time and salt treatment on activity of this enzyme in roots (Tables S3 and S4).
Figure 4. Effects of saline stress on the superoxide dismutase (SOD) activities in the roots and leaves from the silver maple seedlings exposed to different salinity levels (NaCl or CaCl2) after 14 (A, D), 28 (B, E) and 360 (C, F) days.
Values are mean ± SD (n = 4). Different numbers above each bar indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
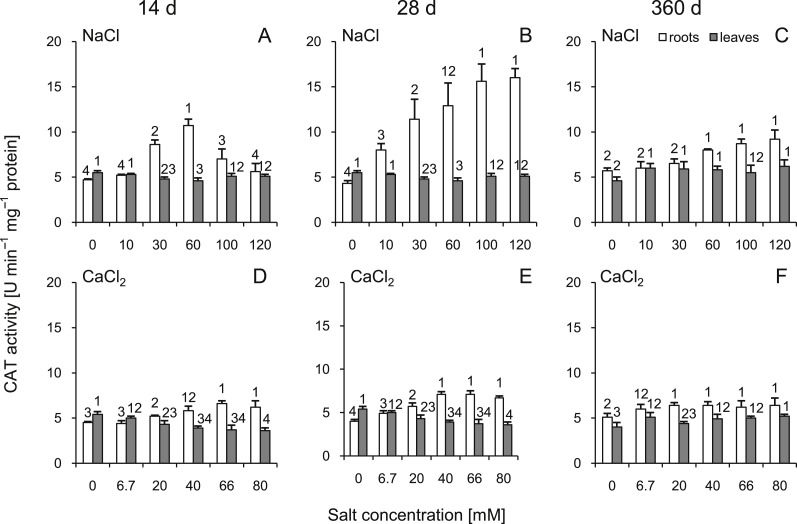
Catalase activity
After 14 and 28 d, CAT activity significantly increased in the roots of almost all salt-treated plants (except 10 and 120 mM of NaCl at 14 d and 6.7 mM of CaCl2 at 14 and 28 d) (Fig. 5). At 360 d, this parameter was slightly but statistically significantly higher in both treatment variants. In the leaves, at 14 and 28 d after NaCl treatment, CAT activity decreased in groups treated with solutions of 30–60 mM. Significant differences were also observed in CaCl2 treated plants and those changes intensified with increasing salt concentration. At 360 d, slight but significant changes were observed. Interestingly, when compared to 14 and 28 d, the trend of changes reversed at 360 d (Fig. 5). Comparing corresponding salt treatments, differences were observed in roots (30 mM NaCl vs 20 mM CaCl2 and 60 mM NaCl vs 20 mM CaCl2 at 14 d; all comparisons at 28 d and 100 mM NaCl vs 66 mM CaCl2 and 120 mM NaCl vs 80 mM CaCl2 at 360 d) as well as in leaves (100 mM NaCl vs 66 mM CaCl2 and 120 mM NaCl vs 80 mM CaCl2 at 14 and 28 d and 30 mM NaCl vs 20 mM CaCl2 at 360 d). All factors showed significant influence on activity of CAT, except time and salt type interaction in leaves (three-way ANOVA) (Tables S3 and S4).
Figure 5. Effects of saline stress on the catalase (CAT) activities in the roots and leaves from the silver maple seedlings exposed to different salinity levels (NaCl or CaCl2) after 14 (A, D), 28 (B, E) and 360 (C, F) days.
Values are mean ± SD (n = 4). Different numbers above each bar indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
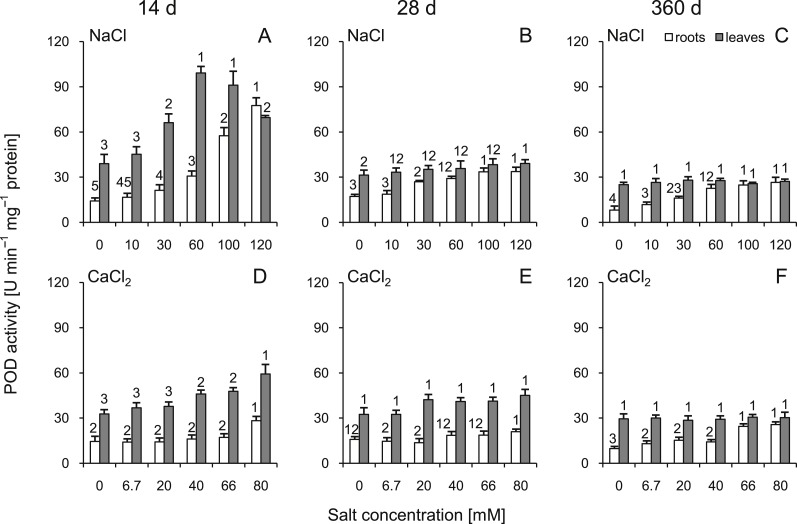
Peroxidase activity
A significant increase in POD activity was observed in the roots throughout the experiment. The highest POD activity (approximately five times greater than the control) was observed at 14 d in the plants treated with 120 mM NaCl solution (Fig. 6). In the leaves, POD activity increased with the increasing salt concentration at 14 and 28 d; however, greater changes were observed at 14 d. At 360 d after treatment, no significant differences in leaves were observed (Fig. 6). Comparing corresponding salt treatments, differences were observed leaves (all comparisons except control groups and the highest dose at 14 d) as well as in roots (30, 60, 100 mM NaCl vs 20, 40, 66 mM CaCl2, respectively, at 14 d, 30, 60, 100, 120 mM NaCl vs 20, 40, 66, 80 mM CaCl2, respectively, at 28 d, 30, 60, 120 mM NaCl vs 20, 40, 80 mM CaCl2, respectively, at 360 d). All factors showed significant influence on activity of POD (three-way ANOVA) (Tables S3 and S4).
Figure 6. Effects of saline stress on the peroxidase (POD) activities in the roots and leaves from the silver maple seedlings exposed to different salinity levels (NaCl or CaCl2) after 14 (A, D), 28 (B, E) and 360 (C, F) days.
Values are mean ± SD (n = 4). Different numbers above each bar indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
Protein content
In the roots watered with 100–120 mM NaCl solution, slight reductions in protein content were recorded in the early phase of the experiment (14 d), while a slight increase was characteristic of the 28 and 360 d. On the other hand, CaCl2 treatment caused an increase in the total protein content at 14, 28 and 360 d. Long-term changes in CaCl2-treated plants were similar to those observed in NaCl-treated plants. The leaves from plants subjected to CaCl2 treatment showed significantly increased protein content at 360 d (Table S1). A three-way ANOVA showed significant influence of all factors on protein content, both in roots and leaves (Tables S3 and S4).
Discussion
Although the reduction in growth parameters were smaller in CaCl2-treated plants than in NaCl-treated plants, its harmful effect was still manifested. Hall, Hofstra & Lumis (1972) showed that increasing salt concentration (changing with distance from a de-iced highway) strongly affected the reduction of plant growth parameters, namely, the weight of buds, needle length and fresh weight, as well as annual radial increments in eastern white pine (Pinus strobus L.). It shows that injury caused by salt-based de-icing agents in woody species not tolerant to salt, such as P. strobus or studied A. saccharinum (Dirr, 1976), is a rather common result of road maintenance. Reduction of growth parameters (shoot length, total leaf area and total DW) was also reported for six olive cultivars treated with a relatively high dose of NaCl (>50 mM) (Chartzoulakis et al., 2002). The authors also indicated that a low dose of salt (25 mM) stimulated olive plant growth. Similar results were observed in our study for CaCl2 treatment (6.7 and 20 mM), while no positive effects of NaCl treatment were observed. Optimal calcium supplementation stimulates growth in many plant species (Fenn & Feagley, 1999). Furthermore, calcium is one of the macronutrients necessary for plants due to its role in cell development and stress response. It is also known to contribute to the physical integrity and functionality of membranes (Hopkins, 1995). It seems that a similar protective effect of low doses of calcium can be observed for silver maple.
It was demonstrated that snowmelting might be a substantial source of chloride ions and could increase the pH of soil, which resulted in its alkalization (Gałuszka et al., 2011). It was also shown that the alkaline pH of soils favored greater bioavailability of boron and reduced bioavailability of zinc for the examined trees. The negative symptoms included loss of photosynthetic activity and decreased vitality. Although in our study perlite was used as substratum (and alkalization was not monitored), such a mode of action is likely in natural conditions and probably enhance negative effect of salinity. Soil salinity causes rapid osmotic stress, which reduces growth of shoots, slows development and accelerates aging of cells in many plant species (Parida & Das, 2005). It is believed that Na+ causes osmotic stress in leaves, affects plant growth (reduces cell expansion and elongation), reduces leaf thickness and disturbs photosynthesis (Tester & Davenport, 2003). Application of salt was showed as agent reducing plant size and the number of leaves of cotton varieties (Gossypium hirsutum L.) (Saleh, 2012). The authors also showed that the number of leaves, chlorophyll a and b content and SPAD (soil plant analyses development) can be used to discriminate between salt-tolerant and salt-sensitive varieties. It was demonstrated that even plants not very susceptible to salinity (six species from the Leguminosae family) reacted to increasing NaCl concentration with a reduction in plant mass (Felker et al., 1981). Another study indicated that citrus (Citrus tangerine Hort. ex Tanaka) seedlings treated with 100 mM NaCl showed a reduction of leaf size, shoot and root length and seedling mass. Furthermore, photosynthesis limitation was observed (Wu, Zou & He, 2010). NaCl treatments (0, 50 and 100 mM) were also shown to decrease chlorophyll a and b contents, CO2 assimilation rate and stomatal conductance in P. cathayana and affected chloroplast functioning (Yang et al., 2009). Described perturbations affecting photosynthesis may have a negative impact on plant primary metabolism and plant growth. Our results showed that the reductions in chlorophyll a content under the influence of NaCl concentrations >60 mM at 14 d coincided with the reduction in RGR, FW and DW accumulation during the experiment, even at 360 d. It can be suggested that lowered chlorophyll content in the early phase of the experiment contributed to disturbances in plant metabolism that could not be fully compensated with time.
It is accepted that all trees are affected by salt stress, but some species are more tolerant than others (Dirr, 1976; Douglas, 2011). Numerous studies showed induced proline accumulation (Munns & Tester, 2008; Aziz, Martin-Tanguy & Larher, 1999; Acosta-Motos et al., 2017) during stress and indicated that it could be a protective mechanism against increased osmotic pressure resulting from salt stress (Hoque, 2008). Previous studies showed a high accumulation of proline in two poplar cultivars under the experimental conditions of salt stress (combined SO2 and NaCl treatment) (Karolewski, 1989). It was also indicated by Marin et al. (2010) that the highest concentration of proline in in vitro-grown roots of selected Prunus species could be observed in groups treated with the highest concentration of NaCl (180 mM), which is similar to the results provided in our study. Proline accumulation in roots may be one of the mechanisms involved in ROS scavenging and may contribute to the enzymatic quenching of ROS (Gill & Tuteja, 2010). This process is mediated by POD and CAT, whose activities increased significantly in salt-treated plants tested in our study at 14 and 28 d, respectively. Furthermore, elevated proline content was shown to protect enzymatic ROS-scavengers such as CAT and POD (Hoque et al., 2007). It can be concluded that coordination of proline accumulation and changing activities of CAT and POD may be treated as an adjustment to salt stress. On the other hand, our results indicate that for silver maple, such a mode of action could not be enough to cope with salt stress, which was manifested in plant growth parameters. Long-term accumulation of proline in leaves was shown in studies on olive (Olea europaea L.) treated with salt (Ben Rouina et al., 2006) and subjected to drought (BenAhmed et al., 2009). The authors suggested a possible beneficial role of proline in improving photosynthetic activity by triggering osmotic adjustment throughout the stress period. Similar results were also shown in our study regarding the coincidental increase in proline and stable chlorophyll contents.
Studies on two poplar species showed increased CAT and SOD activities in leaves and xylem sap of salt-stressed tolerant Euphrates poplar (P. euphratica Oliv.) and sensitive P. popularis ‘35–44’ plants, especially at high salinity levels (up to 250 mM NaCl). Separation of the isoforms of leaf SOD and CAT by polyacrylamide gel electrophoresis revealed that the salt-induced activities of CAT resulted from increased activity of all the detected isoenzymes (Wang et al., 2008). Particularly high SOD and CAT activities were found 18 d after NaCl treatment, which seems to be in agreement with our studies. The authors concluded that P. euphratica plants subjected to saline conditions controlled ROS homeostasis by osmotic control of NaCl-induced ROS production and by rapid upregulation of antioxidant defense to prevent oxidative damage (Wang et al., 2008). In studies on oak, SOD activity increased in young stalks under stress conditions (NaCl treatment). Moreover, the SOD isozyme pattern of oak leaves was altered when compared to the control group (Sehmer, Alaoui-Sosse & Dizengremel, 1995). The effects of different saline water irrigation levels were also studied in olive trees (Olea europaea L.) in which the activities of SOD and CAT in young leaves of salt-treated plants were 2.67 and 1.85 times higher than in the control, respectively. It was concluded that the interaction between the antioxidant defense system and proline content is involved in salt tolerance (BenAhmed et al., 2009). Similar results were shown in a study on physic nut (Jatropha curcas L.) (Silva et al., 2013). The conclusion can be formulated that a similar mechanism is mounted in silver maple subjected to salinity stress and that the activation of SOD is involved in enzymatic antioxidation, especially during intensifying salt stress (>30 mM of NaCl).
We showed that the activity of CAT increased after NaCl and CaCl2 treatment. This increase was marked in the roots at 14 and 28 d after NaCl treatment, while we were not able to detect any greater changes in the leaves during the experiment. In the contrary, studies conducted on salt-resistant brush cherry (Eugenia myrtifolia Sims) watered with saline reclaimed water showed that long-term response (23 weeks) involved increased CAT activity in leaves, but this change seemed not be correlated with salt dose (Acosta-Motos et al., 2017). The authors pointed that the observed CAT changes did not appear to be enough to cope with the stress induced by the long-term exposure to salinity. Based on previous studies of barley (Hordeum vulgare L.) cultivars (Fan et al., 2014), it was concluded that changes in CAT activity in leaves should not be used as a standalone biochemical marker proving salinity tolerance in crop plants. We showed that sequential biochemical testing of roots and leaves accompanied with growth analysis can fulfill the requirements for salt sensitivity testing in trees. CAT was recently suggested as a main factor controlling salt tolerance triggered by H2O2 (Gondim et al., 2012). This is in agreement with experiments conducted on physic nut (Jatropha curcas L.) which is a salt-tolerant species (Gao et al., 2008). It seems that elevated CAT activity in both leaves and roots has a protective effect, while increased activity just in one organ is not enough to trigger adequate response. Surprisingly, in the early stage of our experiments, CAT activity remarkably increased just in the groups treated with the medium NaCl solutions (30 and 60 mM). Increasing concentrations of salt (100 and 120 mM) stopped this effect, which may indicate that plants were not able to cope with severe salt stress. A similar relationship was recorded by Sorkheh et al. (2012) for 8 wild almond species (Prunus spp.) treated with a wide range of NaCl solutions. The gradual increase in CAT activity after 28 d during NaCl-induced stress recorded in the present study is similar to the phenomenon reported before in date palm (Phoenix dactylifera L.) (Al-Qurainy et al., 2017). It is noteworthy that CaCl2 triggered only slight CAT changes in silver maple tissues, which may indicate its lower phytotoxicity.
We observed that POD activity increased dramatically in the roots at 14 d after treatment with NaCl and then decreased with time. Moreover, a positive relationship between POD activity level and salt concentration was observed in both NaCl and CaCl2. The profiles of changes were similar; however, they were markedly more pronounced in the NaCl variant. Some studies indicate that POD is involved in a salinity stress response in Kashgar tamarisk (Tamarix hispida Willd.) and is regulated by ABA-dependent signaling pathways covering salt tolerance in many plant species (Gao et al., 2010). Due to the multifunctional activity of POD, it is believed that its activity is a good marker of plant stress response; however, its role during abiotic stress is still not fully elucidated (Passardi et al., 2005). It was also proposed that peroxidases may play a protective role acting as scavengers of H2O2, which overproduction took place under salt stress (Novo-Uzal et al., 2014). No greater changes in the leaves and roots were observed 360 d after treatment. Therefore, it can be concerned that one year is enough to cope with salt stress and to start recovery. On the other hand, long-lasting stress under urban conditions is severe, and its cyclicality may make the plant unable to cope with it.
Conclusion
It was shown in this study that the plant growth parameters, namely, fresh and dry weight, were reduced by high concentrations of NaCl and CaCl2. Furthermore, the response strictly depended on the salt type and dose. Our experiments indicate the existence of short-term stress-coping reactions (increased enzyme activity coincident with increased proline content under salt stress). Restabilization of long-term biochemical traits and inhibited growth suggest that the studied species can survive de-icing treatments, but subsequent recovery is needed. Overall, this indicates that silver maple seedlings should be considered susceptible to long-lasting severe salt stress. Hence, it is recommended to use silver maple plantings on secondary avenues or park alleys, while planting on often de-iced pavements and roads (e.g., highways) should be avoided. Our study also suggests that CaCl2 shows less toxicity to plants and therefore that its use should be considered.
Supplemental Information
Values are mean ± SD (n = 4). Different lower-case letters indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
n.s. –not significant.
n.s. –not significant.
n.s. –not significant.
Each data point indicates value of relative growth rate [g g−1 FW d−1].
Each data point indicates chlorophyll content [mg g−1FW].
Each data point indicates proline content [mg g−1 FW).
Each data point indicates enzymatic activity [U min−1mg protein−1].
Each data point indicates enzymatic activity [U min−1mg protein−1].
Each data point indicates enzymatic activity [U min−1 mg protein−1].
Each data point indicates value of selected weight parameter [mg plant−1].
Each data point indicates protein content [mg g−1FW].
Acknowledgments
We acknowledge American Journal Experts for English language editing of this article.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jacek Patykowski and Jeremi Kołodziejek conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Mateusz Wala performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Acosta-Motos et al. (2017).Acosta-Motos JR, Hernández JA, Álvarez S, Barba-Espín G, Sánchez-Blanco MJ. The long-term resistance mechanisms, critical irrigation threshold and relief capacity shown by Eugenia myrtifolia plants in response to saline reclaimed water. Plant Physiology and Biochemistry. 2017;111:244–256. doi: 10.1016/j.plaphy.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Al-Qurainy et al. (2017).Al-Qurainy F, Khan S, Nadeem M, Tarroum M. Antioxidant system response and cDNA-SCoT marker profiling in Phoenix dactylifera L. International Journal of Genomics. 2017 doi: 10.1155/2017/1537538. Article 1537538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon (1949).Arnon DI. Copper enzymes in isolated chloroplast. Polyphenoloxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf et al. (1994).Ashraf MY, Azmi AR, Khan AH, Ala SA. Effect of water stress on total phenols, peroxides activity and chlorophyll content in wheat (Triticum aestivum L.) Acta Physiologiae Plantarum. 1994;16:1–18. [Google Scholar]
- Aziz, Martin-Tanguy & Larher (1999).Aziz A, Martin-Tanguy J, Larher F. Salt stress-induced proline accumulation and changes in tyramine and poliamine levels are linked to ionic adjustment in tomato leaf discs. Plant Science. 1999;1:83–91. doi: 10.1016/S0168-9452(99)00071-0. [DOI] [Google Scholar]
- Bates, Waldren & Teare (1973).Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant and Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp & Fridovich (1971).Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Ben-Ahmed et al. (2009).Ben-Ahmed C, Ben Rouina B, Ben Sensoy S, Boukhris M, Ben Abdalla F. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environmental and Experimental Botany. 2009;67:345–352. doi: 10.1016/j.envexpbot.2009.07.006. [DOI] [Google Scholar]
- Ben Rouina et al. (2006).Ben Rouina B, Ben Ahmed C, Athar HUR, Boukhriss M. Water relations, proline accumulation and photosynthetic activity in olive tree (Olea europaea L. cv ‘Chemlali’) in response to salt stress. Pakistan Journal of Botany. 2006;38:1397–1406. [Google Scholar]
- Bradford (1976).Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryson & Barker (2002).Bryson MG, Barker AV. Sodium accumulation in soils and plants along Massachusetts roadsides. Communications in Soil Science and Plant Analysis. 2002;33:67–78. doi: 10.1081/CSS-120002378. [DOI] [Google Scholar]
- Buschbom (1968).Buschbom U. Salt resistance of aerial shoots of woody plants: 1. Effects of chlorides on shoot surfaces. Flora. 1968;157:527–561. [Google Scholar]
- Chartzoulakis et al. (2002).Chartzoulakis K, Loupassaki M, Bertaki M, Androulakis I. Effects of NaCl salinity on growth, ion content and CO2 assimilation rate of six olive cultivars. Scientia Horticultura. 2002;96:235–247. doi: 10.1016/S0304-4238(02)00067-5. [DOI] [Google Scholar]
- Choudhary, Sairam & Tyagi (2005).Choudhary NL, Sairam RK, Tyagi A. Expression of delta(1)-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.) Indian Journal of Biochemistry and Biophysics. 2005;42:366–370. [PubMed] [Google Scholar]
- Cunningham et al. (2008).Cunningham MA, Snyder E, Yonkin D, Ross M, Elsen T. Accumulation of deicing salts in soils in an urban environment. Urban Ecosystems. 2008;11:17–31. doi: 10.1007/s11252-007-0031-x. [DOI] [Google Scholar]
- Czarna (2013).Czarna M. Overview of chemicals applied to winter road maintenance in Poland. Quarterly of Environmental Engineering and Design. 2013;151:18–25. [Google Scholar]
- Czerniawska-Kusza, Kusza & Duzyński (2004).Czerniawska-Kusza I, Kusza G, Duzyński M. Effect of de-icing salts on urban soils and health status of roadside trees in the Opole region. Environmental Toxicology. 2004;19:296–301. doi: 10.1002/tox.20037. [DOI] [PubMed] [Google Scholar]
- Davison (1971).Davison AW. The effects of de-icing salt on roadside verges. 1. Soil and plant analysis. Journal of Applied Ecology. 1971;8:555–561. doi: 10.2307/2402891. [DOI] [Google Scholar]
- Day, Seiler & Persaud (2000).Day SD, Seiler JR, Persaud N. A comparison of root growth dynamics of silver maple and flowering dogwood in compacted soil at differing soil water contents. Tree Physiology. 2000;20:257–263. doi: 10.1093/treephys/20.4.257. [DOI] [PubMed] [Google Scholar]
- Delauney & Verma (1993).Delauney AJ, Verma DPS. Proline biosynthesis and osmoregulation in plants. Plant Journal. 1993;4:215–223. doi: 10.1046/j.1365-313X.1993.04020215.x. [DOI] [Google Scholar]
- Dhindsa, Plumb-Dhindsa & Thorpe (1981).Dhindsa RS, Plumb-Dhindsa P, Thorpe TA. Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Dirr (1976).Dirr MA. Selection of trees for tolerance to salt injury. Journal of Arboriculture. 1976;2:209–216. [Google Scholar]
- Dmuchowski, Baczewska & Bra̧goszewska (2011).Dmuchowski W, Baczewska AH, Bra̧goszewska P. Reaction of street trees to adverse environmental conditions in the centre of Warsaw. Ecological Questions. 2011;15:97–105. doi: 10.12775/v10090-011-0041-4. [DOI] [Google Scholar]
- Dmuchowski, Brogowski & Baczewska (2011).Dmuchowski W, Brogowski Z, Baczewska AH. Evaluation of vigour and health of ‘street’ trees in Warsaw using the foliar ionic status. Polish Journal of Environmental Studies. 2011;20:489–496. [Google Scholar]
- Douglas (2011).Douglas SM. De-icing salts: damage to woody ornamentals. 2011. http://www.ct.gov/caes/lib/caes/documents/publications/fact_sheets/plant_pathology_and_ecology/de-icing_salts-_damage_to_woody_ornamentals_05-13-11.pdf. [24 July 2018]. http://www.ct.gov/caes/lib/caes/documents/publications/fact_sheets/plant_pathology_and_ecology/de-icing_salts-_damage_to_woody_ornamentals_05-13-11.pdf
- Fan et al. (2014).Fan Y, Zhu M, Shabala S, Li CD, Johnson P, Zhou MX. Antioxidant activity in salt-stressed barley leaves: evaluating time- and age-dependence and suitability for the use as a biochemical marker in breeding programs. Journal of Agronomy and Crop Science. 2014;200:261–272. doi: 10.1111/jac.12068. [DOI] [Google Scholar]
- Felker et al. (1981).Felker P, Clark PR, Laag AE, Pratt PF. Salinity tolerance of the tree legumes: Mesquite (Prosopis glandulosa var. torreyana, P. velutina and P. articulata) Algarrobo (P. chilensis), Kiawe (P. pallida) and Tamarugo (P. tamarugo) grown in sand culture on nitrogen-free media. Plant and Soil. 1981;61:311–317. doi: 10.1007/BF02182012. [DOI] [Google Scholar]
- Fenn & Feagley (1999).Fenn LB, Feagley S. Review of beneficial uses of calcium and ammonium salts for stimulating plant growth and metabolite translocation. Communications in Soil Science and Plant Analysis. 1999;30:2627–2641. doi: 10.1080/00103629909370401. [DOI] [Google Scholar]
- Gałuszka et al. (2011).Gałuszka A, Migaszewski ZM, Podlaski R, Dołęgowska S, Michalik A. The influence of chloride de-icers on mineral nutrition and the health status of roadside trees in the city of Kielce, Poland. Environmental Monitoring and Assessment. 2011;176:451–464. doi: 10.1007/s10661-010-1596-z. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2010).Gao C, Wang Y, Liu G, Wang C, Jiang J, Yang C. Cloning of ten peroxidase (POD) genes from Tamarix hispida and characterization of their responses to abiotic stress. Plant Molecular Biology Reporter. 2010;28:77–89. doi: 10.1007/s11105-009-0129-9. [DOI] [Google Scholar]
- Gao et al. (2008).Gao S, Ouyang C, Wang S, Xu Y, Tang L, Chen F. Effects of salt stress on growth, antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L. seedlings. Plant, Soil and Environment. 2008;54:374–381. doi: 10.17221/410-PSE. [DOI] [Google Scholar]
- Gill & Tuteja (2010).Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gondim et al. (2012).Gondim FA, Gomes-Filho E, Costa JH, Mendes Alencar NL, Prisco JT. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiology and Biochemistry. 2012;56:62–71. doi: 10.1016/j.plaphy.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Hall, Hofstra & Lumis (1972).Hall R, Hofstra G, Lumis GP. Effects of deicing salt on eastern white pine: foliar injury, growth suppression and seasonal changes in foliar concentrations of sodium and chloride. Canadian Journal of Forest Research. 1972;2:244–249. doi: 10.1139/x72-040. [DOI] [Google Scholar]
- Hoagland & Arnon (1950).Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circular 347, University of California, Agricultural Experimental Station; Berkley: 1950. [Google Scholar]
- Hofstra, Hall & Lumis (1979).Hofstra G, Hall R, Lumis GP. Studies of salt-induced damage to roadside plants in Ontario. Journal of Arboriculture. 1979;5:25–31. [Google Scholar]
- Hopkins (1995).Hopkins WG. Introduction to plant physiology. 2nd ed Wiley; New York: 1995. [Google Scholar]
- Hoque et al. (2007).Hoque A, Okuma E, Banu MN, Nakamura Y, Shimoishi Y, Murata Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. Journal of Plant Physiology. 2007;164:553–561. doi: 10.1016/j.jplph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hoque (2008).Hoque MA. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification sy shoot and reduce NaCl-induced damage in cultured tobacco cell. Journal of Plant Physiology. 2008;165:813–825. doi: 10.1016/j.jplph.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Karolewski (1989).Karolewski P. Free proline content and susceptibility of poplar (Populus) cuttings to the action of SO2, NaCl and PEG at different temperatures. Environmental Pollution. 1989;57:307–315. doi: 10.1016/0269-7491(89)90086-9. [DOI] [PubMed] [Google Scholar]
- Khan, Unagr & Showater (2000).Khan MA, Unagr IA, Showater AM. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Annals of Botany. 2000;85:225–232. doi: 10.1006/anbo.1999.1022. [DOI] [Google Scholar]
- Kozłowski (1997).Kozłowski TT. Responses of woody plants to flooding and salinity. Tree Physiology. 1997;17:1–9. doi: 10.1093/treephys/17.7.490. [DOI] [Google Scholar]
- Langille (1976).Langille AR. One season’s salt accumulation in soil and trees adjacent to a highway. HortScience. 1976;11:575–576. [Google Scholar]
- Lee et al. (2007).Lee Y-P, Kim S-H, Bang J-W, Lee H-S, Kwak S-S, Kwon S-Y. Enchanced tolerance to oxidatibe stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Reports. 2007;26:591–598. doi: 10.1007/s00299-006-0253-z. [DOI] [PubMed] [Google Scholar]
- Levitt (1980).Levitt J. Responses of plants to environmental stresses. II. Water, radiation, salt, and other stresses. Academic Press; New York: 1980. [Google Scholar]
- Maas (1985).Maas EV. Crop tolerance to saline sprinkling water. Plant and Soil. 1985;89:273–284. doi: 10.1007/978-94-009-5111-2_18. [DOI] [Google Scholar]
- Maehly & Chance (1954).Maehly AC, Chance B. The assay of catalases and peroxidases. In: Glick D, editor. Methods of biochemical analysis. Interscience Publishers, Inc; New York: 1954. pp. 357–425. [DOI] [PubMed] [Google Scholar]
- Mansour (1998).Mansour MMF. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiology and Biochemistry. 1998;36:767–72. doi: 10.1016/S0981-9428(98)80028-4. [DOI] [Google Scholar]
- Marin et al. (2010).Marin JA, Andreu P, Carrasco A, Arbeloa A. Determination of proline concentration, an abiotic stress marker, in root exudates of excised root cultures of fruit tree rootstocks under salt stress. Revue des Régions Arides—Numérospécial. 2010;24:722–727. [Google Scholar]
- Mekdaschi et al. (1988).Mekdaschi R, Horlacher D, Schulz R, Marschner H. Streusalzschäden und Sanierungsmassnahmenzur Verminderung der Streusalzbelastung von Strassenbäumrn in Stuttgart, Angew. Botanik. 1988;62:355–371. [Google Scholar]
- Miller et al. (2010).Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell and Environment. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Munns & Tester (2008).Munns R, Tester M. Mechanisms of salt tolerance. Annual Review in Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Naidu et al. (1991).Naidu BP, Paleg LG, Aspinall D, Jennings AC, Jones GP. Amino-acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry. 1991;30:407–409. doi: 10.1016/0031-9422(91)83693-F. [DOI] [Google Scholar]
- Nixon (2008).Nixon WA. Economics of using calcium chloride vs. sodium chloride for deicing/anti-Icing. 2008. http://publications.iowa.gov/20047/ [22 July 2018]. http://publications.iowa.gov/20047/
- Novo-Uzal et al. (2014).Novo-Uzal E, Gutiérrez J, Martínez-Cortés T, Pomar F. Molecular cloning of two novel peroxidases and their response to salt stress and salicylic acid in the living fossil Ginkgo biloba. Annals of Botany. 2014;114:923–936. doi: 10.1093/aob/mcu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida & Das (2005).Parida AK, Das B. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety. 2005;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Passardi et al. (2005).Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Paul, Rocher & Impens (1987).Paul R, Rocher M, Impens R. Influence of winter de-icing with CaCl2 on Sorbus, Acer, Tilia, and Platanus. Science of Total Environment. 1987;59:277–282. doi: 10.1016/0048-9697(87)90449-9. [DOI] [Google Scholar]
- Peng, Lu & Verma (1997).Peng Z, Lu Q, Verma DPS. Reciprocal regulation of delta-1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes control levels during and after osmotic stress in plants. Molecular and General Genetics. 1997;253:334–341. doi: 10.1007/PL00008600. [DOI] [PubMed] [Google Scholar]
- Poland Ministry of the Environment (2013).Poland Ministry of the Environment Act of 16 April 2004 on Nature Conservation (cons. text) 2013. [24 July 2018]. Journal of Laws of 2018, item 142. https://www.infor.pl/akt-prawny/672349,ustawa-o-ochronie-przyrody.html .
- Poland Ministry of the Environment (2014).Poland Ministry of the Environment Regulation of the Minister of the Environment of 09 October 2014 on species protection of plants. 2014. Journal of Laws of 2014, item 1409.
- Ramakrishna & Viraraghavan (2005).Ramakrishna DM, Viraraghavan T. Environmental impact of chemical deicers—a review. Water, Air and Soil Pollution. 2005;166:49–63. doi: 10.1007/s11270-005-8265-9. [DOI] [Google Scholar]
- Raveh & Levy (2005).Raveh E, Levy Y. Analysis of xylem water as an indicator of current chloride uptake status in citrus tree. Scientia Horticulturae. 2005;103:317–327. doi: 10.1016/j.scienta.2004.06.007. [DOI] [Google Scholar]
- Saeki et al. (2011).Saeki I, Dick CW, Barnes BV, Murakami N. Comparative phylogeography of red maple (Acer rubrum L.) and silver maple (Acer saccharinum L.): impacts of habitat specialization, hybridization and glacial history. Journal of Biogeography. 2011;38:992–1005. doi: 10.1111/j.1365-2699.2010.02462.x. [DOI] [Google Scholar]
- Saleh (2012).Saleh B. Effect of salt stress on growth and chlorophyll content of some cultivated cotton varieties grown in Syria. Communications in Soil Science and Plant Analysis. 2012;43:1976–1983. doi: 10.1080/00103624.2012.693229. [DOI] [Google Scholar]
- Sehmer, Alaoui-Sosse & Dizengremel (1995).Sehmer L, Alaoui-Sosse B, Dizengremel P. Effect of salt stress on growth and on the detoxifying pathway of pedunculate oak seedlings (Quercus robur L.) Journal of Plant Physiology. 1995;147:144–151. doi: 10.1016/S0176-1617(11)81427-6. [DOI] [Google Scholar]
- Silva et al. (2013).Silva EN, Vieira SA, Ribeiro RV, Ponte LFA, Ferreira-Silva SL, Silveira JAG. Contrasting physiological responses of Jatropha curcas plants to single and combined stresses of salinity and heat. Journal of Plant Growth Regulation. 2013;32:159–169. doi: 10.1007/s00344-012-9287-3. [DOI] [Google Scholar]
- Simini & Leone (1986).Simini M, Leone IA. Studies on the effects of de-icing salts on roadside trees. Arboric Journal. 1986;10:221–231. doi: 10.1080/03071375.1986.9746754. [DOI] [Google Scholar]
- Siripornadulsil et al. (2002).Siripornadulsil S, Traina S, Verma DP, Sayre RT. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. The Plant Cell. 2002;14:2837–2847. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkheh et al. (2012).Sorkheh K, Shiran B, Khodambashi M, Rouhi V, Mosavei S, Sofo A. Exogenous proline alleviates the effects of H2O2 induced oxidative stress in wild almond species. Russian Journal of Plant Physiology. 2012;59:788–798. doi: 10.1134/S1021443712060167. [DOI] [Google Scholar]
- Soshinkova et al. (2013).Soshinkova TN, Radyukina NL, Korolkova DV, Nosov AV. Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russian Journal of Plant. Physiology. 2013;60:41–54. doi: 10.1134/S1021443713010093. [DOI] [Google Scholar]
- Sucoff, Hong & Wood (1976).Sucoff E, Hong SG, Wood A. NaCl and twig dieback along highways and cold hardiness of highway versus garden twigs. Canadian Journal of Botany. 1976;54:2268–2274. doi: 10.1139/b76-243. [DOI] [Google Scholar]
- Székely et al. (2008).Székely G, Abrahám E, Cséplo A, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik J, Schmelzer E, Koncz C, Szabados L. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant Journal. 2008;53:11–28. doi: 10.1111/j.1365-313X.2007.03318.x. [DOI] [PubMed] [Google Scholar]
- Tester & Davenport (2003).Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkova, Papadantonakis & Savvas (2006).Trajkova F, Papadantonakis N, Savvas D. Comparative effects of NaCl and CaCl2 salinity on cucumber grown in a closed hydroponic system. HortScience. 2006;41:437–441. [Google Scholar]
- Wang et al. (2008).Wang R, Chen S, Zhou X, Shen X, Deng L, Zhu H, Shao J, Shi Y, Dai S, Fritz E, Hüttermann A, Polle A. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiology. 2008;28:947–957. doi: 10.1093/treephys/28.6.947. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2010).Wang YC, Qu GZ, Li HY, Wu YJ, Wang C, Liu GF, Yang CP. Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Molecular Biology Reports. 2010;37 doi: 10.1007/s11033-009-9884-9. Article 1119. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2005).Wang Y, Wisniewski M, Meilan R, Cui M, Webb R, Fuchigami L. Overexpression of cytosolic ascorbate peroxidase in tomato confers tolerance to chilling and salt stress. Journal of the American Society for Horticultural Science. 2005;130:167–173. [Google Scholar]
- White & Broadley (2001).White PJ, Broadley MR. Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany. 2001;88:967–988. doi: 10.1006/anbo.2001.1540. [DOI] [Google Scholar]
- Wu, Zou & He (2010).Wu QS, Zou YN, He XH. Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiologiae Plantarum. 2010;32:297–304. doi: 10.1007/s11738-009-0407-z. [DOI] [Google Scholar]
- Yang et al. (2009).Yang F, Xiao X, Zhang S, Korpelainen H, Li C. Salt stress responses in Populus cathayana Rehder. Plant Science. 2009;176:669–677. doi: 10.1016/j.plantsci.2009.02.008. [DOI] [Google Scholar]
- Yang, Lan & Gong (2009).Yang S-L, Lan S-S, Gong M. Hydrogen peroxide induced proline and metabolic pathway of its accumulation in maize seedlings. Journal of Plant Physiology. 2009;166:1694–1699. doi: 10.1016/j.jplph.2009.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are mean ± SD (n = 4). Different lower-case letters indicate significant differences by ANOVA followed by Tukey’s test at P < 0.05.
n.s. –not significant.
n.s. –not significant.
n.s. –not significant.
Each data point indicates value of relative growth rate [g g−1 FW d−1].
Each data point indicates chlorophyll content [mg g−1FW].
Each data point indicates proline content [mg g−1 FW).
Each data point indicates enzymatic activity [U min−1mg protein−1].
Each data point indicates enzymatic activity [U min−1mg protein−1].
Each data point indicates enzymatic activity [U min−1 mg protein−1].
Each data point indicates value of selected weight parameter [mg plant−1].
Each data point indicates protein content [mg g−1FW].
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.